Abstract
Commensal Escherichia coli with broad repertoire of virulence and antimicrobial resistance (AMR) genes pose serious public health risks as reservoirs of AMR and virulence. This study undertook whole genome characterization of commensal E. coli from food-producing animals in Uganda to investigate their genome variability (resistome and virulome). We established that the E. coli had high genomic diversity with 38 sequence types, 24 FimH types, and 33 O-antigen serotypes randomly distributed within three phylogroups (A, B1, and E). A greater proportion (≥93.65%) of the E. coli were resistant to amoxicillin/clavulanate and ampicillin antibiotics. The isolates were AmpC beta-lactamase producers dominated by blaEC-15 (71.88%) and tet(A) (20.31%) antimicrobial resistant genes besides a diverse armory of virulence-associated genes in the class of exotoxin, adhesins, iron uptake, and serine protease autotransporters which varied by host species. Cattle were found to be the major source of E. coli carrying Shiga toxin genes, whereas swine was the main source of E. coli carrying colicin-like Usp toxin gene. The study underscores the importance of livestock as the carrier of E. coli with antimicrobial resistance and a large repertoire of virulence traits with a potential of causing disease in animals and humans by acquiring more genetic traits.
1. Introduction
Escherichia coli is a very versatile bacterium that is known to survive in different niches including in warm blooded mammals and the environment due to its genomic plasticity. It comprises pathogenic and non-pathogenic strains which sometimes co-exist in specific niches such as the gut. To survive in the gut, they must be able to withstand the conditions therein, including the assault by the host gut immune system and other chemicals that reach the gut, such as antimicrobial agents. Those that are susceptible to the host immune system and antibacterial agents will be eliminated. The gut commensal bacteria must therefore possess minimal protective attributes such as virulence traits and antimicrobial resistance to the antimicrobials they are frequently exposed to. These traits enable the bacteria to survive the assault and competition with other microbes within those systems [1].
E. coli are generally known to belong to A, B1, B2, C, D, E, and F phylogenetic groups. The phylogroup classification is based on the presence or absence of four genes (chuA, yjaA, TspE4.C2, and arpA) [2]. Phylogroups A and B2 are predominant among animals, while phylogroup B2 and D are responsible for most infections in humans [3]. The pathogenic strains are highly adapted, with specific virulence attributes which enable them to cause disease in different parts of the body, which has come to define their pathotypes, namely, intestinal (or diarrheagenic) pathogenic E. coli (IPEC) and extra-intestinal pathogenic E. coli (ExPEC). There are several pathotypes based on the disease and/or pathogenesis, including uropathogenic E. coli (UPEC), neonatal meningitis E. coli (NMEC, meningitis-associated E. coli (MAEC), avian pathogenic E. coli (APEC), enteropathogenic E. coli (EPEC), Shiga toxin-producing E. coli (STEC); enterohemorrhagic E. coli (EHEC); enterotoxigenic E. coli (ETEC); enteroinvasive E. coli (EIEC); and enteroaggregative E. coli (EAEC), diffuse adhering E. coli (DAEC), adherent-invasive E. coli (AIEC), sepsis-associated E. coli (SEPEC) and unspecified ExPEC, as previously described [4].
Several of these pathotypes, such as APEC, EPEC, EHEC, and ETEC, cause disease in animals with similar virulence factors as in humans. The EHEC strains of E. coli produce “Shiga toxins”, encoded by the stx1 and/or stx2 gene common to all EHEC bacteria, and are also pathogenic for animals and humans [5]. The differentiation between commensal E. coli and others has been challenged because of the frequent genomic alterations and genetic exchanges within and between strains, where non-pathogenic strains acquire virulent genes gradually gaining specific traits for specific pathotypes [6].
E. coli has been found to be the largest contributor to the global burden of antimicrobial resistance causing death in humans [7]. For a long time, these bacteria have been exposed to and have become used to antimicrobial agents. The antimicrobial exposure exerted selective pressure that led to the emergence of E. coli strains that have acquired resistance to multiple antibiotics and a diverse array of virulence factors that have been successfully disseminated globally. Strains that produce extended-spectrum β-lactamases (ESBL), in particular, often result in longer hospitalization, higher morbidity, and increased health-care costs [8,9]. E. coli have a broad habitat, including the gastrointestinal tract of most warm-blooded animals as commensals, soil, water, and sewage, where they are frequently exposed to antibiotics and other chemicals that have made them develop survival mechanisms. They are, therefore, frequently used as resistance indicators [10]. A few of the E. coli that cause intestinal and extraintestinal infections have been reported both in animals and humans.
A lot of interest has been focused on the burden of multi-drug resistant (MDR) strains in humans [11] and their potential sources, such as environmental and animal reservoirs. However, less consideration has been given to humans as potential reservoirs of MDR for animals and the environment [12]. Besides the pathogenic E. coli, commensal bacterial are known to harbor and exchange resistance and virulence loci and can provide a reservoir of resistance genes, which may be transferred between bacterial species, including pathogens [13]. The resistance genes and other fitness attributes are successfully disseminated and shared among E. coli strains within the ecological environment where they survive. The sharing of genetic information among E. coli strains is a threat to public health, animal health, and environmental health from one health perspective [14]. Several factors including antibiotic use, limited infection and prevention, and biosecurity on farms, both in animals and humans, are the major drivers that enhance the development, persistence, and dissemination of resistant organisms [15]. The predominant use of beta-lactams and tetracyclines antibiotics to treat animals in Uganda has driven the increased resistance to the drugs, which is presenting a serious clinical challenge. In particular, β-lactamases of class C are widely distributed on the chromosomes of many Gram-negative species typically annotated as ampC, which under normal conditions are not expressed but high-level expression is often induced by mutation or induction by specific β-lactams that lead to clinical resistance [16].
Human studies have more frequently reported highly successful globally disseminated pandemic high-risk clones, such as ST-131, ST-648, ST-10, and others [17], which are responsible for many health care associated and community infections, and treatment failures. Many of these clones are increasingly being reported in animals and the environment, which enhances the hypothesis that animals may act as reservoirs of E. coli strains pathogenic to human [18]. This study aimed to establish the genomic diversity and existence of high-risk clones, resistome, and virulome within E. coli isolates from livestock in Uganda.
2. Materials and Methods
2.1. Sample Collection
A total of 729 samples were collected from: cattle (n = 225), swine (n = 245), and poultry (n = 257) from September 2020 to April 2021, using a series of cross-sectional studies at swine slaughter places, cattle abattoirs, and live poultry markets in Kampala and Wakiso districts. For cattle and swine, fecal samples were collected, while for poultry, fecal droppings were collected in 275 mL of peptone water.
2.2. Bacteria Isolation
Samples were suspended in peptone water, incubated overnight, and inoculated directly onto Xylose/Lysine/Deoxycholate (XLD) selective agar and incubated at 37 °C overnight. Yellow colonies that were catalase positive and oxidase negative were confirmed as E. coli with API-20E kits (bioMérieux, Inc., 100 Rodolphe Street, Durham, NC, USA). Isolates were subjected to antibiotic susceptibility testing by standard disk diffusion test according to CLSI [19]. Multi-drug resistant isolates were selected for whole genome sequencing and sent to Walter Reed Army Institute of Research (WRAIR)’s Multi-Drug Resistant Organism Repository and Surveillance Network (MRSN) in the USA.
2.3. DNA Library Preparation
A total of 63 multidrug resistant isolates were selected and grown on MacConkey agar for 48 h at 37 °C. The isolates were collected in 1.5 mL tubes and extraction of DNA was completed with DNeasy UltraClean microbial kit (Qiagen, Germantown, MD, USA). DNA libraries were prepared using Kapa HyperPlus kit (Roche Diagnostics, Indianapolis, IN, USA). Libraries’ concentrations were evaluated in a CFX96 real-time iCycler machine (Bio-Rad, Hercules, CA, USA) using KAPA™ Library Quantification Kits (Roche Diagnostics). Paired-end sequencing was performed at MRSN on the Illumina NextSeq machine (Illumina, Inc., San Diego, CA, USA).
2.4. Sequence Analysis
Removal of sequence adapters from raw reads was performed using Btrims [20]. Denovo assembly of raw reads was performed with Newbler (v2.9) [21]. Taxonomic assignment of the isolates using assembled genome sequences was performed by matching against typed reference genomes in the Genome Taxonomy Database (GTDB). A close match with an Average Nucleotide Identity (ANI) value ≥ 95% was used to infer the species of the isolates. High-quality SNPs were generated from concatenated alignment of core genome genes which was then used to build a phylogenetic tree using CSI Phylogeny. The tree was imported into Interactive Tree of Life (https://itol.embl.de/, accessed on 1 March 2023) for viewing and annotation. The in silico ClermonTyping was used to group the isolates into different phylogroups [22]. Assigning the isolates into different Multilocus Sequence Types (MLST) was performed by comparison to sequences available in MLST v2.0 database [23]. SeqSphere+ was used to generate the minimum spanning tree from E. coli isolates based on the MLST scheme [24]. Serotype prediction was performed using SerotypeFinder v2.0 database [25] and FimType prediction was performed using FimTyper v1.0 database [26]. mlplasmids v2.1.0 was used to identify contigs that were of plasmid origin with posterior probability set at 0.9 and minimum contig length of 1000 bp [27]. Contigs identified as plasmids were further confirmed using NCBI nucleotide blast. Antibiotic-resistant genes were identified and confirmed among the isolates using ResFinder 4.1, AMRFinder, and CARD databases within the plasmid and chromosomal DNA [28]. VirulenceFinder v2.0 was used to predict the occurrence of various virulent determinants among the isolates [29]. For all the software used, default parameters were considered unless otherwise indicated.
3. Results
3.1. Antimicrobial Susceptibility Test
The antimicrobial susceptibility test was performed on nine antibiotics. Generally, there were significantly high levels of antibiotic resistance observed with amoxicillin/clavulanate and ampicillin (≥93.65%) antibiotics. Antibiotics with moderate levels of resistance were ceftazidime (47.62%), ciprofloxacin (57.14%), gentamicin (49.21%), and trimethoprim/sulfamethoxazole (53.97%), whereas the majority of isolates were susceptible to chloramphenicol (79.37%), cefotaxime (63.49%), and meropenem (80.95%) antibiotics. Resistance to amoxicillin/clavulanate was significantly high in E. coli isolated from chickens, swine, and cattle, whereas resistance to ampicillin antibiotics was particularly high among E. coli isolated from cattle and swine. All E. coli isolated from chicken were found to be resistant to ciprofloxacin. Resistance to ciprofloxacin was equally high among E. coli isolated from swine.
3.2. Phylogroups, STs, FimH Types, and Serogroup of the E. coli Isolates
Phylogroups B1 (57.81%) and A (31.25%) were most commonly associated with the three host species, while phylogroups E, C, and D were marginally detected. Phylogroups’ compositions were largely dependent on the animal species. For example, phylogroup A was principally isolated from swine and chicken, whereas phylogroup B1 was predominantly isolated from cattle. A total of 39 known MLST groups were detected among the 63 isolates (Figure 1). ST10 (9.38%), ST-206 (9.38%), and ST-2280 (6.25%) were the commonest E. coli sequence types associated with the animals.
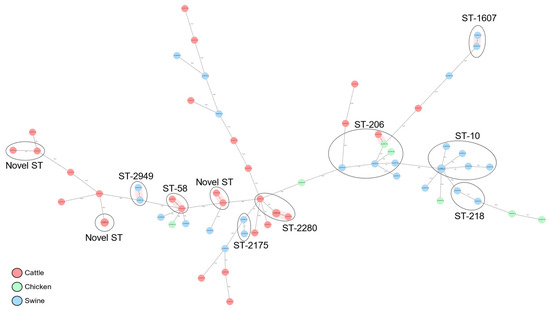
Figure 1.
cgMLST-based minimum spanning tree of 63 commensal E. coli isolates recovered from cattle, swine, and chicken slaughter places in Kampala. Isolates belonging to the same dominant sequence types (ST) are circled and labeled and the hosts of origin are shown in different colors.
Most of the MLST groups were detected only marginally, whereas a significant proportion of the E. coli isolates (10.94%) were from novel STs. The distribution of the different MLST groups were determined by the different phylogroups and animal species. The ST-206 and ST-10 were essentially detected only in phylogroup A (Figure 2). Whereas ST-206 was distributed in all three animal species, ST-10 was restricted within the swine host. Other STs associated with phylogroup A were ST-218, ST-48, ST-484, ST-5019, ST-5277, and ST-77. All novel STs and ST-2280 uniquely cluster within phylogroup B1/cattle host (Figure 2).
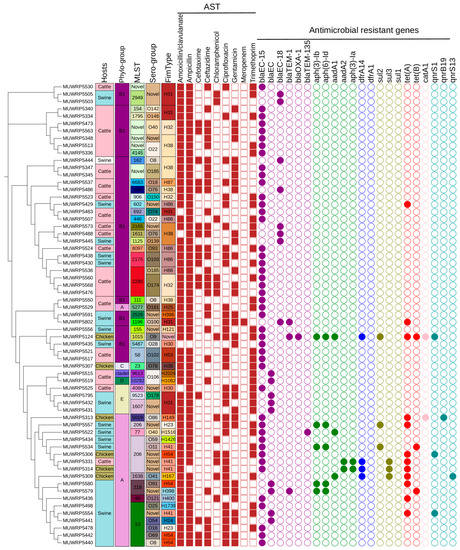
Figure 2.
SNP-based phylogenetic tree of 63 E. coli strains analyzed in this study characterized by phylogroup, sequence type (ST), isolation sources, and sample location with the corresponding antimicrobial resistance genes (absent, open circle; present, filled colored circle according to the class of AMR genes).
The E. coli clustered into 24 different FimH types. FimH31, H38, H32, and H38 were the most common (Figure 2). The majority of fimtypes were marginally detected and not present in more than one isolate. Fim H38 and H32 were majorly detected in E. coli isolated from cattle which reflects their host dependency. Meanwhile, H31 and H86 fimtypes were restricted within cattle and swine hosts. Only H41 was found to have a broader host range as it occurs in all three animal host species. Serogroups O174, O185, O22, and O76 were detected only in cattle and were the commonest, whereas other serogroups were only marginally detected. A greater proportion of the isolate could not be typed into known serogroups (Figure 2).
3.3. Presence of Antimicrobial Resistant Genes
A limited occurrence of different antimicrobial-resistant genes within the genomes of the E. coli isolates was observed. More than half of the isolates (76.19%) had only one antimicrobial-resistant gene within their genome. The most-frequent antibiotic-resistant gene detected was the blaEC-15, which occurs among (71.88%) of the E. coli isolates. The other beta-lactam genes detected were blaEC (14.06%), blaEC-18 (12.50%), and blaTEM-1 (6.25%). Tetracycline-resistant, tet(A) gene was detected among 20.31% of isolates. Only a limited proportion (4.69%) of isolates were found to carry the tet(B) gene. The quinolone group of antibiotics occurs only within a limited number of E. coli isolates (n = 5). Among the quinolone genes detected were the qnrS1 (4.36%), qnrB19 (1.56%), and qnrS13 (1.56%). The folate pathway antagonist genes detected were dfrA14 (6.25%), sul2 (6.25%), sul3 (4.6%), and sul1 (1.56%). The aph-(3)-Ib and aph-(6)-Id aminoglycoside-resistant genes were both detected at a frequency of 9.38%. Other rare aminoglycoside genes were aadA1 (6.25%), aadA2 (3.13%), and aph(3)-Ia (3.13%). The O9-serogroup isolate was associated with multiple antibiotic-resistant genes including blaEC-15, blaTEM-1, (aph (3)-Ib, aph (6)-Id), sul2, tet(A), catA1, and qnrS1. The blaEC-15 and tet(A) genes were predominantly located within phylogroups (B1 and A), whereas the blaEC gene was restricted among phylogroups (B1 and E). The aminoglycoside genes, aph (3)-Ib and aph (6)-Id, were associated with phylogroup A.
None of the blaEC-15 genes were located within the plasmid. However, the blaEC-15 genes were bordered by the fumarate reductase gene cluster upstream and the lipocalin (Figure 3). The blaEC-15 gene was also in close association with the gene for quaternary ammonium compound efflux (Figure 3). Only two isolates were detected with plasmid contigs carrying antimicrobial-resistant genes. The isolate MUWRP5802 was found to have a plasmid contig with blaTEM-1B gene. The second isolate MUWRP5306 had a plasmid fragment that carries tet(A) and qnrS1 antimicrobial resistant genes.
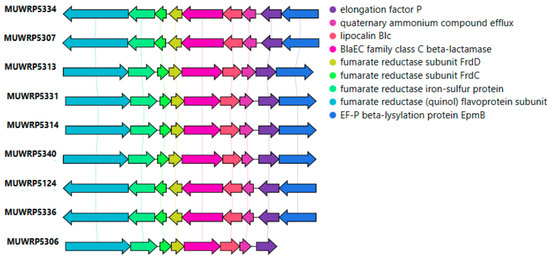
Figure 3.
Gene arrangement around blaEC-15 resistant gene.
3.4. Characteristics of Virulence Determinants among the E. coli Isolates
The virulent gene classes detected were those belonging to exotoxin, adhesins, iron uptake, serine protease autotransporters (SAT), and more. The distribution of the different virulent genes largely depended on the host species, phylogroups, and serogroups (Figure 4). A predominantly high proportion of the virulence-associated genes was detected from E. coli isolated from cattle compared to those isolated from swine and chicken. Two classes of exotoxin virulent genes were detected: the colicin-like Usp toxin gene was rare and occurred within E. coli isolated from swine (phylogroup E and serogroup-O178). Meanwhile, cattle (phylogroup B1) were the major source of E. coli carrying Shiga toxin genes. The stx2B (23.44%) and stx2A (21.88%) were the most common Shiga toxin genes carried by most E. coli isolated from cattle (Figure 4). Additionally, phylogroup B1 was associated with many different types of adhesins genes (Figure 4). For example, the adhesin gene lpfA for long polar fimbria protein occurs in most of phylogroup B1, except those of serogroup (O102). Among the genes that regulate iron uptake, the ireA gene was limited to two isolates of phylogroup B1 (serogroup O22), whereas the iha gene was widely distributed among isolates of phylogroup B1. Another iron uptake gene, chuA, had limited occurrence in isolates of phylogroup E. Other virulence genes found exclusively among phylogroup E were eilA and air gene. The only members of the serine protease autotransporters gene family detected were espP and Tsh genes. The espP gene occurs mainly within cattle, whereas the Tsh gene was from E. coli isolated from chicken. Swine was the major carrier of E. coli isolates with katP gene for plasmid-encoded catalase-peroxidase protein and was encoded within the plasmid. Overall, most of the virulence-associated genes were detected at moderate proportion (20%-29%), whereas others, such as terC, gad, iss, lpfA, traT, and ompT, were very common (>55%) among E. coli from animal sources (Figure 4). The ompT and hlyF genes were found to occur in the same plasmid in six isolates. Additionally, afaD virulence gene was detected in only one isolate (MUWRP5313) and was encoded by plasmid.
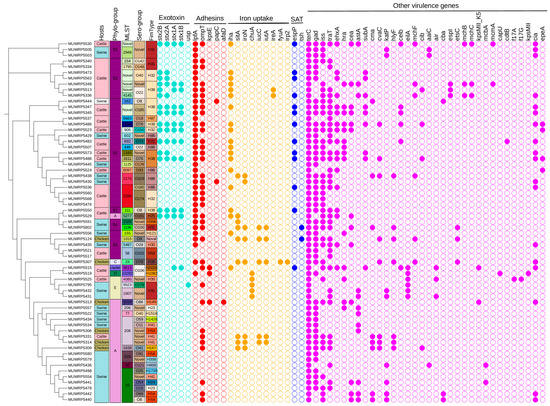
Figure 4.
A SNP-based phylogenetic tree showing the distribution of virulent genes by phylogroup, sequence type (ST), serogroup, fimtype, and host (absent, open circle; present, filled colored circle according to the virulent genes).
4. Discussion
The current study examined the genomic diversity of commensal E. coli in view of their resistome and virulome, which may be of relevance to transmission of these pathogenic strains. Many studies have tended to focus on pathogens, with limited focus on non-pathogenic bacteria that are regularly excluded from surveillance programs, yet may serve as reservoirs of AMR in the environment and the food chains, underscoring the need for more comprehensive analyses and monitoring of food environmental and animal reservoirs of AMR [30,31]. With the threat of antimicrobial resistance and untreatable infections as a big global concern, the role of animals and environment reservoirs in sustaining and disseminating AMR can no longer be ignored, as recent evidence suggests that AMR genes in animal and environmental bacteria can be rapidly acquired by human-associated and pathogenic bacteria [32,33,34].
The commensal isolates in this study showed a high genetic diversity with as many sequence types as the isolates themselves. This phenomenon of high genomic plasticity within E. coli has been reported several times in most parts of the world, including Uganda [35]. The plasticity seen in this species is likely due to horizontal gene transfer, gene loss, as well as other genomic modifications, such as DNA rearrangements and point mutations, which constantly alter the genome content and thus the fitness and competitiveness of individual variants in certain niches [36]. We identified potential zoonotic sequence types, especially ST-10, which was only in isolates from swine and not the other species. ST-10 E. coli is among the international, globally disseminated high-risk pandemic clones that have been frequently incriminated in several human ExPEC infections. This ST clone has been reported in synanthropic animals carrying several resistance genes to antibiotics frequently used in humans [37]. Similarly, this sequence type has been common in food-producing animals [38,39], consistent with our findings. The isolates belonged to two main phylogroups, A and B1, unlike the predominance of B2 which is commonly reported in human pathogenic E. coli, especially the ExPEC but also those of intestinal disease agents. These findings are consistent with other studies [18,40,41] that have reported predominance of phylogroups A and B1 in food animals.
While the isolates in the current study demonstrated carriage of a limited number of antimicrobial resistance genes, they were all AmpC beta-lactamase producers carrying mainly β-lactamase class blaEC genes, with 71.88% carrying the blaEC-15 gene. Similar findings have been reported [18]. The class C β-lactamases comprise the second most abundant group of enzymes and are found solely in Gram-negative bacteria, especially members of the Enterobacteriaceae [42]. Although they were originally identified as chromosomally encoded, more recently, genes encoding class C enzymes have been found mobilized on plasmids in the Enterobacteriaceae [43]. A smaller number of our isolates carried other resistances against tetracyclines tet(A) and tet(B) genes; quinolone resistance genes qnrS1, qnrB19, and qnrS13; as well as a few isolates with resistance genes for folate pathway antagonist genes and aminoglycoside-resistant genes. Resistance often follows antibiotic use patterns in animal populations [44]. The main antibiotics used in animal production in Uganda include penicillins, tetracylines, and sulphonamides [15,45,46,47].
A broad repertoire of virulence genes with invasion/adherence, exotoxin, and siderophore factors among the isolates obtained was demonstrated. Enteric E.coli consists of commensals that are not associated with disease but could cause disease under certain circumstances. To survive in the gut, E. coli strains frequently carry and are capable of toxin secretion, aggregative colonization, and multiplying in the gastrointestinal tract, and with the acquisition of additional genes could cause damage to different environments through the adaptation of key genetic elements, resulting in the formation of new pathotypes [42].
Most of the isolates had the terC, gad, iss, lpfA, and ompT virulence genes commonly found across the different animal hosts while the Shiga toxin genes (stx2B, stx2A, Stx1A, stx1B) occur mainly among E. coli of cattle origin. All isolates had the terC gene, which is part of the tellurite resistance gene operon (ter) that is known to exist widely and in many bacteria, particularly pathogenic species. The terC is part of the ter operon that is known to confer resistance to the oxyanion form of the rare nonessential trace element tellurium, namely, tellurite oxide (TeO3−2), and is implicated in tellurite resistance, phage inhibition, colicine resistance, and pathogenicity [48]. It has been said that the ter operon is required for fitness to survive in the gut where colicins from other competing bacteria and host antimicrobial peptides limit their survival. In Klebsiella pnemoniae, the terC has been reported to offer tolerance to ofloxacin, polymyxin B, and cetylpyridinium chloride as well [49]. The ter operon has also been demonstrated to be associated with stress tolerance factor and enhancing fitness in the gut, particularly against stress induced by the indigenous gut microbiota during colonization [50]. This confirms our observation that commensal bacteria need these anti-stress genes for survival within the microbial community in the gut. Similarly, we found most isolates in possession of the gad gene which encodes the glutamate decarboxylase pathway (GDP), which is a major acid resistance mechanism enabling microorganisms’ survival in low pH environments. The glutamate-dependent acid resistance system is said to be the most potent acid resistance system in commensal and pathogenic Escherichia coli. These gut bacteria need these traits to overcome the acidic environment during transit through the host stomach to successfully colonize the gut.
The Isolates had several other genes directly involved in pathogenicity. The Shiga toxin virulence genes, in particular, mediate the production of Shiga toxin among the enteric pathogenic E. coli strains (the Shiga toxin-producing Escherichia coli) which causes hemorrhagic colitis and hemolytic uremic syndrome [51]. Possession of the stx genes by animal E.coli does not necessarily indicate that they cause disease. It has been suggested that ruminants in particular do not have vascular globotriaosylceramide receptors where Shiga toxin binds and are always asymptomatic [52]. However, the ruminants can easily shed Shiga toxin producing E. coli in their feces and are considered reservoirs for Shiga toxin producing E.coli for humans.
The similarity in virulence factors between human ExPEC and E. coli from animal sources highlights the role of livestock in perpetuating infections in humans, including Shiga toxin-producing E. coli strain as reservoirs. While commensal E. coli commonly reside in human and animal gut without causing disease, they may occasionally cause opportunistic infections when the host immunity is impaired or injured. Their ability to acquire and transmit virulence and AMR genes within the gut both to non-virulent bacteria and pathogenic ones poses a serious threat that needs routine monitoring. They have been particularly found to be efficient in acquiring and transmitting these genes through mobile genetic elements such as transposons and plasmids [53].
5. Conclusions
Taken together, this study established the convergence of a limited resistome and a wide virulome among commensal AmpC beta-lactamase producing E. coli isolates in livestock in the country with a limited number of the international high-risk clones of pandemic importance. These clones have the potential to share both virulence and/or resistance genes to pathogenic E. coli or other pathogenic bacteria within the gastroenteric system.
Author Contributions
Conceptualization, D.K.B., F.W.-M., E.A.M., H.K. and F.N.; sample collection and analysis, B.E. and S.A.; sequencing and bioinformatics, G.W.; writing—original draft preparation, D.K.B. and G.W.; writing, review and editing, F.W.-M., B.E., H.K. and E.A.M.; project administration, D.K.B., F.W.-M. and H.K.; funding acquisition, D.K.B. and F.W.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the United States Armed Forces Health Surveillance Division (AFHSD) Global Emerging Infections Surveillance (GEIS) Branch under PROMIS ID P0119_18_KY_013.01 to DKB. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Data Availability Statement
The nucleotide sequences were submitted to the NCBI database and are available under Bioproject ID: PRJNA977092.
Acknowledgments
The authors acknowledge the support from staff and management of abattoir and slaughter points for facilitating the sample collections. Melissa Martin, Patrick McGann, and Anjali Sapre at the Walter Reed Army Research Institute (WRAIR)’s Multi-Drug-Resistant Organism Repository and Surveillance Network (MRSN) are acknowledged for the whole genome sequencing of the isolates and data analysis support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The views expressed are those of the authors and do not reflect the official policy or position of the Department of Defense or the US Government.
References
- Tawfick, M.M.; Elshamy, A.A.; Mohamed, K.T.; El Menofy, N.G. Gut Commensal Escherichia coli, a High-Risk Reservoir of Transferable Plasmid-Mediated Antimicrobial Resistance Traits. Infect. Drug Resist. 2022, 15, 1077–1091. [Google Scholar] [CrossRef]
- Clermont, O.; Christenson, J.K.; Denamur, E.; Gordon, D.M. The Clermont Escherichia coli phylo-typing method revisited: Improvement of specificity and detection of new phylo-groups. Environ. Microbiol. Rep. 2013, 5, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.; Tyson, G.H.; Strain, E.; Lindsey, R.L.; Strockbine, N.; Ceric, O.; Fortenberry, G.Z.; Harris, B.; Shaw, S.; Tillman, G.; et al. Use of Large-Scale Genomics to Identify the Role of Animals and Foods as Potential Sources of Extraintestinal Pathogenic Escherichia coli That Cause Human Illness. Foods 2022, 11, 1975. [Google Scholar] [CrossRef] [PubMed]
- Geurtsen, J.; de Been, M.; Weerdenburg, E.; Zomer, A.; McNally, A.; Poolman, J. Genomics and pathotypes of the many faces of Escherichia coli. FEMS Microbiol. Rev. 2022, 46, fuac031. [Google Scholar] [CrossRef] [PubMed]
- Tahamtan, Y.; Hayati, M.; Namavari, M.M. Prevalence and distribution of the stx1, stx2 genes in Shiga toxin producing E. coli (STEC) isolates from cattle. Iran. J. Microbiol. 2010, 2, 8–13. [Google Scholar]
- Braz, V.S.; Melchior, K.; Moreira, C.G. Escherichia coli as a Multifaceted Pathogenic and Versatile Bacterium. Front. Cell. Infect. Microbiol. 2020, 10, 548492. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Schwaber, M.J.; Carmeli, Y. Mortality and delay in effective therapy associated with extended-spectrum β-lactamase production in Enterobacteriaceae bacteraemia: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2007, 60, 913–920. [Google Scholar] [CrossRef]
- Tumbarello, M.; Sanguinetti, M.; Montuori, E.; Trecarichi, E.M.; Posteraro, B.; Fiori, B.; Citton, R.; D’Inzeo, T.; Fadda, G.; Cauda, R.; et al. Predictors of mortality in patients with bloodstream infections caused by extended-spectrum-β-lactamase-producing Enterobacteriaceae: Importance of inadequate initial antimicrobial treatment. Antimicrob. Agents Chemother. 2007, 51, 1987–1994. [Google Scholar] [CrossRef]
- Anastasi, E.M.; Matthews, B.; Stratton, H.M.; Katouli, M. Pathogenic Escherichia coli found in sewage treatment plants and environmental waters. Appl. Environ. Microbiol. 2012, 78, 5536–5541. [Google Scholar] [CrossRef]
- Gomi, R.; Matsuda, T.; Matsumur, Y.; Yamamoto, M.; Tanaka, M.; Ichiyam, S.; Yoneda, M. Whole-genome analysis of antimicrobialresistant and extraintestinal pathogenic Escherichia coli in river water. Appl. Environ. Microbiol. 2017, 83, e02703-16. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A. Animal reservoirs for extended spectrum β-lactamase producers. Clin. Microbiol. Infect. 2008, 14, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Javadi, M.; Bouzari, S.; Oloomi, M. Horizontal Gene Transfer and the Diversity of Escherichia coli. In Escherichia coli—Recent Advances on Physiology, Pathogenesis and Biotechnological Applications; InTech: London, UK, 2017. [Google Scholar]
- Manges, A.R.; Johnson, J.R. Reservoirs of Extraintestinal Pathogenic Escherichia coli. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.; Fredrick Wabwire, T.; Tumwine, G.; Waiswa, P. Antimicrobial Usage by Small-Scale Commercial Poultry Farmers in Mid-Western District of Masindi Uganda: Patterns, Public Health Implications, and Antimicrobial Resistance of E. coli. Vet. Med. Int. 2023, 2023, 6644271. Available online: https://www.hindawi.com/journals/vmi/2023/6644271/ (accessed on 5 May 2023). [CrossRef]
- Tooke, C.L.; Hinchliffe, P.; Bragginton, E.C.; Colenso, C.K.; Hirvonen, V.H.A.; Takebayashi, Y.; Spencer, J. β-Lactamases and β-Lactamase Inhibitors in the 21st Century. J. Mol. Biol. 2019, 431, 3472–3500. [Google Scholar] [CrossRef]
- de Lagarde, M.; Vanier, G.; Arsenault, J.; Fairbrother, J.M. High risk clone: A proposal of criteria adapted to the one health context with application to enterotoxigenic Escherichia coli in the pig population. Antibiotics 2021, 10, 244. [Google Scholar] [CrossRef]
- Iramiot, J.S.; Kajumbula, H.; Bazira, J.; De Villiers, E.P.; Asiimwe, B.B. Whole genome sequences of multi-drug resistant Escherichia coli isolated in a Pastoralist Community of Western Uganda: Phylogenomic changes, virulence and resistant genes. PLoS ONE 2020, 15, e0231852. [Google Scholar] [CrossRef]
- Bowden, R.; Israel, B. AST-VAST Liaison Report CLSI Subcommittee on VAST Update. 2020. Available online: https://clsi.org/meetings/ast-file-resources (accessed on 21 February 2021).
- Kong, Y. Btrim: A fast, lightweight adapter and quality trimming program for next-generation sequencing technologies. Genomics 2011, 98, 152–153. [Google Scholar] [CrossRef]
- Nederbragt, A.J. On the middle ground between open source and commercial software—The case of the Newbler program. Genome Biol. 2014, 15, 113. [Google Scholar] [CrossRef]
- Beghain, J.; Bridier-Nahmias, A.; Le Nagard, H.; Denamur, E.; Clermont, O. ClermonTyping: An easy-to-use and accurate in silico method for Escherichia genus strain phylotyping. Microb. Genom. 2018, 4, e000192. [Google Scholar] [CrossRef]
- Larsen, M.V.; Cosentino, S.; Rasmussen, S.; Friis, C.; Hasman, H.; Marvig, R.L.; Jelsbak, L.; Sicheritz-Pontén, T.; Ussery, D.W.; Aarestrup, F.M.; et al. Multilocus sequence typing of total-genome-sequenced bacteria. J. Clin. Microbiol. 2012, 50, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Jünemann, S.; Sedlazeck, F.J.; Prior, K.; Albersmeier, A.; John, U.; Kalinowski, J.; Mellmann, A.; Goesmann, A.; von Haeseler, A.; Stoye, J.; et al. Updating benchtop sequencing performance comparison. Nat. Biotechnol. 2013, 31, 294–296. [Google Scholar] [CrossRef]
- Joensen, K.G.; Tetzschner, A.M.M.; Iguchi, A.; Aarestrup, F.M.; Scheutz, F. Rapid and easy in silico serotyping of Escherichia coli isolates by use of whole-genome sequencing data. J. Clin. Microbiol. 2015, 53, 2410–2426. [Google Scholar] [CrossRef] [PubMed]
- Roer, L.; Tchesnokova, V.; Allesøe, R.; Muradova, M.; Chattopadhyay, S.; Ahrenfeldt, J.; Thomsen, M.C.F.; Lund, O.; Hansen, F.; Hammerum, A.M.; et al. Development of a Web Tool for Escherichia coli Subtyping Based on fimH Alleles. J. Clin. Microbiol. 2017, 55, 2538–2543. Available online: https://journals.asm.org/journal/jcm (accessed on 6 June 2023). [CrossRef] [PubMed]
- Arredondo-Alonso, S.; Rogers, M.R.C.; Braat, J.C.; Verschuuren, T.D.; Top, J.; Corander, J.; Willems, R.J.L.; Schürch, A.C. mlplasmids: A user-friendly tool to predict plasmid-and chromosome-derived sequences for single species. Microb. Genom. 2018, 4, e000224. [Google Scholar] [CrossRef]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef]
- Joensen, K.G.; Scheutz, F.; Lund, O.; Hasman, H.; Kaas, R.S.; Nielsen, E.M.; Aarestrup, F.M. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J. Clin. Microbiol. 2014, 52, 1501–1510. [Google Scholar] [CrossRef]
- Najjuka, C.F.; Kateete, D.P.; Kajumbula, H.M.; Joloba, M.L.; Essack, S.Y. Antimicrobial susceptibility profiles of Escherichia coli and Klebsiella pneumoniae isolated from outpatients in urban and rural districts of Uganda. BMC Res. Notes 2016, 9, 235. [Google Scholar] [CrossRef]
- Masiga, F.; Kigozi, E.; Najjuka, C.F.; Kajumbula, H.; Kateete, D.P. Diarrhoeagenic Escherichia coli isolated from children with acute diarrhoea at Rakai hospital, Southern Uganda. Afr. Health Sci. 2022, 22, 581–588. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, X.; Li, J.; Lv, N.; Liu, F.; Wu, J.; Lin, I.Y.C.; Wu, N.; Weimer, B.C.; Gao, G.F.; et al. The bacterial mobile resistome transfer network connecting the animal and human microbiomes. Appl. Environ. Microbiol. 2016, 82, 6672–6681. [Google Scholar] [CrossRef]
- Westphal-Settele, K.; Konradi, S.; Balzer, F.; Schönfeld, J.; Schmithausen, R. The environment as a reservoir for antimicrobial resistance: A growing problem for public health? Bundesgesundheitsblatt Gesundheitsforsch. Gesundheitsschutz 2018, 61, 533–542. [Google Scholar] [CrossRef]
- Aarestrup, F.M. The livestock reservoir for antimicrobial resistance: A personal view on changing patterns of risks, effects of interventions and the way forward. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140085. [Google Scholar] [CrossRef] [PubMed]
- Kocsis, B.; Gulyás, D.; Szabó, D. Emergence and Dissemination of Extraintestinal Pathogenic High-Risk International Clones of Escherichia coli. Life 2022, 12, 2077. [Google Scholar] [CrossRef] [PubMed]
- Leimbach, A.; Hacker, J.; Dobrindt, U. E. coli as an all-rounder: The thin line between commensalism and pathogenicity. Curr. Top. Microbiol. Immunol. 2013, 358, 3–32. [Google Scholar]
- Sano, E.; Esposito, F.; Fontana, H.; Fuga, B.; Cardenas-Arias, A.; Moura, Q.; Cardoso, B.; Costa, G.C.V.; Bosqueiro, T.C.M.; Sinhorini, J.A.; et al. One health clones of multidrug-resistant Escherichia coli carried by synanthropic animals in Brazil. One Health 2023, 16, 100476. [Google Scholar] [CrossRef] [PubMed]
- Muloi, D.M.; Wee, B.A.; McClean, D.M.H.; Ward, M.J.; Pankhurst, L.; Phan, H.; Ivens, A.C.; Kivali, V.; Kiyong’a, A.; Ndinda, C.; et al. Population genomics of Escherichia coli in livestock-keeping households across a rapidly developing urban landscape. Nat. Microbiol. 2022, 7, 581–589. [Google Scholar] [CrossRef]
- Bojesen, A.M.; Ahmed, U.; Skaarup, H.; Espinosa-Gongora, C. Recurring outbreaks by the same Escherichia coli ST10 clone in a broiler unit during 18 months. Vet. Res. 2022, 53, 2. [Google Scholar] [CrossRef]
- Karakaya, E.; Aydin, F.; Kayman, T.; Abay, S. Escherichia coli in different animal feces: Phylotypes and virulence genes. World J. Microbiol. Biotechnol. 2023, 39, 14. [Google Scholar] [CrossRef]
- Stojević, D.; Humski, A.; Mikulić, M.; Dobranić, V.; Reil, I.; Duvnjak, S.; Benić, M.; Beck, R.; Cvetnić, Ž. Prevalence of virulence genes among Escherichia coli strains isolated from food and carcass swabs of different animal origins in Croatia. J. Vet. Res. 2022, 66, 395–402. [Google Scholar] [CrossRef]
- Jacoby, G.A. AmpC Β-Lactamases. Clin. Microbiol. Rev. 2009, 22, 161–182. [Google Scholar] [CrossRef]
- Philippon, A.; Arlet, G.; Jacoby, G.A. Plasmid-determined AmpC-type β-lactamases. Antimicrob. Agents Chemother. 2002, 46, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A Review on Antibiotic Resistance: Alarm Bells are Ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef] [PubMed]
- Nayiga, S.; Kayendeke, M.; Nabirye, C.; Willis, L.D.; Chandler, C.I.R.; Staedke, S.G. Use of antibiotics to treat humans and animals in Uganda: A cross-sectional survey of households and farmers in rural, urban and peri-urban settings. JAC Antimicrob. Resist. 2020, 2, dlaa082. [Google Scholar] [CrossRef] [PubMed]
- Mikecz, O.; Pica-Ciamarra, U.; Felis, A.; Nizeyimana, G.; Okello, P.; Brunelli, C. Data on antimicrobial use in livestock: Lessons from Uganda. One Health 2020, 10, 100165. [Google Scholar] [CrossRef]
- Paintsil, E.K.; Ofori, L.A.; Akenten, C.W.; Fosu, D.; Ofori, S.; Lamshöft, M.; May, J.; Danso, K.O.; Krumkamp, R.; Dekker, D. Antimicrobial usage in commercial and domestic poultry farming in two communities in the ashanti region of ghana. Antibiotics 2021, 10, 800. [Google Scholar] [CrossRef]
- Turkovicova, L.; Smidak, R.; Jung, G.; Turna, J.; Lubec, G.; Aradska, J. Proteomic analysis of the TerC interactome: Novel links to tellurite resistance and pathogenicity. J. Proteom. 2016, 136, 167–173. [Google Scholar] [CrossRef]
- Mason, S.; Vornhagen, J.; Smith, S.N.; Mike, L.A.; Mobley, H.L.T.; Bachman, M.A. The Klebsiella pneumoniae ter Operon Enhances Stress Tolerance. Infect. Immun. 2023, 91, e0055922. [Google Scholar] [CrossRef]
- Vornhagen, J.; Bassis, C.M.; Ramakrishnan, S.; Hein, R.; Mason, S.; Bergman, Y.; Sunshine, N.; Fan, Y.; Holmes, C.L.; Timp, W.; et al. A plasmid locus associated with Klebsiella clinical infections encodes a microbiomedependent gut fitness factor. PLoS Pathog. 2021, 17, e1009537. [Google Scholar] [CrossRef]
- Huang, X.; Yang, X.; Shi, X.; Erickson, D.L.; Nagaraja, T.G.; Meng, J. Whole-genome sequencing analysis of uncommon Shiga toxin-producing Escherichia coli from cattle: Virulence gene profiles, antimicrobial resistance predictions, and identification of novel O-serogroups. Food Microbiol. 2021, 99, 103821. [Google Scholar] [CrossRef]
- Reyes-Rodríguez, N.E.; Barba-León, J.; Navarro-Ocaña, A.; Vega-Sánchez, V.; de Anda, F.R.G.; Talavera-González, J.M.; Talavera-Rojas, M. Serotypes and Stx2 subtyping of Shiga toxin producing Escherichia coli isolates from cattle carcasses and feces. Rev. Mex. Cienc. Pecu. 2021, 11, 1030–1044. [Google Scholar]
- Bien, J.; Sokolova, O.; Bozko, P. Role of uropathogenic Escherichia coli virulence factors in development of urinary tract infection and kidney damage. Int. J. Nephrol. 2012, 2012, 681473. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).