Abstract
Pathogens that play a role in the development and progression of periodontitis have gained significant attention due to their implications in the onset of various systemic diseases. Periodontitis is characterized as an inflammatory disease of the gingival tissue that is mainly caused by bacterial pathogens. Among them, Porphyromonas gingivalis, Treponema denticola, Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans, and Tannerella forsythia are regarded as the main periodontal pathogens. These pathogens elicit the release of cytokines, which in combination with their virulence factors induce chronic systemic inflammation and subsequently impact neural function while also altering the permeability of the blood–brain barrier. The primary objective of this review is to summarize the existing information regarding periodontal pathogens, their virulence factors, and their potential association with neuroinflammation and neurodegenerative diseases. We systematically reviewed longitudinal studies that investigated the association between periodontal disease and the onset of neurodegenerative disorders. Out of the 24 studies examined, 20 showed some degree of positive correlation between periodontal disease and neurodegenerative disorders, with studies focusing on cognitive function demonstrating the most robust effects. Therefore, periodontal pathogens might represent an exciting new approach to develop novel preventive treatments for neurodegenerative diseases.
1. Introduction
The oral cavity comprises a diverse array of microbiological environments, owing to the local dynamic mechano-chemical conditions. These fluc.tuations in the environment give rise to multiple ecological niches that support the growth of a wide range of oral pathogens. To date, over 700 species of microorganisms have been identified in the oral cavity, out of which 500 are bacterial species [1]. These species are mostly comprised of several phyla including Actinobacteria, Proteobacteria, Firmicutes, Bacteroidetes, Tenericutes, Euryarchaeota, Chlamydiae, and Spirochaetes [2].
One location within the oral cavity that is particularly conducive to bacterial growth is the gingiva, the tissue surrounding the base of the teeth [3]. The gingiva, owing to its proximity to the teeth and its rich nutrient supply, can be susceptible to bacterial overpopulation in the event of poor oral hygiene. Such bacterial proliferation in the gingival sulcus can lead to the development of gingivitis, a common oral disease [4].
Gingivitis and Its Progression to Periodontitis
Gingivitis is a common form of gum disease that can cause irritation and inflammation of the gingival tissue. The onset of gingivitis is multifactorial and can be caused by the formation of plaque, as well as changes in nutrition, hormone levels, and drug abuse [4]. Specific species are implicated in the initiation and progression of gingivitis and include species of Streptococci, Fusobacteria, Actinomyces, Veillonella, Leptotrichia, Prevotella, Treponema, with Bacteroides, Capnocytophaga, and Eikenella also possibly playing an important role [5].
The disease has four stages of progression, with each stage increasing in severity and microbial diversity. As characterized by Page and Schroeder, the stages of gingivitis are “initial lesion” “early lesion”, “established lesion”, and “advanced lesion” [4]. The initial lesion is an acute inflammation that is followed by increased gingival fluid flow and recruitment of neutrophils. The early lesion is characterized by lymphoid cell infiltration (predominantly of T cells). This is also the stage where clinical signs of gingivitis, such as redness and bleeding, start appearing. Progressive worsening of the clinical condition leads to the formation of established lesions called gingival pockets, in which B lymphocytes and plasma cells predominate. This phase can either persist indefinitely, revert to an earlier stage, or progress to an advanced lesion, which is a transition phase to another disease called periodontitis [4,6]. Currently, 20–50% of the global population is affected by some form of periodontal disease, with 10.8% of them displaying symptoms of severe chronic periodontitis [7,8].
It is not well understood how changes in the oral microbiota contribute to the progression of gingivitis, but evidence suggests that some hosts are more susceptible to the disease. These individuals show a tendency to more rapid clinical deterioration due to the differences in their baseline healthy oral microbiota. Such hosts had an increase in certain bacteria from the genera Selenomonas, Lachnospiraceae, Peptococci, Bacteroidaceae, Peptostreptococci, Oribacteria, and Veillonellaceae, as well as a decrease in Abiotrophia [9].
The progression of the disease to periodontitis is affected by multiple local and systemic etiological factors. For that reason, the presence of microbial biofilm is not always sufficient for the pathogenesis of periodontitis. Instead, the disease arises from an imbalance between the host and the microbial biofilm. Further understanding of the imbalance that presets the disease is challenging, given the large variation in the oral microbiota, dental plaque, and the host genetic and immune system profiles [10]. Studies suggest that during gingivitis there is an increase in both the richness and diversity of the subgingival microbiota, and while microbiome diversity in periodontitis remains increased, some species become dominant, reducing the overall diversity when compared to gingivitis [11].
The untreated inflammation caused by periodontal pathogens spreads to the deeper tissue of the teeth and causes tissue attachment loss, followed by the migration and withdrawal of the junctional epithelium. These changes result in the formation of periodontal ‘pockets’, which are considered a hallmark of periodontitis [4]. Prolonged inflammation of the deeper tissues causes an alteration in bone homeostasis and can result in the complete disintegration of the connecting tissue followed by tooth loss [4,10].
2. Periodontal Pathogens, Virulence Factors and Host Response
The prevalence of certain bacterial species and their virulence factors have been shown to be directly related to susceptibility, installation, and progression of periodontitis. Bacterial species present in the subgingival biofilms were sorted by color into six bacterial complexes [12]. The discussion of which species are particularly virulent and initiate the disease is still ongoing, but a specific group of bacteria characterized as the red complex is considered to encompass the most important pathogens in adult periodontal disease. This complex comprises of Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia [10,13]. Additionally, some bacteria from outside this complex, such as Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans, Prevotella species, Eikenella corrodens, Peptostreptococcus micros, and Campylobacter rectus, have been shown to play a possible role in disease pathogenesis [10,13,14]. As of now, P. gingivalis, T. denticola, T. forsythia, A. actinomycetemcomitans, and F. nucleatum are regarded as the principal periodontal pathogens [10,14].
2.1. Porphyromonas gingivalis
P. gingivalis is a Gram-negative anaerobic bacteria that has been identified as a keystone pathogen in the development of periodontitis. Its prevalence is directly associated with the severity of the disease, leading to extensive research on the bacteria’s role in oral health as well as its association with other diseases [15,16].
P. gingivalis has several specialized properties and virulence factors that contribute to its unique impact on the host [15]. One such property is the shedding of outer membrane vesicles (OMVs), which contain the bacterium’s virulence factors, notably gingipains and lipopolysaccharide (LPS) [17,18]. Gingipains are cell surface trypsin-like cysteine proteinases that are crucial for the pathogenicity of P. gingivalis, consisting of lysine-specific (Kgp) and arginine-specific (RgpA and RgpB) proteinases [19]. These enzymes are potent pro-inflammatory factors that trigger the release of the IL-1β and TNF-α via NLR family pyrin domain-containing protein 3 (NLRP3) while also regulating the host’s response by cleaving IL-1β and TNF-α and degrading macrophage CD14 which is crucial for LPS-induced activation of the leukocytes [19,20].
In addition to their pro-inflammatory effects, gingipains also inhibit the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathway, facilitate bacterial coaggregation with other oral bacteria. They also dysregulate the complement cascade by cleavage of C3 into C3a and C3b followed by subsequent degradation of C3a and C3b, preventing the formation of C5 convertase [20]. Gingipains play a particularly important role in periodontitis by enhancing vascular permeability through the activation of the kallikrein/kinin pathway. Kgp is primarily involved in gingival bleeding, while RgpA and RgpB activate coagulation factors and degrade fibrinogen/fibrin [19,20]. LPS also plays a role in promoting tissue destruction through its pro-inflammatory response upon binding with TLR-2 and TLR-4 [20]. This causes a downstream activation of signaling pathways such as activator protein 1 (AP-1), nuclear factor kappa B (NF-κB), mitogen-activated protein kinase (MAPK), c-Jun N-terminal kinases (JNK), and PI3K/Akt, which, in turn, promotes the release of pro-inflammatory factors such as IL-1β, IL-6, and IL-8 and up-regulation of Th17 cell differentiation with LPS derived from a more virulent strain, having a higher impact [15,20,21,22,23]. P. gingivalis also causes SerB phosphatase-dependent alveolar bone loss, partially through stimulation of macrophages [24,25]. Some other important factors of P. gingivalis are peptidylarginine deiminase (PADs), heat-shock proteins (GroEL), and an unidentified soluble heat-stable component that was shown to promote lymphocyte apoptosis in peripheral blood mononuclear cells [15,26]. Besides its virulence factors, P. gingivalis is also able to directly cause a pro-inflammatory response through a fimbria-dependent infection of dendritic cells. This causes dendritic cells to undergo maturation, causing a secretion of IL-1β, IL-6, TNF-α, IL-10, IL-12, IFN-γ, and dendritic cell-induced proliferation of autologous CD4(+) T cells [27].
P. gingivalis is also able to evade the immune system through a number of mechanisms, including the production of immunoglobulin-binding proteins and the suppression and evasion of phagocytosis [28,29]. To further avoid the host immune system, the bacteria can replicate inside autophagosome vacuoles in human coronary artery endothelial cells [30].
It is difficult to determine the exact impact that P. gingivalis has on the host due to its genetic variability between strains. To date, genomic sequences of 94 strains have been determined, with a high degree of variation in their pathogenicity and functional properties [31]. Most of the observed differences in virulence between strains result from differences in the presence of the capsule and its composition, hemagglutinins, fimbriae composition and abundance, and gingipain production [32,33]. Notably, certain P. gingivalis strains are predominantly found in healthy individuals, while others are highly associated with the development and severity of periodontitis. In particular, the CP3 strain of P. gingivalis, isolated from chronic periodontitis patients, has been found to exhibit six-fold higher invasion capabilities than the H3 strain, which in contrast was isolated from healthy individuals [32]. The main strains that are currently used in research are the less virulent ATCC 33277 and FDC 381, and the more virulent W50 and W83 Table 1 [34].

Table 1.
Comparison and summary of the main differences between widely used strains of P. gingivalis.
2.2. Tannerella forsythia
T. forsythia (formerly referred to as Bacteroides forsythia) is a Gram-negative anaerobic pathogen and a principal agent of periodontitis. However, due to difficulties in cultivating this organism, research on T. forsythia has been limited until recently [41]. In vitro studies have demonstrated that T. forsythia can cause alveolar bone reabsorption and cutaneous abscesses in mice [41].
Unlike some other pathogens, T. forsythia utilizes proteins as a source of energy and is unable to breakdown sugars [42]. To break the peptides, the bacteria employ trypsin-like proteases and cysteine-like proteases (PrtH) [41]. While trypsin-like proteases contribute little to the disease, PrtH is the major primary virulence factor of T. forsythia studied to date. PrtH was initially described as Forsythia detaching factor because of its role in cell separation and destruction of the subgingival epithelium [41,43]. Additionally, PrtH has been shown to increase the mitochondrial oxidative membrane potential in vitro, thereby stimulating the production of IL-8 [44]. The leucine-rich repeat cell-surface and secreted protein, BspA, has been shown to be required for attachment to and invasion of epithelial cells and can induce chemokine expression via binding with TLR2 [45]. It was also observed that BspA can alter the progression of atherosclerotic lesions in ApoE(−/−) mice [46]. T. forsythia also produces an electrophilic compound, methylglyoxal, which can generate inflammatory adducts by covalent modification of amino acid side chains to create advanced glycation end-products (AGEs). The resulting AGEs increase pro inflammatory and pro-osteoclastogenic activity [47]. Other interesting virulence factors of T. forsythia include GroEL and karilysin. GroEL is a heat shock protein that synergizes with cytokine IL-17, stimulating inflammatory bone resorption [48]. Karilysin is a matrix metalloprotease-like enzyme that can regulate TNF-α concentration in serum by promoting functional TNF-α shedding from macrophage surfaces through proteolytic cleavage, increasing the total TNF-α concentration [49,50].
Besides proteolytic activity, T. forsythia possesses immune evasion capabilities, in part thanks to its S-layer, which is a unique surface layer formed of the glycosylated proteins TfsA-GP and TfsB-GP [51]. It impacts the adhesion and invasion of cells, bacterial coaggregation and attenuates the expression of pro-inflammatory cytokine by evading immune recognition [52,53,54]. Furthermore, the metalloproteinase karilysin inhibits all pathways of the complement system and is involved in the bacteria’s serum resistance [55]. Interestingly, T. forsythia also exhibits cytopathic activity arresting cells at G2. However, the underlying factor remains to be identified [43].
The impact of strain variances on the behavior of T. forsythia remains poorly understood. Even so, some differences have been noted between the primary T. forsythia strains, which mostly involve changes in biofilm formation and the bacterium’s specificity and binding to P. gingivalis [56,57]. Nonetheless, some strain-dependent changes in host immunological responses have also been observed. For instance, the T. forsythia UB20 strain was shown to induce higher secretion of chemokine protein CXCL10 than the ATCC 43037 strain, possibly due to the difference in LPS [58].
2.3. Treponema denticola
T. denticola is Gram-negative anaerobic bacteria from the Spirochetes family with high motility and proteolytic ability. It triggers an immunological response mainly via TLR2, predominantly through its periplasmic flagellum [59]. The outer membrane of the bacteria, also known as the outer sheath, contains two main pro-inflammatory factors: major sheath protein (MSP) and lipooligosaccharide (LOS). MSP was shown to have a cytotoxic pore-forming activity and induce a pro-inflammatory response (TNF-α, IL-1β, IL-6, and MMP-9) while also being able to disrupt the intracellular regulatory pathways of infected host cells [60,61,62]. LOS, on the other hand, can bind to extracellular matrix proteins, mucosal cells, and oral bacteria [63]. It can also affect binding to other bacteria, thereby increasing their pro-inflammatory potential [63]. Macrophage exposure to LOS and MSP induces tolerance to further stimulation with enterobacterial LPS [62]. Another major virulence factor of T. denticola is the prolyl phenylalanine-specific peptidase dentilisin, which is a surface-expressed protease complex comprised of three lipoproteins. It degrades host cell proteins, stimulates tissue destruction in a TLR2/MyD88/Sp1-dependent fashion, and activates polymorphonuclear neutrophils via the complement C3 pathway [64,65]. Furthermore, dentilisin contributes to the motility of the bacterium by facilitating crawling-dependent surface spreading [66].
Interestingly, T. denticola has a pronounced ability to evade and suppress immunological responses even when compared within its own genus [67]. T. denticola fails to induce RANTES, IL-8, and human beta-defensin-2 messenger RNA response in human gingival epithelial cells [68]. Instead, it reduces protein expression of human beta-defensins (HBDs, reduced by 40%), TNF-α and IL-8. This is in part due to the inhibition of the TLR2 axis by unknown heat-labile inhibitor(s) and dentilisin-dependent cleavage of TNF-α which subsequently also causes a downregulation of IL-8, HBD-2, and HBD-3, significantly dampening the host’s immunological response [68,69,70]. Dentilisin is also responsible for the direct degradation of IL-8 and some other immunological factors, most importantly IgG [71,72]. Despite this, the bacteria still induces minimal macrophage-mediated inflammation [72].
Recent studies have identified significant strain variation in the biofilm formation capabilities of T. denticola. This could be partially dependent on the bacterium’s mobility, which was shown to differ greatly between strains [73].
2.4. Fusobacterium nucleatum
F. nucleatum is a Gram-negative, anaerobic oral commensal, that plays a crucial role in the formation of dental plaque through its ability to strongly coaggregate with other bacteria [74]. Importantly, F. nucleatum and its subspecies have been found to be among the most abundant bacteria in cases of periodontitis [11]. While the bacteria has primarily been implicated in this oral disease, recent evidence has also linked it to a variety of systemic illnesses, including adverse pregnancy outcomes, rheumatoid arthritis, organ abscesses, and gastrointestinal disorders, most importantly colorectal cancer [74]. Experimental data also suggests that F. nucleatum is capable of causing systemic inflammation, promoting cancer metastasis, and increasing drug resistance [74,75,76].
In the context of periodontitis, F. nucleatum has been shown to increase the expression of cytokine and chemokine genes, such as those encoding for TNF-α, IL-8, IL-6, CCL2, and CXCL1 while also being capable of significantly inhibiting cell proliferation of gingival-derived mesenchymal stem cells and gingival fibroblasts [74,77]. Additionally, in gingival fibroblasts, the bacteria induces ROS generation and apoptosis through activation of the AKT/MAPK and NF-κB signaling pathways [78]. Its LPS is known to cause inflammatory degradation of host tissue and bone resorption, mainly by stimulating MMP-9 secretion from macrophages through the TLR4/MyD88 axis [79].
The primary virulence factor of F. nucleatum is FadA, an adhesin protein that is required for the invasion and adhesion of the bacterium to host cells, while also increasing the permeability of endothelial cells to other bacteria [80]. The bacteria also secrete fusolisin, a serine protease that degrades extracellular matrix proteins and IgA [81]. As mentioned previously, an important property of the bacteria is assistance in bacterial coaggregation, in which the outer membrane proteins RadD and Fap2 function as adhesins, binding to a variety of bacteria while also inducing lymphocyte apoptosis [74,80]. Furthermore, F. nucleatum has been shown to have the capability to modulate the host cell transcriptome and epigenome. On exposure to the bacteria, human colonic epithelial cells display changes in gene expression associated with p53 degradation-induced proliferation and release of ROS, while in human carotid artery endothelial cells, exposure causes overexpression of EFNA1 and LIF, two genes connected to colorectal cancer and down regulation of multiple histone modifications related genes [82]. It was also shown that F. nucleatum can modulate the osteogenic and dentinogenic potential of human stem cells from the Apical Papilla (tissue located at the base of the developing dental root) in vitro [83].
F. nucleatum is a heterogenous species, exhibiting considerable genetic variability, with the number of F. nucleatum-specific genes varying in count up to ten-fold between strains [80,84]. For that reason, F. nucleatum is currently subdivided into four subspecies: animalis, nucleatum, polymorphum, and vincentii (which includes fusiforme) [80]. These subspecies exhibit varying production of virulence factors, as well as differing in the ability to form biofilms and attach to and invade cells due to changes in their surface-expressed glycans and proteins [80,84,85]. For instance, F. nucleatum ssp. polymorphum is the only subspecies that failed to form a single-subspecies biofilm in vitro, likely due to its less conserved adhesion proteins CmpA and Fap2 [85].
2.5. Aggregatibacter actinomycetemcomitans
Another significant periodontal pathogen is A. actinomycetemcomitans, a Gram-negative non-motile anaerobe. It is mainly known for its early colonization of the gingiva and its high association with the severity and progression of periodontitis [86]. The bacteria’s LPS stimulates collagen phagocytosis, alongside IL-8 and IL-6 production from gingival fibroblasts and an increase in IL-12, IFN-γ, TNF-α, IL-1β, and IL-6 production in dendritic cells [87,88,89]. Its LPS has also been shown to be a potent stimulant of ROS in neutrophils [90]. In addition to LPS, A. actinomycetemcomitans produces several other virulence factors, including leukotoxins and cytolethal distending toxins (CDTs). Leukotoxins, which are localized on the outer membrane of the bacterium and are secreted into the serum, bind to the lymphocyte function-associated antigen 1 (LFA-1) CD18 subunit and cause a suppressed immune response, allowing for the progression of periodontitis [91]. The effects of leukotoxins on different leukocyte populations vary with neutrophils, monocytes, and other cells being affected differently [91]. There is a great difference in genetic diversity of A. actinomycetemcomitans, with substantially different virulence properties, partially owing to a difference in leukotoxin production [86]. CDTs are bacterial protein exotoxins that are expressed by several Gram-negative species. They are genotoxins that cause DNA damage, cell cycle arrest, and eventually apoptosis and are highly toxic to immune cells, including T cells, B cells, and mononuclear cells [92]. CDTs were also shown to stimulate the production of pro-inflammatory factors in peripheral blood mononuclear cells and osteolytic cytokines in periodontal connective tissue cells, such as RANKL, which leads to bone resorption in periodontitis [93].
Currently there are seven different serotypes of A. actinomycetemcomitans (a–g), which are differentiated based on their surface antigens, with certain serotypes exhibiting higher pathogenicity than others [94]. This variation in pathogenicity is believed to be primarily due to changes and deletions within the promoter region of a gene that regulates leukotoxin expression [94,95]. Notably, some highly pathogenic strains that belong to the group of serotype b strains (designated as JP2 clone) exhibit a 530 base pair deletion in the promotor region, resulting in a 10 to 20-fold increased production of leukotoxin in comparison with other strains [94,96].
A summary of the main virulence mechanisms of each bacterium can be seen in Table 2.

Table 2.
Summary of the main virulence factors of the principle periodontal pathogens.
2.6. Synergistic Behavior
Periodontal bacteria rarely exist in isolation and require the presence of other bacterial species for optimal growth and function [102]. Understanding the underlying synergistic interactions among periodontal bacteria is crucial for comprehending oral microbial pathogenesis and its implications for neurodegenerative disorders. Co-aggregation, modulation of virulence factors, and the impact of nutrient availability, all play an important role in the formation of the disease’s phenotype [103].
Among the main periodontal pathogens, P. gingivalis has been extensively studied for its interactions with other bacteria, demonstrating synergistic interactions with all of them. For instance, it directly co-aggregates with F. nucleatum and T. denticola forming complex biofilms [104]. Gingipains that are released from P. gingivalis support the growth of various microorganisms by increasing nutrient availability while also protecting the bacteria from the immunological response of the host [14]. Additionally, gingipains enhance the attachment and invasion of T. forsythia to epithelial cells [105]. P. gingivalis increases the free glycine in the surrounding media, which was shown to support T. denticola growth [106,107]. The bacteria also enhances biofilm formation by F. nucleatum by releasing diffusible signaling molecules [108].
On the other hand, F. nucleatum was shown to enhance the growth of P. gingivalis in the presence of oxygen, which suggests that F. nucleatum is capable of lowering local redox potential, thus benefiting the growth of more oxygen-sensitive anaerobic organisms [109]. Mixed infection with F. nucleatum was also shown to strengthen the invasion capacity of P. gingivalis in oral epithelial cells [110]. Co-culture with T. denticola results in an increase in P. gingivalis biomass, possibly through succinate released from T. denticola, which has been shown to alleviate the heme requirement of P. gingivalis when grown in a heme-limited environment [107]. Sonicated extracts of T. forsythia stimulate growth of P. gingivalis in nutrition-decreased medium, possibly due to a protein acting as a growth-promoting factor [111]. While the extent of interactions between A. actinomycetemcomitans and P. gingivalis are not yet fully studied, a synergistic effect on pathogenicity has been reported [112].
Other periodontal pathogens, although less studied, also exhibit various synergistic interactions. T. denticola MSP binds to F. nucleatum and facilitates bacterial coaggregation, while F. nucleatum promotes bacterial growth [104,113]. It was also noted that F. nucleatum and T. forsythia develop synergistic interactions when co-cultured [114]. Hydrolyzed β-glucan released by T. forsythia β-glucanase supports F. nucleatum growth, while T. forsythia can utilize muropeptides derived from F. nucleatum [115,116]. Co-culture of T. forsythia along with other periodontal pathogens enhanced the formation of abscesses in rabbits and mice, while also inducing a synergistic effect on alveolar bone loss [117]. Some of the more prominent interactions can be seen in Figure 1.
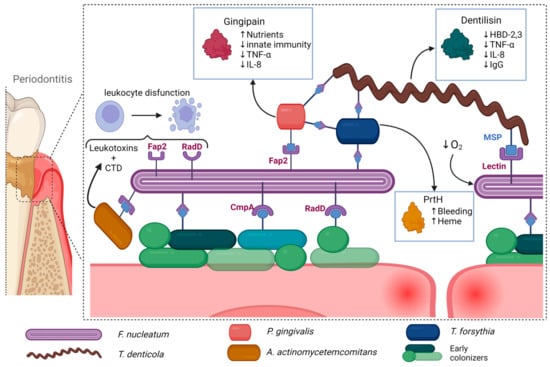
Figure 1.
Periodontal pathogens exhibit a range of synergistic interactions that facilitate further bacterial growth and proinflammatory pathways. For instance, certain species such as F. nucleatum and T. denticola act as scaffolding bacteria, binding to other periodontal pathogens and allowing for the formation of complex biofilms. T. denticola also exhibits a strong immunoregulatory effect, reducing the efficiency of the host’s immune response, while F. nucleatum decreases local oxygen concentration, further favoring the growth of periodontal pathogens. Other species such as P. gingivalis produce gingipains that increase nutrient availability, while also reducing the effectiveness of the host’s immunological response. A. actinomycetemcomitans also exhibits a strong immunoregulatory effect through leukotoxin and CTD, which inhibit the function of leukocytes. T. forsythia further degrades epithelial cells, increasing nutrient and heme availability. Taken together, these interactions contribute to the progression of periodontitis and its potential systemic consequences. Created with BioRender.com, accessed on 3 July 2023.
2.7. Mechanisms of Systemic Inflammation and Its Correlation to Periodontitis
The infection of periodontal pathogens and subsequent release of cytokines have been shown to induce low-grade, chronic systemic inflammation in patients. Elevations of pro-inflammatory mediators, such as IL-1, IL-6, C-reactive protein (CRP), and fibrinogen, as well as increased neutrophil count in the blood, have been observed in individuals with periodontal disease [118,119,120]. Additionally, successful periodontal treatment has been shown to reverse systemic inflammatory marker levels in the blood, suggesting a causal relationship between periodontal disease and systemic inflammation [119].
The underlying mechanism of this phenomenon is believed to be the increase of bacterial load, which leads to a weakening of the barrier separating the biofilm from the blood stream, allowing for the dissemination of pro-inflammatory factors from the periodontal bacteria and infected tissue [121,122]. This process may also permit bacterial products, such as lipopolysaccharides, outer membrane vesicles, and proteases, to reach the circulation, resulting in bacteremia as shown in Figure 2 [121]. Furthermore, certain periodontal bacteria possess the ability to disseminate to other parts of the body, likely via the bloodstream, although recent evidence suggests that other bacteria, like T. denticola, may utilize the lymphatic system instead [16,123].
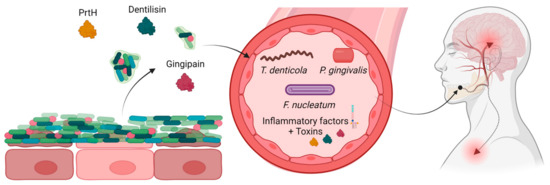
Figure 2.
Periodontitis produces bacterial toxins and proinflammatory factors, which alongside detached bacteria can enter the blood stream, disseminating and possibly causing neural inflammation. Created with BioRender.com, accessed on 12 May 2023.
This capability to disseminate further enhances systemic inflammation and may help explain the link between periodontitis and a range of systemic diseases. Such diseases include cardiovascular disease, rheumatoid arthritis, respiratory diseases, chronic kidney disease, metabolic disease (such as type II diabetes, and metabolic syndrome-related obesity), renal disease, non-alcoholic fatty liver disease, gut microbiome-related disorder (such as adverse pregnancy outcomes, celiac disease, inflammatory bowel disease, and irritable bowel syndrome), impairment of cognitive function, asthma, allergy, wound closure, cancers, aneurysms, stroke, microvascular defects, age-related disorders, regenerative, and stem cell dysfunction [122,124]. In recent years, exploring the linkages between periodontal pathogens and chronic diseases has been a focal point of extensive research endeavors. In addition to the well-documented impact of the inflammatory response, several theories have been proposed to clarify the underlying mechanisms driving this intricate relationship, including the release of specific factors that facilitate disease progression, cellular infections leading to alterations in protein expression that favor pathogenesis, interactions with receptors, and immunomodulatory effects [125].
The relationship between inflammation and altered brain function can be seen through elevated serum levels of pro-inflammatory cytokines, such as IL-1, IL-6, and TNF-α, in patients with depression [126]. Tricyclic antidepressants can inhibit cytokine release in immune cells, while therapies involving IFN- α, and IL-2 can result in depression-like symptoms [127,128,129].
It is noteworthy that periodontal pathogens have consistently been detected in the interdental biofilm of periodontally healthy subjects, challenging the assumption that these bacteria are exclusive to those with clinical inflammation [130,131,132]. Observed periodontal bacteria, specifically from the red complex, have been positively linked to an increased risk of caries through the disruption of the local microbial flora [132]. Moreover, a reduction in the levels of red and orange Socransky complexes bacteria in subjects that display no signs of gingivitis has been associated with a significant decrease in interdental inflammation [131]. This suggests that the pathogenic processes of periodontal bacteria can be triggered in healthy subjects and cause a state of para-inflammation, preceding the manifestation of clinical symptoms by several years or decades.
3. Neurodegenerative and Periodontal Disease
Neurodegenerative diseases are a diverse array of conditions that affect the central and peripheral nervous system and includes Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS), amyloid lateral sclerosis (ALS), Huntington’s disease, and various other neurodegenerative disorders [133]. It is characterized by impaired and eventual loss of neuronal function, often culminating in cognitive decline, intellectual deterioration, and dementia. While neurodegenerative diseases all have different pathways of onset and progression, they all share similar mechanisms which can in the majority be grouped in one of four categories: 1—Genetic Predisposition and Environmental Factors; 2—Protein Dysregulation, Aggregation, and Neurodegeneration; 3—Neuroinflammation and Inflammatory Factors; 4—Blood–brain barrier Dysfunction and Peripheral Immune Activation [134]. Over the past decade, neuroinflammation has been getting more attention due to discoveries of its vast implications for all neurodegenerative diseases. It is characterized by the activation of glial cells and the release of inflammatory factors [134]. Probably the best known neuroinflammatory disorder is multiple sclerosis which is considered a T-cell mediated autoimmune disease and is characterized by continuous neuroinflammation and demyelination [135]. In Alzheimer’s disease, increased levels of pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, have been observed in the brains of affected individuals [136]. Moreover, studies have shown that these inflammatory factors can contribute to the production of amyloid plaques and tau hyperphosphorylation, further exacerbating neuronal dysfunction [136]. In Parkinson’s disease, microglial activation and subsequent release of inflammatory mediators, including TNF-α, IL-1β, and prostaglandins, contribute to the neuroinflammatory response and progressive neurodegeneration [137].
Emerging evidence suggests that blood–brain barrier (BBB) dysfunction and peripheral immune activation play crucial roles in the initiation and progression of neurodegenerative diseases [138]. Studies utilizing advanced imaging techniques and biomarker analysis have revealed BBB disruption in individuals with AD and MS, characterized by increased permeability, and altered expression of tight junction proteins [139,140]. Furthermore, peripheral immune cells, including monocytes and T cells, can infiltrate the CNS in response to chronic inflammation, releasing inflammatory factors that contribute to neuroinflammation and neurodegeneration [141].
3.1. Periodontitis and Neurodegenerative Disorders
In recent years, there has been growing interest in the potential link between periodontitis and the onset and progression of neurodegenerative disorders. Variations in testing methods and demographics make it challenging to establish a clear causal connection. However, evidence suggests that pro-inflammatory mediators produced in response to periodontal pathogens can alter the permeability of the blood–brain barrier and pass through it, which leads to neuroinflammation and synaptic impairment [22,142,143,144]. For this reason, multiple studies have tried to observe the possible interaction periodontitis would have with neurodegenerative disorders as shown in Table 3. Criteria and the process of selecting studies that were then included in Table 3 can be seen in Appendix A.

Table 3.
Summary of the longitudinal studies that attempted to correlate periodontitis with neurodegenerative disorders.
The currently available and acquired longitudinal studies are as follows: 1 study that focuses on mild memory impairment, 5 studies for mild cognitive impairment, 14 studies for Alzheimer’s and other dementias, and 4 studies that focus on Parkinson’s disease. Out of the 24 studies, 20 found some form of positive correlation between periodontal disease and neurodegenerative disorders, with the highest consistent effect being observed in studies that focused on cognitive function.
Additional studies have revealed a higher number of positive IgG antibody tests against A. actinomycetemcomitans, P. gingivalis, and T. forsythia in subjects with AD [169]. Furthermore, some periodontal pathogens, such as P. gingivalis, T. denticola, and F. nucleatum, were shown to be able to directly enter the central nervous system and trigger neuroinflammation [16,123,170,171]. Postmortem studies showed that 8 out of 10 AD patients had P. gingivalis DNA present in their cerebrospinal fluid, with the correlation between gingipains load in the brain and AD diagnosis and pathology [16]. Another study used brain imaging to observe the impact of periodontal treatment on imaging markers of AD and found a moderate to strong protective effect [172].
Animal research was conducted to further investigate this correlation, using models of periodontitis in rats and mice. When subjected to these models, animals exhibited cognitive impairment and a significant reduction in a number of neurons, as well as an increase of pro-inflammatory factors (IL-1β and TNF-α), in the hippocampus [173,174,175]. This was accompanied by an increase in Tau phosphorylation, as well as expression of the glial fibrillary acidic protein, IL-6, cyclooxygenase-2, iNOS, AβPP, and β-secretase 1 and a decrease in A disintegrin and metalloproteinase domain-containing protein 10 expression [173,174]. Additionally, oral administration of P. gingivalis alone also leads to the activation of microglial cells and overexpression of pro-inflammatory cytokine (IL-6, TNF-α, and IL-1β), followed by neuronal death in the cortex, hippocampus, and substantia nigra [16,176,177]. Infected mice exhibit pathological features similar to those of Alzheimer’s disease, including elevated expression of the APP and BACE1 genes and an increase in tau cleavage that is dependent on gingipain. This results in elevated production of the Aβ1-42 peptide [16,177].
In one study, mice treated with P. gingivalis displayed depression-like behavior possibly caused by LPS-TLR4-dependent downregulation of p75NTR in astrocites [178]. Administration of LPS alone in the gingival tissue of mice was also shown to stimulate glial cell production of inflammatory cytokines which then afflicted the cortex and hippocampus resulting in significantly impaired spatial learning and memory [22]. P. gingivalis was also shown to regulate the neuronal cell cycle by upregulating E2F1 and downregulating CDK11, and iNOS gene expression [179].
The idea that oral bacteria can affect neuronal health is still relatively new and, therefore, not much is known about the specific impact of other periodontal pathogens. However, similar findings have been made for T. denticola, which was shown to be capable of entering the brain and directly impacting nerve cells. This resulted in the accumulation of intra and extracellular Aβ and neuronal apoptosis [180,181].
3.2. Bidirectional Interactions, Causation, and Critical Overview
Neurodegenerative disorders are amongst the most complex and enigmatic of diseases and are influenced by multiple systemic, genetic, and environmental factors which all modify the onset and progression of the disease. The relationship between periodontitis and neurodegenerative disorders is similarly complex and not yet fully understood, with no direct causal relationship established to date.
Studies investigating the relationship between periodontitis and dementia have yielded mixed results as shown in Table 3. Regrettably, there were no longitudinal studies that fit the criteria regarding MS, ALS, and Huntington’s disease. Particularly in the context of MS, investigating the potential influence of periodontal disease on its onset and progression holds considerable interest, given the established association with neuroinflammation. However, an investigation conducted in 2015, employing a case–control design with 756 affected patients, failed to establish a significant correlation between periodontitis and MS after adjusting for confounding factors such as smoking [182]. Furthermore, a comprehensive review of 17 studies in 2021 that explored the relationship between MS and oral health yielded no evidence of an association [183]. It is possible that the inflammatory processes that occur in MS may supersede the modest influence exerted by the introduction of further inflammation through periodontitis. To gain deeper insights into this interplay, further investigation is required.
While periodontitis and neurodegenerative diseases are etiologically different, they share multiple common risk factors such as hypertension and diabetes. Another such unaccounted for confounding variable may be responsible for the observed correlation. As Thomson et al. pointed out people with better childhood cognitive function have better oral health which makes them more likely to have better cognition in old age [151]; therefore, it is difficult to ascertain if periodontitis is a risk factor for neurodegeneration or if both conditions are coexisting due to shared risk factors. If it is established that periodontitis does directly impact neurodegeneration, it is unlikely to be a clear-cut relationship. It is possible that periodontal bacteria only impact neurodegeneration after disseminating to the brain, which would make the pathology invisible as long as the blood–brain barrier remains intact.
The significant variation in the behavior of different bacterial strains and their local interactions with other species further complicates the issue. While some strains may not impact neurodegeneration, others could have a significant effect. This could also vary depending on local synergistic interactions the pathogenic strain forms with the surrounding species, which could again be strain dependent. However, current testing methods do not account for strain type, leading to inconsistency. Nonetheless, further research is required to fully comprehend the relationship between periodontitis and neurodegeneration.
4. Conclusions
Periodontal disease is a preventable and prevalent condition that could have an impact on neurodegenerative function through the induction of chronic, low-grade systemic inflammation. The resulting pro-inflammatory factors can increase the permeability of the blood–brain barrier, allowing for the infiltration of pathogens or pro-inflammatory agents into the brain, leading to neuroinflammation and synaptic impairment, which could contribute to neurodegenerative disorders such as Alzheimer’s disease. However, it should be emphasized that although there is a significant amount of clinical evidence indicating a potential association between periodontitis and neurodegenerative disorders, proving causation, and determining the strength of this association is challenging. To better understand this relationship, further research is required.
Author Contributions
Conceptualization, D.V. and Ž.M.; methodology, D.V. and Ž.M.; investigation, D.V.; writing—original draft preparation, D.V. and Ž.M.; writing—review and editing, D.V., I.G. and Ž.M.; visualization, D.V. and Ž.M.; supervision, Ž.M.; funding acquisition, I.G. and Ž.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Rijeka, grant numbers uniri-prirod-18-302 to Ž.M. and uniri-biomed-18-17 to I.G.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We would like to express our sincere gratitude to Nicholas J. Bradshaw and Stribor Marković for their invaluable input and, along with Elizabeth Bradshaw, reviewing and proof reading the final version of this paper. Their expertise and feedback have significantly enhanced the quality of this work. Additionally, BioRender was used to produce the figures included in the paper.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A
Appendix A.1. Systemic Extraction of Data
The only section that required a specific approach was Table 3. Data curation was performed in alignment to PRISMA guidelines.
This was conducted by two authors using the PubMed advanced filter with the search terms: periodontitis; periodontal disease; dementia; motor neuron disease; amyotrophic lateral sclerosis; multiple sclerosis; Parkinson; Parkinson disease; Alzheimer disease; neurodegenerative disease; neurodegenerative. These search terms were then mixed using Boolean operators (AND, OR, and NOT) and MeSH terms. The results of the search are shown below Figure A1.
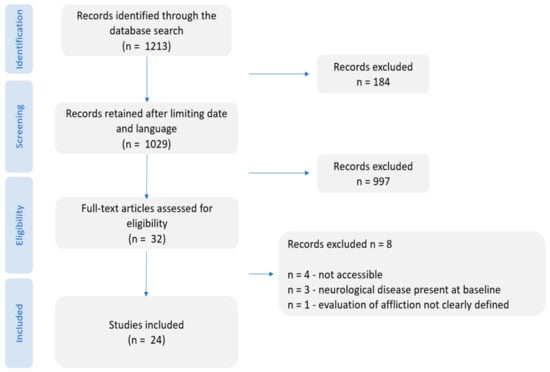
Figure A1.
PRISMA flow diagram indicating the steps followed during the scientific literature review process of this work.
Appendix A.2. Eligibility Criteria
We focused on studies written in English that were published between 1993 and 5 May 2023. The inclusion criteria were as follows: 1—human studies; 2—the analysis of the link between periodontal disease and neurodegeneration was one of the objectives of the study; 3—Evaluation methods for periodontal disease was clearly defined; 3—Evaluation methods for neurodegenerative disease was clearly defined; 5—studies observed the longitudinal relationship between periodontal disease and neurodegeneration; 6—subjects were not afflicted by the observed disease when examined at baseline.
References
- Raizada, M.K.; Joe, B.; Bryan, N.S.; Chang, E.B.; Dewhirst, F.E.; Borisy, G.G.; Galis, Z.S.; Henderson, W.; Jose, P.A.; Ketchum, C.J.; et al. Report of the National Heart, Lung, and Blood Institute Working Group on the Role of Microbiota in Blood Pressure Regulation: Current Status and Future Directions. Hypertension 2017, 70, 479–485. [Google Scholar] [CrossRef]
- Reynolds-Campbell, G.; Nicholson, A.; Thoms-Rodriguez, C.-A. Oral Bacterial Infections: Diagnosis and Management. Dent. Clin. N. Am. 2017, 61, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Definition of Gingiva—NCI Dictionary of Cancer Terms—NCI. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/gingiva (accessed on 24 November 2022).
- Rathee, M.; Jain, P. Gingivitis; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Nowicki, E.M.; Shroff, R.; Singleton, J.A.; Renaud, D.E.; Wallace, D.; Drury, J.; Zirnheld, J.; Colleti, B.; Ellington, A.D.; Lamont, R.J.; et al. Microbiota and Metatranscriptome Changes Accompanying the Onset of Gingivitis. mBio 2018, 9, e00575-18. [Google Scholar] [CrossRef] [PubMed]
- Page, R.C. Gingivitis. J. Clin. Periodontol. 1986, 13, 345–359. [Google Scholar] [CrossRef]
- Nazir, M.A. Prevalence of Periodontal Disease, Its Association with Systemic Diseases and Prevention. Int. J. Health Sci. 2017, 11, 72–80. [Google Scholar]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global Burden of Severe Periodontitis in 1990–2010. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef]
- Huang, S.; Li, R.; Zeng, X.; He, T.; Zhao, H.; Chang, A.; Bo, C.; Chen, J.; Yang, F.; Knight, R.; et al. Predictive Modeling of Gingivitis Severity and Susceptibility via Oral Microbiota. ISME J. 2014, 8, 1768–1780. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal Diseases. Nat. Rev. Dis. Prim. 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- Abusleme, L.; Hoare, A.; Hong, B.-Y.; Diaz, P.I. Microbial Signatures of Health, Gingivitis, and Periodontitis. Periodontology 2000 2021, 86, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L. Microbial Complexes in Subgingival Plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Guthmiller, J.M.; Novak, K.F. Periodontal Diseases; ASM Press: Washington, DC, USA, 2002. [Google Scholar]
- Suzuki, N.; Yoneda, M.; Hirofuji, T. Mixed Red-Complex Bacterial Infection in Periodontitis. Int. J. Dent. 2013, 2013, 587279. [Google Scholar] [CrossRef]
- How, K.Y.; Song, K.P.; Chan, K.G. Porphyromonas Gingivalis: An Overview of Periodontopathic Pathogen below the Gum Line. Front. Microbiol. 2016, 7, 53. [Google Scholar] [CrossRef]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas Gingivalis in Alzheimer’s Disease Brains: Evidence for Disease Causation and Treatment with Small-Molecule Inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef]
- Okamura, H.; Hirota, K.; Yoshida, K.; Weng, Y.; He, Y.; Shiotsu, N.; Ikegame, M.; Uchida-Fukuhara, Y.; Tanai, A.; Guo, J. Outer Membrane Vesicles of Porphyromonas Gingivalis: Novel Communication Tool and Strategy. Jpn. Dent. Sci. Rev. 2021, 57, 138–146. [Google Scholar] [CrossRef]
- Veith, P.D.; Chen, Y.-Y.; Gorasia, D.G.; Chen, D.; Glew, M.D.; O’Brien-Simpson, N.M.; Cecil, J.D.; Holden, J.A.; Reynolds, E.C. Porphyromonas Gingivalis Outer Membrane Vesicles Exclusively Contain Outer Membrane and Periplasmic Proteins and Carry a Cargo Enriched with Virulence Factors. J. Proteome Res. 2014, 13, 2420–2432. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Collyer, C.A. Gingipains from Porphyromonas Gingivalis—Complex Domain Structures Confer Diverse Functions. Eur. J. Microbiol. Immunol. (Bp) 2011, 1, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Han, N.; Du, J.; Guo, L.; Luo, Z.; Liu, Y. Pathogenesis of Important Virulence Factors of Porphyromonas Gingivalis via Toll-Like Receptors. Front. Cell. Infect. Microbiol. 2019, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chen, L.; Li, S.; Gu, Z.; Yan, J. Lipopolysaccharide (LPS) of Porphyromonas Gingivalis Induces IL-1beta, TNF-Alpha and IL-6 Production by THP-1 Cells in a Way Different from That of Escherichia Coli LPS. Innate Immun. 2008, 14, 99–107. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, C.; Zhang, X.; Chen, H.; Dong, J.; Lu, W.; Song, Z.; Zhou, W. Porphyromonas Gingivalis Lipopolysaccharide Induces Cognitive Dysfunction, Mediated by Neuronal Inflammation via Activation of the TLR4 Signaling Pathway in C57BL/6 Mice. J. Neuroinflamm. 2018, 15, 37. [Google Scholar] [CrossRef]
- Groeger, S.; Jarzina, F.; Domann, E.; Meyle, J. Porphyromonas Gingivalis Activates NFκB and MAPK Pathways in Human Oral Epithelial Cells. BMC Immunol. 2017, 18, 1. [Google Scholar] [CrossRef]
- Hienz, S.A.; Paliwal, S.; Ivanovski, S. Mechanisms of Bone Resorption in Periodontitis. J. Immunol. Res. 2015, 2015, 615486. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Yoshizawa-Smith, S.; Glowacki, A.; Maltos, K.; Pacheco, C.; Shehabeldin, M.; Mulkeen, M.; Myers, N.; Chong, R.; Verdelis, K.; et al. Induction of M2 Macrophages Prevents Bone Loss in Murine Periodontitis Models. J. Dent. Res. 2019, 98, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Geatch, D.R.; Harris, J.I.; Heasman, P.A.; Taylor, J.J. In Vitro Studies of Lymphocyte Apoptosis Induced by the Periodontal Pathogen Porphyromonas Gingivalis. J. Periodontal Res. 1999, 34, 70–78. [Google Scholar] [CrossRef]
- Jotwani, R.; Cutler, C.W. Fimbriated Porphyromonas Gingivalis Is More Efficient than Fimbria-Deficient P. gingivalis in Entering Human Dendritic Cells in Vitro and Induces an Inflammatory Th1 Effector Response. Infect. Immun. 2004, 72, 1725–1732. [Google Scholar] [CrossRef]
- Werheim, E.R.; Senior, K.G.; Shaffer, C.A.; Cuadra, G.A. Oral Pathogen Porphyromonas Gingivalis Can Escape Phagocytosis of Mammalian Macrophages. Microorganisms 2020, 8, 1432. [Google Scholar] [CrossRef]
- Vincents, B.; Guentsch, A.; Kostolowska, D.; von Pawel-Rammingen, U.; Eick, S.; Potempa, J.; Abrahamson, M. Cleavage of IgG1 and IgG3 by Gingipain K from Porphyromonas Gingivalis May Compromise Host Defense in Progressive Periodontitis. FASEB J. 2011, 25, 3741–3750. [Google Scholar] [CrossRef] [PubMed]
- Dorn, B.R.; Dunn, W.A.; Progulske-Fox, A. Porphyromonas Gingivalis Traffics to Autophagosomes in Human Coronary Artery Endothelial Cells. Infect. Immun. 2001, 69, 5698–5708. [Google Scholar] [CrossRef]
- Porphyromonas Gingivalis (ID 714)—Genome—NCBI. Available online: https://www.ncbi.nlm.nih.gov/genome/714 (accessed on 7 April 2023).
- Mendez, K.N.; Hoare, A.; Soto, C.; Bugueño, I.; Olivera, M.; Meneses, C.; Pérez-Donoso, J.M.; Castro-Nallar, E.; Bravo, D. Variability in Genomic and Virulent Properties of Porphyromonas Gingivalis Strains Isolated From Healthy and Severe Chronic Periodontitis Individuals. Front. Cell. Infect. Microbiol. 2019, 9, 246. [Google Scholar] [CrossRef]
- Rocha, F.G.; Berges, A.; Sedra, A.; Ghods, S.; Kapoor, N.; Pill, L.; Davey, M.E.; Fairman, J.; Gibson, F.C. A Porphyromonas Gingivalis Capsule-Conjugate Vaccine Protects From Experimental Oral Bone Loss. Front. Oral Health 2021, 2, 686402. [Google Scholar] [CrossRef]
- Dahlén, G.; Gmür, R.; Yoshino, T. Phenotypes, Serotypes and Antibiotic Susceptibility of Swedish Porphyromonas Gingivalis Isolates from Periodontitis and Periodontal Abscesses. Oral Microbiol. Immunol. 2007, 22, 80–86. [Google Scholar] [CrossRef]
- Naito, M.; Hirakawa, H.; Yamashita, A.; Ohara, N.; Shoji, M.; Yukitake, H.; Nakayama, K.; Toh, H.; Yoshimura, F.; Kuhara, S.; et al. Determination of the Genome Sequence of Porphyromonas Gingivalis Strain ATCC 33277 and Genomic Comparison with Strain W83 Revealed Extensive Genome Rearrangements in P. gingivalis. DNA Res. 2008, 15, 215–225. [Google Scholar] [CrossRef]
- Biyikoğlu, B.; Ricker, A.; Diaz, P.I. Strain-Specific Colonization Patterns and Serum Modulation of Multi-Species Oral Biofilm Development. Anaerobe 2012, 18, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.E.; Duncan, M.J. Enhanced Biofilm Formation and Loss of Capsule Synthesis: Deletion of a Putative Glycosyltransferase in Porphyromonas Gingivalis. J. Bacteriol. 2006, 188, 5510–5523. [Google Scholar] [CrossRef] [PubMed]
- Seers, C.A.; Mahmud, A.S.M.; Huq, N.L.; Cross, K.J.; Reynolds, E.C. Porphyromonas Gingivalis Laboratory Strains and Clinical Isolates Exhibit Different Distribution of Cell Surface and Secreted Gingipains. J. Oral Microbiol. 2020, 13, 1858001. [Google Scholar] [CrossRef]
- Chastain-Gross, R.P.; Xie, G.; Bélanger, M.; Kumar, D.; Whitlock, J.A.; Liu, L.; Raines, S.M.; Farmerie, W.G.; Daligault, H.E.; Han, C.S.; et al. Genome Sequence of Porphyromonas Gingivalis Strain 381. Genome Announc. 2017, 5, e01467-16. [Google Scholar] [CrossRef]
- Aduse-Opoku, J.; Joseph, S.; Devine, D.A.; Marsh, P.D.; Curtis, M.A. Molecular Basis for Avirulence of Spontaneous Variants of Porphyromonas Gingivalis: Genomic Analysis of Strains W50, BE1 and BR1. Mol. Oral Microbiol. 2022, 37, 122–132. [Google Scholar] [CrossRef]
- Sharma, A. Virulence Mechanisms of Tannerella Forsythia. Periodontol 2000 2010, 54, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, B.; Węsierska, A.; Zalewska, K.; Sokołowska, M.M.; Bursiewicz, W.; Socha, M.; Ozorowski, M.; Pawlak-Osińska, K.; Wiciński, M. The Role of Tannerella Forsythia and Porphyromonas Gingivalis in Pathogenesis of Esophageal Cancer. Infect. Agents Cancer 2019, 14, 3. [Google Scholar] [CrossRef]
- Nakajima, T.; Tomi, N.; Fukuyo, Y.; Ishikura, H.; Ohno, Y.; Arvind, R.; Arai, T.; Ishikawa, I.; Arakawa, S. Isolation and Identification of a Cytopathic Activity in Tannerella Forsythia. Biochem. Biophys. Res. Commun. 2006, 351, 133–139. [Google Scholar] [CrossRef]
- Tomi, N.; Fukuyo, Y.; Arakawa, S.; Nakajima, T. Pro-Inflammatory Cytokine Production from Normal Human Fibroblasts Is Induced by Tannerella Forsythia Detaching Factor. J. Periodontal Res. 2008, 43, 136–142. [Google Scholar] [CrossRef]
- Onishi, S.; Honma, K.; Liang, S.; Stathopoulou, P.; Kinane, D.; Hajishengallis, G.; Sharma, A. Toll-Like Receptor 2-Mediated Interleukin-8 Expression in Gingival Epithelial Cells by the Tannerella Forsythia Leucine-Rich Repeat Protein BspA. Infect. Immun. 2008, 76, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.R.; Jun, H.K.; Choi, B.K. Tannerella Forsythia BspA Increases the Risk Factors for Atherosclerosis in ApoE(-/-) Mice. Oral Dis. 2014, 20, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Settem, R.P.; Honma, K.; Shankar, M.; Li, M.; LaMonte, M.; Xu, D.; Genco, R.J.; Browne, R.W.; Sharma, A. Tannerella Forsythia-Produced Methylglyoxal Causes Accumulation of Advanced Glycation Endproducts to Trigger Cytokine Secretion in Human Monocytes. Mol. Oral Microbiol. 2018, 33, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.-J.; Choi, Y.-J.; An, S.-J.; Lee, H.-R.; Jun, H.-K.; Choi, B.-K. Tannerella Forsythia GroEL Induces Inflammatory Bone Resorption and Synergizes with Interleukin-17. Mol. Oral Microbiol. 2017, 32, 301–313. [Google Scholar] [CrossRef]
- Bryzek, D.; Ksiazek, M.; Bielecka, E.; Karim, A.Y.; Potempa, B.; Staniec, D.; Koziel, J.; Potempa, J. A Pathogenic Trace of Tannerella Forsythia—Shedding of Soluble Fully Active Tumor Necrosis Factor α from the Macrophage Surface by Karilysin. Mol. Oral Microbiol. 2014, 29, 294–306. [Google Scholar] [CrossRef]
- Karim, A.Y.; Kulczycka, M.; Kantyka, T.; Dubin, G.; Jabaiah, A.; Daugherty, P.S.; Thogersen, I.B.; Enghild, J.J.; Nguyen, K.-A.; Potempa, J. A Novel Matrix Metalloprotease-like Enzyme (Karilysin) of the Periodontal Pathogen Tannerella Forsythia ATCC 43037. Biol. Chem. 2010, 391, 105–117. [Google Scholar] [CrossRef]
- Oh, Y.J.; Sekot, G.; Duman, M.; Chtcheglova, L.; Messner, P.; Peterlik, H.; Schäffer, C.; Hinterdorfer, P. Characterizing the S-Layer Structure and Anti-S-Layer Antibody Recognition on Intact Tannerella Forsythia Cells by Scanning Probe Microscopy and Small Angle X-Ray Scattering. J. Mol. Recognit. 2013, 26, 542–549. [Google Scholar] [CrossRef]
- Sekot, G.; Posch, G.; Messner, P.; Matejka, M.; Rausch-Fan, X.; Andrukhov, O.; Schäffer, C. Potential of the Tannerella Forsythia S-Layer to Delay the Immune Response. J. Dent. Res. 2011, 90, 109–114. [Google Scholar] [CrossRef]
- Shimotahira, N.; Oogai, Y.; Kawada-Matsuo, M.; Yamada, S.; Fukutsuji, K.; Nagano, K.; Yoshimura, F.; Noguchi, K.; Komatsuzawa, H. The Surface Layer of Tannerella Forsythia Contributes to Serum Resistance and Oral Bacterial Coaggregation. Infect. Immun. 2013, 81, 1198–1206. [Google Scholar] [CrossRef]
- Bloch, S.; Zwicker, S.; Bostanci, N.; Sjöling, Å.; Boström, E.A.; Belibasakis, G.N.; Schäffer, C. Immune Response Profiling of Primary Monocytes and Oral Keratinocytes to Different Tannerella Forsythia Strains and Their Cell Surface Mutants. Mol. Oral Microbiol. 2018, 33, 155–167. [Google Scholar] [CrossRef]
- Jusko, M.; Potempa, J.; Karim, A.Y.; Ksiazek, M.; Riesbeck, K.; Garred, P.; Eick, S.; Blom, A.M. A Metalloproteinase Karilysin Present in the Majority of Tannerella Forsythia Isolates Inhibits All Pathways of the Complement System. J. Immunol. 2012, 188, 2338–2349. [Google Scholar] [CrossRef] [PubMed]
- Bloch, S.; Thurnheer, T.; Murakami, Y.; Belibasakis, G.N.; Schäffer, C. Behavior of Two Tannerella Forsythia Strains and Their Cell Surface Mutants in Multispecies Oral Biofilms. Mol. Oral Microbiol. 2017, 32, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, V.; Janesch, B.; Windwarder, M.; Maresch, D.; Braun, M.L.; Megson, Z.A.; Vinogradov, E.; Goneau, M.-F.; Sharma, A.; Altmann, F.; et al. Tannerella Forsythia Strains Display Different Cell-Surface Nonulosonic Acids: Biosynthetic Pathway Characterization and First Insight into Biological Implications. Glycobiology 2017, 27, 342–357. [Google Scholar] [CrossRef]
- Chinthamani, S.; Settem, R.P.; Honma, K.; Stafford, G.P.; Sharma, A. Tannerella Forsythia Strains Differentially Induce Interferon Gamma-Induced Protein 10 (IP-10) Expression in Macrophages Due to Lipopolysaccharide Heterogeneity. Pathog. Dis. 2022, 80, ftac008. [Google Scholar] [CrossRef] [PubMed]
- Ruby, J.; Martin, M.; Passineau, M.J.; Godovikova, V.; Fenno, J.C.; Wu, H. Activation of the Innate Immune System by Treponema Denticola Periplasmic Flagella through Toll-Like Receptor 2. Infect. Immun. 2017, 86, e00573-17. [Google Scholar] [CrossRef] [PubMed]
- Gaibani, P.; Caroli, F.; Nucci, C.; Sambri, V. Major Surface Protein Complex of Treponema Denticola Induces the Production of Tumor Necrosis Factor Alpha, Interleukin-1 Beta, Interleukin-6 and Matrix Metalloproteinase 9 by Primary Human Peripheral Blood Monocytes. J. Periodontal Res. 2010, 45, 361–366. [Google Scholar] [CrossRef]
- Fenno, J.C.; Hannam, P.M.; Leung, W.K.; Tamura, M.; Uitto, V.-J.; McBride, B.C. Cytopathic Effects of the Major Surface Protein and the Chymotrypsinlike Protease of Treponema Denticola. Infect. Immun. 1998, 66, 1869–1877. [Google Scholar] [CrossRef]
- Nussbaum, G.; Ben-Adi, S.; Genzler, T.; Sela, M.; Rosen, G. Involvement of Toll-like Receptors 2 and 4 in the Innate Immune Response to Treponema Denticola and Its Outer Sheath Components. Infect. Immun. 2009, 77, 3939–3947. [Google Scholar] [CrossRef]
- Grenier, D. Binding Properties of Treponema Denticola Lipooligosaccharide. J. Oral Microbiol. 2013, 5, 21517. [Google Scholar] [CrossRef]
- Ganther, S.; Radaic, A.; Malone, E.; Kamarajan, P.; Chang, N.-Y.N.; Tafolla, C.; Zhan, L.; Fenno, J.C.; Kapila, Y.L. Treponema Denticola Dentilisin Triggered TLR2/MyD88 Activation Upregulates a Tissue Destructive Program Involving MMPs via Sp1 in Human Oral Cells. PLoS Pathog. 2021, 17, e1009311. [Google Scholar] [CrossRef]
- Yamazaki, T.; Miyamoto, M.; Yamada, S.; Okuda, K.; Ishihara, K. Surface Protease of Treponema Denticola Hydrolyzes C3 and Influences Function of Polymorphonuclear Leukocytes. Microbes Infect. 2006, 8, 1758–1763. [Google Scholar] [CrossRef] [PubMed]
- Kokubu, E.; Kikuchi, Y.; Okamoto-Shibayama, K.; Nakamura, S.; Ishihara, K. Crawling Motility of Treponema Denticola Modulated by Outer Sheath Protein. Microbiol. Immunol. 2021, 65, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Nixon, C.S.; Steffen, M.J.; Ebersole, J.L. Cytokine Responses to Treponema Pectinovorum and Treponema Denticola in Human Gingival Fibroblasts. Infect. Immun. 2000, 68, 5284–5292. [Google Scholar] [CrossRef]
- Brissette, C.A.; Pham, T.-T.T.; Coats, S.R.; Darveau, R.P.; Lukehart, S.A. Treponema Denticola Does Not Induce Production of Common Innate Immune Mediators from Primary Gingival Epithelial Cells. Oral Microbiol. Immunol. 2008, 23, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.E.; Choi, Y. Treponema Denticola Suppresses Expression of Human Beta-Defensin-2 in Gingival Epithelial Cells through Inhibition of TNFalpha Production and TLR2 Activation. Mol. Cells 2010, 29, 407–412. [Google Scholar] [CrossRef]
- Shin, J.E.; Kim, Y.S.; Oh, J.-E.; Min, B.-M.; Choi, Y. Treponema Denticola Suppresses Expression of Human {beta}-Defensin-3 in Gingival Epithelial Cells through Inhibition of the Toll-like Receptor 2 Axis. Infect. Immun. 2010, 78, 672–679. [Google Scholar] [CrossRef]
- Miyamoto, M.; Ishihara, K.; Okuda, K. The Treponema Denticola Surface Protease Dentilisin Degrades Interleukin-1β (IL-1β), IL-6, and Tumor Necrosis Factor Alpha. Infect. Immun. 2006, 74, 2462–2467. [Google Scholar] [CrossRef]
- Okuda, T.; Kimizuka, R.; Miyamoto, M.; Kato, T.; Yamada, S.; Okuda, K.; Ishihara, K. Treponema Denticola Induces Interleukin-8 and Macrophage Chemoattractant Protein 1 Production in Human Umbilical Vein Epithelial Cells. Microbes Infect. 2007, 9, 907–913. [Google Scholar] [CrossRef]
- Dos Santos, P.B.D.R.E.; De Lima, P.M.N.; Palma, A.L.D.R.; Abu Hasna, A.; Rossoni, R.D.; Junqueira, J.C.; De Oliveira, L.D. Review- The Periodontal Pathogen Treponema Denticola: An Atherosclerosis Risk Factor. Res. Soc. Dev. 2021, 10, e25810111637. [Google Scholar] [CrossRef]
- Han, Y.W. Fusobacterium Nucleatum: A Commensal-Turned Pathogen. Curr. Opin. Microbiol. 2015, 23, 141–147. [Google Scholar] [CrossRef]
- Xu, C.; Fan, L.; Lin, Y.; Shen, W.; Qi, Y.; Zhang, Y.; Chen, Z.; Wang, L.; Long, Y.; Hou, T.; et al. Fusobacterium Nucleatum Promotes Colorectal Cancer Metastasis through MiR-1322/CCL20 Axis and M2 Polarization. Gut Microbes 2021, 13, 1980347. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium Nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 2017, 170, 548–563.e16. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Ji, X.; Zhang, X.; Tang, D.; Feng, Q. Persistent Exposure to Fusobacterium Nucleatum Triggers Chemokine/Cytokine Release and Inhibits the Proliferation and Osteogenic Differentiation Capabilities of Human Gingiva-Derived Mesenchymal Stem Cells. Front. Cell. Infect. Microbiol. 2019, 9, 429. [Google Scholar] [CrossRef]
- Fusobacterium Nucleatum Facilitates Apoptosis, ROS Generation, and Inflammatory Cytokine Production by Activating AKT/MAPK and NF- κ B Signaling Pathways in Human Gingival Fibroblasts—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/31737164/ (accessed on 2 March 2023).
- Yin, L.; Li, X.; Hou, J. Macrophages in Periodontitis: A Dynamic Shift between Tissue Destruction and Repair. Jpn. Dent. Sci. Rev. 2022, 58, 336–347. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Z.; Tang, Z.; Huang, Y.; Huang, M.; Liu, H.; Ziebolz, D.; Schmalz, G.; Jia, B.; Zhao, J. More Than Just a Periodontal Pathogen—The Research Progress on Fusobacterium Nucleatum. Front. Cell. Infect. Microbiol. 2022, 12, 64. [Google Scholar] [CrossRef] [PubMed]
- Bachrach, G.; Rosen, G.; Bellalou, M.; Naor, R.; Sela, M.N. Identification of a Fusobacterium Nucleatum 65 KDa Serine Protease. Oral Microbiol. Immunol. 2004, 19, 155–159. [Google Scholar] [CrossRef]
- Despins, C.A.; Brown, S.D.; Robinson, A.V.; Mungall, A.J.; Allen-Vercoe, E.; Holt, R.A. Modulation of the Host Cell Transcriptome and Epigenome by Fusobacterium Nucleatum. mBio 2021, 12, e0206221. [Google Scholar] [CrossRef]
- Razghonova, Y.; Zymovets, V.; Wadelius, P.; Rakhimova, O.; Manoharan, L.; Brundin, M.; Kelk, P.; Romani Vestman, N. Transcriptome Analysis Reveals Modulation of Human Stem Cells from the Apical Papilla by Species Associated with Dental Root Canal Infection. Int. J. Mol. Sci. 2022, 23, 14420. [Google Scholar] [CrossRef]
- Ang, M.Y.; Dutta, A.; Wee, W.Y.; Dymock, D.; Paterson, I.C.; Choo, S.W. Comparative Genome Analysis of Fusobacterium Nucleatum. Genome Biol. Evol. 2016, 8, 2928–2938. [Google Scholar] [CrossRef]
- Muchova, M.; Balacco, D.L.; Grant, M.M.; Chapple, I.L.C.; Kuehne, S.A.; Hirschfeld, J. Fusobacterium Nucleatum Subspecies Differ in Biofilm Forming Ability in Vitro. Front. Oral Health 2022, 3, 853618. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Maula, T.; Bao, K.; Lindholm, M.; Bostanci, N.; Oscarsson, J.; Ihalin, R.; Johansson, A. Virulence and Pathogenicity Properties of Aggregatibacter Actinomycetemcomitans. Pathogens 2019, 8, 222. [Google Scholar] [CrossRef]
- Naqvi, A.R.; Fordham, J.B.; Khan, A.; Nares, S. MicroRNAs Responsive to Aggregatibacter Actinomycetemcomitans and Porphyromonas Gingivalis LPS Modulate Expression of Genes Regulating Innate Immunity in Human Macrophages. Innate Immun. 2014, 20, 540–551. [Google Scholar] [CrossRef]
- Ohguchi, Y.; Ishihara, Y.; Ohguchi, M.; Koide, M.; Shirozu, N.; Naganawa, T.; Nishihara, T.; Noguchi, T. Capsular Polysaccharide from Actinobacillus Actinomycetemcomitans Inhibits IL-6 and IL-8 Production in Human Gingival Fibroblast. J. Periodontal Res. 2003, 38, 191–197. [Google Scholar] [CrossRef]
- Vernal, R.; Leon, R.; Herrera, D.; Garcia-Sanz, J.A.; Silva, A.; Sanz, M. Variability in the Response of Human Dendritic Cells Stimulated with Porphyromonas Gingivalis or Aggregatibacter Actinomycetemcomitans. J. Periodontal Res. 2008, 43, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Guentsch, A.; Puklo, M.; Preshaw, P.M.; Glockmann, E.; Pfister, W.; Potempa, J.; Eick, S. Neutrophils in Chronic and Aggressive Periodontitis in Interaction with Porphyromonas Gingivalis and Aggregatibacter Actinomycetemcomitans. J. Periodontal Res. 2009, 44, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A. Aggregatibacter Actinomycetemcomitans Leukotoxin: A Powerful Tool with Capacity to Cause Imbalance in the Host Inflammatory Response. Toxins 2011, 3, 242–259. [Google Scholar] [CrossRef]
- Pons, B.J.; Vignard, J.; Mirey, G. Cytolethal Distending Toxin Subunit B: A Review of Structure–Function Relationship. Toxins 2019, 11, 595. [Google Scholar] [CrossRef]
- Guerra, L.; Cortes-Bratti, X.; Guidi, R.; Frisan, T. The Biology of the Cytolethal Distending Toxins. Toxins 2011, 3, 172–190. [Google Scholar] [CrossRef]
- Akrivopoulou, C.; Green, I.M.; Donos, N.; Nair, S.P.; Ready, D. Aggregatibacter Actinomycetemcomitans Serotype Prevalence and Antibiotic Resistance in a UK Population with Periodontitis. J. Glob. Antimicrob. Resist. 2017, 10, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Åberg, C.H.; Kelk, P.; Johansson, A. Aggregatibacter Actinomycetemcomitans: Virulence of Its Leukotoxin and Association with Aggressive Periodontitis. Virulence 2014, 6, 188–195. [Google Scholar] [CrossRef]
- Brígido, J.A.; da Silveira, V.R.S.; Rego, R.O.; Nogueira, N.A.P. Serotypes of Aggregatibacter Actinomycetemcomitans in Relation to Periodontal Status and Geographic Origin of Individuals-a Review of the Literature. Med. Oral Patol. Oral Cir. Bucal 2014, 19, e184–e191. [Google Scholar] [CrossRef]
- Lin, F.-Y.; Hsiao, F.-P.; Huang, C.-Y.; Shih, C.-M.; Tsao, N.-W.; Tsai, C.-S.; Yang, S.-F.; Chang, N.-C.; Hung, S.-L.; Lin, Y.-W. Porphyromonas Gingivalis GroEL Induces Osteoclastogenesis of Periodontal Ligament Cells and Enhances Alveolar Bone Resorption in Rats. PLoS ONE 2014, 9, e102450. [Google Scholar] [CrossRef]
- Song, H.; Bélanger, M.; Whitlock, J.; Kozarov, E.; Progulske-Fox, A. Hemagglutinin B Is Involved in the Adherence of Porphyromonas Gingivalis to Human Coronary Artery Endothelial Cells. Infect. Immun. 2005, 73, 7267–7273. [Google Scholar] [CrossRef]
- Posch, G.; Andrukhov, O.; Vinogradov, E.; Lindner, B.; Messner, P.; Holst, O.; Schäffer, C. Structure and Immunogenicity of the Rough-Type Lipopolysaccharide from the Periodontal Pathogen Tannerella Forsythia. Clin. Vaccine Immunol. 2013, 20, 945–953. [Google Scholar] [CrossRef]
- Ikegami, A.; Honma, K.; Sharma, A.; Kuramitsu, H.K. Multiple Functions of the Leucine-Rich Repeat Protein LrrA of Treponema Denticola. Infect. Immun. 2004, 72, 4619–4627. [Google Scholar] [CrossRef] [PubMed]
- Nørskov-Lauritsen, N.; Claesson, R.; Jensen, A.B.; Åberg, C.H.; Haubek, D. Aggregatibacter Actinomycetemcomitans: Clinical Significance of a Pathobiont Subjected to Ample Changes in Classification and Nomenclature. Pathogens 2019, 8, 243. [Google Scholar] [CrossRef] [PubMed]
- Berezow, A.B.; Darveau, R.P. Microbial Shift and Periodontitis. Periodontol 2000 2011, 55, 36–47. [Google Scholar] [CrossRef]
- Holt, S.C.; Bramanti, T.E. Factors in Virulence Expression and Their Role in Periodontal Disease Pathogenesis. Crit. Rev. Oral Biol. Med. 1991, 2, 177–281. [Google Scholar] [CrossRef]
- Rosen, G.; Genzler, T.; Sela, M.N. Coaggregation of Treponema Denticola with Porphyromonas Gingivalis and Fusobacterium Nucleatum Is Mediated by the Major Outer Sheath Protein of Treponema Denticola. FEMS Microbiol. Lett. 2008, 289, 59–66. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Inagaki, S.; Onishi, S.; Kuramitsu, H.K.; Sharma, A. Porphyromonas Gingivalis Vesicles Enhance Attachment, and the Leucine-Rich Repeat BspA Protein Is Required for Invasion of Epithelial Cells by “Tannerella Forsythia”. Infect. Immun. 2006, 74, 5023–5028. [Google Scholar] [CrossRef]
- Kin, L.X.; Butler, C.A.; Slakeski, N.; Hoffmann, B.; Dashper, S.G.; Reynolds, E.C. Metabolic Cooperativity between Porphyromonas Gingivalis and Treponema Denticola. J. Oral Microbiol. 2020, 12, 1808750. [Google Scholar] [CrossRef]
- Tan, K.H.; Seers, C.A.; Dashper, S.G.; Mitchell, H.L.; Pyke, J.S.; Meuric, V.; Slakeski, N.; Cleal, S.M.; Chambers, J.L.; McConville, M.J.; et al. Porphyromonas Gingivalis and Treponema Denticola Exhibit Metabolic Symbioses. PLoS Pathog. 2014, 10, e1003955. [Google Scholar] [CrossRef]
- Saito, Y.; Fujii, R.; Nakagawa, K.-I.; Kuramitsu, H.K.; Okuda, K.; Ishihara, K. Stimulation of Fusobacterium Nucleatum Biofilm Formation by Porphyromonas Gingivalis. Oral Microbiol. Immunol. 2008, 23, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.P.; Fitzsimonds, Z.R.; Lamont, R.J. Metabolic Signaling and Spatial Interactions in the Oral Polymicrobial Community. J. Dent. Res. 2019, 98, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Inagaki, S.; Kimizuka, R.; Okuda, K.; Hosaka, Y.; Nakagawa, T.; Ishihara, K. Fusobacterium Nucleatum Enhances Invasion of Human Gingival Epithelial and Aortic Endothelial Cells by Porphyromonas Gingivalis. FEMS Immunol. Med. Microbiol. 2008, 54, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, M.; Yoshikane, T.; Motooka, N.; Yamada, K.; Hisama, K.; Naito, T.; Okada, I.; Yoshinaga, M.; Hidaka, K.; Imaizumi, K.; et al. Stimulation of Growth of Porphyromonas Gingivalis by Cell Extracts from Tannerella Forsythia. J. Periodontal Res. 2005, 40, 105–109. [Google Scholar] [CrossRef]
- Chen, P.B.; Davern, L.B.; Katz, J.; Eldridge, J.H.; Michalek, S.M. Host Responses Induced by Co-Infection with Porphyromonas Gingivalis and Actinobacillus Actinomycetemcomitans in a Murine Model. Oral Microbiol. Immunol. 1996, 11, 274–281. [Google Scholar] [CrossRef]
- ter Steeg, P.F.; van der Hoeven, J.S. Growth Stimulation of Treponema Denticola by Periodontal Microorganisms. Antonie Van Leeuwenhoek 1990, 57, 63–70. [Google Scholar] [CrossRef]
- Sharma, A.; Inagaki, S.; Sigurdson, W.; Kuramitsu, H.K. Synergy between Tannerella Forsythia and Fusobacterium Nucleatum in Biofilm Formation. Oral Microbiol. Immunol. 2005, 20, 39–42. [Google Scholar] [CrossRef]
- Honma, K.; Ruscitto, A.; Sharma, A. β-Glucanase Activity of the Oral Bacterium Tannerella Forsythia Contributes to the Growth of a Partner Species, Fusobacterium Nucleatum, in Cobiofilms. Appl. Environ. Microbiol. 2017, 84, e01759-17. [Google Scholar] [CrossRef]
- Ruscitto, A.; Honma, K.; Veeramachineni, V.M.; Nishikawa, K.; Stafford, G.P.; Sharma, A. Regulation and Molecular Basis of Environmental Muropeptide Uptake and Utilization in Fastidious Oral Anaerobe Tannerella Forsythia. Front. Microbiol. 2017, 8, 648. [Google Scholar] [CrossRef] [PubMed]
- Settem, R.P.; El-Hassan, A.T.; Honma, K.; Stafford, G.P.; Sharma, A. Fusobacterium Nucleatum and Tannerella Forsythia Induce Synergistic Alveolar Bone Loss in a Mouse Periodontitis Model. Infect. Immun. 2012, 80, 2436–2443. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Meng, H.; Xu, L.; Zhang, L.; Chen, Z.; Feng, X.; Lu, R.; Sun, X.; Ren, X. Systemic Inflammation Markers in Patients With Aggressive Periodontitis: A Pilot Study. J. Periodontol. 2008, 79, 2340–2346. [Google Scholar] [CrossRef]
- D’Aiuto, F.; Parkar, M.; Andreou, G.; Suvan, J.; Brett, P.M.; Ready, D.; Tonetti, M.S. Periodontitis and Systemic Inflammation: Control of the Local Infection Is Associated with a Reduction in Serum Inflammatory Markers. J. Dent. Res. 2004, 83, 156–160. [Google Scholar] [CrossRef]
- El-Shinnawi, U.; Soory, M. Associations between Periodontitis and Systemic Inflammatory Diseases: Response to Treatment. Recent Pat. Endocr. Metab. Immune Drug Discov. 2013, 7, 169–188. [Google Scholar] [CrossRef]
- Dhotre, S.V.; Davane, M.S.; Nagoba, B.S. Periodontitis, Bacteremia and Infective Endocarditis: A Review Study. Arch. Pediatr. Infect. Dis. 2017, 5, 1–5. [Google Scholar] [CrossRef]
- Martínez-García, M.; Hernández-Lemus, E. Periodontal Inflammation and Systemic Diseases: An Overview. Front. Physiol. 2021, 12, 709438. [Google Scholar] [CrossRef]
- Pisani, F.; Pisani, V.; Arcangeli, F.; Harding, A.; Singhrao, S.K. The Mechanistic Pathways of Periodontal Pathogens Entering the Brain: The Potential Role of Treponema Denticola in Tracing Alzheimer’s Disease Pathology. Int. J. Environ. Res. Public Health 2022, 19, 9386. [Google Scholar] [CrossRef]
- Local and Systemic Mechanisms Linking Periodontal Disease and Inflammatory Comorbidities|Nature Reviews Immunology. Available online: https://www.nature.com/articles/s41577-020-00488-6 (accessed on 6 March 2023).
- Bourgeois, D.; Inquimbert, C.; Ottolenghi, L.; Carrouel, F. Periodontal Pathogens as Risk Factors of Cardiovascular Diseases, Diabetes, Rheumatoid Arthritis, Cancer, and Chronic Obstructive Pulmonary Disease—Is There Cause for Consideration? Microorganisms 2019, 7, 424. [Google Scholar] [CrossRef]
- Lee, C.-H.; Giuliani, F. The Role of Inflammation in Depression and Fatigue. Front. Immunol. 2019, 10, 1696. [Google Scholar] [CrossRef]
- Xia, Z.; Depierre, J.W.; Nässberger, L. Tricyclic Antidepressants Inhibit IL-6, IL-1β and TNF-α Release in Human Blood Monocytes and IL-2 and Interferon-γ in T Cells. Immunopharmacology 1996, 34, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Su, K.-P.; Lai, H.-C.; Peng, C.-Y.; Su, W.-P.; Chang, J.P.-C.; Pariante, C.M. Interferon-Alpha-Induced Depression: Comparisons between Early- and Late-Onset Subgroups and with Patients with Major Depressive Disorder. Brain. Behav. Immun. 2019, 80, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Capuron, L.; Ravaud, A.; Dantzer, R. Early Depressive Symptoms in Cancer Patients Receiving Interleukin 2 and/or Interferon Alfa-2b Therapy. J. Clin. Oncol. 2000, 18, 2143–2151. [Google Scholar] [CrossRef] [PubMed]
- Carrouel, F.; Viennot, S.; Santamaria, J.; Veber, P.; Bourgeois, D. Quantitative Molecular Detection of 19 Major Pathogens in the Interdental Biofilm of Periodontally Healthy Young Adults. Front. Microbiol. 2016, 7, 840. [Google Scholar] [CrossRef]
- Bourgeois, D.; Bravo, M.; Llodra, J.-C.; Inquimbert, C.; Viennot, S.; Dussart, C.; Carrouel, F. Calibrated Interdental Brushing for the Prevention of Periodontal Pathogens Infection in Young Adults—A Randomized Controlled Clinical Trial. Sci. Rep. 2019, 9, 15127. [Google Scholar] [CrossRef] [PubMed]
- Inquimbert, C.; Bourgeois, D.; Bravo, M.; Viennot, S.; Tramini, P.; Llodra, J.C.; Molinari, N.; Dussart, C.; Giraudeau, N.; Carrouel, F. The Oral Bacterial Microbiome of Interdental Surfaces in Adolescents According to Carious Risk. Microorganisms 2019, 7, 319. [Google Scholar] [CrossRef]
- Alkahtani, S.; AL-Johani, N.S.; Alarifi, S. Mechanistic Insights, Treatment Paradigms, and Clinical Progress in Neurological Disorders: Current and Future Prospects. Int. J. Mol. Sci. 2023, 24, 1340. [Google Scholar] [CrossRef]
- Wareham, L.K.; Liddelow, S.A.; Temple, S.; Benowitz, L.I.; Di Polo, A.; Wellington, C.; Goldberg, J.L.; He, Z.; Duan, X.; Bu, G.; et al. Solving Neurodegeneration: Common Mechanisms and Strategies for New Treatments. Mol. Neurodegener. 2022, 17, 23. [Google Scholar] [CrossRef]
- Bjelobaba, I.; Savic, D.; Lavrnja, I. Multiple Sclerosis and Neuroinflammation: The Overview of Current and Prospective Therapies. Curr. Pharm. Des. 2017, 23, 693–730. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and Microglial Activation in Alzheimer Disease: Where Do We Go from Here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Reale, M.; Iarlori, C.; Thomas, A.; Gambi, D.; Perfetti, B.; Di Nicola, M.; Onofrj, M. Peripheral Cytokines Profile in Parkinson’s Disease. Brain. Behav. Immun. 2009, 23, 55–63. [Google Scholar] [CrossRef]
- Dadas, A.; Washington, J.; Marchi, N.; Janigro, D. Chapter 2—Blood–Brain Barrier in Disease States. In Nervous System Drug Delivery; Lonser, R.R., Sarntinoranont, M., Bankiewicz, K., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 21–37. ISBN 978-0-12-813997-4. [Google Scholar]
- Kirk, J.; Plumb, J.; Mirakhur, M.; McQuaid, S. Tight Junctional Abnormality in Multiple Sclerosis White Matter Affects All Calibres of Vessel and Is Associated with Blood-Brain Barrier Leakage and Active Demyelination. J. Pathol. 2003, 201, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Shinohara, M.; Shinohara, M.; Yamazaki, A.; Murray, M.E.; Liesinger, A.M.; Heckman, M.G.; Lesser, E.R.; Parisi, J.E.; Petersen, R.C.; et al. Selective Loss of Cortical Endothelial Tight Junction Proteins during Alzheimer’s Disease Progression. Brain 2019, 142, 1077–1092. [Google Scholar] [CrossRef]
- Berriat, F.; Lobsiger, C.S.; Boillée, S. The Contribution of the Peripheral Immune System to Neurodegeneration. Nat. Neurosci. 2023, 26, 942–954. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Selvakumar, G.P.; Zaheer, S.; Ahmed, M.E.; Raikwar, S.P.; Zahoor, H.; Saeed, D.; Natteru, P.A.; Iyer, S.; et al. Brain and Peripheral Atypical Inflammatory Mediators Potentiate Neuroinflammation and Neurodegeneration. Front. Cell. Neurosci. 2017, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ran, M.; Li, H.; Lin, Y.; Ma, K.; Yang, Y.; Fu, X.; Yang, S. New Insight into Neurological Degeneration: Inflammatory Cytokines and Blood–Brain Barrier. Front. Mol. Neurosci. 2022, 15, 1013933. [Google Scholar] [CrossRef] [PubMed]
- Galea, I. The Blood–Brain Barrier in Systemic Infection and Inflammation. Cell. Mol. Immunol. 2021, 18, 2489–2501. [Google Scholar] [CrossRef]
- Kaye, E.K.; Valencia, A.; Baba, N.; Spiro, A.; Dietrich, T.; Garcia, R.I. Tooth Loss and Periodontal Disease Predict Poor Cognitive Function in Older Men. J. Am. Geriatr. Soc. 2010, 58, 713–718. [Google Scholar] [CrossRef]
- Okamoto, N.; Morikawa, M.; Tomioka, K.; Yanagi, M.; Amano, N.; Kurumatani, N. Association between Tooth Loss and the Development of Mild Memory Impairment in the Elderly: The Fujiwara-Kyo Study. J. Alzheimer’s Dis. 2015, 44, 777–786. [Google Scholar] [CrossRef]
- Nilsson, H.; Sanmartin Berglund, J.; Renvert, S. Longitudinal Evaluation of Periodontitis and Development of Cognitive Decline among Older Adults. J. Clin. Periodontol. 2018, 45, 1142–1149. [Google Scholar] [CrossRef]
- Saito, S.; Ohi, T.; Murakami, T.; Komiyama, T.; Miyoshi, Y.; Endo, K.; Satoh, M.; Asayama, K.; Inoue, R.; Kikuya, M.; et al. Association between Tooth Loss and Cognitive Impairment in Community-Dwelling Older Japanese Adults: A 4-Year Prospective Cohort Study from the Ohasama Study. BMC Oral Health 2018, 18, 142. [Google Scholar] [CrossRef]
- Iwasaki, M.; Kimura, Y.; Ogawa, H.; Yamaga, T.; Ansai, T.; Wada, T.; Sakamoto, R.; Ishimoto, Y.; Fujisawa, M.; Okumiya, K.; et al. Periodontitis, Periodontal Inflammation, and Mild Cognitive Impairment: A 5-Year Cohort Study. J. Periodontal Res. 2019, 54, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Huang, X.; Gong, Y.; Sun, J. Association between Tooth Loss Rate and Risk of Mild Cognitive Impairment in Older Adults: A Population-Based Longitudinal Study. Aging 2021, 13, 21599–21609. [Google Scholar] [CrossRef]
- Thomson, W.M.; Barak, Y. Tooth Loss and Dementia: A Critical Examination. J. Dent. Res. 2021, 100, 226–231. [Google Scholar] [CrossRef]
- Arrivé, E.; Letenneur, L.; Matharan, F.; Laporte, C.; Helmer, C.; Barberger-Gateau, P.; Miquel, J.L.; Dartigues, J.F. Oral Health Condition of French Elderly and Risk of Dementia: A Longitudinal Cohort Study. Community Dent. Oral Epidemiol. 2012, 40, 230–238. [Google Scholar] [CrossRef]
- Tzeng, N.-S.; Chung, C.-H.; Yeh, C.-B.; Huang, R.-Y.; Yuh, D.-Y.; Huang, S.-Y.; Lu, R.-B.; Chang, H.-A.; Kao, Y.-C.; Chiang, W.-S.; et al. Are Chronic Periodontitis and Gingivitis Associated with Dementia? A Nationwide, Retrospective, Matched-Cohort Study in Taiwan. Neuroepidemiology 2016, 47, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-T.; Lee, H.-C.; Hu, C.-J.; Huang, L.-K.; Chao, S.-P.; Lin, C.-P.; Su, E.C.-Y.; Lee, Y.-C.; Chen, C.-C. Periodontitis as a Modifiable Risk Factor for Dementia: A Nationwide Population-Based Cohort Study. J. Am. Geriatr. Soc. 2017, 65, 301–305. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Hu, H.-Y.; Huang, L.-Y.; Chou, P.; Chu, D. Periodontal Disease Associated with Higher Risk of Dementia: Population-Based Cohort Study in Taiwan. J. Am. Geriatr. Soc. 2017, 65, 1975–1980. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Ohara, T.; Furuta, M.; Takeshita, T.; Shibata, Y.; Hata, J.; Yoshida, D.; Yamashita, Y.; Ninomiya, T. Tooth Loss and Risk of Dementia in the Community: The Hisayama Study. J. Am. Geriatr. Soc. 2017, 65, e95–e100. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kim, K.; Chang, J.; Kim, S.M.; Kim, S.J.; Cho, H.-J.; Park, S.M. Association of Chronic Periodontitis on Alzheimer’s Disease or Vascular Dementia. J. Am. Geriatr. Soc. 2019, 67, 1234–1239. [Google Scholar] [CrossRef]
- Kim, D.-H.; Jeong, S.-N.; Lee, J.-H. Severe Periodontitis with Tooth Loss as a Modifiable Risk Factor for the Development of Alzheimer, Vascular, and Mixed Dementia: National Health Insurance Service-National Health Screening Retrospective Cohort 2002–2015. J. Periodontal Implant. Sci. 2020, 50, 303–312. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Chang, C.-C.; Lin, C.-S.; Yeh, C.-C.; Hu, C.-J.; Wu, C.-Z.; Chen, T.-L.; Liao, C.-C. Risk of Dementia in Patients with Periodontitis and Related Protective Factors: A Nationwide Retrospective Cohort Study. J. Clin. Periodontol. 2020, 47, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-R.; Son, M.; Kim, Y.-R.; Kang, H.-K. Risk of Dementia According to the Severity of Chronic Periodontitis in Korea: A Nationwide Retrospective Cohort Study. Epidemiol. Health 2022, 44, e2022077. [Google Scholar] [CrossRef] [PubMed]
- Holmer, J.; Eriksdotter, M.; Häbel, H.; Hed Myrberg, I.; Jonsson, A.; Pussinen, P.J.; Garcia-Ptacek, S.; Jansson, L.; Sandborgh-Englund, G.; Buhlin, K. Periodontal Conditions and Incident Dementia: A Nationwide Swedish Cohort Study. J. Periodontol. 2022, 93, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Kiuchi, S.; Cooray, U.; Kusama, T.; Yamamoto, T.; Abbas, H.; Nakazawa, N.; Kondo, K.; Osaka, K.; Aida, J. Oral Status and Dementia Onset: Mediation of Nutritional and Social Factors. J. Dent. Res. 2022, 101, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Ide, M.; Harris, M.; Stevens, A.; Sussams, R.; Hopkins, V.; Culliford, D.; Fuller, J.; Ibbett, P.; Raybould, R.; Thomas, R.; et al. Periodontitis and Cognitive Decline in Alzheimer’s Disease. PLoS ONE 2016, 11, e0151081. [Google Scholar] [CrossRef]
- Chen, C.-K.; Wu, Y.-T.; Chang, Y.-C. Association between Chronic Periodontitis and the Risk of Alzheimer’s Disease: A Retrospective, Population-Based, Matched-Cohort Study. Alzheimer’s Res. Ther. 2017, 9, 56. [Google Scholar] [CrossRef]
- Liu, T.C.; Sheu, J.J.; Lin, H.C.; Jensen, D.A. Increased Risk of Parkinsonism Following Chronic Periodontitis: A Retrospective Cohort Study. Mov. Disord. 2013, 28, 1307–1308. [Google Scholar] [CrossRef]
- Chen, C.-K.; Wu, Y.-T.; Chang, Y.-C. Periodontal Inflammatory Disease Is Associated with the Risk of Parkinson’s Disease: A Population-Based Retrospective Matched-Cohort Study. PeerJ 2017, 5, e3647. [Google Scholar] [CrossRef]
- Woo, H.G.; Chang, Y.; Lee, J.S.; Song, T.-J. Association of Tooth Loss with New-Onset Parkinson’s Disease: A Nationwide Population-Based Cohort Study. Park. Dis. 2020, 2020, 4760512. [Google Scholar] [CrossRef]
- Jeong, E.; Park, J.-B.; Park, Y.-G. Evaluation of the Association between Periodontitis and Risk of Parkinson’s Disease: A Nationwide Retrospective Cohort Study. Sci. Rep. 2021, 11, 16594. [Google Scholar] [CrossRef]
- Kamer, A.R.; Craig, R.G.; Pirraglia, E.; Dasanayake, A.P.; Norman, R.G.; Boylan, R.J.; Nehorayoff, A.; Glodzik, L.; Brys, M.; de Leon, M.J. TNF-α and Antibodies to Periodontal Bacteria Discriminate between Alzheimer’s Disease Patients and Normal Subjects. J. Neuroimmunol. 2009, 216, 92–97. [Google Scholar] [CrossRef]
- Chen, K.-P.; Lee, Y.-P.; Hwang, M.-J.; Chiang, C.-P. Fusobacterium Nucleatum-Caused Brain Abscess—Case Report. J. Dent. Sci. 2021, 16, 776–777. [Google Scholar] [CrossRef] [PubMed]
- Foschi, F.; Izard, J.; Sasaki, H.; Sambri, V.; Prati, C.; Müller, R.; Stashenko, P. Treponema Denticola in Disseminating Endodontic Infections. J. Dent. Res. 2006, 85, 761–765. [Google Scholar] [CrossRef]
- Schwahn, C.; Frenzel, S.; Holtfreter, B.; Van der Auwera, S.; Pink, C.; Bülow, R.; Friedrich, N.; Völzke, H.; Biffar, R.; Kocher, T.; et al. Effect of Periodontal Treatment on Preclinical Alzheimer’s Disease—Results of a Trial Emulation Approach. Alzheimer’s Dement. 2022, 18, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Zhang, S.; Duan, L.; Yang, F.; Zhang, K.; Yan, F.; Ge, S. Periodontitis Deteriorates Cognitive Function and Impairs Neurons and Glia in a Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 79, 1785–1800. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Qian, X.; Wang, Q.; Huang, L.; Ge, S. Experimental Periodontitis Deteriorates Cognitive Function and Impairs Insulin Signaling in a Streptozotocin-Induced Alzheimer’s Disease Rat Model. J. Alzheimer’s Dis. 2022, 88, 57–74. [Google Scholar] [CrossRef]
- Wang, X.; Tong, Y.; Zhang, J.; Khan, N.; Zhang, K.; Bai, H.; Zhang, Q.; Chen, Y. Neuroinflammation Changes with Periodontal Inflammation Status during Periodontitis in Wild-Type Mice. Oral Dis. 2021, 27, 1001–1011. [Google Scholar] [CrossRef]
- Feng, Y.-K.; Wu, Q.-L.; Peng, Y.-W.; Liang, F.-Y.; You, H.-J.; Feng, Y.-W.; Li, G.; Li, X.-J.; Liu, S.-H.; Li, Y.-C.; et al. Oral P. gingivalis Impairs Gut Permeability and Mediates Immune Responses Associated with Neurodegeneration in LRRK2 R1441G Mice. J. Neuroinflamm. 2020, 17, 347. [Google Scholar] [CrossRef]
- Ilievski, V.; Zuchowska, P.K.; Green, S.J.; Toth, P.T.; Ragozzino, M.E.; Le, K.; Aljewari, H.W.; O’Brien-Simpson, N.M.; Reynolds, E.C.; Watanabe, K. Chronic Oral Application of a Periodontal Pathogen Results in Brain Inflammation, Neurodegeneration and Amyloid Beta Production in Wild Type Mice. PLoS ONE 2018, 13, e0204941. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Kang, X.-N.; Cao, Y.; Zheng, D.-X.; Lu, Y.-M.; Pang, C.-F.; Wang, Z.; Cheng, B.; Peng, Y. Porphyromonas Gingivalis Induces Depression via Downregulating P75NTR-Mediated BDNF Maturation in Astrocytes. Brain Behav. Immun. 2019, 81, 523–534. [Google Scholar] [CrossRef]
- Bachtiar, E.W.; Septiwidyati, T.R. Possible Role of Porphyromonas Gingivalis in the Regulation of E2F1, CDK11, and INOS Gene Expression in Neuronal Cell Cycle: A Preliminary Study. J. Int. Soc. Prev. Community Dent. 2021, 11, 582–587. [Google Scholar] [CrossRef]
- Su, X.; Tang, Z.; Lu, Z.; Liu, Y.; He, W.; Jiang, J.; Zhang, Y.; Wu, H. Oral Treponema Denticola Infection Induces Aβ1–40 and Aβ1–42 Accumulation in the Hippocampus of C57BL/6 Mice. J. Mol. Neurosci. 2021, 71, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Su, X.; Tang, Z.; Jian, L.; Zhu, H.; Cheng, X.; Wu, H. Treponema Denticola Induces Neuronal Apoptosis by Promoting Amyloid-β Accumulation in Mice. Pathogens 2022, 11, 1150. [Google Scholar] [CrossRef]
- Gustavsen, M.W.; Celius, E.G.; Moen, S.M.; Bjølgerud, A.; Berg-Hansen, P.; Nygaard, G.O.; Sandvik, L.; Lie, B.A.; Harbo, H.F. No Association between Multiple Sclerosis and Periodontitis after Adjusting for Smoking Habits. Eur. J. Neurol. 2015, 22, 588–590. [Google Scholar] [CrossRef]
- Al-Ansari, A. Is There an Association between Multiple Sclerosis and Oral Health? Evid. Based Dent. 2021, 22, 44–45. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).