Shiga-Toxin-Producing Strains of Escherichia coli O104:H4 and a Strain of O157:H7, Which Can Cause Human Hemolytic Uremic Syndrome, Differ in Biofilm Formation in the Presence of CO2 and in Their Ability to Grow in a Novel Cell Culture Medium
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Reagents, Growth Conditions, and Antibiotic Susceptibility
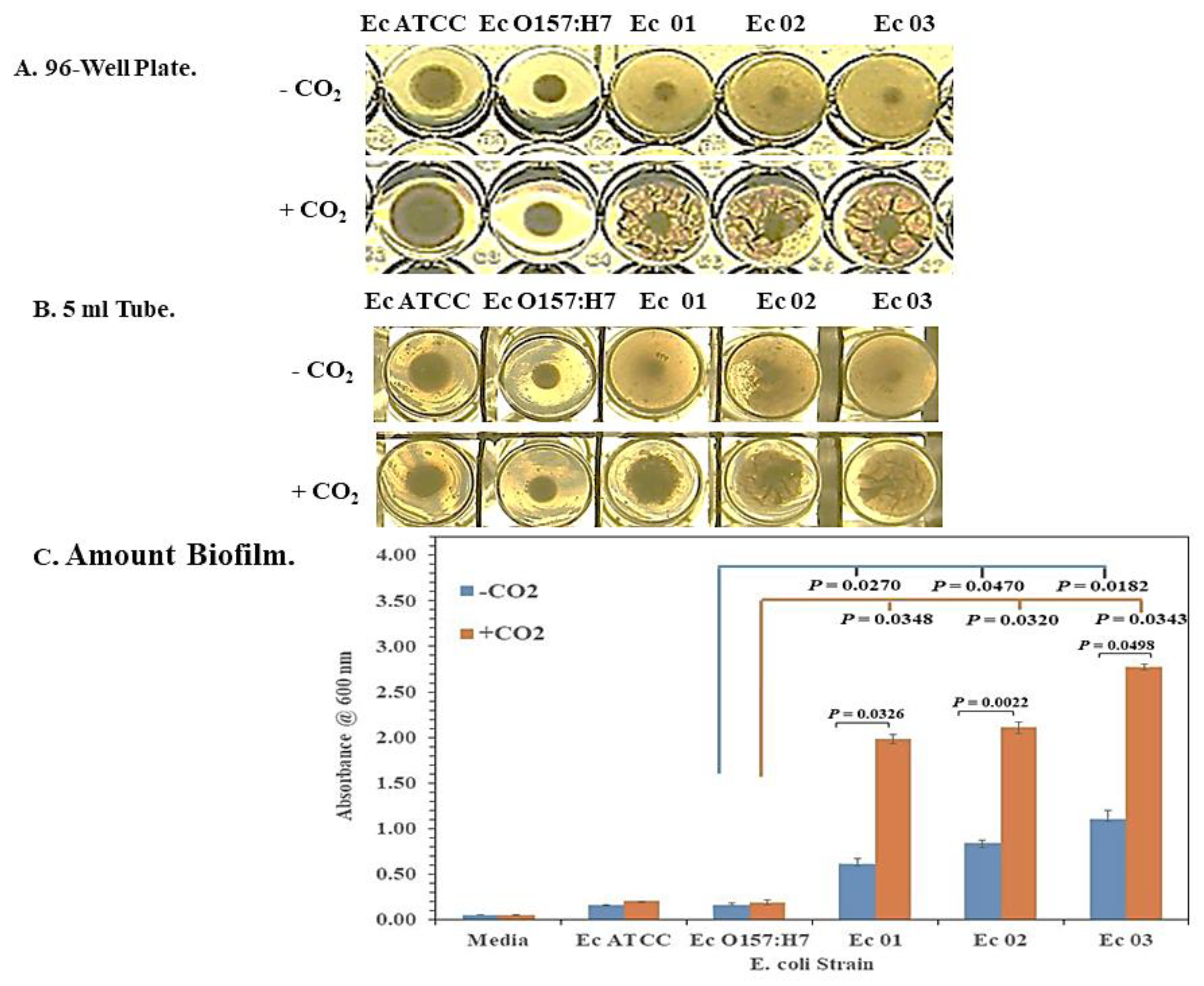
2.2. Biofilm Formation
2.3. Human Embryonic Kidney (HEK) 293 Toll-like Receptor (TLR) Cell Cultures
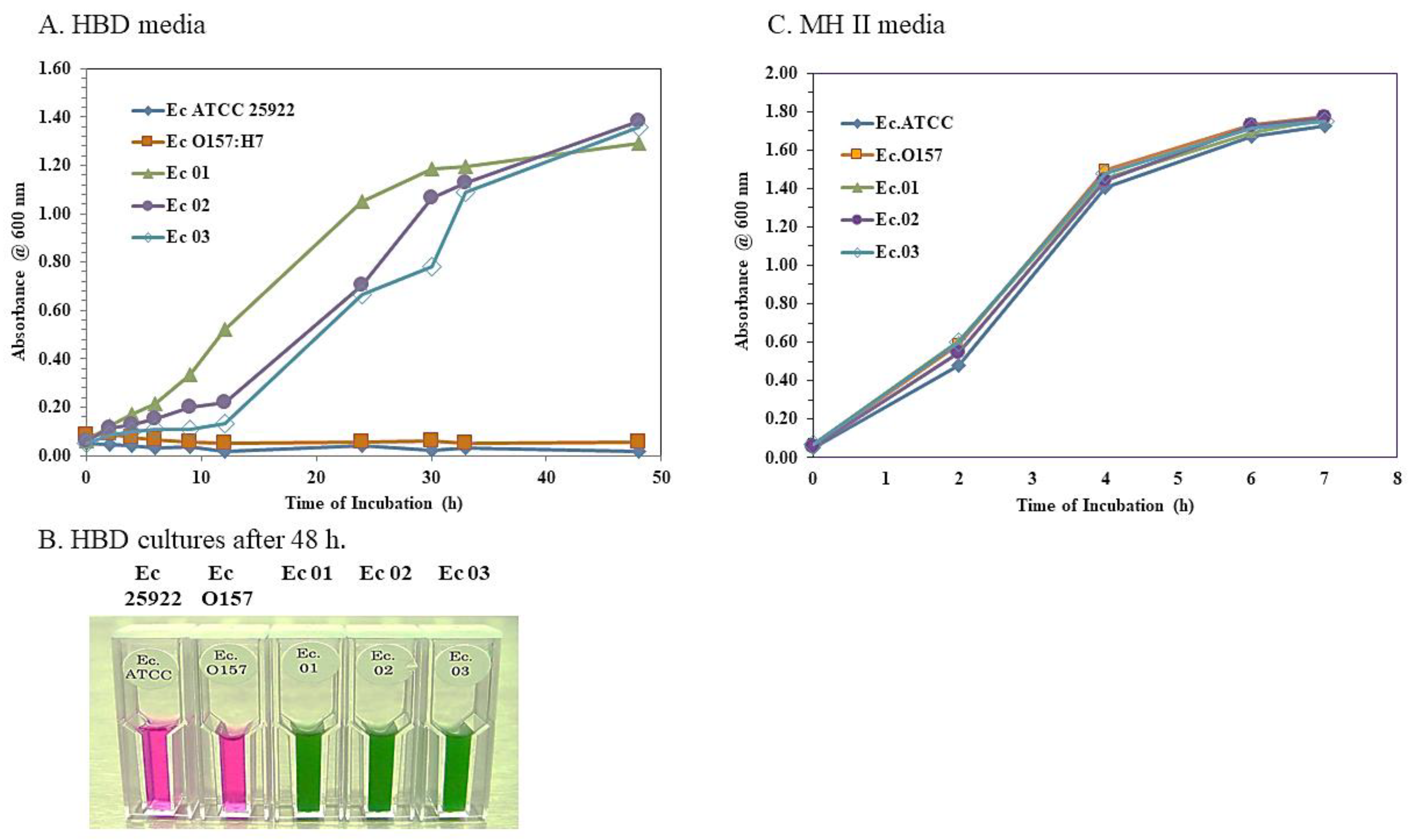
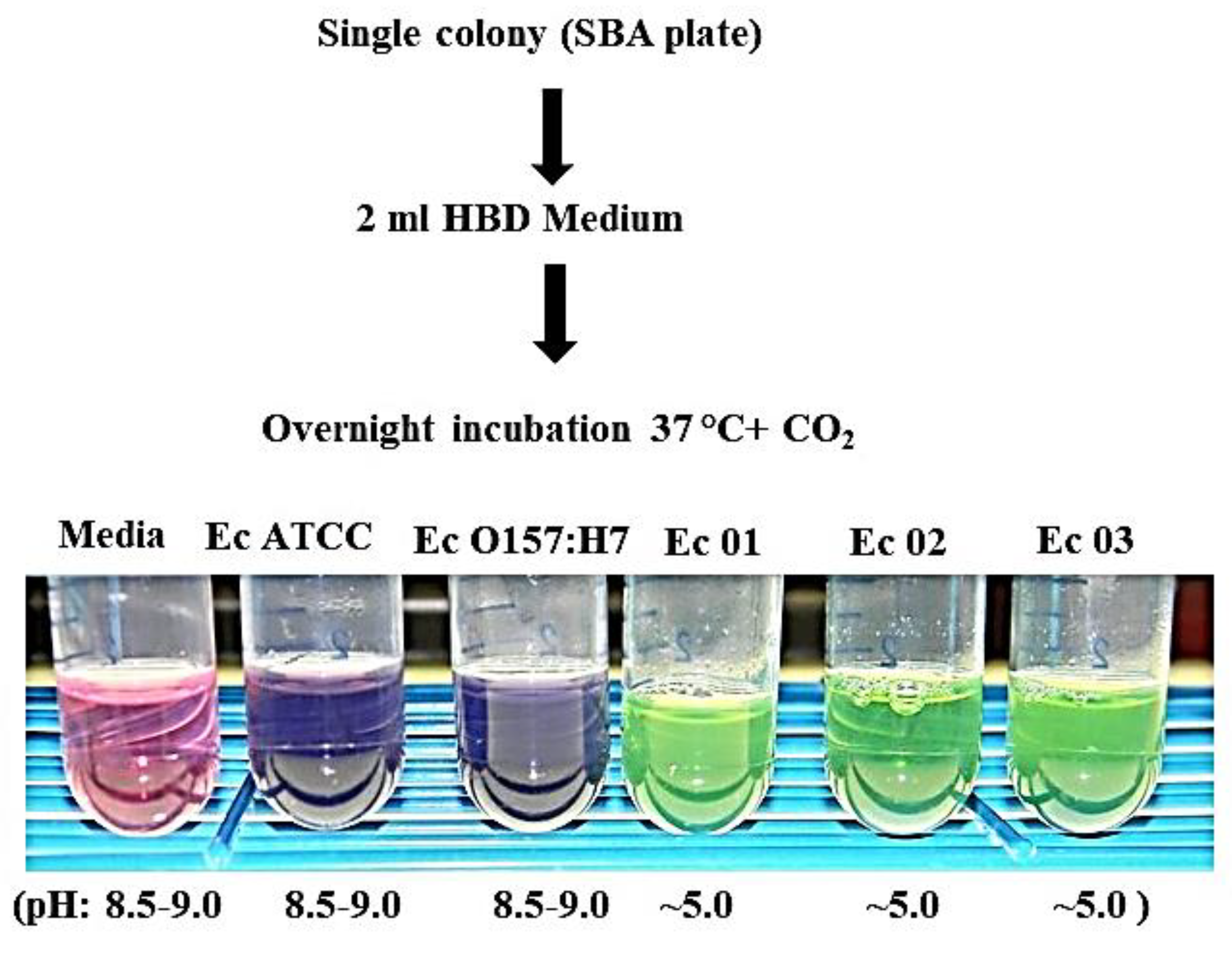
2.4. E. coli Strains in an HBD Cell Culture Medium
2.5. Comparison of Amino Acid Sequence of AAF/I Protein Components
2.6. Statistical Analysis
3. Results
3.1. Enhanced Biofilm Formation by E. coli O104:H4 in 5% CO2
3.2. Interaction of the Live E. coli Strains with Innate Immune TLR2 and 4
3.3. Growth of the E. coli O104:H4 Strains but Not the O157:H7 Strain in an HBD Cell Culture Medium
3.4. Growth of the E. coli O104:H4 Strains in an HBD Cell Culture Medium Starting with a Single Colony
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; de Silva, N.-R.; Gargouri, N.; et al. World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef] [PubMed]
- Pires, S.M.; Majowicz, S.; Gill, A.; Devleesschauwer, B. Global and regional source attribution of Shiga toxin-producing Escherichia coli infections using analysis of outbreak surveillance data. Epidemiol. Infect. 2019, 147, e236. [Google Scholar] [CrossRef] [PubMed]
- Tarr, P.I. Escherichia coli O157:H7: Clinical, diagnostic, and epidemiological aspects of human infection. Clin. Infect. Dis. 1995, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tarr, P.I.; Gordon, C.A.; Chandler, W.L. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 2005, 365, 1073–1086. [Google Scholar] [CrossRef]
- Kavanagh, D.; Raman, S.; Sheerin, N.S. Management of hemolytic uremic syndrome. F1000Prime Rep. 2014, 6, 119. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.S.; Jelacic, S.; Habeeb, R.L.; Watkins, S.L.; Tarr, P.I. The risk of the hemolytic-uremi syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N. Eng. J. Med. 2000, 342, 1930–1936. [Google Scholar] [CrossRef] [PubMed]
- Bitzan, M. Treatment options for HUS secondary to Escherichia coli O157:H7. Kidney Internat. 2009, 75, S62–S66. [Google Scholar] [CrossRef]
- Holtz, L.R.; Tarr, P.I. Shiga Toxin-Producing Escherichia coli: Microbiology, Pathogenesis, Epidemiology, and Prevention. Available online: https://www.uptodate.com (accessed on 7 January 2023).
- O’Brian, A.D.; Tesh, V.L.; Donohue-Rolfe, A.; Jackson, M.P.; Olsenes, S.; Sandvig, K.; Lindberg, A.A.; Keusch, G.T. Shiga toxin: Biochemistry, genetics, mode of action, and role in pathogenesis. Curr. Top. Microbiol. Immunol. 1992, 180, 65–94. [Google Scholar]
- Gyles, C.L. Shiga toxin-producing Escherichia coli: An overview. J. Anim. Sci. 2007, 85 (Suppl. SE), E45–E62. [Google Scholar] [CrossRef]
- Strockbine, N.A.; Marques, L.R.M.; Newland, J.W.; Smith, H.W.; Holmes, R.K.; O’Brien, A.D. Two toxin-converting phages from Escherichia coli O157:H strain 933 encode antigenically distinct toxins with similar biologic activities. Infect. Immun. 1986, 53, 135–140. [Google Scholar] [CrossRef]
- Ostroff, S.M.; Tarr, P.I.; Neill, M.A.; Lewis, J.; Hargrett-Bean, N.; Kobayashi, J.M. Toxin genotypes and plasmid profiles as determinants of systemic sequelae in Escherichia coli O157:H7 infections. J. Infect. Dis. 1989, 160, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Proulx, F.; Seidman, E.G.; Karpman, D. Pathogenesis of Shiga toxin-associated hemolytic uremic syndrome. Pediatr. Res. 2001, 50, 163. [Google Scholar] [CrossRef]
- Bielaszewska, M.; Tarr, P.I.; Karch, H.; Zhang, W.; Mathys, W. Phenotypic and molecular analysis of tellurite resistance among enterohemorrhagic Escherichia coli O157:H7 and sorbitol-fermenting O157:NM clinical isolates. J. Clin. Microbiol. 2005, 43, 452–454. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Frank, C.; Werber, D.; Cramer, J.P.; Askar, M.; Faber, M.; an der Heiden, M.; Berhard, H.; Fruth, A.; Prager, R.; Spode, A.; et al. Epidemic profile of shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N. Engl. J. Med. 2011, 365, 1771–1780. [Google Scholar] [CrossRef]
- Gault, G.; Weill, F.X.; Mariani-Kurkdjian, P.; Silva, N.J.; King, L.; Aldabe, B.; Charron, M.; Ong, N.; Castor, C.; Mace, E.; et al. Outbreak of haemolytic uraemic syndrome and bloody diarrhoea due to Escherichia coli O104:H;4, South-West France, June 2011. Eurosurveillance 2011, 16, 19950. [Google Scholar] [CrossRef] [PubMed]
- Scheutz, F.; Nielsen, E.M.; Frimodt-Moller, J.; Boisen, N.; Morabito, S.; Tozzoli, R.; Nataro, J.P.; Caprioli, A. Characteristics of the enteroaggregative Shiga toxin/verotoxin-producing Escherichia coli O10f4:H4 strain causing the outbreak of haemolytic uraemic syndrome in Germany, May to June 2011. Eurosurveillance 2011, 16, 19889. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, U.; Bernard, H.; Werber, D.; Böhmer, M.M.; Remschmidt, C.; Wilking, H.; Defere, Y.; an der Heiden, M.; Adlhoch, C.; Dreesman, J.; et al. German outbreak of Escherichia coli O104:H4 associated with sprouts. N. Engl. J. Med. 2011, 365, 1763–1770. [Google Scholar] [CrossRef]
- Brzuszkiewicz, E.; Thürmer, A.; Schuldes, J.; Leimbach, A.; Liesegang, H.; Meyer, F.-D.; Boelter, J.; Petersen, H.; Gottschalk, G.; Daniel, R. Genome sequence analyses of two isolates from the recent Escherichia coli outbreak in Germany reveal the emergence of a new pathotype: Entero-aggregative-haemorrhgic Escherichia coli EAHEC. Arch. Microbiol. 2011, 193, 883–891. [Google Scholar] [CrossRef]
- Nataro, J.P.; Deng, Y.; Maneval, D.R.; German, A.L.; Martin, W.C.; Levine, M.M. Aggregative adherence fimbriae I of enteroaggregative Escherichia coli mediate adherence to Hep-2 cells and hemagglutination of human erythrocytes. Infect. Immun. 1992, 60, 2297–2304. [Google Scholar] [CrossRef]
- Rasko, D.A.; Webster, D.R.; Sahl, J.W.; Bashir, A.; Boisen, N.; Scheutz, F.; Paxino, E.E.; Sebra, R.; Chin, C.-S.; Iliopoulos, D.; et al. Origins of the E. coli strain causing an outbreak of hemolytic-uremic syndrome in Germany. N. Engl. J. Med. 2011, 365, 709–717. [Google Scholar] [CrossRef]
- Rohde, H.; Qin, J.; Cui, Y.; Li, D.; Loman, N.J.; Hentschke, M.; Chen, W.; Pu, F.; Peng, Y.; Li, J.; et al. Open-source genomic analysis of Shiga toxin-producing E. coli O104:H4. N. Engl. J. Med. 2011, 365, 718–724. [Google Scholar]
- Nataro, J.P.; Kaper, J.B. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 1998, 11, 142–201. [Google Scholar] [CrossRef]
- Moon, H.W.; Whipp, S.C.; Argenzio, R.A.; Levine, M.M.; Giannella, R.A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect. Immun. 1983, 41, 1340–1351. [Google Scholar] [CrossRef]
- Jerse, A.E.; Yu, J.; Tall, B.D.; Kaper, J.B. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc. Natl. Acad. Sci. USA 1990, 87, 7839–7843. [Google Scholar] [CrossRef]
- Vogeleer, P.; Tremblay, Y.D.N.; Mafu, A.A.; Jacques, M.; Harel, J. Life on the outside: Role of biofilms in environmental persistence of Shiga-toxin producing Escherichia coli. Front. Microbiol. 2014, 5, 317. [Google Scholar] [CrossRef] [PubMed]
- Pratt, L.A.; Kolter, R. Genetic analysis of Escherichia coli biofilm formation: Roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 1998, 30, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Hofmann, C.S.; Cottrell, B.J.; Strobaugh, T.P.; Paoli, G.C.; Nguyen, L.-H.; Yan, X.; Uhlich, G.A. Phenotypic and genotypic characterization of biofilm forming capabilities in non-O157 Shiga toxin-producing Escherichia coli strains. PLoS ONE 2013, 8, e84863. [Google Scholar] [CrossRef] [PubMed]
- Katani, R.; Kudva, I.T.; Srinivasan, S.; Stasko, J.B.; Schilling, M.; Li, L.; Cote, R.; DebRoy, C.; Arthur, T.M.; Sokurenko, E.V.; et al. Strain and host-cell dependent role of type-1 fimbriae in the adherence phenotype of super-shed Escherichia coli O157:H7. Int. J. Med. Microbiol. 2021, 311, 151511. [Google Scholar] [CrossRef]
- Berry, A.A.; Yang, Y.; Pakharukova, N.; Garnett, J.A.; Lee, W.-C.; Cota, E.; Marchant, J.; Roy, S.; Tuittila, M.; Liu, B.; et al. Structural insight into host recognition by aggregative adherence fimbriae of enteroaggregative Escherichia coli. PloS Path. 2014, 10, e1004404. [Google Scholar] [CrossRef]
- Berger, P.; Knodler, M.; Forstner, K.U.; Berger, M.; Bertling, C.; Sharma, C.M.; Vogel, J.; Karch, H.; Dobrindt, U.; Mellmann, A. The primary transcriptome of the Escherichia coli O104:H4 pAA plasmid and novel insights into its virulence gene expression and regulation. Sci. Rep. 2016, 6, 35307. [Google Scholar] [CrossRef]
- Nagy, A.; Xu, Y.; Bauchan, G.R.; Shelton, D.R.; Nou, X. Aggregative adherence fimbriae I (AAF/I) mediate colonization of fresh produce and abiotic surface by Shiga toxigenic enteroaggregative Escherichia coli O104:H4. Internat. J. Food Microbiol. 2016, 229, 44–51. [Google Scholar] [CrossRef]
- Schiller, P.; Knodler, M.; Berger, P.; Greune, L.; Fruth, A.; Mellmann, A.; Dersch, P.; Berger, M.; Dobrindt, U. The superior adherence phenotype of E. coli O104:H4 is directly mediated by the aggregative adherence fimbriae type I. Virulence 2021, 12, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.M.; Povolotsky, T.L.; Wieler, L.H.; Hengge, R. Cyclic-di-GMP signalling and biofilm-related properties of the Shiga toxin-producing 2011 German outbreak Escherichia coli O104:H4. EMBO Mol. Med. 2014, 6, 1622–1637. [Google Scholar] [CrossRef]
- Yan, Z.; Yin, M.; Chen, J.; Li, X. Assembly and substrate recognition of curli biogenesis system. Nat. Commun. 2020, 11, 241. [Google Scholar] [CrossRef]
- Elpers, L.; Hensel, M. Expression and functional characterization of various chaperon-usher fimbriae, curli fimbriae, and type 4 pili of enterohemorrhagic Escherichia coli O157:H7 Sakai. Front. Microbiol. 2020, 11, 378. [Google Scholar] [CrossRef] [PubMed]
- Farfan, M.J.; Cantero, L.; Vidal, R.; Botkin, D.J.; Torres, A.G. Long polar fimbriae of enterohemorrhagic Escherichia coli O157:H7 bind to extracellular matrix proteins. Infect. Immun. 2011, 79, 3744–3750. [Google Scholar] [CrossRef] [PubMed]
- Ross, B.N.; Rojas-Lopez, M.; Cieza, R.J.; McWilliams, B.D.; Torres, A.G. The role of long polar fimbriae in Escherichia coli O104:H4 adhesion and colonization. PLoS ONE 2015, 10, e0141845. [Google Scholar] [CrossRef]
- Low, A.S.; Dziva, F.; Torres, A.G.; Martinez, J.L.; Rosser, T.; Naylor, S.; Spears, K.; Holden, N.; Mahajan, A.; Findlay, J.; et al. Cloning, expression, and characterization of fimbrial operon F9 from enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 2006, 74, 2233–2244. [Google Scholar] [CrossRef]
- Holmes, A.; Rossez, Y.; Wright, K.M.; Hedley, P.E.; Morris, J.; Willats, W.G.T.; Holden, N.J. Escherichia coli O157:H7 F9 fimbriae recognize plant xyloglucan and elicit a response in Arabisopsis thaliana. Int. J. Mol. Sci. 2020, 21, 9720. [Google Scholar] [CrossRef]
- Beloin, C.; Roux, A.; Ghigo, J.-M. Escherichia coli biofilms. Curr. Top. Microbiol. Immunol. 2008, 322, 249–289. [Google Scholar]
- Kaplan, J.B. Biofilm dispersal: Mechanisms, clinical implications, and potential therapeutic uses. J. Dent. Res. 2010, 89, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, G.; Hayden, M.S.; Greenblatt, M.B.; Bussey, C.; Flavell, R.A.; Ghosh, S. A toll-like receptor that prevents infection by uropathogenic bacteria. Science 2004, 303, 1522–1526. [Google Scholar] [CrossRef] [PubMed]
- Amemiya, K.; Dankmeyer, J.L.; Bernhards, R.C.; Fetterer, D.P.; Waag, D.M.; Worsham, P.L.; DeShazer, D. Activation of Toll-like receptors by live Gram-negative bacterial pathogens reveals mitigation of TLR4 responses and activation of TLR5 by flagella. Front. Cell. Infect. Microbiol. 2021, 11, 745325. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Kang, J.Y.; Nanm, X.; Jin, M.S.; Youn, S.-J.; Ryu, Y.H.; Mah, S.; Han, S.H.; Lee, H.; Paik, S.-G.; Lee, J.-O. Recognition of lipopeptide patterns by Toll-like receptor 2-Toll-like receptor 6 heterodimer. Immunity 2009, 31, 873–884. [Google Scholar] [CrossRef]
- Kurokawa, K.; Ryu, K.-H.; Ichikawa, R.; Masuda, A.; Kim, M.-S.; Lee, H.; Chae, J.-H.; Shimizu, T.; Saitoh, T.; Kuwano, K.; et al. Novel bacterial lipoprotein structures conserved in low-GC content Gram-positive bacteria are recognized by Toll-like receptor 2. J. Biol. Chem. 2012, 287, 13170–13181. [Google Scholar] [CrossRef]
- Nakayama, H.; Kurokawa, K.; Lee, B.L. Lipoproteins in bacteria: Structures and biosynthetic pathways. FEBS J. 2012, 279, 4247–4268. [Google Scholar] [CrossRef]
- Poltorak, A.; He, X.; Smirnova, I.; Liu, M.-Y.; van Huffel, C.; Du, X.; Birdwell, D.; Alejos, E.; Silva, M.; Galanos, C.; et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science 1998, 282, 2085–2088. [Google Scholar] [CrossRef]
- Shimazu, R.; Akashi, S.; Ogata, H.; Nagai, Y.; Fukudome, K.; Miyake, K.; Kimoto, M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 1999, 189, 1777–1782. [Google Scholar] [CrossRef]
- Hayashi, F.; Smith, K.D.; Ozinsky, A.; Hawn, T.R.; Yi, E.C.; Goodlett, D.R.; Eng, J.K.; Akira, S.; Underhill, D.M.; Aderem, A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 2001, 410, 1099–1103. [Google Scholar] [CrossRef]
- Donnelly, M.A.; Steiner, T.S. Two nonadjacent regions in enteroaggregative Escherichia coli flagellin are required for activation of Toll-like receptor 5. J. Biol. Chem. 2002, 277, 40456–40461. [Google Scholar] [CrossRef]
- Smith, K.D.; Andersen-Nissen, E.; Hayashi, F.; Strobe, K.; Bergman, M.A.; Barrett, S.L.B.; Cookson, B.T.; Aderem, A. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat. Immunol. 2003, 4, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Heine, H.S.; Hershfield, J.; Marchand, C.; Miller, L.; Halasohoris, S.; Prucell, B.K.; Worsham, P.L. In vitro antibiotic susceptibilities of Yersinia pestis determined by broth microdilution following CLSI methods. Antimicrobial. Agents Chemother. 2015, 59, 1919–1921. [Google Scholar] [CrossRef]
- Surgalla, M.J.; Beesley, E.D. Congo Red-agar plating medium for detecting pigmentation in Pasteurella pestis. Appl. Microbiol. 1969, 18, 834–837. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Melton-Celsa, A.R.; Sinclair, J.F.; Carvalho, H.M.; Robinson, C.; O’Brian, A.D. Monoclonal antibody 11E10, which neutralizes Shiga toxin type 2 (Stx2), recognizes three regions on the Stx2 A subunit, blocks the enzymatic action of the toxin in vitro, and alters the overall cellular distribution of the toxin. Infect. Immun. 2009, 77, 2730–2740. [Google Scholar] [CrossRef] [PubMed]
- Fass, R.J.; Barnishan, J. Minimal inhibitory concentrations of 34 antimicrobial agents for control strains Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853. Antimicrobial. Agents Chemother. 1979, 16, 622–624. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Awosika, J.; Baldwin, C.; Bishop-Lilly, K.A.; Biswas, B.; Broomall, S.; Chain, P.S.G.; Chertkov, O.; Chokoshvili, O.; Coyne, S.; et al. Genomic comparison of Escherichia coli O104:H4 isolates from 2009 and 2011 reveals plasmid, and prophage heterogeneity, including Shiga toxin encoding phage stx2. PLoS ONE 2012, 7, e48228. [Google Scholar] [CrossRef]
- Chokoshvili, O.; Lomashvili, K.; Malakmadze, N.; Geleishvil, M.; Brant, J.; Imnadze, P.; Chitadze, N.; Tevzadze, L.; Chanturia, G.; Tevdoradze, T.; et al. Investigation of an outbreak of bloody diarrhea complicated with hemolytic uremic syndrome. J. Epidemiol. Glob. Health 2014, 4, 249–259. [Google Scholar] [CrossRef]
- Merritt, J.H.; Kadouri, D.E.; O’Toole, G.A. Growing and analyzing static biofilms. Curr. Protoc. Microbiol. 2005, 1, unit-1B.1. [Google Scholar]
- Safadi, R.A.; Abu-Ali, G.S.; Sloup, R.E.; Rudrik, J.T.; Waters, C.M.; Eaton, K.A.; Manning, S.D. Correlation between in vivo biofilm formation and virulence gene expression in Escherichia coli O104:H4. PLoS ONE 2012, 7, e41628. [Google Scholar] [CrossRef]
- Heppel, L.A. Selective release of enzymes from bacteria. Science 1967, 156, 1451–1455. [Google Scholar] [CrossRef] [PubMed]
- MacAlister, T.J.; Costerton, J.W.; Thompson, L.; Thompson, J.; Ingram, J.M. Distribution of alkaline phosphatase within the periplasmic space of Gram-negative bacteria. J. Bacteriol. 1972, 111, 827–832. [Google Scholar] [CrossRef]
- Cummins, E.P.; Selfridge, A.C.; Sporn, P.H.; Sznajer, J.I.; Taylor, C.T. Carbon dioxide-sensing in organisms and its implications for human disease. Cell Mol. Life Sci. 2014, 71, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Bielaszewska, M.; Mellmann, A.; Zhang, W.; Köck, R.; Fruth, A.; Bauwens, A.; Peters, G.; Karch, H. Characterization of the Escherichia coli strain associated with an outbreak of heamolytic uraemic syndrome in Germany, 2011: A microbiology study. Lancet Infect. Dis. 2011, 11, 671–678. [Google Scholar] [CrossRef]
- Boisen, N.; Hansen, A.-M.; Melton-Celsa, A.R.; Zangari, T.; Mortensen, N.P.; Kaper, J.B.; O’Brien, A.D.; Nataro, J.P. The presence of the pAA plasmid in the German O104:H4 Shiga toxin type 2a (Stx2A)-producing enteroaggregative Escherichia coli strain promotes the translocation of Stx2a across an epithelial cell monolayer. J. Infect. Dis. 2014, 210, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- Boisen, N.; Melton-Celsa, A.R.; Hansen, A.-M.; Zangari, T.; Smith, M.A.; Russo, L.M.; Scheutz, F.; O’Brian, A.D.; Nataro, J.P. The role of the AggR regulon in the virulence of the Shiga toxin-producing enteroaggregative Escherichia coli epidemic O104:H4 strain in mice. Front. Microbiol. 2019, 10, 1824. [Google Scholar] [CrossRef] [PubMed]
- Hüttener, M.; Prieto, A.; Espelt, J.; Bernabeu, M.; Juárez, A. Stringent response and AggR-dependent virulence regulation in the enteroaggregative Escherichia coli strain 042. Front. Microbiol. 2018, 9, 717. [Google Scholar] [CrossRef] [PubMed]
- Jønsson, R.; Struve, C.; Boisen, N.; Mateiu, R.V.; Santiago, A.E.; Jenssen, H.; Nataro, J.P.; Krogflelt, K.A. Novel aggregative adherence fimbria variant of enteroaggregative Escherichia coli. Infect. Immun. 2015, 83, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Busch, A.; Waksman, G. Chaperone-usher pathways: Diversity and pilus assembly mechanism. Phil. Trans. R. Soc. B 2012, 367, 1112–1122. [Google Scholar] [CrossRef]
- Jønssonc, R.; Struve, C.; Boll, E.J.; Boisen, N.; Joensen, K.G.; Sørensen, C.A.; Jensen, B.H.; Scheutz, F.; Jenssen, H.; Krogfelt, K.A. A novel pAA virulence plasmid encoding toxins and two distinct variants of the fimbriae of enteroaggregative Escherichia coli. Front. Microbiol. 2017, 8, 263. [Google Scholar]
- Ferdous, M.; Zhou, K.; de Boer, R.F.; Friedrich, A.W.; Kooistra-Smid, A.M.D.; Rossen, J.W.A. Comprehensive characterizatiom of Escherichia coli O104:H4 isolated from patients in the Netherlands. Front. Microbiol. 2015, 6, 1348. [Google Scholar] [CrossRef]
- Uhlich, G.A.; Keen, J.E.; Elder, R.O. Mutations in the csgD promoter associated with variations in curli expression in certain strains of Escherichia coli O157:H7. Appl. Environ. Microbiol. 2001, 67, 2367–2370. [Google Scholar] [CrossRef] [PubMed]
- Uhlich, G.A.; Chen, C.-Y.; Cottrell, B.J.; Hofmann, C.S.; Yan, X.; Nguyen, L. Stx1 prophage excision in Escherichia coli strain PA20 confers strong curli and biofilm formation by restoring native mlrA. FEMS Microbiol. Lett. 2016, 363, fnw123. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sheng, H.; Xue, Y.; Zhao, W.; Hovde, C.J.; Minnich, S.A. Escherichia coli O157:H7 curli fimbriae promotes biofilm formation, epithelial cell invasion, and persistence in cattle. Microorganisms 2020, 8, 580. [Google Scholar] [CrossRef]
- Azam, M.W.; Zuberi, A.; Khan, A.U. bolA gene involved in curli amyloids and fimbriae production in E. coli: Exploring pathways to inhibit biofilm and amyloid formation. J. Biol. Res.-Thessaloniki. 2020, 27, 10. [Google Scholar] [CrossRef]
- Brown, P.K.; Dozois, C.M.; Nickerson, C.A.; Zuppardo, A.; Terlonge, J.; Curtiss III, R. MlrA, a novel regulator of curli (AgF) and extracellular matrix synthesis by Escherichia coli and Salmonella enterica serovar Typhimurium. Mol. Microbiol. 2001, 41, 349–363. [Google Scholar] [CrossRef]
- Uhlich, G.A.; Chen, C.-Y.; Cottrell, B.J.; Hofmann, C.S.; Dudley, E.G.; Strobaugh, T.P., Jr.; Nguyen, L.-H. Phage insertion in mlrA and variations in rpoS limit curli expression and biofilm formation in Escherichia coli serotype O157:H7. Microbiology 2013, 159, 1586–1596. [Google Scholar] [CrossRef]
- Ogasawara, H.; Yamamoto, K.; Ishihama, A. Regulatory role of MlrA in transcription activation of csgD, the master regulator of biofilm formation in Escherichia coli. FEMS Micribiol. Lett. 2010, 312, 160–168. [Google Scholar] [CrossRef]
- Naves, P.; del Prado, G.; Huelves, L.; Gracia, M.; Ruiz, V.; Blanco, J.; Dahbi, G.; Blanco, M.; del Carmen Ponte, M.; Soriano, F. Correlation between virulence of factors and in vitro biofilm formation by Escherichia coli strains. Microb. Pathog. 2008, 45, 86–91. [Google Scholar] [CrossRef]
- Cremét, L.; Corvec, S.; Batard, E.; Auger, M.; Lopez, I.; Pagniez, F.; Dauvergne, S.; Caroff, N. Comparison of three methods to study biofilm formation by clinical strains of Escherichia coli. Diag. Microbiol. Infect. Dis. 2013, 75, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Cepas, V.; Ballén, V.; Gabasa, Y.; Ramirez, M.; López, Y.; Soto, S.M. Transposon insertion in the purL gene induces biofilm depletion in Escherichia coli ATCC 25922. Pathogens 2020, 9, 774. [Google Scholar] [CrossRef] [PubMed]
- Butnick, N.Z.; Miyamoto, C.; Chizzonite, R.; Cullen, B.R.; Ju, G.; Skalka, A.M. Regulation of the human c-myc gene: 5′ noncoding sequences do not affect translation. Mol. Cell. Biol. 1985, 5, 3009–3016. [Google Scholar] [PubMed]
- Berger, J.; Howard, A.D.; Gerber, L.; Cullen, B.R.; Udenfriend, S. Expression of active, membrane-bound human placental alkaline phosphatase by transfected simian cells. Proc. Natl. Acad. Sci. USA 1987, 84, 4885–4889. [Google Scholar] [CrossRef] [PubMed]

| Strains a | Source/Clinical History | Phenotype/Genotype b | Reference |
|---|---|---|---|
| E. coli ATCC 25922 | Clinical isolate, Seattle, WA, 1946 | sorbitol (+), β-glucuronidase (+), tellurite (−), penicillin (r), ampicillin (s), streptomycin (s), motile (+), Congo Red (±), Stx2a (−) | [58], this study |
| E. coli O157:H7 | Clinical isolate, 2000 | sorbitol (−), β-glucuronisase (−), tellurite (+), penicillin (r), ampicillin (s), streptomycin (s), motile (+), Congo Red (±), Stx2a (+) | this study |
| E. coli O104:H4, 2011C 3493 (Ec 01) | HUS in US 2011, traveled from Germany, May 2011 | sorbitol (+), β-glucuronisase (+), tellurite (+), penicillin (r), ampicillin (r), streptomycin (r), motile (+), Congo Red (+), Stx2a (+); stx1 (−), stx2 (+), eae (−), aatA (+), agg A (+), aggR (+) | [59,60], this study |
| E. coli O104:H4, 2009EL 2050 (Ec 02) | Bloody diarrhea, Republic of Georgia, 2009 | sorbitol (+), β-glucuronisase (+), tellurite (+), penicillin (r), ampicillin (r), streptomycin (r), motile (+), Congo Red (+), Stx2a (+); stx1 (−), stx 2 (+), eae (−), aatA (+), aggA (+), aggR (+) | [59,60], this study |
| E. coli O104:H4, 2009EL 2071 (Ec 03) | Bloody diarrhea, Republic of Georgia, 2009 | sorbitol (+), β-glucuronisase (+), tellurite (+), penicillin (r), ampicillin (r), streptomycin (r), motile (+), Congo Red (+), Stx2a (+); stx1 (−), stx2 (+), eae (−), aatA (+), aggA (+), aggR (+) | [59,60], this study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amemiya, K.; Rozak, D.A.; Dankmeyer, J.L.; Dorman, W.R.; Marchand, C.; Fetterer, D.P.; Worsham, P.L.; Purcell, B.K. Shiga-Toxin-Producing Strains of Escherichia coli O104:H4 and a Strain of O157:H7, Which Can Cause Human Hemolytic Uremic Syndrome, Differ in Biofilm Formation in the Presence of CO2 and in Their Ability to Grow in a Novel Cell Culture Medium. Microorganisms 2023, 11, 1744. https://doi.org/10.3390/microorganisms11071744
Amemiya K, Rozak DA, Dankmeyer JL, Dorman WR, Marchand C, Fetterer DP, Worsham PL, Purcell BK. Shiga-Toxin-Producing Strains of Escherichia coli O104:H4 and a Strain of O157:H7, Which Can Cause Human Hemolytic Uremic Syndrome, Differ in Biofilm Formation in the Presence of CO2 and in Their Ability to Grow in a Novel Cell Culture Medium. Microorganisms. 2023; 11(7):1744. https://doi.org/10.3390/microorganisms11071744
Chicago/Turabian StyleAmemiya, Kei, David A. Rozak, Jennifer L. Dankmeyer, William R. Dorman, Charles Marchand, David P. Fetterer, Patricia L. Worsham, and Brett K. Purcell. 2023. "Shiga-Toxin-Producing Strains of Escherichia coli O104:H4 and a Strain of O157:H7, Which Can Cause Human Hemolytic Uremic Syndrome, Differ in Biofilm Formation in the Presence of CO2 and in Their Ability to Grow in a Novel Cell Culture Medium" Microorganisms 11, no. 7: 1744. https://doi.org/10.3390/microorganisms11071744
APA StyleAmemiya, K., Rozak, D. A., Dankmeyer, J. L., Dorman, W. R., Marchand, C., Fetterer, D. P., Worsham, P. L., & Purcell, B. K. (2023). Shiga-Toxin-Producing Strains of Escherichia coli O104:H4 and a Strain of O157:H7, Which Can Cause Human Hemolytic Uremic Syndrome, Differ in Biofilm Formation in the Presence of CO2 and in Their Ability to Grow in a Novel Cell Culture Medium. Microorganisms, 11(7), 1744. https://doi.org/10.3390/microorganisms11071744






