Effects of Bacterial Metabolites on the Wnt4 Protein in Dental-Pulp-Stem-Cells-Based Endodontic Pulpitis Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Retrieval of Protein Sequence
2.2. Validation of the Structure
2.3. Retrieval and Preparation of Microbial Metabolites
2.4. Molecular Docking
2.5. Molecular Dynamics Simulations (MDS)
3. Results
3.1. WNT4 Protein Sequence and Structure Generation
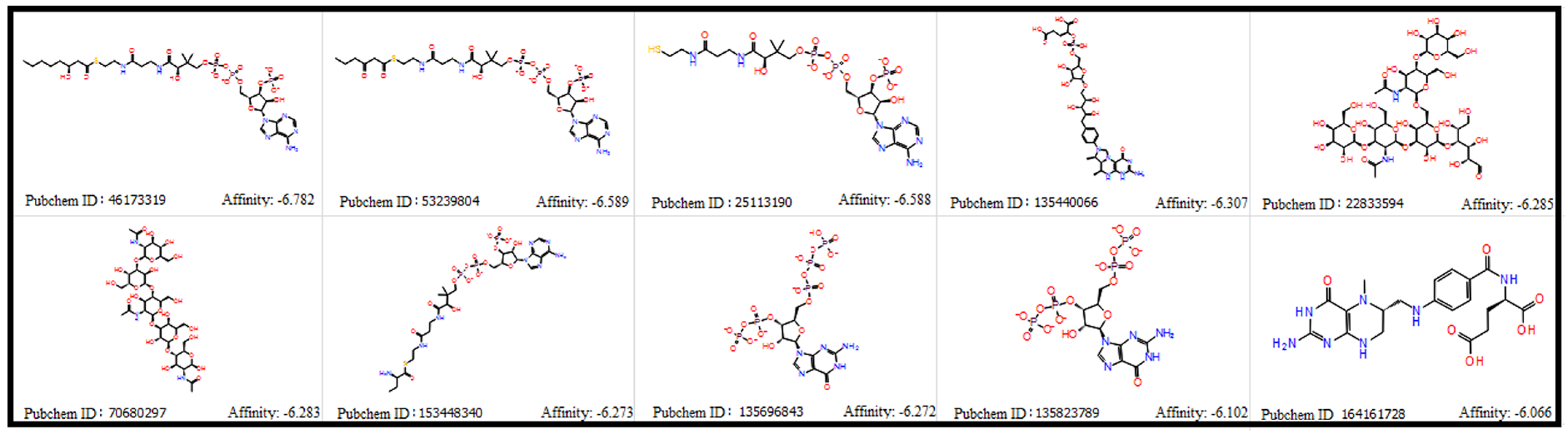
3.2. Porphyromonas gingivalis—Derived Metabolites and Molecular Docking
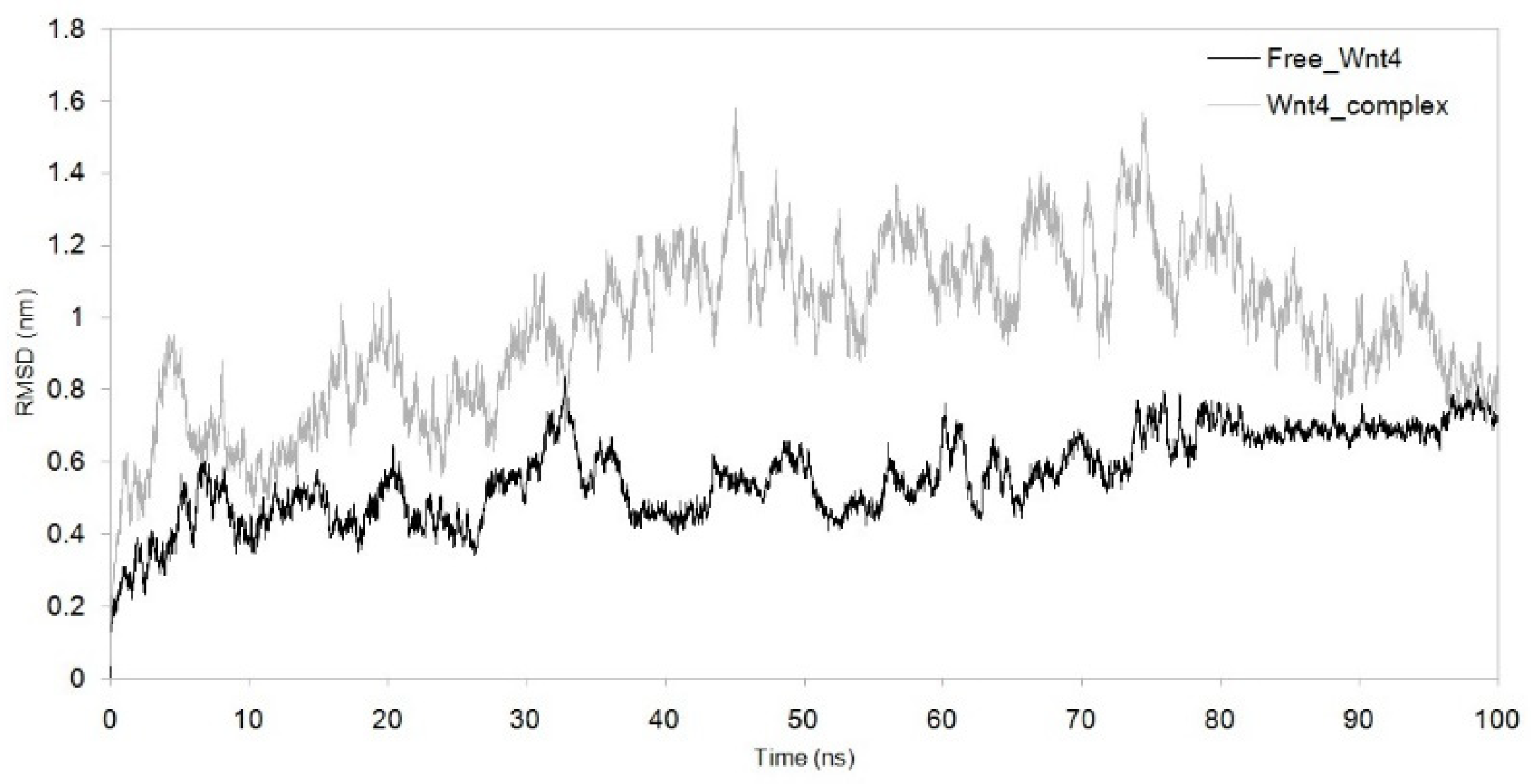
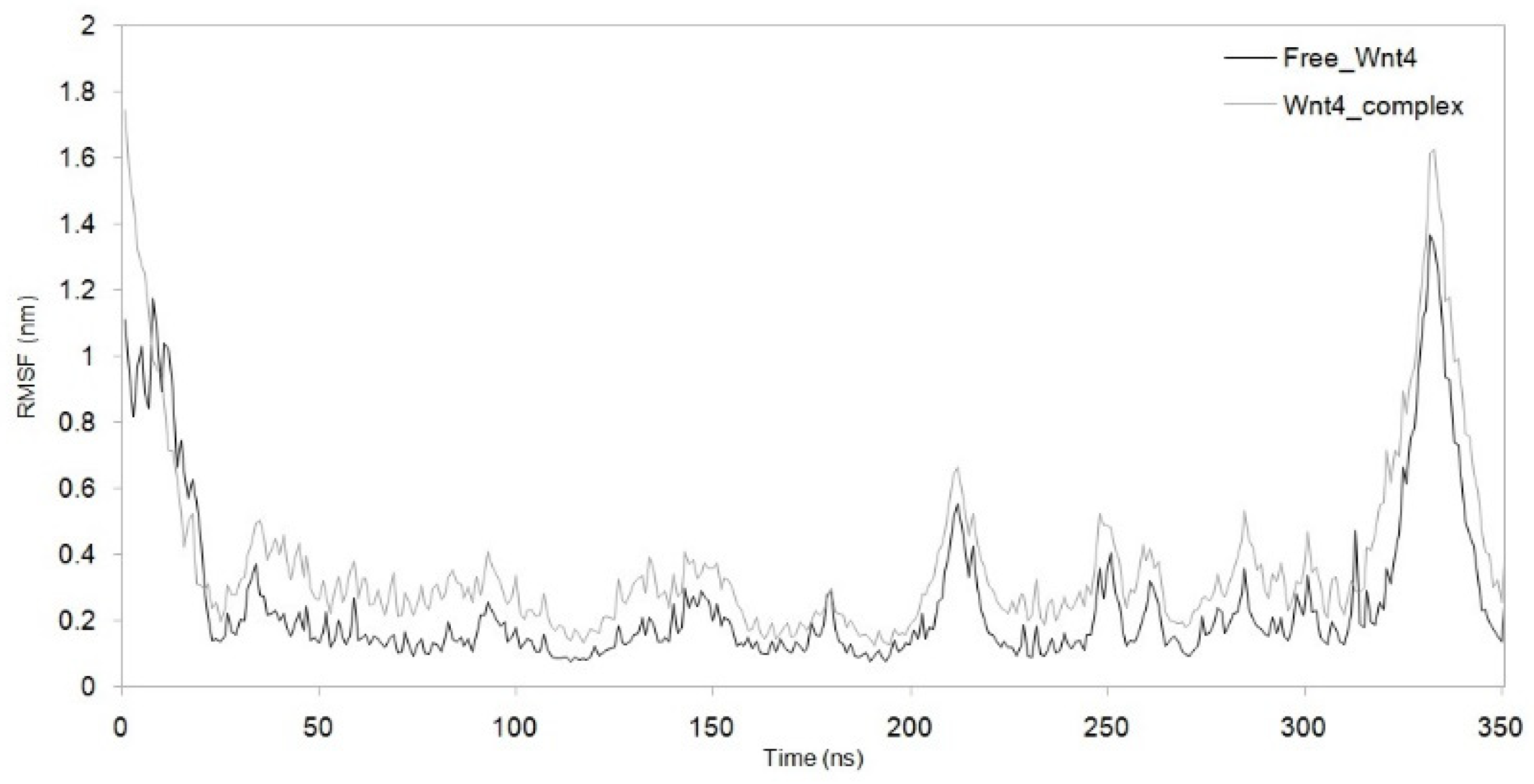
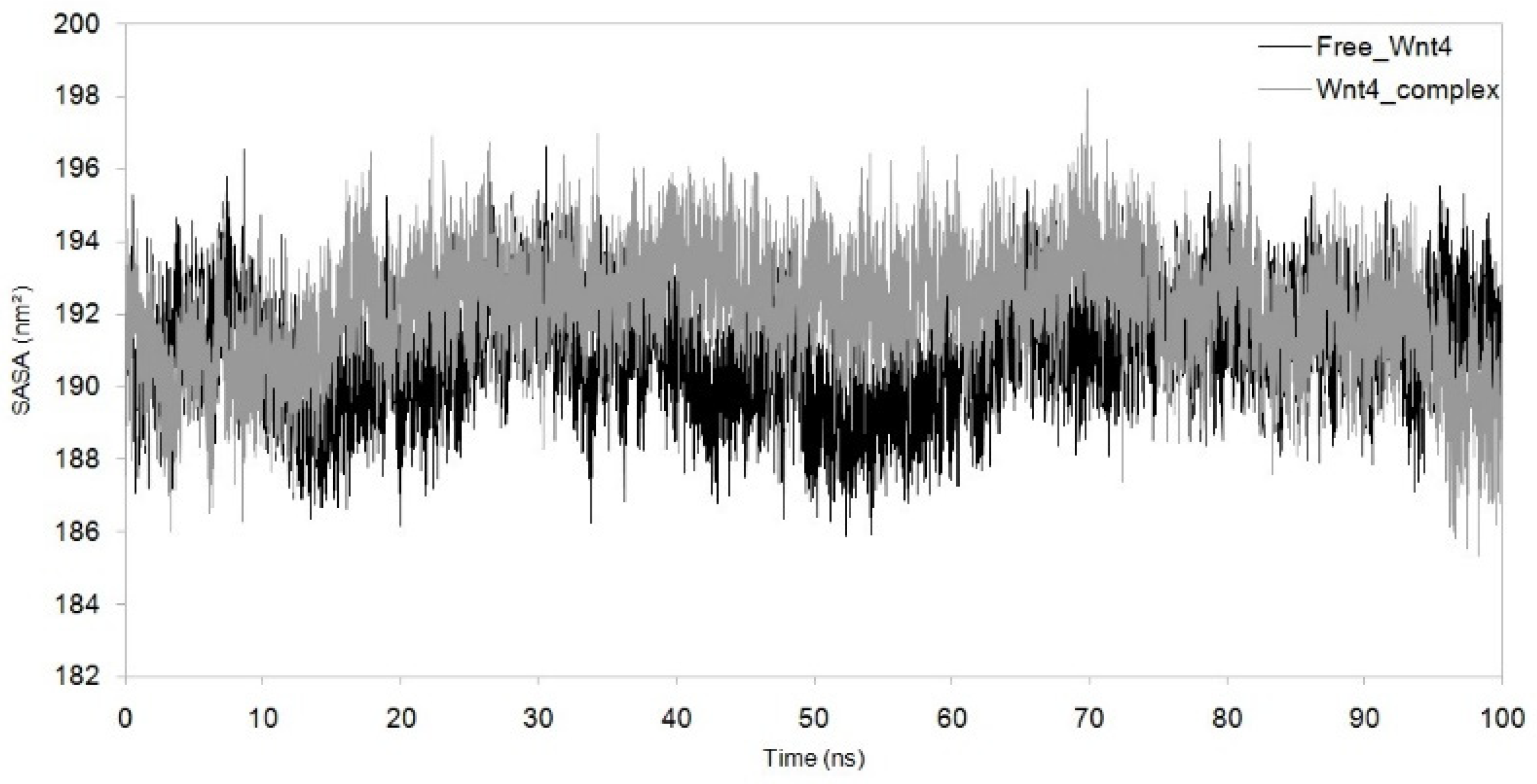
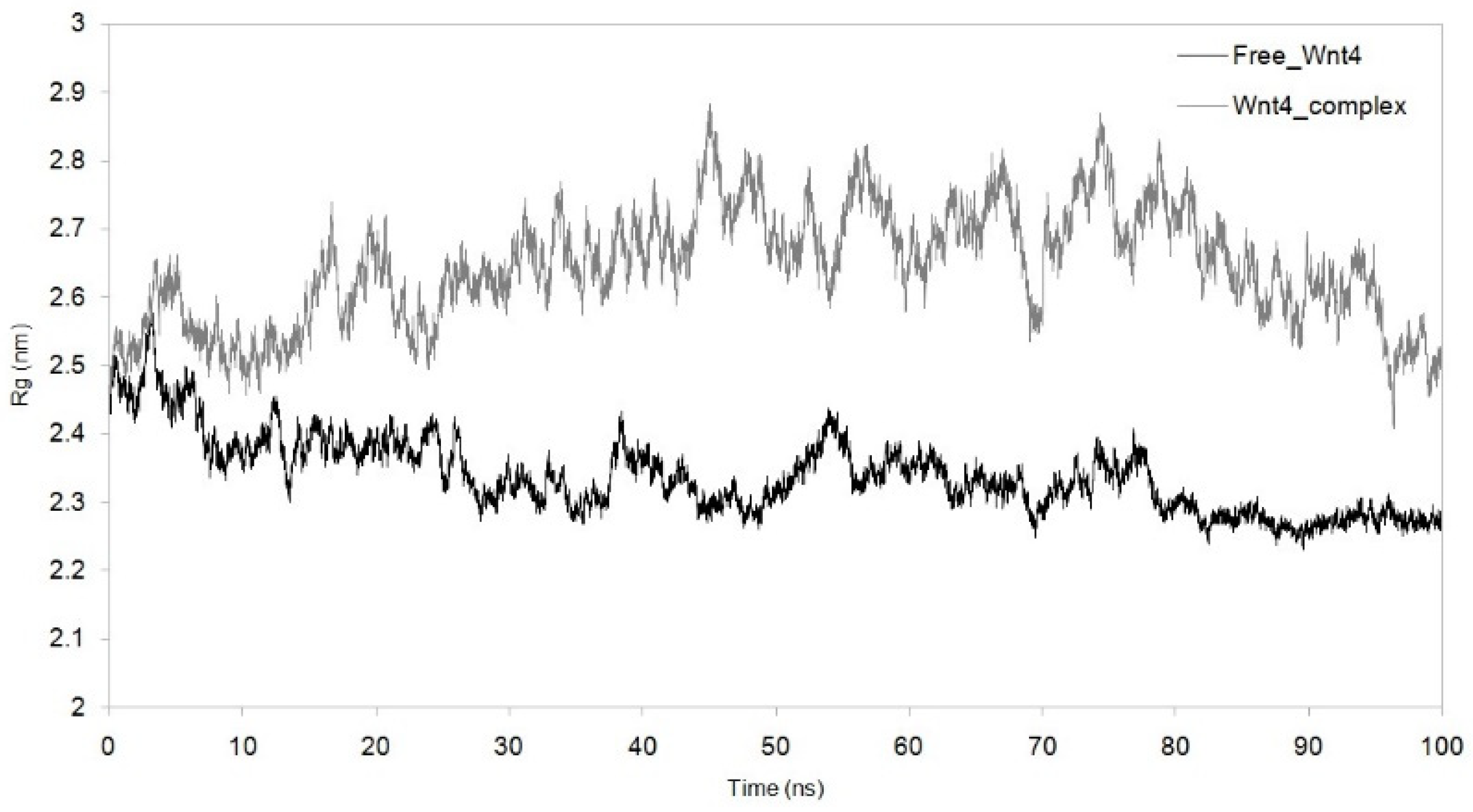
3.3. Dynamic Simulation of WNT4 with a Microbial Inhibitor
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bernabe, E.; Marcenes, W.; Hernandez, C.R.; Bailey, J.; Abreu, L.G.; Alipour, V.; Amini, S.; Arabloo, J.; Arefi, Z.; Arora, A.; et al. Global, Regional, and National Levels and Trends in Burden of Oral Conditions from 1990 to 2017: A Systematic Analysis for the Global Burden of Disease 2017 Study. J. Dent. Res. 2020, 99, 362–373. [Google Scholar] [CrossRef]
- Cimilli, H.; Karacayli, U.; Şişman, N.; Kartal, N.; Mumcu, G. Comparison of the Oral Health-Related Quality of Life and Dental Pain in Symptomatic Irreversible Pulpitis and Pericoronitis. J. Dent. Sci. 2012, 7, 250–260. [Google Scholar] [CrossRef]
- Sundqvist, G.; Johansson, E.; Sjögren, U. Prevalence of Black-Pigmented Bacteroides Species in Root Canal Infections. J. Endod. 1989, 15, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Van Winkelhoff, A.J.; Carlee, A.W.; De Graaff, J. Bacteroides Endodontalis and Other Black-Pigmented Bacteroides Species in Odontogenic Abscesses. Infect. Immun. 1985, 49, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Griffin, S.O.; Jones, J.A.; Brunson, D.; Griffin, P.M.; Bailey, W.D. Burden of Oral Disease among Older Adults and Implications for Public Health Priorities. Am. J. Public Health 2012, 102, 411–418. [Google Scholar] [CrossRef]
- Volponi, A.A.; Pang, Y.; Sharpe, P.T. Stem Cell-Based Biological Tooth Repair and Regeneration. Trends Cell Biol. 2010, 20, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Baudry, A.; Uzunoglu, E.; Schneider, B.; Kellermann, O.; Goldberg, M. From Pulpal Stem Cells to Tooth Repair: An Emerging Field for Dental Tissue Engineering. Evid. Based Endod. 2016, 1, 2. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Maciejewska, I.; Galler, K.M.; Cavender, A.; D’Souza, R.N. TWIST1 Promotes the Odontoblast-like Differentiation of Dental Stem Cells. Adv. Dent. Res. 2011, 23, 280–284. [Google Scholar] [CrossRef]
- Takahashi, M.; Yamada, Y.; Ozawa, R.; Ohya, M.; Ito, K.; Ueda, M. Expression of Odontoblastic-Related Genes in Human Dental Follicle Cells, Dental Pulp Stem Cells, and Oral Mucosal Cells. Int. J. Oral Med. Sci. 2004, 3, 41–48. [Google Scholar] [CrossRef]
- Sloan, A.J.; Waddington, R.J. Dental Pulp Stem Cells: What, Where, How? Int. J. Paediatr. Dent. 2009, 19, 61–70. [Google Scholar] [CrossRef]
- Tatullo, M.; Marrelli, M.; Shakesheff, K.M.; White, L.J. Dental Pulp Stem Cells: Function, Isolation and Applications in Regenerative Medicine. J. Tissue Eng. Regen. Med. 2015, 9, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Bakopoulou, A. Stem Cells of Dental Origin: Current Research Trends and Key Milestones towards Clinical Application. Stem Cells Int. 2016, 2016, 4209891. [Google Scholar] [CrossRef]
- Liu, Y.; Gan, L.; Cui, D.-X.; Yu, S.-H.; Pan, Y.; Zheng, L.-W.; Wan, M. Epigenetic Regulation of Dental Pulp Stem Cells and Its Potential in Regenerative Endodontics. World J. Stem Cells 2021, 13, 1647. [Google Scholar] [CrossRef]
- Rad, R.M.; Atila, D.; Akgün, E.E.; Evis, Z.; Keskin, D.; Tezcaner, A. Evaluation of Human Dental Pulp Stem Cells Behavior on a Novel Nanobiocomposite Scaffold Prepared for Regenerative Endodontics. Mater. Sci. Eng. C 2019, 100, 928–948. [Google Scholar]
- Diana, R.; Ardhani, R.; Kristanti, Y.; Santosa, P. Dental Pulp Stem Cells Response on the Nanotopography of Scaffold to Regenerate Dentin-Pulp Complex Tissue. Regen. Ther. 2020, 15, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Nuti, N.; Corallo, C.; Chan, B.M.F.; Ferrari, M.; Gerami-Naini, B. Multipotent Differentiation of Human Dental Pulp Stem Cells: A Literature Review. Stem Cell Rev. Rep. 2016, 12, 511–523. [Google Scholar] [CrossRef]
- Niehrs, C. The Complex World of WNT Receptor Signalling. Nat. Rev. Mol. Cell Biol. 2012, 13, 767–779. [Google Scholar] [CrossRef]
- Komiya, Y.; Habas, R. Wnt Signal Transduction Pathways. Organogenesis 2008, 4, 68–75. [Google Scholar] [CrossRef]
- Wang, J.; Wynshaw-Boris, A. The Canonical Wnt Pathway in Early Mammalian Embryogenesis and Stem Cell Maintenance/Differentiation. Curr. Opin. Genet. Dev. 2004, 14, 533–539. [Google Scholar] [CrossRef]
- Zhong, T.-Y.; Zhang, Z.-C.; Gao, Y.-N.; Lu, Z.; Qiao, H.; Zhou, H.; Liu, Y. Loss of Wnt4 Expression Inhibits the Odontogenic Potential of Dental Pulp Stem Cells through JNK Signaling in Pulpitis. Am. J. Transl. Res. 2019, 11, 1819. [Google Scholar]
- Shen, M.; Sali, A. Statistical Potential for Assessment and Prediction of Protein Structures. Protein Sci. 2006, 15, 2507–2524. [Google Scholar] [CrossRef]
- Sudhan, M.; Janakiraman, V.; Patil, R.; Oyouni, A.A.A.; Hasan Mufti, A.; Ahmed, S.S.S.J. Asn215Ser, Ala143Thr, and Arg112Cys Variants in α-Galactosidase A Protein Confer Stability Loss in Fabry’s Disease. J. Biomol. Struct. Dyn. 2022, 1–10. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal Human Dental Pulp Stem Cells (DPSCs) in Vitro and in Vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem Cell Properties of Human Dental Pulp Stem Cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Mao, J.; Liu, Y. Pulp Stem Cells Derived from Human Permanent and Deciduous Teeth: Biological Characteristics and Therapeutic Applications. Stem Cells Transl. Med. 2020, 9, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Choy, K.-T.; Wong, A.Y.-L.; Kaewpreedee, P.; Sia, S.F.; Chen, D.; Hui, K.P.Y.; Chu, D.K.W.; Chan, M.C.W.; Cheung, P.P.-H.; Huang, X. Remdesivir, Lopinavir, Emetine, and Homoharringtonine Inhibit SARS-CoV-2 Replication in Vitro. Antivir. Res. 2020, 178, 104786. [Google Scholar] [CrossRef]
- Bhowmik, D.; Nandi, R.; Jagadeesan, R.; Kumar, N.; Prakash, A.; Kumar, D. Identification of Potential Inhibitors against SARS-CoV-2 by Targeting Proteins Responsible for Envelope Formation and Virion Assembly Using Docking Based Virtual Screening, and Pharmacokinetics Approaches. Infect. Genet. Evol. 2020, 84, 104451. [Google Scholar] [CrossRef]
- Kallithraka, S.; Aliaj, L.; Makris, D.P.; Kefalas, P. Anthocyanin Profiles of Major Red Grape (Vitis vinifera L.) Varieties Cultivated in Greece and Their Relationship with in Vitro Antioxidant Characteristics. Int. J. Food Sci. Technol. 2009, 44, 2385–2393. [Google Scholar] [CrossRef]
- Rienth, M.; Vigneron, N.; Darriet, P.; Sweetman, C.; Burbidge, C.; Bonghi, C.; Walker, R.P.; Famiani, F.; Castellarin, S.D. Grape Berry Secondary Metabolites and Their Modulation by Abiotic Factors in a Climate Change Scenario—A Review. Front. Plant Sci. 2021, 12, 643258. [Google Scholar] [CrossRef]
- Marahatha, R.; Shrestha, A.; Sharma, K.; Regmi, B.P.; Sharma, K.R.; Poudel, P.; Basnyat, R.C.; Parajuli, N. In Silico Study of Alkaloids: Neferine and Berbamine Potentially Inhibit the SARS-CoV-2 RNA-Dependent RNA Polymerase. J. Chem. 2022, 2022, 7548802. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abulhamael, A.M.; Bhandi, S.; Albar, N.H.; Shaiban, A.S.; Bavabeedu, S.S.; Alzahrani, K.J.; Alzahrani, F.M.; Halawani, I.F.; Patil, S. Effects of Bacterial Metabolites on the Wnt4 Protein in Dental-Pulp-Stem-Cells-Based Endodontic Pulpitis Treatment. Microorganisms 2023, 11, 1764. https://doi.org/10.3390/microorganisms11071764
Abulhamael AM, Bhandi S, Albar NH, Shaiban AS, Bavabeedu SS, Alzahrani KJ, Alzahrani FM, Halawani IF, Patil S. Effects of Bacterial Metabolites on the Wnt4 Protein in Dental-Pulp-Stem-Cells-Based Endodontic Pulpitis Treatment. Microorganisms. 2023; 11(7):1764. https://doi.org/10.3390/microorganisms11071764
Chicago/Turabian StyleAbulhamael, Ayman M., Shilpa Bhandi, Nasreen H. Albar, Amal S. Shaiban, Shashit Shetty Bavabeedu, Khalid J. Alzahrani, Fuad M. Alzahrani, Ibrahim F. Halawani, and Shankargouda Patil. 2023. "Effects of Bacterial Metabolites on the Wnt4 Protein in Dental-Pulp-Stem-Cells-Based Endodontic Pulpitis Treatment" Microorganisms 11, no. 7: 1764. https://doi.org/10.3390/microorganisms11071764
APA StyleAbulhamael, A. M., Bhandi, S., Albar, N. H., Shaiban, A. S., Bavabeedu, S. S., Alzahrani, K. J., Alzahrani, F. M., Halawani, I. F., & Patil, S. (2023). Effects of Bacterial Metabolites on the Wnt4 Protein in Dental-Pulp-Stem-Cells-Based Endodontic Pulpitis Treatment. Microorganisms, 11(7), 1764. https://doi.org/10.3390/microorganisms11071764







