Primary Production in the Kara, Laptev, and East Siberian Seas
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources, Study Area, and Samplings
2.2. Freshwater Content
2.3. Chlorophyll a (chl-a) Concentration Measurement
2.4. In-Situ Primary Production Experiments
3. Results
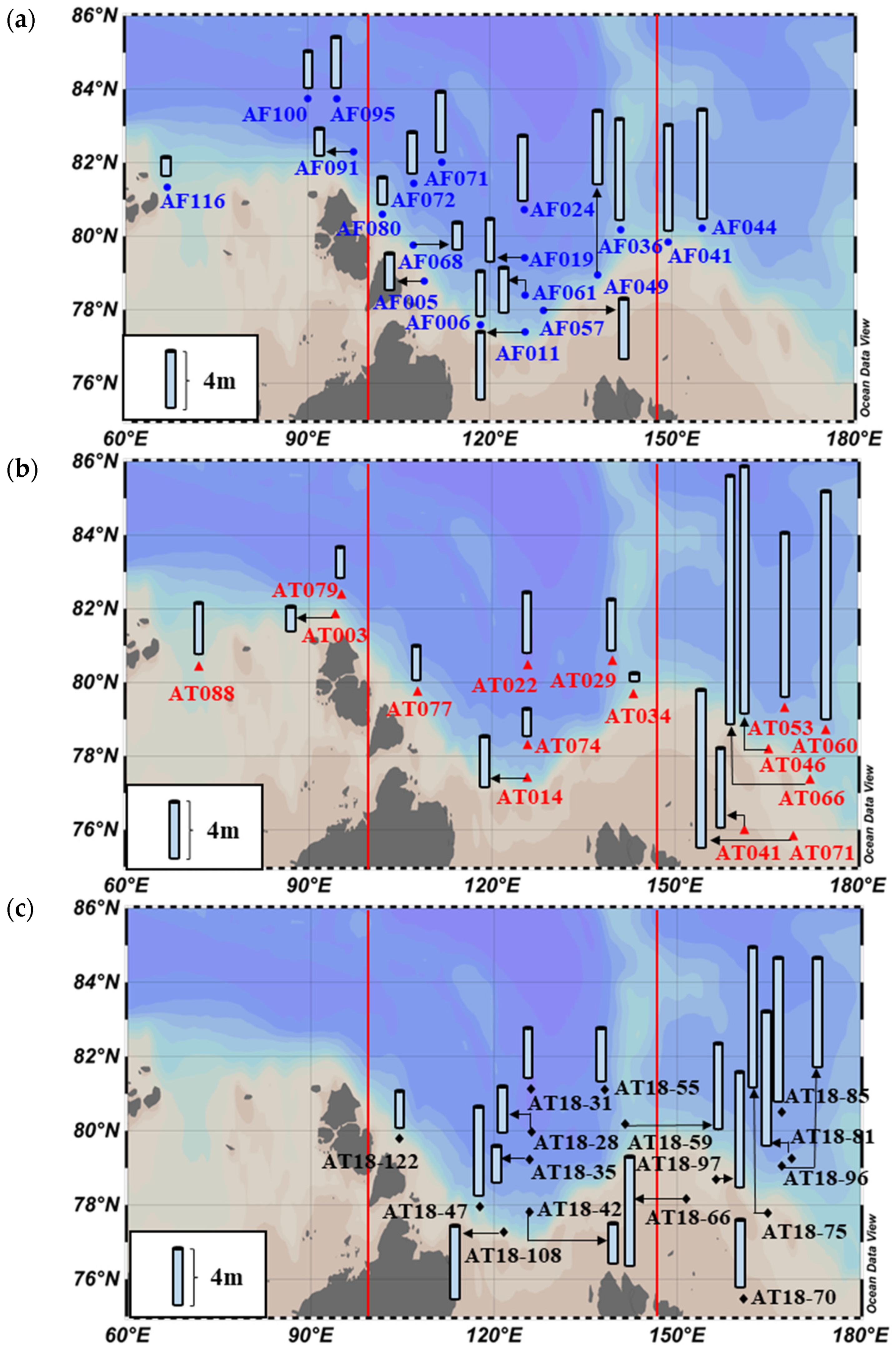
3.1. Hydrographic Environmental Conditions
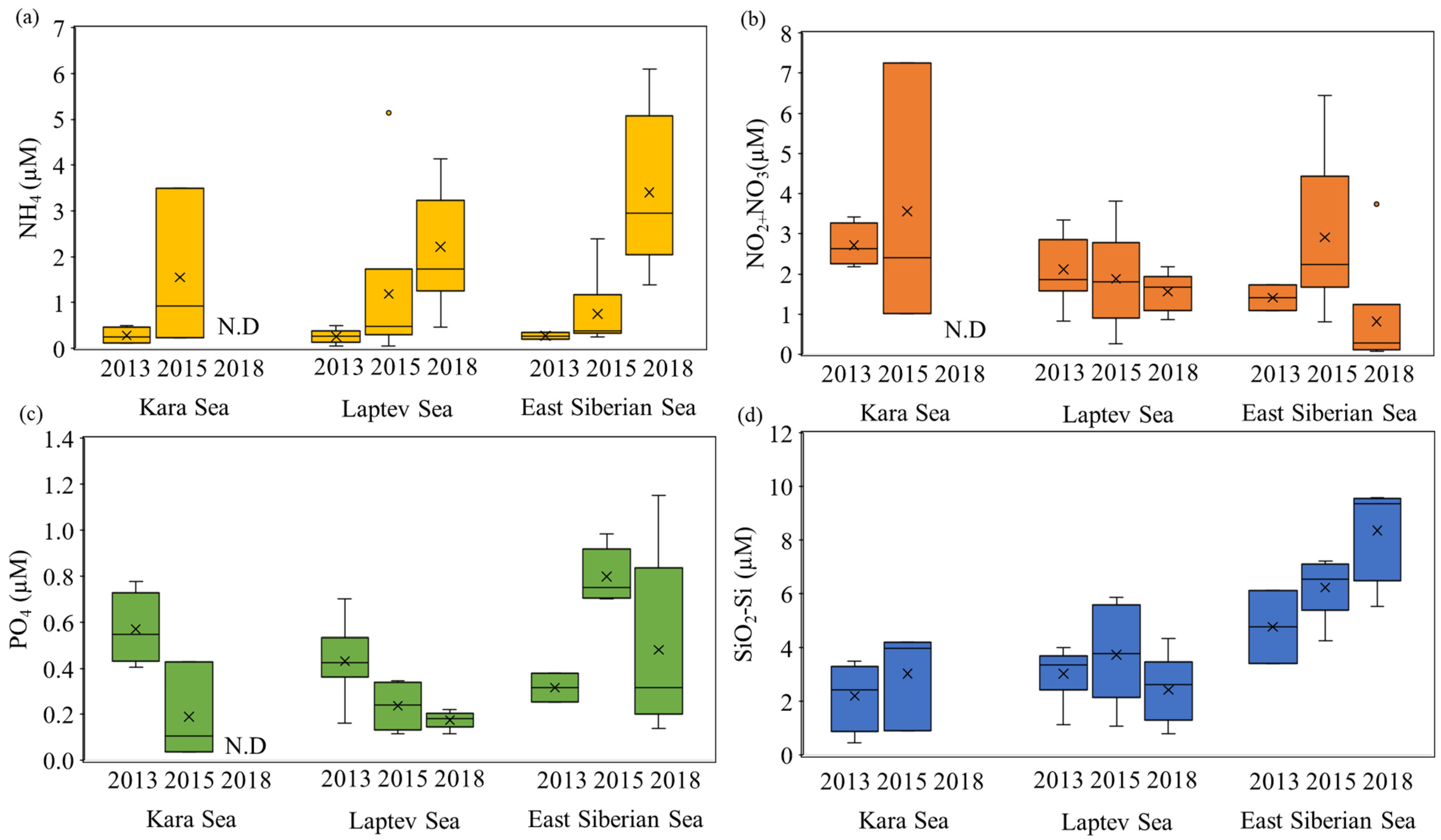
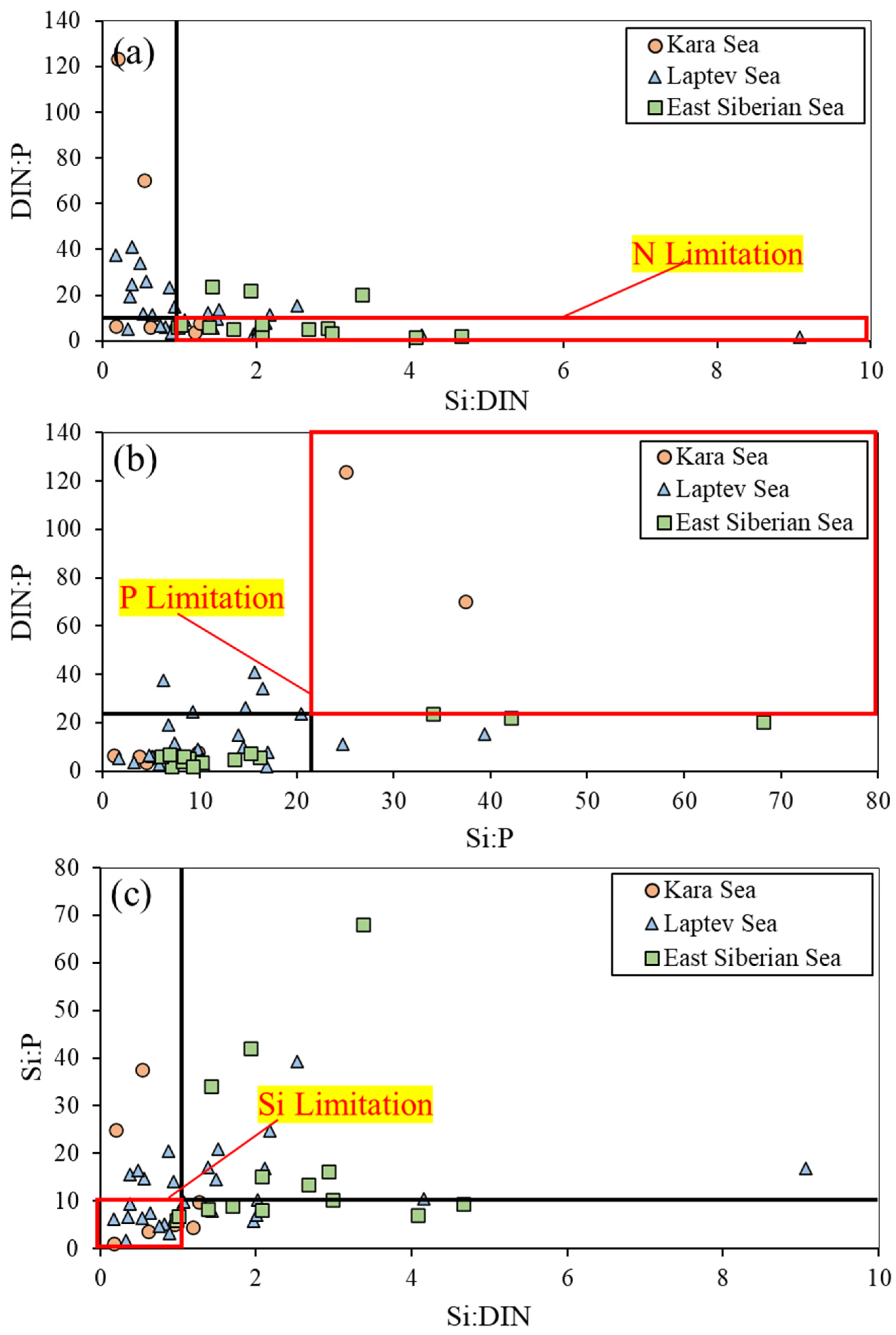
3.2. Nutrient Concentrations
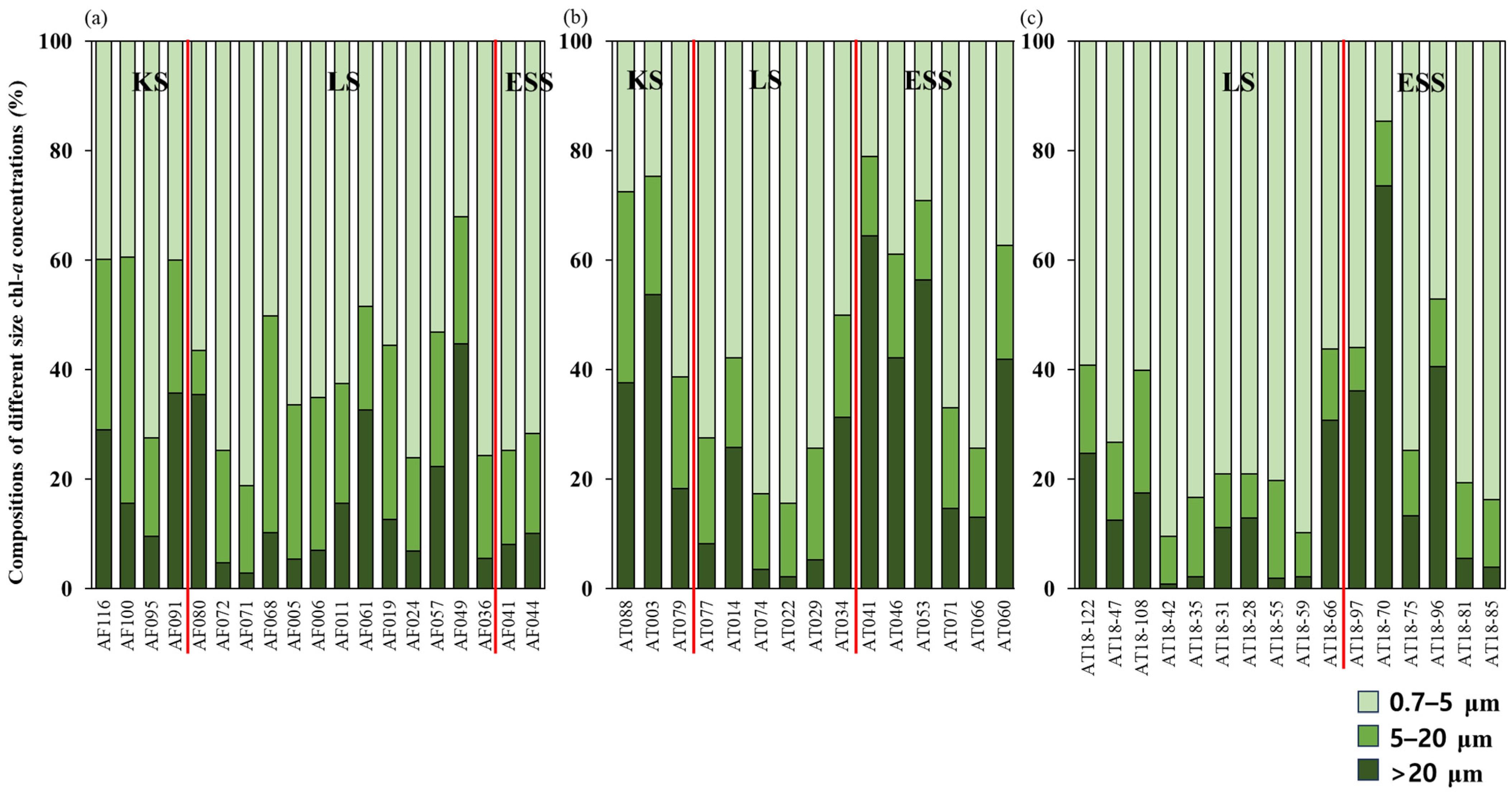
3.3. Chlorophyll-a Concentrations
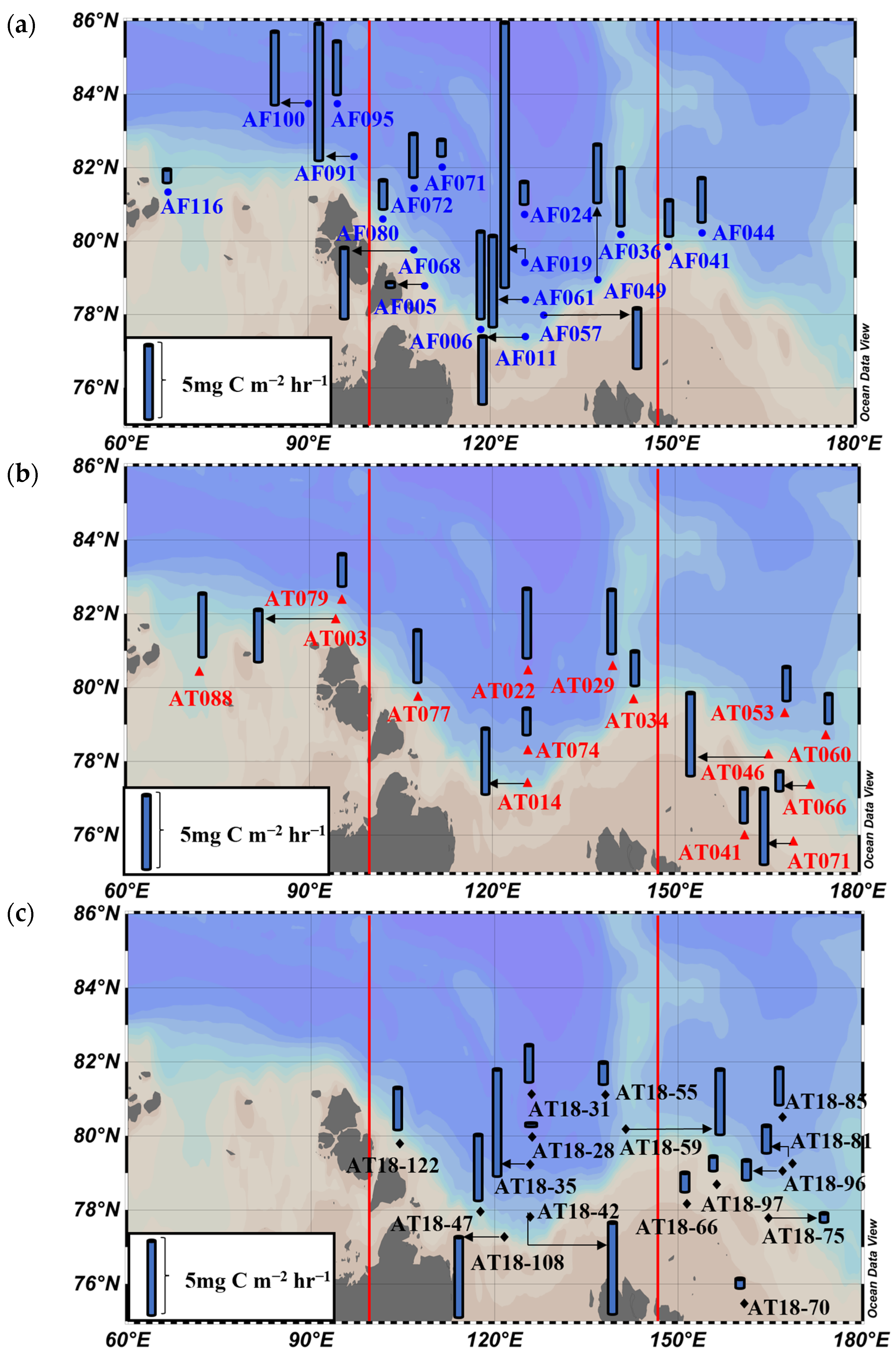
3.4. Primary Production Rates
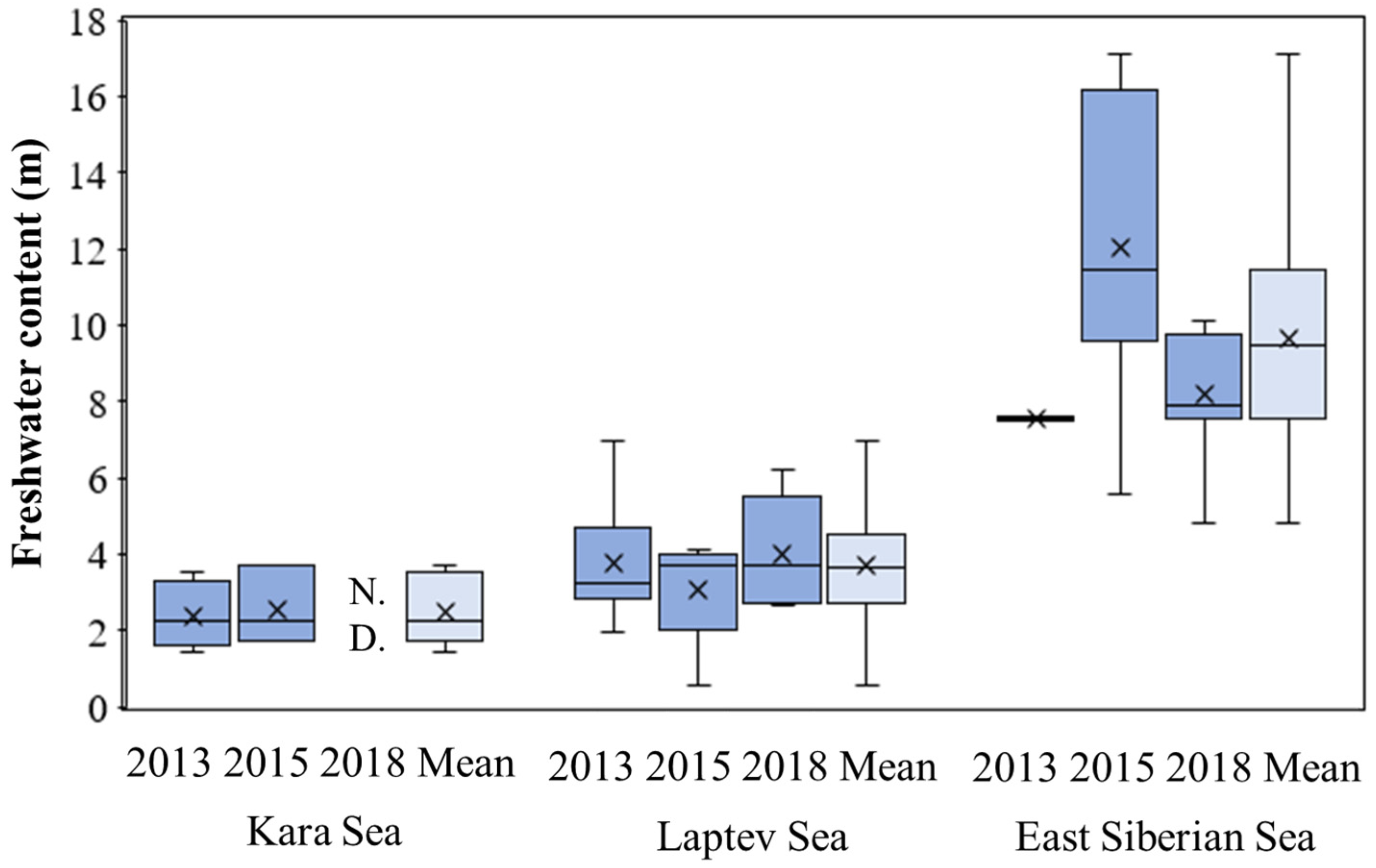
3.5. Freshwater Content and Freshwater Inventory
4. Discussion
4.1. Interannual Variations in Primary Production Rates among the Three Seas
4.2. Comparisons of Primary Production Rates between This and Previous Studies
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Codispoti, L.; Kelly, V.; Thessen, A.; Matrai, P.; Suttles, S.; Hill, V.; Steele, M.; Light, B. Synthesis of primary production in the Arctic Ocean: III: Nitrate and phosphate based estimates of net community production. Prog. Oceanogr. 2013, 110, 126–150. [Google Scholar] [CrossRef]
- Ji, R.; Jin, M.; Varpe, Ø. Sea ice phenology and timing of primary production pulses in the Arctic Ocean. Glob. Chang. Biol. 2013, 19, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Crawford, A.D.; Krumhardt, K.M.; Lovenduski, N.S.; van Dijken, G.L.; Arrigo, K.R. Summer high-wind events and phytoplankton productivity in the Arctic Ocean. J. Geophys. Res. Oceans 2020, 125, e2020JC016565. [Google Scholar] [CrossRef]
- Hays, G.C.; Richardson, A.J.; Robinson, C. Climate change and marine plankton. Trends Ecol. Evol. 2005, 20, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Pörtner, H.; Karl, D.; Boyd, P.; Cheung, W.; Lluch-Cota, S.; Nojiri, Y.; Schmidt, D.; Zavialov, P.; Alheit, J.; Aristegui, J. Climate Change 2014: Impacts, Adaptation, and Vulnerability—Part A: Global and Sectoral Aspects; Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014; pp. 411–484. [Google Scholar]
- Niebauer, H.; Alexander, V.; Henrichs, S. Physical and biological oceanographic interaction in the spring bloom at the Bering Sea marginal ice edge zone. J. Geophys. Res. Oceans 1990, 95, 22229–22241. [Google Scholar] [CrossRef]
- Carmack, E.C.; Aagaard, K.; Swift, J.H.; MacDonald, R.W.; McLaughlin, F.A.; Jones, E.P.; Perkin, R.G.; Smith, J.N.; Ellis, K.M.; Killius, L.R. Changes in temperature and tracer distributions within the Arctic Ocean: Results from the 1994 Arctic Ocean section. Deep Sea Res. Part II Top. Stud. Oceanogr. 1997, 44, 1487–1502. [Google Scholar] [CrossRef]
- Hill, V.J.; Cota, G. Spatial patterns of primary production on the shelf, slope and basin of the Western Arctic in 2002. Deep Sea Res. Part II Top. Stud. Oceanogr. 2005, 52, 3344–3354. [Google Scholar] [CrossRef]
- Popova, E.; Yool, A.; Coward, A.; Aksenov, Y.; Alderson, S.; De Cuevas, B.; Anderson, T. Control of primary production in the Arctic by nutrients and light: Insights from a high resolution ocean general circulation model. Biogeosciences 2010, 7, 3569–3591. [Google Scholar] [CrossRef]
- Hill, V.J.; Matrai, P.A.; Olson, E.; Suttles, S.; Steele, M.; Codispoti, L.A.; Zimmerman, R.C. Synthesis of integrated primary production in the Arctic Ocean: II. In situ and remotely sensed estimates. Prog. Oceanogr. 2013, 110, 107–125. [Google Scholar] [CrossRef]
- Christensen, J.H.; Boberg, F.; Christensen, O.B.; Lucas-Picher, P. On the need for bias correction of regional climate change projections of temperature and precipitation. Geophys. Res. Lett. 2008, 35, L20709. [Google Scholar] [CrossRef]
- Najafi, M.R.; Zwiers, F.W.; Gillett, N.P. Attribution of Arctic temperature change to greenhouse-gas and aerosol influences. Nat. Clim. Chang. 2015, 5, 246–249. [Google Scholar] [CrossRef]
- Rothrock, D.; Zhang, J. Arctic Ocean sea ice volume: What explains its recent depletion? J. Geophys. Res. Oceans 2005, 110, C01002. [Google Scholar] [CrossRef]
- Kwok, R.; Cunningham, G.; Wensnahan, M.; Rigor, I.; Zwally, H.; Yi, D. Thinning and volume loss of the Arctic Ocean sea ice cover: 2003–2008. J. Geophys. Res. Oceans 2009, 114, C07005. [Google Scholar] [CrossRef]
- Kwok, R.; Rothrock, D. Decline in Arctic sea ice thickness from submarine and ICESat records: 1958–2008. Geophys. Res. Lett. 2009, 36, L15501. [Google Scholar] [CrossRef]
- McPhee, M.; Proshutinsky, A.; Morison, J.; Steele, M.; Alkire, M. Rapid change in freshwater content of the Arctic Ocean. Geophys. Res. Lett. 2009, 36, L10602. [Google Scholar] [CrossRef]
- Coupel, P.; Ruiz-Pino, D.; Sicre, M.-A.; Chen, J.; Lee, S.; Schiffrine, N.; Li, H.; Gascard, J.-C. The impact of freshening on phytoplankton production in the Pacific Arctic Ocean. Prog. Oceanogr. 2015, 131, 113–125. [Google Scholar] [CrossRef]
- Carmack, E.C.; Yamamoto-Kawai, M.; Haine, T.W.; Bacon, S.; Bluhm, B.A.; Lique, C.; Melling, H.; Polyakov, I.V.; Straneo, F.; Timmermans, M.L. Freshwater and its role in the Arctic Marine System: Sources, disposition, storage, export, and physical and biogeochemical consequences in the Arctic and global oceans. J. Geophys. Res. Biogeosci. 2016, 121, 675–717. [Google Scholar] [CrossRef]
- Perovich, D.K.; Richter-Menge, J.A. Loss of sea ice in the Arctic. Ann. Rev. Mar. Sci. 2009, 1, 417–441. [Google Scholar] [CrossRef]
- Arrigo, K.R.; van Dijken, G.L. Secular trends in Arctic Ocean net primary production. J. Geophys. Res. Oceans 2011, 116, C09011. [Google Scholar] [CrossRef]
- Lannuzel, D.; Tedesco, L.; Van Leeuwe, M.; Campbell, K.; Flores, H.; Delille, B.; Miller, L.; Stefels, J.; Assmy, P.; Bowman, J. The future of Arctic sea-ice biogeochemistry and ice-associated ecosystems. Nat. Clim. Chang. 2020, 10, 983–992. [Google Scholar] [CrossRef]
- Jones, E.P.; Anderson, L.G.; Jutterström, S.; Mintrop, L.; Swift, J.H. Pacific freshwater, river water and sea ice meltwater across Arctic Ocean basins: Results from the 2005 Beringia Expedition. J. Geophys. Res. Oceans 2008, 113, 1–10. [Google Scholar] [CrossRef]
- Yun, M.S.; Whitledge, T.E.; Kong, M.; Lee, S.H. Low primary production in the Chukchi Sea shelf, 2009. Cont. Shelf Res. 2014, 76, 1–11. [Google Scholar] [CrossRef]
- Yun, M.S.; Whitledge, T.E.; Stockwell, D.; Son, S.; Lee, J.; Park, J.; Lee, D.; Park, J.; Lee, S. Primary production in the Chukchi Sea with potential effects of freshwater content. Biogeosciences 2016, 13, 737–749. [Google Scholar] [CrossRef]
- Yamamoto-Kawai, M.; McLaughlin, F.; Carmack, E.; Nishino, S.; Shimada, K.; Kurita, N. Surface freshening of the Canada Basin, 2003–2007: River runoff versus sea ice meltwater. J. Geophys. Res. Oceans 2009, 114, C00A05. [Google Scholar] [CrossRef]
- Farmer, J.R.; Sigman, D.M.; Granger, J.; Underwood, O.M.; Fripiat, F.; Cronin, T.M.; Martínez-García, A.; Haug, G.H. Arctic Ocean stratification set by sea level and freshwater inputs since the last ice age. Nat. Geosci. 2021, 14, 684–689. [Google Scholar] [CrossRef]
- McLaughlin, F.A.; Carmack, E.C. Deepening of the nutricline and chlorophyll maximum in the Canada Basin interior, 2003–2009. Geophys. Res. Lett. 2010, 37, L24602. [Google Scholar] [CrossRef]
- Slagstad, D.; Ellingsen, I.; Wassmann, P. Evaluating primary and secondary production in an Arctic Ocean void of summer sea ice: An experimental simulation approach. Prog. Oceanogr. 2011, 90, 117–131. [Google Scholar] [CrossRef]
- Semiletov, I.P.; Dudarev, O.; Luchin, V.; Charkin, A.; Shin, K.H.; Tanaka, N. The East Siberian Sea as a transition zone between Pacific-derived waters and Arctic shelf waters. Geophys. Res. Lett. 2005, 32, L10614. [Google Scholar] [CrossRef]
- Anderson, L.G.; Jutterström, S.; Hjalmarsson, S.; Wåhlström, I.; Semiletov, I.P. Out-gassing of CO2 from Siberian Shelf seas by terrestrial organic matter decomposition. Geophys. Res. Lett. 2009, 36, L20601. [Google Scholar] [CrossRef]
- Lee, S.H.; Joo, H.M.; Liu, Z.; Chen, J.; He, J. Phytoplankton productivity in newly opened waters of the Western Arctic Ocean. Deep Sea Res. II Top. Stud. Oceanogr. 2012, 81, 18–27. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.H.; Lee, H.; Kang, J.J.; Lee, J.H.; Lee, D.; An, S.; Stockwell, D.A.; Whitledge, T.E. Field-obtained carbon and nitrogen uptake rates of phytoplankton in the Laptev and East Siberian seas. Biogeosci. Discuss. 2017, 1–32. [Google Scholar] [CrossRef]
- Anderson, L.G.; Björk, G.; Jutterström, S.; Pipko, I.; Shakhova, N.; Semiletov, I.P.; Wåhlström, I. East Siberian Sea, an Arctic region of very high biogeochemical activity. Biogeosciences 2011, 8, 1745–1754. [Google Scholar] [CrossRef]
- Karlsson, E.; Charkin, A.; Dudarev, O.; Semiletov, I.; Vonk, J.; Sánchez-García, L.; Andersson, A.; Gustafsson, Ö. Carbon isotopes and lipid biomarker investigation of sources, transport and degradation of terrestrial organic matter in the Buor-Khaya Bay, SE Laptev Sea. Biogeosciences 2011, 8, 1865–1879. [Google Scholar] [CrossRef]
- Semiletov, I.P.; Shakhova, N.E.; Sergienko, V.I.; Pipko, I.I.; Dudarev, O.V. On carbon transport and fate in the East Siberian Arctic land–shelf–atmosphere system. Environ. Res. Lett. 2012, 7, 015201. [Google Scholar] [CrossRef]
- Osadchiev, A.; Pisareva, M.; Spivak, E.; Shchuka, S.; Semiletov, I. Freshwater transport between the Kara, Laptev, and East-Siberian seas. Sci. Rep. 2020, 10, 13041. [Google Scholar] [CrossRef] [PubMed]
- Hill, V.J.; Ardyna, M.; Lee, S.H.; Varela, D.E. Decadal trends in phytoplankton production in the Pacific Arctic Region from 1950 to 2012. Deep Sea Res. Part II Top. Stud. Oceanogr. 2018, 152, 82–94. [Google Scholar] [CrossRef]
- Bhavya, P.S.; Lee, J.H.; Lee, H.W.; Kang, J.J.; Lee, J.H.; Lee, D.; An, S.H.; Stockwell, D.A.; Whitledge, T.E.; Lee, S.H. First in situ estimations of small phytoplankton carbon and nitrogen uptake rates in the Kara, Laptev, and East Siberian seas. Biogeosciences 2018, 15, 5503–5517. [Google Scholar] [CrossRef]
- Pool, H.J.; Atkins, W.R.G. Photoelectric measurements of submarine illumination throught the year. J. Mar. Biol. Assoc. UK 1929, 16, 297–324. [Google Scholar] [CrossRef]
- Rintoul, S.R.; Trull, T.W. Seasonal evolution of the mixed layer in the Subantarctic Zone south of Australia. J. Geophys. Res. Oceans 2001, 106, 31447–31462. [Google Scholar] [CrossRef]
- Aagaard, K.; Carmack, E.C. The role of sea ice and other fresh water in the Arctic circulation. J. Geophys. Res. Oceans 1989, 94, 14485–14498. [Google Scholar] [CrossRef]
- Anderson, L.G.; Turner, D.B.; Wedborg, M.; Dyrssen, D. Determination of total alkalinity and total dissolved inorganic carbon. In Methods of Seawater Analysis; Grasshoff, K., Kremling, K., Ehrhardt, M., Eds.; Wiley-VCH: Weinheim, Germany, 1999; pp. 127–148. [Google Scholar]
- Lee, S.H.; Joo, H.T.; Lee, J.H.; Lee, J.H.; Kang, J.J.; Lee, H.W.; Lee, D.; Kang, C.K. Seasonal carbon uptake rates of phytoplankton in the northern East/Japan Sea. Deep Res. Part II Top. Stud. Oceanogr. 2017, 143, 45–53. [Google Scholar] [CrossRef]
- Gosselin, M.; Levasseur, M.; Wheeler, P.A.; Horner, R.A.; Booth, B.C. New measurements of phytoplankton and ice algal production in the Arctic Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 1997, 44, 1623–1644. [Google Scholar] [CrossRef]
- Subba Rao, D.; Platt, T. Primary production of Arctic waters. Polar Biol. 1984, 3, 191–201. [Google Scholar] [CrossRef]
- Lee, S.H.; Whitledge, T.E. Primary and new production in the deep Canada Basin during summer 2002. Polar Biol. 2005, 28, 190–197. [Google Scholar] [CrossRef]
- Dortch, Q.; Whitledge, T.E. Does nitrogen or silicon limit phytoplankton production in the Mississippi River plume and nearby regions? Cont. Shelf Res. 1992, 12, 1293–1309. [Google Scholar] [CrossRef]
- Justić, D.; Rabalais, N.N.; Turner, R.E.; Dortch, Q. Changes in nutrient structure of river-dominated coastal waters: Stoichiometric nutrient balance and its consequences. Estuar. Coast. Shelf Sci. 1995, 40, 339–356. [Google Scholar] [CrossRef]
- Demidov, A.B.; Gagarin, V.I.; Eremeeva, E.V.; Artemiev, V.A.; Polukhin, A.A.; Shchuka, S.A.; Grigoriev, A.V.; Khrapko, A.N.; Flint, M.V. Vertical variability of primary production and Chlorophyll a in the Kara Sea in the middle of summer: Contribution of subsurface maxima to the water column values. Oceanology 2021, 61, 645–661. [Google Scholar] [CrossRef]
- Lee, S.H.; Stockwell, D.A.; Joo, H.M.; Son, Y.B.; Kang, C.K.; Whitledge, T.E. Phytoplankton production from melting ponds on Arctic sea ice. J. Geophys. Res. Oceans 2012, 117, C04030. [Google Scholar] [CrossRef]
- Lee, S.H.; Yun, M.S.; Jang, H.K.; Kang, J.J.; Kim, K.; Lee, D.; Jo, N.; Park, S.H.; Lee, J.H.; Ahn, S.H. Size-differential photosynthetic traits of phytoplankton in the Chukchi Sea. Cont. Shelf Res. 2023, 255, 104933. [Google Scholar] [CrossRef]
- Demidov, A.B.; Gagarin, V.I. Primary Production and Associated Environmental Conditions in the East Siberian Sea in Autumn. Dokl. Earth Sci. 2019, 487, 1006–1011. [Google Scholar] [CrossRef]
- Ahn, S.H.; Kim, K.; Jo, N.; Kang, J.J.; Lee, J.H.; Whitledge, T.E.; Stockwell, D.A.; Lee, H.W.; Lee, S.H. Fluvial influence on the biochemical composition of particulate organic matter in the Laptev and Western East Siberian seas during 2015. Mar. Environ. Res. 2020, 155, 104873. [Google Scholar] [CrossRef] [PubMed]
- Demidov, A.B.; Kopelevich, O.V.; Mosharov, S.A.; Sheberstov, S.V.; Vazyulya, S.V. Modelling Kara Sea phytoplankton primary production: Development and skill assessment of regional algorithms. J. Sea Res. 2017, 125, 1–17. [Google Scholar] [CrossRef]
- Rudels, B.; Anderson, L.; Jones, E. Formation and evolution of the surface mixed layer and halocline of the Arctic Ocean. J. Geophys. Res. Oceans 1996, 101, 8807–8821. [Google Scholar] [CrossRef]
- Woodgate, R.A.; Aagaard, K.; Muench, R.D.; Gunn, J.; Björk, G.; Rudels, B.; Roach, A.; Schauer, U. The Arctic Ocean boundary current along the Eurasian slope and the adjacent Lomonosov Ridge: Water mass properties, transports and transformations from moored instruments. Deep Sea Res. I Oceanogr. Res. Pap. 2001, 48, 1757–1792. [Google Scholar] [CrossRef]
- Rudels, B.; Jones, E.P.; Schauer, U.; Eriksson, P. Atlantic sources of the Arctic Ocean surface and halocline waters. Polar Res. 2004, 23, 181–208. [Google Scholar] [CrossRef]
- Lee, S.H.; Whitledge, T.E.; Kang, S.-H. Recent carbon and nitrogen uptake rates of phytoplankton in Bering Strait and the Chukchi Sea. Cont. Shelf Res. 2007, 27, 2231–2249. [Google Scholar] [CrossRef]
- Yun, M.S.; Chung, K.H.; Zimmermann, S.; Zhao, J.; Joo, H.M.; Lee, S.H. Phytoplankton productivity and its response to higher light levels in the Canada Basin. Polar Biol. 2012, 35, 257–268. [Google Scholar] [CrossRef]
- Proshutinsky, A.; Krishfield, R.; Toole, J.M.; Timmermans, M.-L.; Williams, W.; Zimmermann, S.; Yamamoto-Kawai, M.; Armitage, T.W.K.; Dukhovskoy, D.; Golubeva, E.; et al. Analysis of the Beaufort Gyre freshwater content in 2003–2018. J. Geophys. Res. Oceans 2019, 124, 9658–9689. [Google Scholar] [CrossRef]
- Lee, Y.; Min, J.O.; Yang, E.J.; Cho, K.H.; Jung, J.; Park, J.; Moon, J.K.; Kang, S.H. Influence of sea ice concentration on phytoplankton community structure in the Chukchi and East Siberian Seas, Pacific Arctic Ocean. Deep Sea Res. I Oceanogr. Res. Pap. 2019, 147, 54–64. [Google Scholar] [CrossRef]
- Demidov, A.B.; Gagarin, V.; Arashkevich, E.; Makkaveev, P.; Konyukhov, I.; Vorobieva, O.; Fedorov, A. Spatial variability of primary production and chlorophyll in the Laptev Sea in August–September. Oceanology 2019, 59, 678–691. [Google Scholar] [CrossRef]
- Spall, M.A.; Pickart, R.S.; Brugler, E.T.; Moore, G.; Thomas, L.; Arrigo, K.R. Role of shelfbreak upwelling in the formation of a massive under-ice bloom in the Chukchi Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2014, 105, 17–29. [Google Scholar] [CrossRef]
- Gong, D.; Pickart, R.S. Summertime circulation in the eastern Chukchi Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2015, 118, 18–31. [Google Scholar] [CrossRef]
- Demidov, A.B.; Mosharov, S.A.; Makkaveev, P.N. Patterns of the Kara Sea primary production in autumn: Biotic and abiotic forcing of subsurface layer. J. Mar. Syst. 2014, 132, 130–149. [Google Scholar] [CrossRef]
- Sakshaug, E. Primary and Secondary Production in the Arctic Seas. In The Organic Carbon Cycle in the Arctic Ocean; Stein, R., MacDonald, R.W., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 57–81. [Google Scholar]
- Grebmeier, J.M.; Bluhm, B.A.; Cooper, L.W.; Danielson, S.L.; Arrigo, K.R.; Blanchard, A.L.; Clarke, J.T.; Day, R.H.; Frey, K.E.; Gradinger, R.R. Ecosystem characteristics and processes facilitating persistent macrobenthic biomass hotspots and associated benthivory in the Pacific Arctic. Prog. Oceanogr. 2015, 136, 92–114. [Google Scholar] [CrossRef]
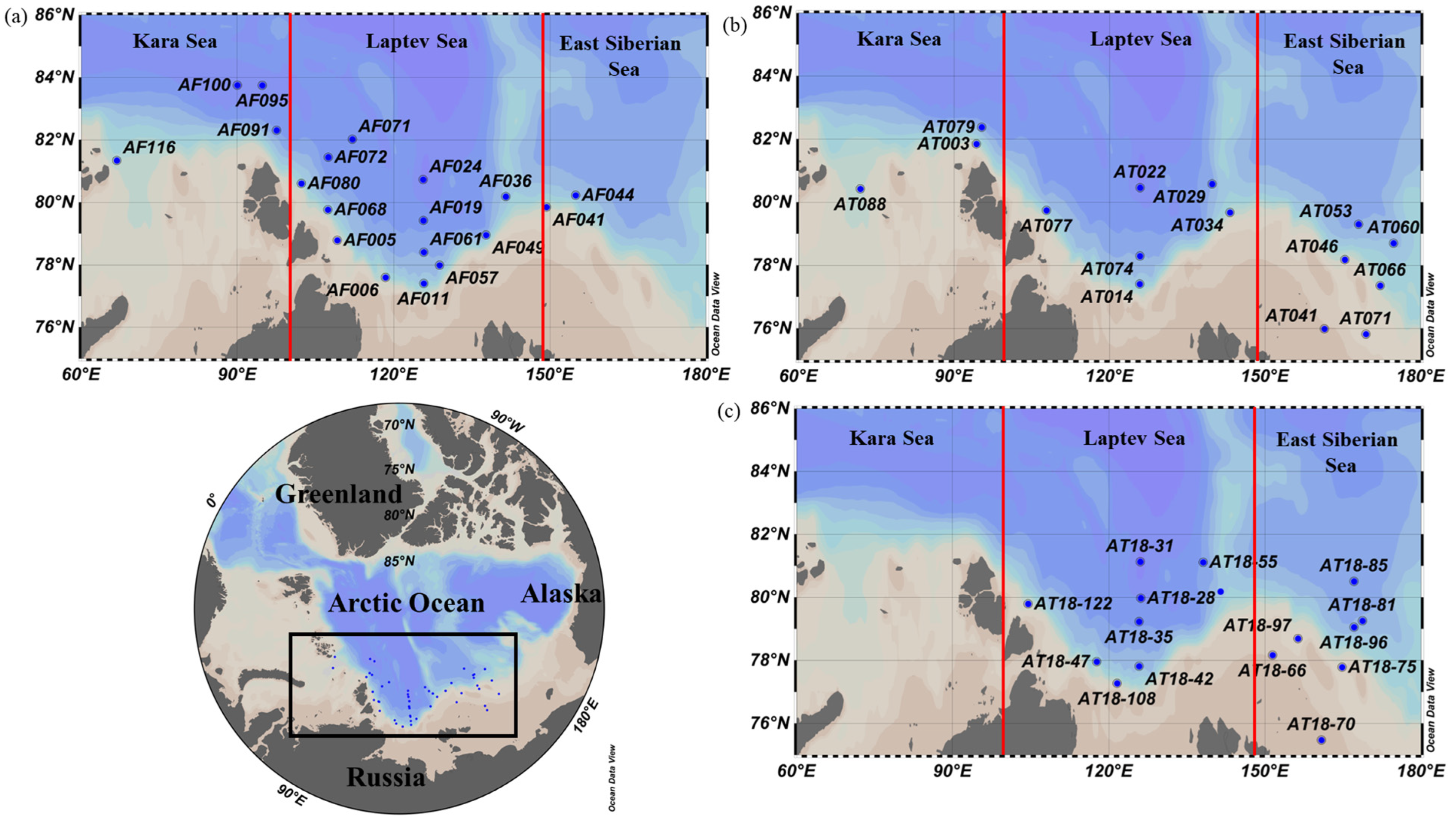




| Sea | Station Name | Date | Latitude | Longitude | Temperature (°C) | Salinity | Euphotic Depth (m) | Mixed Layer Depth (m) | Stratification Index (kg m−3) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 2013 | Kara Sea | AF091 | 14 September | 82.30 | 97.55 | −1.14 ± 0.70 | 34.10 ± 0.52 | 38 | 31 | 0.50 |
| AF095 | 15 September | 83.74 | 94.79 | −1.45 ± 0.32 | 33.69 ± 0.80 | 70 | 19 | 1.39 | ||
| AF100 | 16 September | 83.75 | 90.01 | −1.59 ± 0.06 | 34.08 ± 0.39 | 46 | 17 | 0.88 | ||
| AF116 | 19 September | 81.34 | 66.87 | 0.42 ± 0.83 | 34.28 ± 0.57 | 46 | 26 | 0.82 | ||
| Laptev Sea | AF005 | 25 August | 78.78 | 109.20 | −1.16 ± 0.41 | 33.88 ± 1.13 | 38 | 13 | 2.83 | |
| AF006 | 26 August | 77.59 | 118.45 | −0.91 ± 0.89 | 33.73 ± 1.06 | 50 | 10 | 2.95 | ||
| AF011 | 27 August | 77.40 | 125.80 | −0.91 ± 1.20 | 33.20 ± 1.55 | 51 | 14 | 4.16 | ||
| AF019 | 28 August | 79.42 | 125.74 | −1.43 ± 0.33 | 33.82 ± 0.71 | 60 | 15 | 1.33 | ||
| AF024 | 29 August | 80.72 | 125.69 | −1.47 ± 0.32 | 33.31 ± 1.17 | 51 | 9 | 2.47 | ||
| AF036 | 1 September | 80.18 | 141.56 | −1.58 ± 0.15 | 32.59 ± 1.96 | 54 | 9 | 4.77 | ||
| AF049 | 5 September | 78.95 | 137.77 | −0.91 ± 1.01 | 33.21 ± 1.71 | 51 | 7 | 4.68 | ||
| AF057 | 5 September | 77.98 | 128.83 | −1.26 ± 0.83 | 33.43 ± 1.17 | 51 | 5 | 3.83 | ||
| AF061 | 6 September | 78.40 | 125.83 | −1.31 ± 0.45 | 33.79 ± 0.87 | 51 | 9 | 2.59 | ||
| AF068 | 10 September | 79.76 | 107.39 | −1.32 ± 0.43 | 34.12 ± 0.59 | 33 | 6 | 1.64 | ||
| AF071 | 11 September | 82.02 | 112.10 | −1.44 ± 0.36 | 33.41 ± 1.00 | 43 | 27 | 1.68 | ||
| AF072 | 12 September | 81.44 | 107.48 | −1.39 ± 0.35 | 33.83 ± 0.80 | 49 | 14 | 1.50 | ||
| AF080 | 13 September | 80.60 | 102.31 | −0.14 ± 0.55 | 34.11 ± 0.71 | 76 | 22 | 1.12 | ||
| East Siberian Sea | AF041 | 2 September | 79.85 | 149.38 | −1.49 ± 0.18 | 32.41 ± 1.47 | 51 | 18 | 3.24 | |
| AF044 | 3 September | 80.22 | 154.98 | −1.57 ± 0.13 | 32.42 ± 1.25 | 35 | 28 | 2.46 |
| Period | Sea | Station Name | Date | Latitude | Longitude | Temperature (°C) | Salinity | Euphotic Depth (m) | Mixed Layer Depth (m) | Stratification Index (kg m−3) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | Kara Sea | AT003 | 28 August | 81.84 | 94.31 | −0.77 ± 0.50 | 34.18 ± 0.50 | 30 | 25 | 0.85 |
| AT079 | 24 September | 87.42 | 95.33 | −1.60 ± 0.25 | 34.03 ± 0.81 | 56 | 15 | 1.55 | ||
| AT088 | 26 September | 80.42 | 71.91 | −0.07 ± 1.34 | 33.50 ± 2.77 | 26 | 10 | 7.22 | ||
| Laptev Sea | AT014 | 1 September | 77.40 | 125.80 | −0.80 ± 1.35 | 33.65 ± 1.23 | 30 | 8 | 4.18 | |
| AT022 | 4 September | 80.46 | 125.89 | −1.31 ± 0.65 | 33.47 ± 1.17 | 40 | 18 | 2.73 | ||
| AT029 | 6 September | 80.57 | 139.78 | −1.13 ± 0.84 | 33.65 ± 0.90 | 40 | 10 | 2.37 | ||
| AT034 | 8 September | 79.68 | 143.24 | N.D. | N.D. | 28 | N.D. | N.D. | ||
| AT074 | 19 September | 78.29 | 125.87 | −0.92 ± 1.40 | 33.50 ± 1.51 | 30 | 21 | 3.76 | ||
| AT077 | 22 September | 79.74 | 107.82 | −1.61 ± 0.11 | 33.06 ± 1.62 | 34 | 11 | 3.11 | ||
| East Siberian Sea | AT041 | 10 September | 75.98 | 161.43 | −1.47 ± 0.09 | 30.19 ± 1.23 | 52 | 18 | 2.18 | |
| AT046 | 10 September | 78.18 | 165.37 | −1.44 ± 0.22 | 31.43 ± 1.55 | 56 | 8 | 3.90 | ||
| AT053 | 12 September | 79.30 | 168.03 | −1.41 ± 0.22 | 31.53 ± 1.41 | 54 | 10 | 3.89 | ||
| AT060 | 14 September | 78.70 | 174.77 | −1.21 ± 0.33 | 30.64 ± 2.04 | 57 | 15 | 4.56 | ||
| AT066 | 14 September | 77.36 | 172.19 | −1.14 ± 0.33 | 30.40 ± 2.25 | 70 | 17 | 5.03 | ||
| AT071 | 16 September | 75.82 | 169.46 | −1.21 ± 0.58 | 30.95 ± 2.33 | 60 | 13 | 5.59 |
| Period | Sea | Station Name | Date | Latitude | Longitude | Temperature (°C) | Salinity | Euphotic Depth (m) | Mixed Layer Depth (m) | Stratification Index (kg m−3) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2018 | Laptev Sea | AT18-28 | 31 August | 79.97 | 126.17 | −1.61 ± 0.19 | 33.78 ± 1.10 | 38 | 16 | 2.43 |
| AT18-31 | 1 September | 81.13 | 126.08 | −1.61 ± 0.12 | 33.62 ± 1.26 | 38 | 11 | 2.91 | ||
| AT18-35 | 2 September | 79.23 | 125.84 | −1.18 ± 0.90 | 33.92 ± 0.79 | 48 | 18 | 2.12 | ||
| AT18-42 | 3 September | 77.81 | 125.83 | −0.73 ± 1.38 | 33.85 ± 0.95 | 40 | 17 | 2.87 | ||
| AT18-47 | 4 September | 77.96 | 117.69 | −0.79 ± 0.78 | 33.53 ± 1.34 | 26 | 5 | 4.70 | ||
| AT18-55 | 6 September | 81.11 | 138.14 | −1.70 ± 0.09 | 33.59 ± 1.06 | 36 | 15 | 2.20 | ||
| AT18-59 | 6 September | 80.18 | 141.49 | −0.88 ± 1.33 | 32.83 ± 2.04 | 40 | 22 | 4.81 | ||
| AT18-108 | 21 September | 77.27 | 121.62 | −0.64 ± 0.78 | 33.38 ± 1.40 | 28 | 6 | 4.30 | ||
| AT18-122 | 23 September | 79.79 | 104.51 | −0.37 ± 0.82 | 33.87 ± 1.03 | 34 | 25 | 1.75 | ||
| East Siberian Sea | AT18-66 | 8 September | 78.17 | 151.48 | 0.09 ± 2.14 | 29.86 ± 1.76 | 20 | 21 | 4.77 | |
| AT18-70 | 8 September | 75.47 | 160.87 | −1.46 ± 0.10 | 30.26 ± 1.15 | 46 | 16 | 2.30 | ||
| AT18-75 | 9 September | 77.78 | 164.84 | −1.39 ± 0.15 | 31.70 ± 1.87 | 36 | 11 | 4.21 | ||
| AT18-81 | 10 September | 79.25 | 168.78 | −1.56 ± 0.09 | 31.80 ± 1.44 | 42 | 25 | 3.09 | ||
| AT18-85 | 13 September | 80.50 | 167.15 | −1.60 ± 0.09 | 31.75 ± 1.07 | 40 | 24 | 2.60 | ||
| AT18-96 | 17 September | 79.05 | 167.15 | −0.76 ± 1.29 | 32.22 ± 2.14 | 24 | 15 | 5.68 | ||
| AT18-97 | 17 September | 78.69 | 156.39 | 0.04 ± 1.87 | 31.59 ± 2.54 | 22 | 30 | 5.62 |
| Region | Primary Production Rates (mg C m−2 d−1) | Method | Year | Month | Season | Reference |
|---|---|---|---|---|---|---|
| Kara Sea | 55 ± 20 | 14C | 2007 | September | Summer | [65] |
| 24 ± 71 | 2011 | September–October | Summer–autumn | |||
| 99 ± 62 | 13C | 2013, 2015 | August–September | Summer | This study | |
| Laptev Sea | 64 ± 19 | 14C | 2015 | September | Summer | [62] |
| 23 ± 11 | 2017 | September | ||||
| 55 ± 25 | 2018 | August–September | ||||
| 100 ± 77 | 13C | 2013, 2015, 2018 | August–September | Summer | This study | |
| East Siberian Sea | 33 ± 15 | 13C | 2004–2012 | July | Summer | [37] |
| 93 ± 49 | August | |||||
| 132 ± 44 | September | |||||
| 28 ± 13 | 14C | 2017 | September | Summer | [52] | |
| 56 ± 35 | 13C | 2013, 2015, 2018 | August–September | Summer | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Kim, K.; Jo, N.; Jang, H.-K.; Ahn, S.-H.; Lee, J.; Lee, H.; Park, S.; Lee, D.; Stockwell, D.A.; et al. Primary Production in the Kara, Laptev, and East Siberian Seas. Microorganisms 2023, 11, 1886. https://doi.org/10.3390/microorganisms11081886
Kim S, Kim K, Jo N, Jang H-K, Ahn S-H, Lee J, Lee H, Park S, Lee D, Stockwell DA, et al. Primary Production in the Kara, Laptev, and East Siberian Seas. Microorganisms. 2023; 11(8):1886. https://doi.org/10.3390/microorganisms11081886
Chicago/Turabian StyleKim, Soohyun, Kwanwoo Kim, Naeun Jo, Hyo-Keun Jang, So-Hyun Ahn, Janghan Lee, Howon Lee, Sanghoon Park, Dabin Lee, Dean A. Stockwell, and et al. 2023. "Primary Production in the Kara, Laptev, and East Siberian Seas" Microorganisms 11, no. 8: 1886. https://doi.org/10.3390/microorganisms11081886
APA StyleKim, S., Kim, K., Jo, N., Jang, H.-K., Ahn, S.-H., Lee, J., Lee, H., Park, S., Lee, D., Stockwell, D. A., Whitledge, T. E., & Lee, S.-H. (2023). Primary Production in the Kara, Laptev, and East Siberian Seas. Microorganisms, 11(8), 1886. https://doi.org/10.3390/microorganisms11081886










