Occurrence and Patterns of Enterotoxin Genes, spa Types and Antimicrobial Resistance Patterns in Staphylococcus aureus in Food and Food Contact Surfaces in Singapore
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection, Isolation and Identification of S. aureus
2.2. Detection and Isolation of SE Genes
2.3. Spa Typing
2.4. Antimicrobial Susceptibility Testing
2.5. Statistical Analysis
3. Results
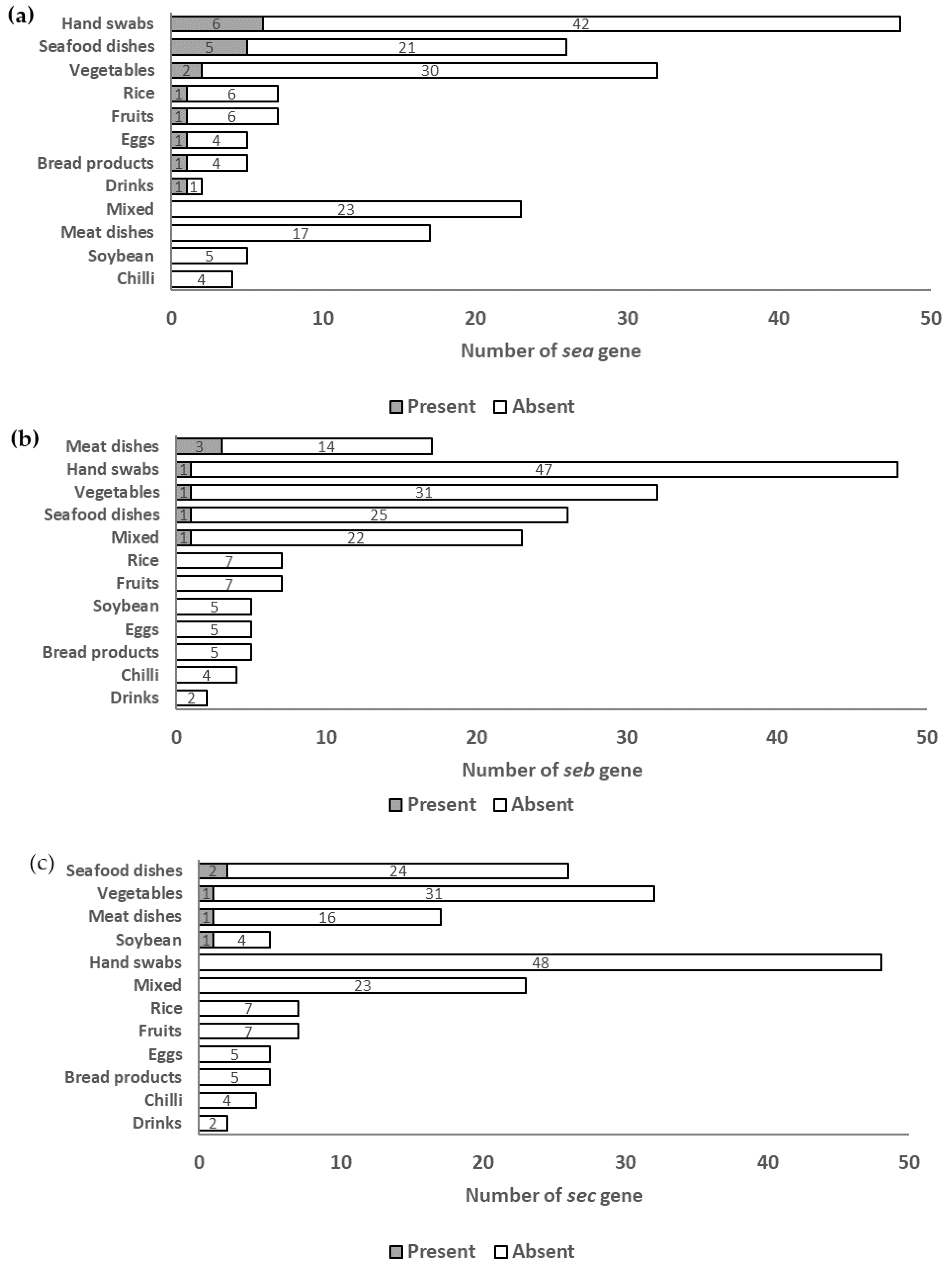
3.1. Occurrences and Distribution of SE Genes
3.2. Occurrence and Distribution of Antimicrobial Resistance (AMR) in Food
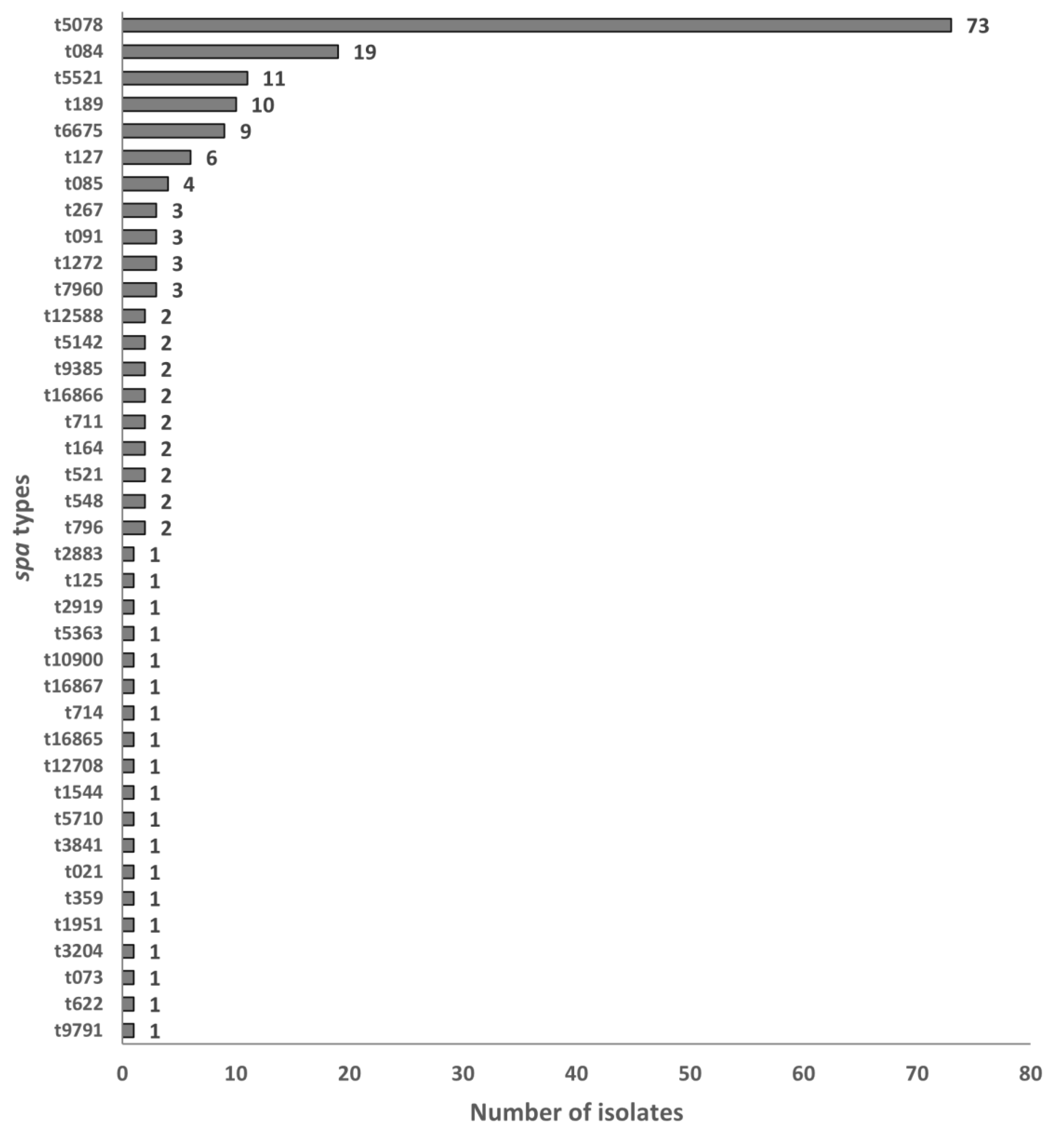
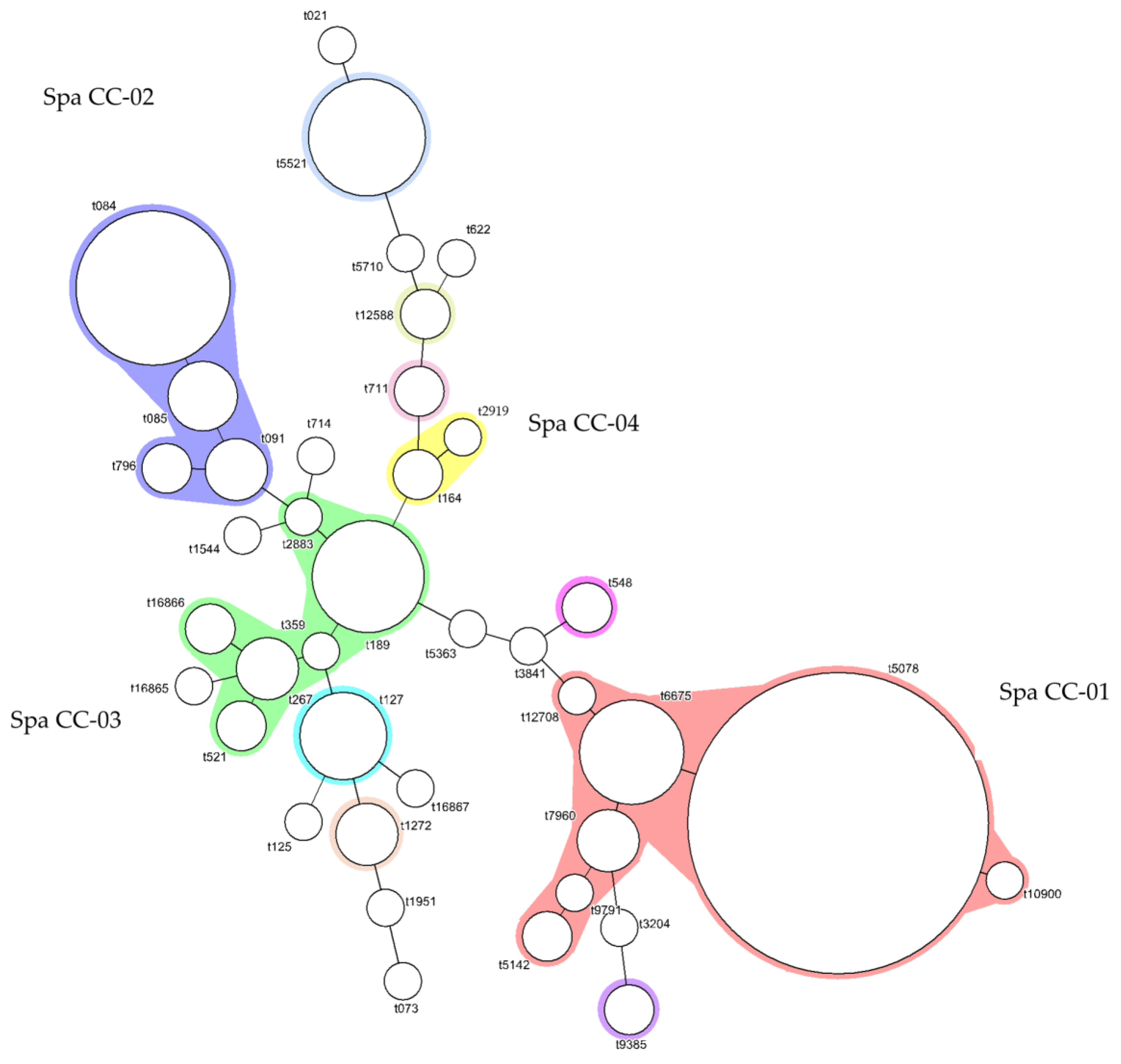
3.3. Distribution of Spa Types
4. Discussion
4.1. Overall Occurrence of SE Genes
4.2. Occurrence of SE Genes according to Food and Food Contact Surface Category
4.3. Distribution of Spa Types
4.4. General Antimicrobial Resistance Patterns
4.5. Antimicrobial Resistance Patterns according to Food and Food Contact Surface Category
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bencardino, D.; Amagliani, G.; Brandi, G. Carriage of Staphylococcus aureus among food handlers: An ongoing challenge in public health. Food Control 2021, 130, 108362. [Google Scholar] [CrossRef]
- Kadariya, J.; Smith, T.C.; Thapaliya, D. Staphylococcus aureus and staphylococcal food-borne disease: An ongoing challenge in public health. BioMed Res. Int. 2014, 2014, 827965. [Google Scholar] [CrossRef] [PubMed]
- Argudín, Á.M.; María, M.C.; María, R.R. Food poisoning and Staphylococcus aureus enterotoxins. Toxins 2010, 2, 1751–1773. [Google Scholar] [CrossRef] [PubMed]
- Shanehbandi, D.; Baradaran, B.; Sadigh-Eteghad, S.; Zarredar, H. Occurrence of methicillin resistant and enterotoxigenic Staphylococcus aureus in traditional cheeses in the north west of Iran. Natl. Sch. Res. Not. 2014, 2014, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mashouf, R.Y.; Hosseini, S.M.; Mousavi, S.M.; Arabestani, M.R. Prevalence of enterotoxin genes and antibacterial susceptibility pattern of Staphylococcus aureus strains isolated from animal originated foods in West of Iran. Oman Med. J. 2015, 30, 283. [Google Scholar] [CrossRef]
- Thaikruea, L.; Pataraarechachai, J.; Savanpunyalert, P.; Naluponjiragul, U. An unusual outbreak of food poisoning. Southeast Asian J. Trop. Med. Public Health 1995, 26, 78–85. [Google Scholar]
- Guo, Y.; Yu, X.; Wang, J.; Hua, D.; You, Y.; Wu, Q.; Ji, Q.; Zhang, J.; Li, L.; Hu, Y. A food poisoning caused by ST7 Staphylococcal aureus harboring sea gene in Hainan province, China. Front. Microbiol. 2023, 14, 1110720. [Google Scholar] [CrossRef]
- Asao, T.; Kumeda, Y.; Kawai, T.; Shibata, T.; Oda, H.; Haruki, K.; Nakazawa, H.; Kozaki, S. An extensive outbreak of staphylococcal food poisoning due to low-fat milk in Japan: Estimation of enterotoxin A in the incriminated milk and powdered skim milk. Epidemiology Infect. 2003, 130, 33. [Google Scholar] [CrossRef]
- Oliveira, R.; Pinho, E.; Almeida, G.; Azevedo, N.F.; Almeida, C. Prevalence and diversity of Staphylococcus aureus and staphylococcal enterotoxins in raw milk from Northern Portugal. Front. Microbiol. 2022, 13, 703. [Google Scholar] [CrossRef]
- Oliveira, D.C.; Tomasz, A.; de Lencastre, H. The evolution of pandemic clones of methicillin-resistant Staphylococcus aureus: Identification of two ancestral genetic backgrounds and the associated mec elements. Microb. Drug Resist. 2001, 7, 349–361. [Google Scholar] [CrossRef]
- Peacock, S.J.; De Silva, G.D.I.; Justice, A.; Cowland, A.; Moore, C.E.; Winearls, C.G.; Day, N.P.J. Comparison of multilocus sequence typing and pulsed-field gel electrophoresis as tools for typing Staphylococcus aureus isolates in a microepidemiological setting. J. Clin. Microbiol. 2002, 40, 3764–3770. [Google Scholar] [CrossRef] [PubMed]
- Salzberg, S.L.; Yorke, J.A. Beware of mis-assembled genomes. Bioinformatics 2005, 21, 4320–4321. [Google Scholar] [CrossRef] [PubMed]
- Sergelidis, D.; Angelidis, A.S. Methicillin-resistant Staphylococcus aureus: A controversial food-borne pathogen. Lett. Appl. Microbiol. 2017, 64, 409–418. [Google Scholar] [CrossRef]
- Cremonesi, P.; Luzzana, M.; Brasca, M.; Morandi, S.; Lodi, R.; Vimercati, C.; Agnellini, D.; Caramenti, G.; Moroni, P.; Castiglioni, B. Development of a multiplex PCR assay for the identification of Staphylococcus aureus enterotoxigenic strains isolated from milk and dairy products. Mol. Cell. Probes 2005, 19, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Rosec, J.P.; Gigaud, O. Staphylococcal enterotoxin genes of classical and new types detected by PCR in France. Int. J. Food Microbiol. 2002, 77, 61–70. [Google Scholar] [CrossRef]
- Strommenger, B.; Kettlitz, C.; Werner, G.; Witte, W. Multiplex PCR assay for simultaneous detection of nine clinically relevant antibiotic resistance genes in Staphylococcus aureus. J. Clin. Microbiol. 2003, 41, 4089–4094. [Google Scholar] [CrossRef]
- Veras, J.F.; do Carmo, L.S.; Tong, L.C.; Shupp, J.W.; Cummings, C.; Dos Santos, D.A.; Cerqueira, M.M.O.P.; Cantini, A.; Nicoli, J.R.; Jett, M. A study of the enterotoxigenicity of coagulase-negative and coagulase-positive staphylococcal isolates from food poisoning outbreaks in Minas Gerais, Brazil. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2008, 12, 410–415. [Google Scholar] [CrossRef]
- CLSI. Zone Diameter and Minimal Inhibitory Concentration Breakpoints for Staphylococcus spp. In Performance Standards for Antimicrobial Susceptibility Testing; CLSI: Wayne, PA, USA, 2017; Volume supplement M100, pp. 56–63. [Google Scholar]
- Gholamzad, M.; Khatami, M.R.; Ghassemi, S.; Malekshahi, Z.V.; Shooshtari, M.B. Detection of Staphylococcus enterotoxin B (SEB) using an immunochromatographic test strip. Jundishapur J. Microbiol. 2015, 8, e26793. [Google Scholar] [CrossRef]
- Strommenger, B.; Kettlitz, C.; Weniger, T.; Harmsen, D.; Friedrich, A.W.; Witte, W. Assignment of Staphylococcus isolates to groups by spa typing, SmaI macrorestriction analysis, and multilocus sequence typing. J. Clin. Microbiol. 2006, 44, 2533–2540. [Google Scholar] [CrossRef]
- Shopsin, B.; Gomez, M.; Montgomery, S.O.; Smith, D.H.; Waddington, M.; Dodge, D.E.; Bost, D.A.; Riehman, M.; Naidich, S.; Kreiswirth, B.N. Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J. Clin. Microbiol. 1999, 37, 3556–3563. [Google Scholar] [CrossRef]
- Mellmann, A.; Weniger, T.; Berssenbrügge, C.; Rothgänger, J.; Sammeth, M.; Stoye, J.; Harmsen, D. Based Upon Repeat Pattern (BURP): An algorithm to characterize the long-term evolution of Staphylococcus aureus populations based on spa polymorphisms. BMC Microbiol. 2007, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Hallin, M.; Friedrich, A.W.; Struelens, M.J. spa typing for epidemiological surveillance of Staphylococcus aureus. Methods Mol. Biol. 2009, 551, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Su Kyung, O.; Nari, L.; Young Sun, C.; Dong-Bin, S.; Soo Young, C.; Minseon, K. Occurrence of toxigenic Staphylococcus aureus in ready-to-eat food in Korea. J. Food Prot. 2007, 70, 1153–1158. [Google Scholar]
- Guoxiang, C.; Guangyu, B.; Yongzhong, C.; Wenguang, Y.; Yan, W.; Xiaorong, Z.; Liping, Z.; Yantao, W. Prevalence and diversity of enterotoxin genes with genetic background of Staphylococcus aureus isolates from different origins in China. Int. J. Food Microbiol. 2015, 211, 142–147. [Google Scholar]
- Normanno, G.; Firinu, A.; Virgilio, S.; Mula, G.; Dambrosio, A.; Poggiu, L.; Decastelli, L.; Mioni, R.; Scuota, S.; Bolzoni, G.; et al. Coagulase-positive Staphylococci and Staphylococcus aureus in food products marketed in Italy. Int. J. Food Microbiol. 2005, 98, 73–79. [Google Scholar] [CrossRef]
- Singapore Statutes Online (Ed.) Food Regulations, 2005th ed.; Singapore Statutes Online: Singapore, 2005. [Google Scholar]
- Yu Cheng, C.; Wan Wen, L.; Chin Ming, F.; Wan Yu, P.; Chien-Shun, C.; Hau-Yang, T. PCR detection of Staphylococcal enterotoxins (SEs) N, O, P, Q, R, U, and survey of SE types in Staphylococcus aureus isolates from food-poisoning cases in Taiwan. Int. J. Food Microbiol. 2008, 121, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Pinchuk, I.V.; Ellen, B.J.; Victor, R.E. Staphylococcal Enterotoxins. Toxins 2010, 2, 2177–2197. [Google Scholar] [CrossRef]
- Hait, J.; Tallent, S.; Melka, D.; Keys, C.; Bennett, R. Prevalence of enterotoxins and toxin gene profiles of S taphylococcus aureus isolates recovered from a bakery involved in a second staphylococcal food poisoning occurrence. J. Appl. Microbiol. 2014, 117, 866–875. [Google Scholar] [CrossRef]
- Tang, J.; Tang, C.; Chen, J.; Du, Y.; Yang, X.-N.; Wang, C.; Zhang, H.; Yue, H. Phenotypic characterization and prevalence of enterotoxin genes in Staphylococcus aureus isolates from outbreaks of illness in Chengdu City. Foodborne Pathog. Dis. 2011, 8, 1317–1320. [Google Scholar] [CrossRef]
- Johler, S.; Petra, G.; Marco, J.; Jörg, H.; Andreas, B.; Roger, S. Further evidence for staphylococcal food poisoning outbreaks caused by egc-encoded enterotoxins. Toxins 2015, 7, 997–1004. [Google Scholar] [CrossRef]
- McLauchlin, J.; Narayanan, G.L.; Mithani, V.; O'neill, G. The detection of enterotoxins and toxic shock syndrome toxin genes in Staphylococcus aureus by polymerase chain reaction. J. Food Prot. 2000, 63, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Omoe, K.; Ishikawa, M.; Shimoda, Y.; Dong-Liang, H.; Ueda, S.; Shinagawa, K. Detection of seg, seh, and sei genes in Staphylococcus aureus isolates and determination of the enterotoxin productivities of S. aureus isolates harboring seg, seh, or sei genes. J. Clin. Microbiol. 2002, 40, 857–862. [Google Scholar] [CrossRef]
- Rall, V.L.M.; Sforcin, J.M.; Augustini, V.C.M.; Watanabe, M.T.; Fernandes Jr, A.; Rall, R.; Silva, M.G.; Araújo Jr, J.P. Detection of enterotoxin genes of Staphylococcus sp isolated from nasal cavities and hands of food handlers. Braz. J. Microbiol. 2010, 41, 59–65. [Google Scholar] [CrossRef]
- Shimamura, Y.; Kidokoro, S.; Murata, M. Survey and properties of Staphylococcus aureus isolated from Japanese-style desserts. Biosci. Biotechnol. Biochem. 2006, 70, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Aung, K.T.; Hsu, L.Y.; Koh, T.H.; Hapuarachchi, H.C.; Chau, M.L.; Gutiérrez, R.A.; Ng, L.C. Prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in retail food in Singapore. Antimicrob. Resist. Infect. Control 2017, 6, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Abdalrahman, L.S.; Wells, H.; Fakhr, M.K. Staphylococcus aureus is more prevalent in retail beef livers than in pork and other beef cuts. Pathogens 2015, 4, 182–198. [Google Scholar] [CrossRef]
- Velasco, V.; Vergara, J.L.; Bonilla, A.M.; Munoz, J.; Mallea, A.; Vallejos, D.; Quezada-Aguiluz, M.; Campos, J.; Rojas-Garcia, P. Prevalence and characterization of Staphylococcus aureus strains in the pork chain supply in Chile. Foodborne Pathog. Dis. 2018, 15, 262–268. [Google Scholar] [CrossRef]
- Şanlıbaba, P. Prevalence, antibiotic resistance, and enterotoxin production of Staphylococcus aureus isolated from retail raw beef, sheep, and lamb meat in Turkey. Int. J. Food Microbiol. 2022, 361, 109461. [Google Scholar] [CrossRef]
- Berger, T.; Eisenkraft, A.; Bar-Haim, E.; Kassirer, M.; Aran, A.A.; Fogel, I. Toxins as biological weapons for terror—Characteristics, challenges and medical countermeasures: A mini-review. Disaster Mil. Med. 2016, 2, 1–7. [Google Scholar] [CrossRef]
- Gill, D.M. Bacterial toxins: A table of lethal amounts. Microbiol. Rev. 1982, 46, 86–94. [Google Scholar] [CrossRef]
- Blaiotta, G.; Ercolini, D.; Pennacchia, C.; Fusco, V.; Casaburi, A.; Pepe, O.; Villani, F. PCR detection of staphylococcal enterotoxin genes in Staphylococcus spp. strains isolated from meat and dairy products. Evidence for new variants of seG and seI in S. aureus AB-8802. J. Appl. Microbiol. 2004, 97, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Felix, B.; Vingadassalon, N.; Grout, J.; Hennekine, J.-A.; Guillier, L.; Auvray, F. Staphylococcus aureus strains associated with food poisoning outbreaks in France: Comparison of different molecular typing methods, including MLVA. Front. Microbiol. 2015, 6, 882. [Google Scholar]
- Alni, R.H.; Mohammadzadeh, A.; Mahmoodi, P. Molecular typing of Staphylococcus aureus of different origins based on the polymorphism of the spa gene: Characterization of a novel spa type. 3 Biotech 2018, 8, 1–7. [Google Scholar]
- Johler, S.; Macori, G.; Bellio, A.; Acutis, P.L.; Gallina, S.; Decastelli, L. Characterization of Staphylococcus aureus isolated along the raw milk cheese production process in artisan dairies in Italy. J. Dairy Sci. 2018, 101, 2915–2920. [Google Scholar] [CrossRef] [PubMed]
- Teo, J.W.; Kum, S.; Jureen, R.; Lin, R.T. Molecular characterization of a catalase-negative Staphylococcus aureus blood culture isolate. J. Clin. Microbiol. 2015, 53, 3699–3701. [Google Scholar] [CrossRef]
- Ho, J.; O’Donoghue, M.M.; Boost, M.V. Occupational exposure to raw meat: A newly-recognized risk factor for Staphylococcus aureus nasal colonization amongst food handlers. Int. J. Hyg. Environ. Health 2014, 217, 347–353. [Google Scholar] [CrossRef]
- Tunsjø, H.S.; Kalyanasundaram, S.; Charnock, C.; Leegaard, T.M.; Moen, A.E. Challenges in the identification of methicillin-resistant Staphylococcus argenteus by routine diagnostics. Apmis 2018, 126, 533–537. [Google Scholar] [CrossRef]
- Aung, M.S.; Urushibara, N.; Kawaguchiya, M.; Sumi, A.; Takahashi, S.; Ike, M.; Ito, M.; Habadera, S.; Kobayashi, N. Molecular epidemiological characterization of Staphylococcus argenteus clinical isolates in Japan: Identification of three clones (ST1223, ST2198, and ST2550) and a novel staphylocoagulase genotype XV. Microorganisms 2019, 7, 389. [Google Scholar] [CrossRef]
- Ilczyszyn, W.M.; Sabat, A.J.; Akkerboom, V.; Szkarlat, A.; Klepacka, J.; Sowa-Sierant, I.; Wasik, B.; Kosecka-Strojek, M.; Buda, A.; Miedzobrodzki, J.; et al. Clonal structure and characterization of Staphylococcus aureus strains from invasive infections in paediatric patients from South Poland: Association between age, spa types, clonal complexes, and genetic markers. PLoS ONE 2016, 11, e0151937. [Google Scholar] [CrossRef]
- Miko, B.A.; Hafer, C.A.; Lee, C.J.; Sullivan, S.B.; Hackel, M.A.; Johnson, B.M.; Whittier, S.; Della-Latta, P.; Uhlemann, A.-C.; Lowy, F.D. Molecular characterization of methicillin-susceptible Staphylococcus aureus clinical isolates in the United States, 2004 to 2010. J. Clin. Microbiol. 2013, 51, 874–879. [Google Scholar] [CrossRef]
- Saffari, F.; Radfar, A.; Sobhanipoor, M.H.; Ahmadrajabi, R. Spa gene-based molecular typing of nasal methicillin-susceptible Staphylococcus aureus from patients and health-care workers in a dialysis center in southeast Iran. Pathog. Glob. Health 2020, 114, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Uhlemann, A.C.; Dumortier, C.; Hafer, C.; Taylor, B.S.; Sánchez, E.; Rodriguez-Taveras, C.; Leon, P.; Rojas, R.; Olive, C.; Lowy, F.D. Molecular characterization of Staphylococcus aureus from outpatients in the Caribbean reveals the presence of pandemic clones. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 31, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Fetsch, A.; Contzen, M.; Hartelt, K.; Kleiser, A.; Maassen, S.; Rau, J.; Kraushaar, B.; Layer, F.; Strommenger, B. Staphylococcus aureus food-poisoning outbreak associated with the consumption of ice-cream. Int. J. Food Microbiol. 2014, 187, 1–6. [Google Scholar] [CrossRef]
- Strommenger, B.; Braulke, C.; Heuck, D.; Schmidt, C.; Pasemann, B.; Nübel, U.; Witte, W. spa Typing of Staphylococcus aureus as a frontline tool in epidemiological typing. J. Clin. Microbiol. 2008, 46, 574–581. [Google Scholar] [CrossRef]
- Ge, B.; Mukherjee, S.; Hsu, C.-H.; Davis, J.A.; Tran, T.T.T.; Yang, Q.; Abbott, J.W.; Ayers, S.L.; Young, S.R.; Crarey, E.T.; et al. MRSA and multidrug-resistant Staphylococcus aureus in US retail meats. Food Microbiol. 2017, 62, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Udo, E.E.; Al-Mufti, S.; Albert, M.J. The prevalence of antimicrobial resistance and carriage of virulence genes in Staphylococcus aureus isolated from food handlers in Kuwait City restaurants. BMC Res. Notes 2009, 2, 1–6. [Google Scholar] [CrossRef]
- Wang, W.; Baloch, Z.; Jiang, T.; Zhang, C.; Peng, Z.; Li, F.; Fanning, S.; Ma, A.; Xu, J. Enterotoxigenicity and antimicrobial resistance of Staphylococcus aureus isolated from retail food in China. Front. Microbiol. 2017, 8, 2256. [Google Scholar] [CrossRef]
- Chaalal, W.; Chaalal, N.; Bourafa, N.; Kihal, M.; Diene, S.M.; Rolain, J.-M. Characterization of Staphylococcus aureus isolated from food products in Western Algeria. Foodborne Pathog. Dis. 2018, 15, 353–360. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Bao, H.; Wei, R.; Zhou, Y.; Zhang, H.; Wang, R. Population structure and antimicrobial profile of Staphylococcus aureus strains associated with bovine mastitis in China. Microb. Pathog. 2016, 97, 103–109. [Google Scholar] [CrossRef]
- Akpaka, P.E.; Roberts, R.; Monecke, S. Molecular characterization of antimicrobial resistance genes against Staphylococcus aureus isolates from Trinidad and Tobago. J. Infect. Public Health 2017, 10, 316–323. [Google Scholar] [CrossRef]
- Lowy, F.D. Antimicrobial resistance: The example of Staphylococcus aureus. J. Clin. Investig. 2003, 111, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.E.; Christensen, H.; Aarestrup, F.M. Diversity and evolution of blaZ from Staphylococcus aureus and coagulase-negative staphylococci. J. Antimicrob. Chemother. 2006, 57, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Aydin, A.; Muratoglu, K.; Sudagidan, M.; Bostan, K.; Okuklu, B.; Harsa, S. Prevalence and antibiotic resistance of foodborne Staphylococcus aureus isolates in Turkey. Foodborne Pathog. Dis. 2011, 8, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, T.M.; De Oliveira, C.R.; Chambers, H.F.; Chatterjee, S.S. BP4: A new perspective on Staphylococcus aureus β-lactam resistance. Microorganisms 2018, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Reading, C.; Cole, M. Clavulanic acid: A beta-lactamase-inhibiting beta-lactam from Streptomyces clavuligerus. Antimicrob. Agents Chemother. 1977, 11, 852–857. [Google Scholar] [CrossRef]
- Achek, R.; Hotzel, H.; Cantekin, Z.; Nabi, I.; Hamdi, T.M.; Neubauer, H.; El-Adawy, H. Emerging of antimicrobial resistance in staphylococci isolated from clinical and food samples in Algeria. BMC Res. Notes 2018, 11, 1–7. [Google Scholar] [CrossRef]
- Xu, J.; Shi, C.; Song, M.; Xu, X.; Yang, P.; Paoli, G.; Shi, X. Phenotypic and genotypic antimicrobial resistance traits of foodborne Staphylococcus aureus isolates from Shanghai. J. Food Sci. 2014, 79, M635–M642. [Google Scholar] [CrossRef]
- Ardic, N.; Ozyurt, M.; Sareyyupoglu, B.; Haznedaroglu, T. Investigation of erythromycin and tetracycline resistance genes in methicillin-resistant staphylococci. Int. J. Antimicrob. Agents 2005, 26, 213–218. [Google Scholar] [CrossRef]
- Chajęcka-Wierzchowska, W.; Zadernowska, A.; Nalepa, B.; SIERPI´ NSKA, M.A.G.D.A.; Łaniewska-Trokenheim, L. Retail ready-to-eat food as a potential vehicle for Staphylococcus spp. harboring antibiotic resistance genes. J. Food Prot. 2014, 77, 993–998. [Google Scholar] [CrossRef]
- Tatini, S.R. Influence of food environments on growth of Staphylococcus aureus and production of various enterotoxins. J. Milk Food Technol. 1973, 36, 559–563. [Google Scholar] [CrossRef]
- Mesbah, A.; Mashak, Z.; Abdolmaleki, Z. A survey of prevalence and phenotypic and genotypic assessment of antibiotic resistance in Staphylococcus aureus bacteria isolated from ready-to-eat food samples collected from Tehran Province, Iran. Trop. Med. Health 2021, 49, 1–12. [Google Scholar] [CrossRef] [PubMed]
| Reagents | Volume of Reagents Used for the Detection of Virulence Genes (µL) | |||
|---|---|---|---|---|
| Multiplex PCR 1 | Multiplex PCR 2 | Singleplex PCR 1 | Singleplex PCR 2 | |
| HF Buffer (5×) | 10 | 10 | 10 | 10 |
| dNTP (10 mM) | 1 | 1 | 1 | 1 |
| ESA F + R primer (10 μM) | 1 | |||
| ESB F + R primer (10 μM) | 1 | |||
| ESC F + R primer (10 μM) | 1 | |||
| ESD F + R primer (10 μM) | 1 | |||
| ESE F + R primer (10 μM) | 1 | |||
| ESG F + R primer (10 μM) | 0.5 | |||
| ESH F + R primer (10 μM) | 0.5 | |||
| ESI F + R primer (10 μM) | 1 | |||
| ESJ F + R primer (10 μM) | 1 | |||
| ESL F + R primer (10 μM) | 1 | |||
| Phusion Taq polymerase | 1 | 0.5 | 0.5 | 0.5 |
| Molecular grade water | 29 | 30.5 | 32.5 | 32.5 |
| Gene | Primer | Nucleotide Sequences | Amplicon Size (bp) | Multiplex/Singleplex PCR |
|---|---|---|---|---|
| sea | ESA′ | 5′-ACGATCAATTTTTACAG′-3′ | 544 | Multiplex PCR 1 |
| ESA′ | 5′-TGCATGTTTTCAGAGTTAAT′-3′ | |||
| seb | ESB′ | 5′-GAATGATATTAATTCGCAT′-3′ | 416 | Multiplex PCR 1 |
| ESB′ | 5′-TCTTTGTCGTAAGATAAACTT′-3′ | |||
| sec | ESC′ | 5′-GACATAAAAGCTAGGAATT′-3′ | 257 | Singleplex PCR 2 |
| ESC′ | 5′-AAATCGGATTAACATTATCC′-3′ | |||
| sed | ESD′ | 5′-TTACTAGTTTGGTAATATCTCCT′-3′ | 334 | Multiplex PCR 1 |
| ESD′ | 5′-CCACCATAACAATTAATG′-3′ | |||
| see | ESE′ | 5′-ATAGATAAAGTTAAAACAAGCA′-3′ | 170 | Multiplex PCR 1 |
| ESE′ | 5′-TAACTTACCGTGGACC′-3′ | |||
| seg | ESG′ | 5′-ACGTCTCCACCTGTTGAAG′-3′ | 400 | Multiplex PCR 2 |
| ESG′ | 5′-TGAGCCAGTGTCTTGCTTT′-3′ | |||
| seh | ESH′ | 5′-TCACATCATATGCGAAAGCA′-3′ | 357 | Multiplex PCR 2 |
| ESH′ | 5′-TAGCACCAATCACCCTTTC′-3′ | |||
| sei | ESI′ | 5′-TGGAACAGGACAAGCTGAA′-3′ | 467 | Multiplex PCR 2 |
| ESI′ | 5′-TAAAGTGGCCCCTCCATAC′-3′ | |||
| sej | ESJ′ | 5′-CAGCGATAGCAAAAATGAAAC′-3′ | 240 | Singleplex PCR 1 |
| ESJ′ | 5′-TCTAGCGGAACAACAGTTCTG′-3′ | |||
| sel | ESL′ | 5′-CACCAGAATCACACCGCTT′-3′ | 426 | Multiplex PCR 2 |
| ESL′ | 5′-CTGTTTGATGCTTGCCATT′-3′ |
| Sample Category | n | % |
|---|---|---|
| Hand swabs | 48 | 26.5 |
| Vegetables | 32 | 17.7 |
| Seafood dishes | 26 | 14.4 |
| Mixed | 23 | 12.7 |
| Meat dishes | 17 | 9.4 |
| Fruits | 7 | 3.8 |
| Rice | 7 | 3.8 |
| Bread products | 5 | 2.8 |
| Eggs | 5 | 2.8 |
| Soybean | 5 | 2.8 |
| Chili | 4 | 2.2 |
| Drinks | 2 | 1.1 |
| Total | 181 | 100% |
| Type of SE Genes | Name of SE Genes | % | n |
|---|---|---|---|
| Classical SE genes | sea | 18.8 | 18 |
| seb | 7.3 | 7 | |
| sec | 5.3 | 5 | |
| sed | 0.0 | 0 | |
| see | 0.0 | 0 | |
| Non-classical SE genes | seg | 26.0 | 25 |
| sei | 26.0 | 25 | |
| seh | 8.3 | 8 | |
| sel | 7.3 | 7 | |
| sej | 1.0 | 1 | |
| Total | 100 | 96 |
| Antimicrobial Class | Antimicrobial Agent Tested in the Study | Percentage of Isolates Showing Resistant Phenotypes (n) |
|---|---|---|
| Aminoglycosides | Amikacin (AK) | 0.0% |
| Gentamicin (CN) | 3.3% (6/181) | |
| Beta-lactams | Amoxycillin/Clavulanic Acid (AMC) | 0.6% (1/181) |
| Ampicillin (AMP) | 54.7% (99/181) | |
| Penicillin G (P) | 54.7% (99/181) | |
| Cephalosporins | Cefoxitin (FOX) | 0.0% |
| Ceftriaxone (CRO) | 0.0% | |
| Chloramphenicols | Chloramphenicol (C) | 0.0% |
| Fluoroquinolones | Ciprofloxacin (CIP) | 1.7% (3/181) |
| Norfloxacin (NOR) | 0.6% (1/181) | |
| Clycopeptides | Vancomycin (VA) | 0.0% |
| Macrolides | Azithromycin (AZM) | 8.8% (16/181) |
| Rifampicin (RD) | 0.0% | |
| Sulphonamides | Sulphamethoxazole/Trimethoprim (SXT) | 0.0% |
| Tetracyclines | Tetracycline (TE) | 14.9% (27/181) |
| Antimicrobial Class | Antimicrobial Agent Tested in the Study | Bread Products (n = 5) | Chilli (n = 4) | Drinks (n = 2) | Eggs (n = 5) | Fruits (n = 7) | Hand Swabs (n = 48) | Meat Dishes (n = 17) | Mixed (n = 23) | Rice (n = 7) | Seafood Dishes (n = 26) | Soybean (n = 5) | Vegetables (n = 32) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aminoglycosides | Amikacin (AK) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gentamicin (CN) | 0 | 25 | 0 | 0 | 14 | 0 | 0 | 0 | 0 | 4 | 0 | 9 | |
| Beta-lactams | Amoxycillin/ Clavulanic Acid (AMC) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 |
| Ampicillin (AMP) | 80 | 25 | 50 | 40 | 71 | 60 | 53 | 57 | 57 | 54 | 40 | 47 | |
| Penicillin G (P) | 80 | 25 | 50 | 40 | 71 | 60 | 53 | 57 | 57 | 54 | 40 | 47 | |
| Cephalosporins | Cefoxitin (FOX) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ceftriaxone (CRO) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Chloramphenicols | Chloramphenicol (C) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fluoroquinolones | Ciprofloxacin (CIP) | 0 | 0 | 0 | 0 | 0 | 2 | 6 | 4 | 0 | 0 | 0 | 0 |
| Norfloxacin (NOR) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | |
| Clycopeptides | Vancomycin (VA) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Macrolides | Azithromycin (AZM) | 0 | 0 | 0 | 20 | 14 | 8 | 0 | 4 | 14 | 12 | 20 | 13 |
| Rifampicin (RD) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Sulphonamides | Sulfamethoxazole/ Trimethoprim (SXT) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tetracyclines | Tetracycline (TE) | 60 | 25 | 0 | 0 | 29 | 15 | 18 | 9 | 0 | 8 | 20 | 19 |
 .
.| Spa Type | sea | seb | sec |
|---|---|---|---|
| t5078 (n = 73) | 0 | 0 | 0 |
| t084 (n = 19) | 0 | 2 | 0 |
| t5521 (n = 11) | 11 | 0 | 0 |
| t189 (n = 10) | 0 | 0 | 0 |
| t6675 (n = 9) | 1 | 0 | 0 |
| t127 (n = 6) | 3 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, K.L.; Khor, W.C.; Ong, K.H.; Timothy, L.; Aung, K.T. Occurrence and Patterns of Enterotoxin Genes, spa Types and Antimicrobial Resistance Patterns in Staphylococcus aureus in Food and Food Contact Surfaces in Singapore. Microorganisms 2023, 11, 1785. https://doi.org/10.3390/microorganisms11071785
Lim KL, Khor WC, Ong KH, Timothy L, Aung KT. Occurrence and Patterns of Enterotoxin Genes, spa Types and Antimicrobial Resistance Patterns in Staphylococcus aureus in Food and Food Contact Surfaces in Singapore. Microorganisms. 2023; 11(7):1785. https://doi.org/10.3390/microorganisms11071785
Chicago/Turabian StyleLim, Ker Li, Wei Ching Khor, Kar Hui Ong, Lois Timothy, and Kyaw Thu Aung. 2023. "Occurrence and Patterns of Enterotoxin Genes, spa Types and Antimicrobial Resistance Patterns in Staphylococcus aureus in Food and Food Contact Surfaces in Singapore" Microorganisms 11, no. 7: 1785. https://doi.org/10.3390/microorganisms11071785
APA StyleLim, K. L., Khor, W. C., Ong, K. H., Timothy, L., & Aung, K. T. (2023). Occurrence and Patterns of Enterotoxin Genes, spa Types and Antimicrobial Resistance Patterns in Staphylococcus aureus in Food and Food Contact Surfaces in Singapore. Microorganisms, 11(7), 1785. https://doi.org/10.3390/microorganisms11071785





