Prevention and Health Benefits of Prebiotics, Probiotics and Postbiotics in Acute Lymphoblastic Leukemia
Abstract
1. Introduction
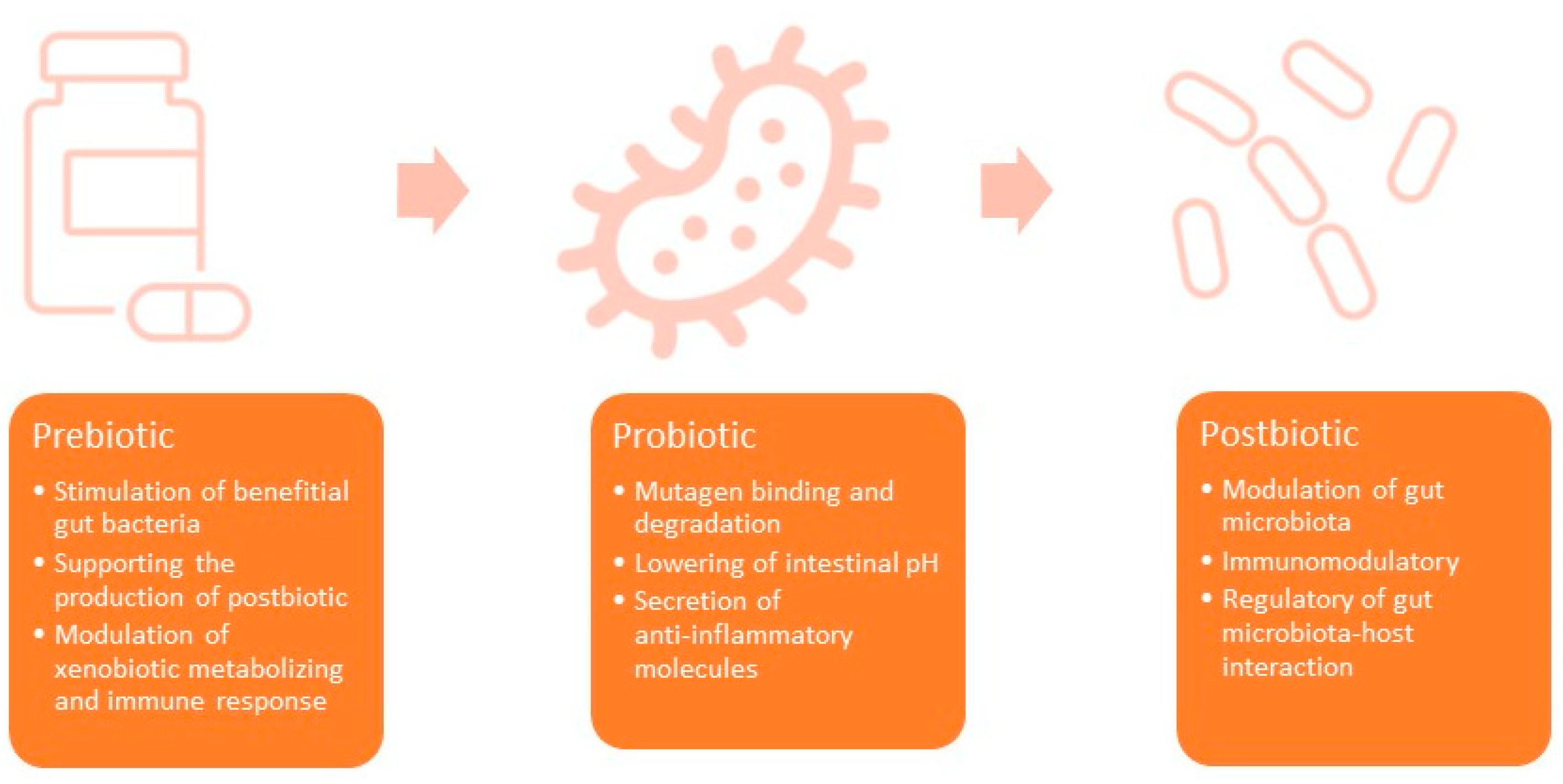
1.1. Prebiotics
1.2. Probiotics
1.3. Postbiotics
1.4. Basic Mechanisms of Anticancer Properties of Prebiotics, Probiotics, and Postbiotics
2. Prebiotics in ALL
3. Probiotics in ALL
3.1. Supplementation of Probiotics in ALL
3.2. Anticancer Activity of Probiotics
3.3. The Effect of Anti-ALL Therapies on Microbiota
4. Postbiotic in ALL
4.1. Short-Chain Fatty Acids
4.2. Tryptophan
4.3. Folic Acid (FA)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bhojwani, D.; Yang, J.J.; Pui, C.-H. Biology of Childhood Acute Lymphoblastic Leukemia. Pediatr. Clin. N. Am. 2015, 62, 47–60. [Google Scholar] [CrossRef]
- Inaba, H.; Mullighan, C.G. Pediatric Acute Lymphoblastic Leukemia. Haematologica 2020, 105, 2524–2539. [Google Scholar] [CrossRef]
- Mueller, K.T.; Waldron, E.; Grupp, S.A.; Levine, J.E.; Laetsch, T.W.; Pulsipher, M.A.; Boyer, M.W.; August, K.J.; Hamilton, J.; Awasthi, R.; et al. Clinical Pharmacology of Tisagenlecleucel in B-Cell Acute Lymphoblastic Leukemia. Clin. Cancer Res. 2018, 24, 6175–6184. [Google Scholar] [CrossRef] [PubMed]
- Bossù, G.; Di Sario, R.; Argentiero, A.; Esposito, S. Antimicrobial Prophylaxis and Modifications of the Gut Microbiota in Children with Cancer. Antibiotics 2021, 10, 152. [Google Scholar] [CrossRef]
- Hollister, E.B.; Riehle, K.; Luna, R.A.; Weidler, E.M.; Rubio-Gonzales, M.; Mistretta, T.-A.; Raza, S.; Doddapaneni, H.V.; Metcalf, G.A.; Muzny, D.M.; et al. Structure and Function of the Healthy Pre-Adolescent Pediatric Gut Microbiome. Microbiome 2015, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Bhuta, R.; DeNardo, B.; Wang, J.; Atoyan, J.; Zhang, Y.; Nelson, D.; Shapiro, J. Durable Changes in the Gut Microbiome in Survivors of Childhood Acute Lymphoblastic Leukemia. Pediatr. Blood Cancer 2021, 68, e29308. [Google Scholar] [CrossRef] [PubMed]
- Bauer, H.; Horowitz, R.E.; Levenson, S.M.; Popper, H. The Response of the Lymphatic Tissue to the Microbial Flora. Studies on Germfree Mice. Am. J. Pathol. 1963, 42, 471–483. [Google Scholar]
- de Vrese, M.; Schrezenmeir, J. Probiotics, Prebiotics, and Synbiotics. Adv. Biochem. Eng. Biotechnol. 2008, 111, 1–66. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Perceval, C.; Szajewska, H.; Indrio, F.; Weizman, Z.; Vandenplas, Y. Prophylactic Use of Probiotics for Gastrointestinal Disorders in Children. Lancet Child Adolesc. Health 2019, 3, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Martyniak, A.; Medyńska-Przęczek, A.; Wędrychowicz, A.; Skoczeń, S.; Tomasik, P.J. Prebiotics, Probiotics, Synbiotics, Paraprobiotics and Postbiotic Compounds in IBD. Biomolecules 2021, 11, 1903. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, H.; Nakase, H.; Inoue, S.; Kawanami, C.; Itani, T.; Ohana, M.; Kusaka, T.; Uose, S.; Hisatsune, H.; Tojo, M.; et al. Efficacy of Probiotic Treatment with Bifidobacterium Longum 536 for Induction of Remission in Active Ulcerative Colitis: A Randomized, Double-Blinded, Placebo-Controlled Multicenter Trial. Dig. Endosc. 2016, 28, 67–74. [Google Scholar] [CrossRef]
- Kok, C.R.; Hutkins, R. Yogurt and Other Fermented Foods as Sources of Health-Promoting Bacteria. Nutr. Rev. 2018, 76, 4–15. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Vinderola, G.; Sanders, M.E.; Salminen, S. The Concept of Postbiotics. Foods 2022, 11, 1077. [Google Scholar] [CrossRef]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Korčok, D.J.; Tršić-Milanović, N.A.; Ivanović, N.D.; Đorđević, B.I. Development of Probiotic Formulation for the Treatment of Iron Deficiency Anemia. Chem. Pharm. Bull. 2018, 66, 347–352. [Google Scholar] [CrossRef]
- González, A.; Gálvez, N.; Martín, J.; Reyes, F.; Pérez-Victoria, I.; Dominguez-Vera, J.M. Identification of the Key Excreted Molecule by Lactobacillus Fermentum Related to Host Iron Absorption. Food Chem. 2017, 228, 374–380. [Google Scholar] [CrossRef]
- Raman, M.; Ambalam, P.; Kondepudi, K.K.; Pithva, S.; Kothari, C.; Patel, A.T.; Purama, R.K.; Dave, J.M.; Vyas, B.R.M. Potential of Probiotics, Prebiotics and Synbiotics for Management of Colorectal Cancer. Gut Microbes 2013, 4, 181–192. [Google Scholar] [CrossRef]
- Romanin, D.E.; Llopis, S.; Genovés, S.; Martorell, P.; Ramón, V.D.; Garrote, G.L.; Rumbo, M. Probiotic Yeast Kluyveromyces Marxianus CIDCA 8154 Shows Anti-Inflammatory and Anti-Oxidative Stress Properties in In Vivo Models. Benef. Microbes 2016, 7, 83–93. [Google Scholar] [CrossRef]
- Song, D.; Wang, X.; Ma, Y.; Liu, N.-N.; Wang, H. Beneficial Insights into Postbiotics against Colorectal Cancer. Front. Nutr. 2023, 10, 1111872. [Google Scholar] [CrossRef] [PubMed]
- Thammarutwasik, P.; Hongpattarakere, T.; Chantachum, S.; Kijroongrojana, K.; Itharat, A.; Reanmongkol, W.; Tewtrakul, S.; Ooraikul, B. Prebiotics—A Review. Songklanakarin J. Sci. Technol. (SJST) 2009, 31, 401–408. [Google Scholar]
- Schoener, C.A.; Carillo-Conde, B.; Hutson, H.N.; Peppas, N.A. An Inulin and Doxorubicin Conjugate for Improving Cancer Therapy. J. Drug Deliv. Sci. Technol. 2013, 23, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Bindels, L.B.; Neyrinck, A.M.; Loumaye, A.; Catry, E.; Walgrave, H.; Cherbuy, C.; Leclercq, S.; Van Hul, M.; Plovier, H.; Pachikian, B.; et al. Increased Gut Permeability in Cancer Cachexia: Mechanisms and Clinical Relevance. Oncotarget 2018, 9, 18224–18238. [Google Scholar] [CrossRef] [PubMed]
- Mazraeh, R.; Azizi-Soleiman, F.; Jazayeri, S.M.H.M.; Noori, S.M.A. Effect of Inulin-Type Fructans in Patients Undergoing Cancer Treatments: A Systematic Review. Pak. J. Med. Sci. 2019, 35, 575–580. [Google Scholar] [CrossRef]
- Carrion, C.C.; Nasrollahzadeh, M.; Sajjadi, M.; Jaleh, B.; Soufi, G.J.; Iravani, S. Lignin, Lipid, Protein, Hyaluronic Acid, Starch, Cellulose, Gum, Pectin, Alginate and Chitosan-Based Nanomaterials for Cancer Nanotherapy: Challenges and Opportunities. Int. J. Biol. Macromol. 2021, 178, 193–228. [Google Scholar] [CrossRef]
- Chandel, V.; Biswas, D.; Roy, S.; Vaidya, D.; Verma, A.; Gupta, A. Current Advancements in Pectin: Extraction, Properties and Multifunctional Applications. Foods 2022, 11, 2683. [Google Scholar] [CrossRef]
- Elshahed, M.S.; Miron, A.; Aprotosoaie, A.C.; Farag, M.A. Pectin in Diet: Interactions with the Human Microbiome, Role in Gut Homeostasis, and Nutrient-Drug Interactions. Carbohydr. Polym. 2021, 255, 117388. [Google Scholar] [CrossRef]
- Mao, Y.; Kasravi, B.; Nobaek, S.; Wang, L.Q.; Adawi, D.; Roos, G.; Stenram, U.; Molin, G.; Bengmark, S.; Jeppsson, B. Pectin-Supplemented Enteral Diet Reduces the Severity of Methotrexate-Induced Enterocolitis in Rats. Scand. J. Gastroenterol. 1996, 31, 558–567. [Google Scholar] [CrossRef]
- Murphy, E.J.; Rezoagli, E.; Major, I.; Rowan, N.J.; Laffey, J.G. β-Glucan Metabolic and Immunomodulatory Properties and Potential for Clinical Application. J. Fungi 2020, 6, 356. [Google Scholar] [CrossRef]
- Ruszkowski, J.; Witkowski, J.M. Lactulose: Patient- and Dose-Dependent Prebiotic Properties in Humans. Anaerobe 2019, 59, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Elkington, S.G. Lactulose. Gut 1970, 11, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Geuking, M.B.; Köller, Y.; Rupp, S.; McCoy, K.D. The Interplay between the Gut Microbiota and the Immune System. Gut Microbes 2014, 5, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Chua, L.L.; Rajasuriar, R.; Azanan, M.S.; Abdullah, N.K.; Tang, M.S.; Lee, S.C.; Woo, Y.L.; Lim, Y.A.L.; Ariffin, H.; Loke, P.N. Reduced Microbial Diversity in Adult Survivors of Childhood Acute Lymphoblastic Leukemia and Microbial Associations with Increased Immune Activation. Microbiome 2017, 5, 35. [Google Scholar] [CrossRef]
- Ekert, H.; Jurk, I.H.; Waters, K.D.; Tiedemann, K. Prophylactic Co-Trimoxazole and Lactobacilli Preparation in Neutropenic Patients. Med. Pediatr. Oncol. 1980, 8, 47–51. [Google Scholar] [CrossRef]
- Wada, M.; Nagata, S.; Saito, M.; Shimizu, T.; Yamashiro, Y.; Matsuki, T.; Asahara, T.; Nomoto, K. Effects of the Enteral Administration of Bifidobacterium Breve on Patients Undergoing Chemotherapy for Pediatric Malignancies. Support. Care Cancer 2010, 18, 751–759. [Google Scholar] [CrossRef]
- Reyna-Figueroa, J.; Barrón-Calvillo, E.; García-Parra, C.; Galindo-Delgado, P.; Contreras-Ochoa, C.; Lagunas-Martínez, A.; Campos-Romero, F.H.; Silva-Estrada, J.A.; Limón-Rojas, A.E. Probiotic Supplementation Decreases Chemotherapy-Induced Gastrointestinal Side Effects in Patients With Acute Leukemia. J. Pediatr. Hematol. Oncol. 2019, 41, 468–472. [Google Scholar] [CrossRef]
- Oldenburg, M.; Rüchel, N.; Janssen, S.; Borkhardt, A.; Gössling, K.L. The Microbiome in Childhood Acute Lymphoblastic Leukemia. Cancers 2021, 13, 4947. [Google Scholar] [CrossRef] [PubMed]
- Ambesh, P.; Stroud, S.; Franzova, E.; Gotesman, J.; Sharma, K.; Wolf, L.; Kamholz, S. Recurrent Lactobacillus Bacteremia in a Patient With Leukemia. J. Investig. Med. High Impact Case Rep. 2017, 5, 2324709617744233. [Google Scholar] [CrossRef]
- Park, K.B.; Oh, S.H.; Kim, N.S.; Oh, C.H.; Jeon, J.I. Kimchi Fermented in a Kimchi Refrigerator Showed Enhanced Anti-cancer Effects on Human Leukemia and Gastric Cancer Cells (LB405). FASEB J. 2014, 28, LB405. [Google Scholar] [CrossRef]
- El-Nezami, H.S.; Polychronaki, N.N.; Ma, J.; Zhu, H.; Ling, W.; Salminen, E.K.; Juvonen, R.O.; Salminen, S.J.; Poussa, T.; Mykkänen, H.M. Probiotic Supplementation Reduces a Biomarker for Increased Risk of Liver Cancer in Young Men from Southern China. Am. J. Clin. Nutr. 2006, 83, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Nami, Y.; Abdullah, N.; Haghshenas, B.; Radiah, D.; Rosli, R.; Khosroushahi, A.Y. Assessment of Probiotic Potential and Anticancer Activity of Newly Isolated Vaginal Bacterium Lactobacillus Plantarum 5BL. Microbiol. Immunol. 2014, 58, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Tarrah, A.; de Castilhos, J.; Rossi, R.C.; da Silva Duarte, V.; Ziegler, D.R.; Corich, V.; Giacomini, A. In Vitro Probiotic Potential and Anti-Cancer Activity of Newly Isolated Folate-Producing Streptococcus Thermophilus Strains. Front. Microbiol. 2018, 9, 2214. [Google Scholar] [CrossRef]
- Mangrolia, U.; Osborne, W.J. Staphylococcus Xylosus VITURAJ10: Pyrrolo [1,2α] Pyrazine-1,4-Dione, Hexahydro-3-(2-Methylpropyl) (PPDHMP) Producing, Potential Probiotic Strain with Antibacterial and Anticancer Activity. Microbial. Pathog. 2020, 147, 104259. [Google Scholar] [CrossRef]
- Badgeley, A.; Anwar, H.; Modi, K.; Murphy, P.; Lakshmikuttyamma, A. Effect of Probiotics and Gut Microbiota on Anti-Cancer Drugs: Mechanistic Perspectives. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188494. [Google Scholar] [CrossRef]
- Pagani, I.S.; Poudel, G.; Wardill, H.R. A Gut Instinct on Leukaemia: A New Mechanistic Hypothesis for Microbiota-Immune Crosstalk in Disease Progression and Relapse. Microorganisms 2022, 10, 713. [Google Scholar] [CrossRef]
- Hakim, H.; Dallas, R.; Wolf, J.; Tang, L.; Schultz-Cherry, S.; Darling, V.; Johnson, C.; Karlsson, E.A.; Chang, T.-C.; Jeha, S.; et al. Gut Microbiome Composition Predicts Infection Risk During Chemotherapy in Children With Acute Lymphoblastic Leukemia. Clin. Infect. Dis. 2018, 67, 541–548. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, W.; Liu, H.; Duan, J.; Zhang, Y.; Liu, M.; Li, H.; Hou, Z.; Wu, K.K. Effect of High-Dose Methotrexate Chemotherapy on Intestinal Bifidobacteria, Lactobacillus and Escherichia Coli in Children with Acute Lymphoblastic Leukemia. Exp. Biol. Med. 2012, 237, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Wong, W.S.W.; Saadon, R.; Vilboux, T.; Deeken, J.; Niederhuber, J.; Hourigan, S.K.; Yang, E. Gut Microbial Composition Difference between Pediatric ALL Survivors and Siblings. Pediatr. Hematol. Oncol. 2020, 37, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Rajagopala, S.V.; Singh, H.; Yu, Y.; Zabokrtsky, K.B.; Torralba, M.G.; Moncera, K.J.; Frank, B.; Pieper, R.; Sender, L.; Nelson, K.E. Persistent Gut Microbial Dysbiosis in Children with Acute Lymphoblastic Leukemia (ALL) During Chemotherapy. Microb. Ecol. 2020, 79, 1034–1043. [Google Scholar] [CrossRef]
- Nearing, J.T.; Connors, J.; Whitehouse, S.; Van Limbergen, J.; Macdonald, T.; Kulkarni, K.; Langille, M.G.I. Infectious Complications Are Associated With Alterations in the Gut Microbiome in Pediatric Patients with Acute Lymphoblastic Leukemia. Front. Cell. Infect. Microbiol. 2019, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Chua, L.L.; Rajasuriar, R.; Lim, Y.A.L.; Woo, Y.L.; Loke, P.; Ariffin, H. Temporal Changes in Gut Microbiota Profile in Children with Acute Lymphoblastic Leukemia Prior to Commencement-, during-, and Post-Cessation of Chemotherapy. BMC Cancer 2020, 20, 151. [Google Scholar] [CrossRef]
- van Vliet, M.J.; Tissing, W.J.E.; Dun, C.A.J.; Meessen, N.E.L.; Kamps, W.A.; de Bont, E.S.J.M.; Harmsen, H.J.M. Chemotherapy Treatment in Pediatric Patients with Acute Myeloid Leukemia Receiving Antimicrobial Prophylaxis Leads to a Relative Increase of Colonization with Potentially Pathogenic Bacteria in the Gut. Clin. Infect. Dis. 2009, 49, 262–270. [Google Scholar] [CrossRef]
- Tunyapanit, W.; Chelae, S.; Laoprasopwattana, K. Does Ciprofloxacin Prophylaxis during Chemotherapy Induce Intestinal Microflora Resistance to Ceftazidime in Children with Cancer? J. Infect. Chemother. 2018, 24, 358–362. [Google Scholar] [CrossRef]
- Lähteenmäki, K.; Wacklin, P.; Taskinen, M.; Tuovinen, E.; Lohi, O.; Partanen, J.; Mättö, J.; Vettenranta, K. Haematopoietic Stem Cell Transplantation Induces Severe Dysbiosis in Intestinal Microbiota of Paediatric ALL Patients. Bone Marrow Transplant. 2017, 52, 1479–1482. [Google Scholar] [CrossRef]
- Simms-Waldrip, T.R.; Sunkersett, G.; Coughlin, L.A.; Savani, M.R.; Arana, C.; Kim, J.; Kim, M.; Zhan, X.; Greenberg, D.E.; Xie, Y.; et al. Antibiotic-Induced Depletion of Anti-Inflammatory Clostridia Is Associated with the Development of Graft-versus-Host Disease in Pediatric Stem Cell Transplantation Patients. Biol. Blood Marrow Transplant. 2017, 23, 820–829. [Google Scholar] [CrossRef]
- Hur, Y.-M.; Kim, S.-H.; Park, K.-Y. Inhibitory Effects of Kimchi Extracts on the Growth and DNA Synthesis of Human Cancer Cells. Prev. Nutr. Food Sci. 1999, 4, 107–112. [Google Scholar]
- Garbacz, K. Anticancer Activity of Lactic Acid Bacteria. Semin. Cancer Biol. 2022, 86, 356–366. [Google Scholar] [CrossRef]
- Kato-Mori, Y.; Orihashi, T.; Kanai, Y.; Sato, M.; Sera, K.; Hagiwara, K. Fermentation Metabolites from Lactobacillus Gasseri and Propionibacterium Freudenreichii Exert Bacteriocidal Effects in Mice. J. Med. Food 2010, 13, 1460–1467. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Akedo, I.; Otani, T.; Suzuki, T.; Nakamura, T.; Takeyama, I.; Ishiguro, S.; Miyaoka, E.; Sobue, T.; Kakizoe, T. Randomized Trial of Dietary Fiber and Lactobacillus Casei Administration for Prevention of Colorectal Tumors. Int. J. Cancer 2005, 116, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Zhang, X.; Covasa, M. Emerging Roles of Lactic Acid Bacteria in Protection against Colorectal Cancer. World J. Gastroenterol. 2014, 20, 7878–7886. [Google Scholar] [CrossRef] [PubMed]
- Chuah, L.-O.; Foo, H.L.; Loh, T.C.; Mohammed Alitheen, N.B.; Yeap, S.K.; Abdul Mutalib, N.E.; Abdul Rahim, R.; Yusoff, K. Postbiotic Metabolites Produced by Lactobacillus Plantarum Strains Exert Selective Cytotoxicity Effects on Cancer Cells. BMC Complement. Altern. Med. 2019, 19, 114. [Google Scholar] [CrossRef]
- Nakkarach, A.; Foo, H.L.; Song, A.A.-L.; Mutalib, N.E.A.; Nitisinprasert, S.; Withayagiat, U. Anti-Cancer and Anti-Inflammatory Effects Elicited by Short Chain Fatty Acids Produced by Escherichia Coli Isolated from Healthy Human Gut Microbiota. Microbial. Cell Factories 2021, 20, 36. [Google Scholar] [CrossRef]
- Microbial and Host Factors Contribute to Bloodstream Infection in a Pediatric Acute Lymphocytic Leukemia Mouse Model—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/36345525/ (accessed on 6 March 2023).
- Bollmann, L.M.; Skerhut, A.J.; Asfaha, Y.; Horstick, N.; Hanenberg, H.; Hamacher, A.; Kurz, T.; Kassack, M.U. The Novel Class IIa Selective Histone Deacetylase Inhibitor YAK540 Is Synergistic with Bortezomib in Leukemia Cell Lines. Int. J. Mol. Sci. 2022, 23, 13398. [Google Scholar] [CrossRef]
- Utsunomiya, A.; Izutsu, K.; Jo, T.; Yoshida, S.; Tsukasaki, K.; Ando, K.; Choi, I.; Imaizumi, Y.; Kato, K.; Kurosawa, M.; et al. Oral Histone Deacetylase Inhibitor Tucidinostat (HBI-8000) in Patients with Relapsed or Refractory Adult T-Cell Leukemia/Lymphoma: Phase IIb Results. Cancer Sci. 2022, 113, 2778–2787. [Google Scholar] [CrossRef]
- Pulliam, S.R.; Pellom, S.T.; Shanker, A.; Adunyah, S.E. Butyrate Regulates the Expression of Inflammatory and Chemotactic Cytokines In Human Acute Leukemic Cells During Apoptosis. Cytokine 2016, 84, 74–87. [Google Scholar] [CrossRef]
- Korecka, A.; Dona, A.; Lahiri, S.; Tett, A.J.; Al-Asmakh, M.; Braniste, V.; D’Arienzo, R.; Abbaspour, A.; Reichardt, N.; Fujii-Kuriyama, Y.; et al. Bidirectional Communication between the Aryl Hydrocarbon Receptor (AhR) and the Microbiome Tunes Host Metabolism. NPJ Biofilms Microbiomes 2016, 2, 16014. [Google Scholar] [CrossRef]
- Marsland, B.J. Regulating Inflammation with Microbial Metabolites. Nat. Med. 2016, 22, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Rooks, M.G.; Garrett, W.S. Gut Microbiota, Metabolites and Host Immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Silk, J.D.; Lakhal, S.; Laynes, R.; Vallius, L.; Karydis, I.; Marcea, C.; Boyd, C.A.R.; Cerundolo, V. IDO Induces Expression of a Novel Tryptophan Transporter in Mouse and Human Tumor Cells. J. Immunol. 2011, 187, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.; Chang, N.; Xu, B.; Qiu, Y.; Wang, S.; Zhou, L.; He, Y.; Xie, X.; Li, Y. Amino Acid Metabolism: Challenges and Opportunities for the Therapeutic Treatment of Leukemia and Lymphoma. Immunol. Cell Biol. 2022, 100, 507–528. [Google Scholar] [CrossRef]
- Sun, J.-X.; Zhang, W.-G.; Chen, Y.-X.; Zhao, W.-H.; Tian, W.; Yang, Y.; Liu, S.-H. Indoleamine 2, 3-Dioxygenase Expression in Cells of Human Acute Monocyte Leukemia (M(5)) and Acute Lymphocyte Leukemia and Therapeutic Effect of Its Inhibitor 1-Methyl Tryptophan. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2007, 15, 478–482. [Google Scholar]
- Chen, X.-L.; Guo, J.-M.; Zhang, Y.; Niu, X.-N.; Pei, X.-H.; Zhang, W.-H. Expression of Indoleamine 2,3-Dioxygenase in Acute Leukemic Cells and the Clinical Significance. Int. J. Clin. Exp. Med. 2016, 9, 8605–8609. [Google Scholar]
- Levit, R.; Savoy de Giori, G.; de Moreno de LeBlanc, A.; LeBlanc, J.G. Recent Update on Lactic Acid Bacteria Producing Riboflavin and Folates: Application for Food Fortification and Treatment of Intestinal Inflammation. J. Appl. Microbiol. 2021, 130, 1412–1424. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Daily Iron and Folic Acid Supplementation in Pregnant Women; World Health Organization: Geneva, Switzerland, 2012; ISBN 978-92-4-150199-6. [Google Scholar]
- Greaves, M. Infection, Immune Responses and the Aetiology of Childhood Leukaemia. Nat. Rev. Cancer 2006, 6, 193–203. [Google Scholar] [CrossRef]
- Wan Ismail, W.R.; Abdul Rahman, R.; Rahman, N.A.A.; Atil, A.; Nawi, A.M. The Protective Effect of Maternal Folic Acid Supplementation on Childhood Cancer: A Systematic Review and Meta-Analysis of Case-Control Studies. J. Prev. Med. Public Health 2019, 52, 205–213. [Google Scholar] [CrossRef]
- Shaw, A.K.; Infante-Rivard, C.; Morrison, H.I. Use of Medication during Pregnancy and Risk of Childhood Leukemia (Canada). Cancer Causes Control 2004, 15, 931–937. [Google Scholar] [CrossRef]
- Schüz, J.; Weihkopf, T.; Kaatsch, P. Medication Use during Pregnancy and the Risk of Childhood Cancer in the Offspring. Eur. J. Pediatr. 2007, 166, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Amigou, A.; Rudant, J.; Orsi, L.; Goujon-Bellec, S.; Leverger, G.; Baruchel, A.; Bertrand, Y.; Nelken, B.; Plat, G.; Michel, G.; et al. Folic Acid Supplementation, MTHFR and MTRR Polymorphisms, and the Risk of Childhood Leukemia: The ESCALE Study (SFCE). Cancer Causes Control 2012, 23, 1265–1277. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.W.; Selvin, S.; Block, G.; Golden, C.; Carmichael, S.L.; Metayer, C. Maternal Prenatal Intake of One-Carbon Metabolism Nutrients and Risk of Childhood Leukemia. Cancer Causes Control 2016, 27, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Milne, E.; Royle, J.A.; Miller, M.; Bower, C.; de Klerk, N.H.; Bailey, H.D.; van Bockxmeer, F.; Attia, J.; Scott, R.J.; Norris, M.D.; et al. Maternal Folate and Other Vitamin Supplementation during Pregnancy and Risk of Acute Lymphoblastic Leukemia in the Offspring. Int. J. Cancer 2010, 126, 2690–2699. [Google Scholar] [CrossRef]
- Ajrouche, R.; Rudant, J.; Orsi, L.; Petit, A.; Baruchel, A.; Nelken, B.; Pasquet, M.; Michel, G.; Bergeron, C.; Ducassou, S.; et al. Maternal Reproductive History, Fertility Treatments and Folic Acid Supplementation in the Risk of Childhood Acute Leukemia: The ESTELLE Study. Cancer Causes Control 2014, 25, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Linabery, A.M.; Puumala, S.E.; Hilden, J.M.; Davies, S.M.; Heerema, N.A.; Roesler, M.A.; Ross, J.A. Maternal Vitamin and Iron Supplementation and Risk of Infant Leukaemia: A Report from the Children’s Oncology Group. Br. J. Cancer 2010, 103, 1724–1728. [Google Scholar] [CrossRef] [PubMed]
| Study | Probiotic | Effect | Therapy | Study Group |
|---|---|---|---|---|
| Study on human | ||||
| Ekert et al. [38] | Lactobacilli spp. | Diminished vomiting and nausea | No information about dose and time | 68 children with leukemia and solid tumors |
| Wada et al. [39] | Bifidobacterium breve | Prevent infections | 109 freeze-dried, living BBG-01, 8 weeks | 42 patients with malignancies |
| Reyna-Figueroa et al. [40] | Lactobacillus rhamnosus GG | Reducing side effects of chemotherapy | 5 × 109 CFU twice daily, one week | 60 children with acute leukemia |
| Study on cell line | ||||
| Nami et al. [45] | Lactobacillus plantarum | Anticancer activity—cytotoxic effect | No data about CFU, 12, 24/48 h incubation | 5 human cell line MCF-7, AGS, HeLa, HT-29, and HUVEC |
| Tarrah et al. [46] | Streptococcus thermophilus | Stimulate of folate productions | 107 cells/mL, 24/48 h incubation | HT-29 cell line |
| Mangrolia et al. [47] | Staphylococcus xylosus | Antibacterial and anticancer activity | No data about CFU, 24/48 h incubation | MCF-7 cell line |
| Study | Description | Results |
|---|---|---|
| Shaw et al. [82] | FA supplementation before and during pregnancy | Reduced the risk of ALL in children. OR 0.9 (CI: 0.7–1.1) |
| Schüz et al. [83] | FA supplementation during pregnancy | Reduced risk of ALL in children > 5 years. 0.67 (CI: 0.5–0.9) |
| Amigou et al. [84] | FA supplementation before and during pregnancy | Reduced the risk of ALL in childhood. OR 0.4 (CI: 0.3–0.6) |
| Singer et al. [85] | Maternal intake of multivitamin complex | Reduced the risk of ALL in their children. OR 0.91 (CI: 0.84–0.99) |
| Milne et al. [86] | Summarized some FA supplementation studies | No strong evidence for positive effects. OR 1.06 (CI: 0.77–1.48) |
| Ajrouche et al. [87] | FA supplementation before and during pregnancy | Minimal positive effect of FA supplementation. OR 0.7 (CI: 0.5–1.0) |
| Linabery et al. [88] | FA supplementation during pregnancy | No associations between FA supplementation and ALL. OR 0.63 (CI: 0.34–1.18) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martyniak, A.; Zakrzewska, Z.; Schab, M.; Zawartka, A.; Wędrychowicz, A.; Skoczeń, S.; Tomasik, P.J. Prevention and Health Benefits of Prebiotics, Probiotics and Postbiotics in Acute Lymphoblastic Leukemia. Microorganisms 2023, 11, 1775. https://doi.org/10.3390/microorganisms11071775
Martyniak A, Zakrzewska Z, Schab M, Zawartka A, Wędrychowicz A, Skoczeń S, Tomasik PJ. Prevention and Health Benefits of Prebiotics, Probiotics and Postbiotics in Acute Lymphoblastic Leukemia. Microorganisms. 2023; 11(7):1775. https://doi.org/10.3390/microorganisms11071775
Chicago/Turabian StyleMartyniak, Adrian, Zuzanna Zakrzewska, Magdalena Schab, Aleksandra Zawartka, Andrzej Wędrychowicz, Szymon Skoczeń, and Przemysław J. Tomasik. 2023. "Prevention and Health Benefits of Prebiotics, Probiotics and Postbiotics in Acute Lymphoblastic Leukemia" Microorganisms 11, no. 7: 1775. https://doi.org/10.3390/microorganisms11071775
APA StyleMartyniak, A., Zakrzewska, Z., Schab, M., Zawartka, A., Wędrychowicz, A., Skoczeń, S., & Tomasik, P. J. (2023). Prevention and Health Benefits of Prebiotics, Probiotics and Postbiotics in Acute Lymphoblastic Leukemia. Microorganisms, 11(7), 1775. https://doi.org/10.3390/microorganisms11071775






