Responses of Zooplankton Community Pattern to Environmental Factors along the Salinity Gradient in a Seagoing River in Tianjin, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Sampling and Laboratory Analyses
2.3. Data Analysis
3. Results
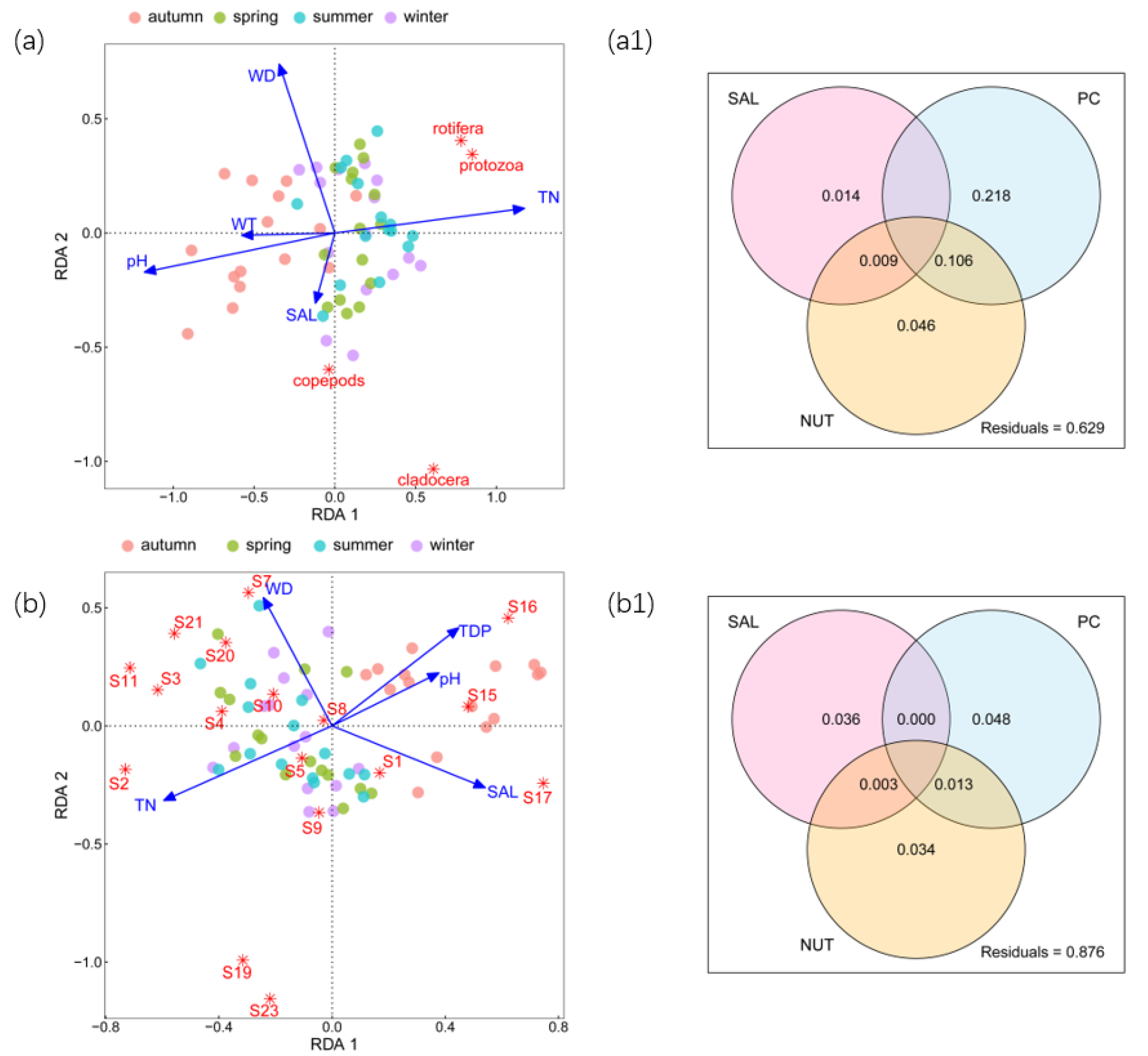
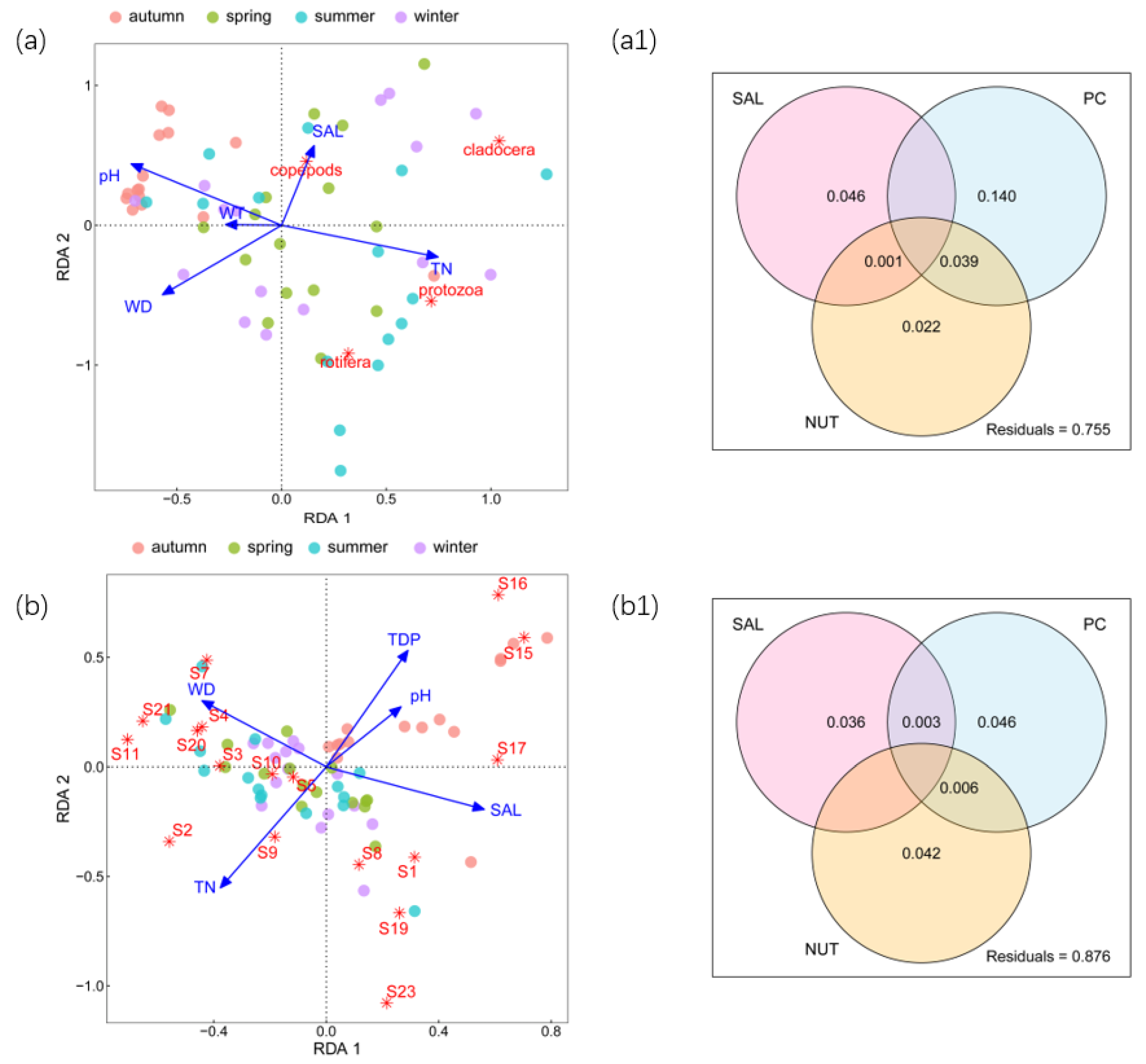
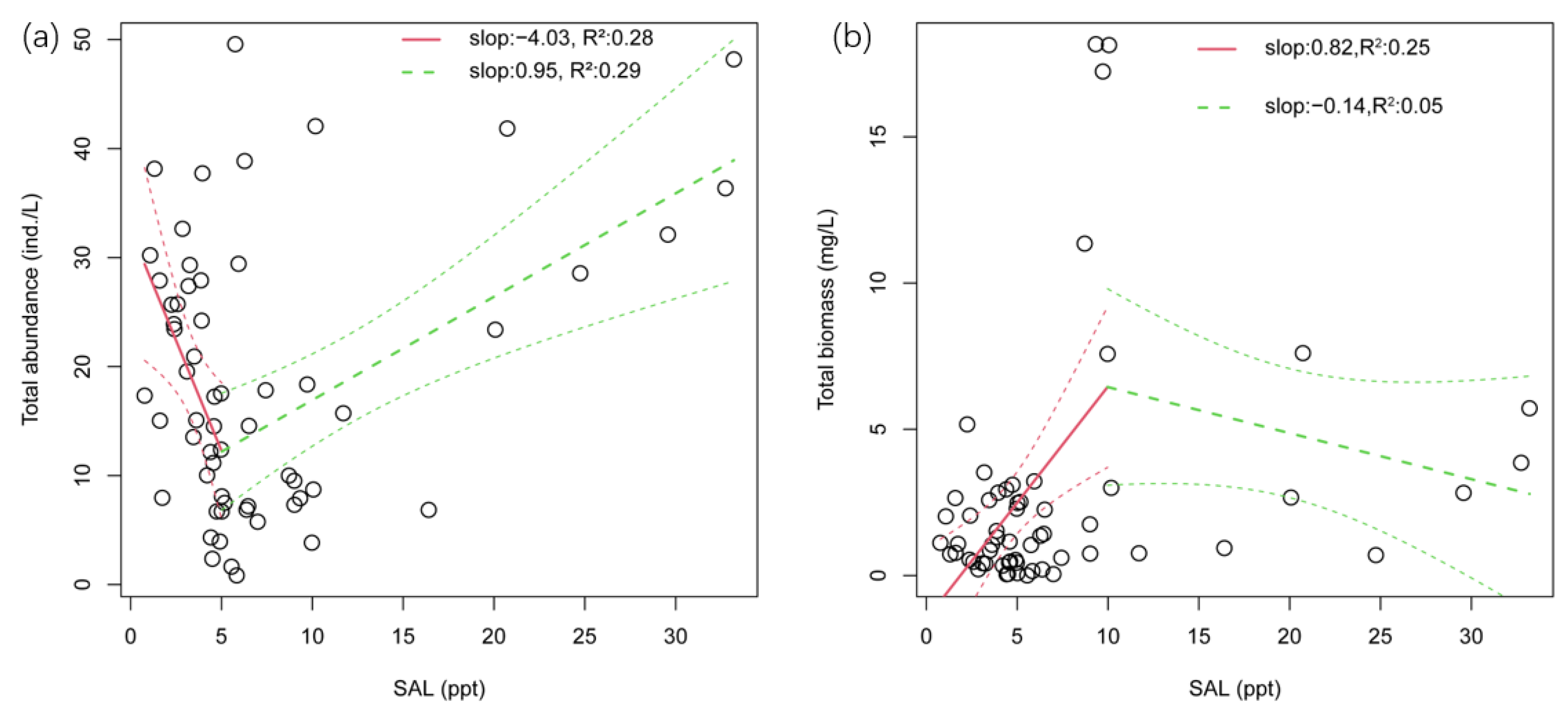
3.1. Zooplankton Abundance and Relationships with Environmental Factors
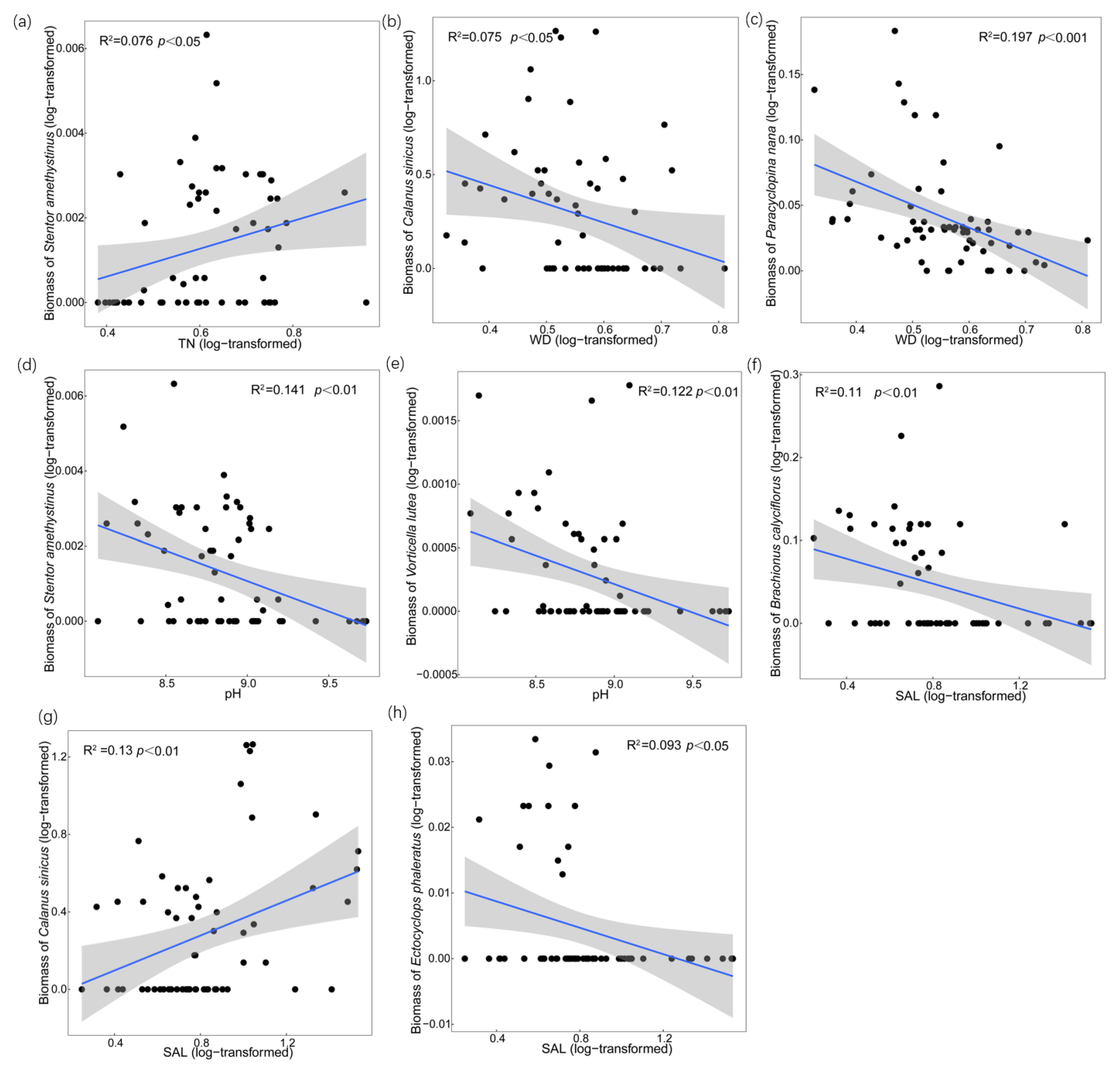
3.2. Zooplankton Biomass and Relationships with Environmental Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Zhao, K.; Cao, Y.; Wu, B.; Pang, W.; You, Q.; Wang, Q. Zooplankton Community Structure and Its Relationship with Environmental Factors in Poyang Lake. Acta Ecol. Sin. 2020, 40, 6644. [Google Scholar]
- Li, C.; Feng, W.; Chen, H.; Li, X.; Song, F.; Guo, W.; Giesy, J.P.; Sun, F. Temporal Variation in Zooplankton and Phytoplankton Community Species Composition and the Affecting Factors in Lake Taihu—A Large Freshwater Lake in China. Environ. Pollut. 2019, 245, 1050–1057. [Google Scholar] [CrossRef]
- Shin, S.S.; Choi, S.Y.; Seo, M.H.; Lee, S.J.; Soh, H.Y.; Youn, S.H. Spatiotemporal Distribution Characteristics of Copepods in the Water Masses of the Northeastern East China Sea. J. Mar. Sci. Eng. 2022, 10, 754. [Google Scholar] [CrossRef]
- Sun, Y.; Li, H.; Yang, Q.; Liu, Y.; Fan, J.; Guo, H. Disentangling Effects of River Inflow and Marine Diffusion in Shaping the Planktonic Communities in a Heavily Polluted Estuary. Environ. Pollut. 2020, 267, 115414. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, J.M.; Bozelli, R.L.; de Melo Rocha, A.; de Assis Esteves, F. Effects of Slight Salinity Increases on Moina Micrura (Cladocera) Populations: Field and Laboratory Observations. Mar. Freshwater Res. 2008, 59, 808. [Google Scholar] [CrossRef]
- Heine-Fuster, I.; Vega-Retter, C.; Sabat, P.; Ramos-Jiliberto, R. Osmoregulatory and Demographic Responses to Salinity of the Exotic Cladoceran Daphnia Exilis. J. Plankton Res. 2010, 32, 1405–1411. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Vila-Gispert, A.; Quintana, X.D.; Van Hoa, A.; Nguyen, T.P.; Ut Vu, N. Effects of Salinity on Species Composition of Zooplankton on Hau River, Mekong Delta, Vietnam. Ann. Limnol. Int. J. Lim. 2020, 56, 20. [Google Scholar] [CrossRef]
- Li, D.; Wen, Y.; Zhang, G.; Zhang, G.; Sun, J.; Xu, W. Effects of Terrestrial Inputs on Mesozooplankton Community Structure in Bohai Bay, China. Diversity 2022, 14, 410. [Google Scholar] [CrossRef]
- Zhao, W.; Dai, L.; Chen, X.; Wu, Y.; Sun, Y.; Zhu, L. Characteristics of Zooplankton Community Structure and Its Relationship with Environmental Factors in the South Yellow Sea. Mar. Pollut. Bull. 2022, 176, 113471. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, H.; Wang, Z.; Huang, T.; Huang, H. Phytoplankton Community Response to Environmental Factors along a Salinity Gradient in a Seagoing River, Tianjin, China. Microorganisms 2022, 11, 75. [Google Scholar] [CrossRef]
- Duggan, I.C.; Green, J.D.; Shiel, R.J. Distribution of Rotifer Assemblages in North Island, New Zealand, Lakes: Relationships to Environmental and Historical Factors: New Zealand Rotifer Distribution. Freshw. Biol. 2002, 47, 195–206. [Google Scholar] [CrossRef]
- Xiong, W.; Li, J.; Chen, Y.; Shan, B.; Wang, W.; Zhan, A. Determinants of Community Structure of Zooplankton in Heavily Polluted River Ecosystems. Sci. Rep. 2016, 6, 22043. [Google Scholar] [CrossRef]
- Paturej, E.; Gutkowska, A. The Effect of Salinity Levels on the Structure of Zooplankton Communities. Arch. Biol. Sci. 2015, 67, 483–492. [Google Scholar] [CrossRef]
- Lin, Q.; Xu, L.; Hou, J.; Liu, Z.; Jeppesen, E.; Han, B.-P. Responses of Trophic Structure and Zooplankton Community to Salinity and Temperature in Tibetan Lakes: Implication for the Effect of Climate Warming. Water Res. 2017, 124, 618–629. [Google Scholar] [CrossRef]
- Gutierrez, M.F.; Tavşanoğlu, Ü.N.; Vidal, N.; Yu, J.; Teixeira-de Mello, F.; Çakiroglu, A.I.; He, H.; Liu, Z.; Jeppesen, E. Salinity Shapes Zooplankton Communities and Functional Diversity and Has Complex Effects on Size Structure in Lakes. Hydrobiologia 2018, 813, 237–255. [Google Scholar] [CrossRef]
- Yu, J.; Jiang, Z.; Li, T.; Zhou, W.; Dou, Y.; Gao, J.; Jia, X. Research on Plankton in Tianjin Section of Downstream of Haihe River. Hebei Fish. 2016, 5, 22–23. [Google Scholar]
- Xu, M.; Cao, H.; Xie, P.; Deng, D.; Feng, W.; Xu, J. Use of PFU Protozoan Community Structural and Functional Characteristics in Assessment of Water Quality in a Large, Highly Polluted Freshwater Lake in China. J. Environ. Monit. 2005, 7, 670. [Google Scholar] [CrossRef]
- Huang, J.; Lin, W.; Shi, C.; Wu, S.; Xu, R. The Effects of Restoration Techniques on Protozoan Communities in Mandarin Fish Culture Ponds, Based on an Artificial Substrate. Aquacult. Int. 2010, 18, 339–348. [Google Scholar] [CrossRef]
- Fermani, P.; Diovisalvi, N.; Torremorell, A.; Lagomarsino, L.; Zagarese, H.E.; Unrein, F. The Microbial Food Web Structure of a Hypertrophic Warm-Temperate Shallow Lake, as Affected by Contrasting Zooplankton Assemblages. Hydrobiologia 2013, 714, 115–130. [Google Scholar] [CrossRef]
- Park, G.S.; Marshall, H.G. The Trophic Contributions of Rotifers in Tidal Freshwater and Estuarine Habitats. Estuar. Coast. Shelf Sci. 2000, 51, 729–742. [Google Scholar] [CrossRef]
- Wang, S.; Xie, P.; Geng, H. The Relative Importance of Physicochemical Factors and Crustacean Zooplankton as Determinants of Rotifer Density and Species Distribution in Lakes Adjacent to the Yangtze River, China. Limnologica 2010, 40, 1–7. [Google Scholar] [CrossRef]
- Gu, Y.-L.; Huang, Q.; Xu, L.; Rizo, E.Z.; Alonso, M.; Dumont, H.J.; Han, B.-P. Species Diversity and Community Assembly of Cladocera in the Sand Ponds of the Ulan Buh Desert, Inner Mongolia of China. Diversity 2021, 13, 502. [Google Scholar] [CrossRef]
- Voutilainen, A.; Jurvelius, J.; Lilja, J.; Viljanen, M.; Rahkola-Sorsa, M. Associating Spatial Patterns of Zooplankton Abundance with Water Temperature, Depth, Planktivorous Fish and Chlorophyll. Boreal Env. Res. 2016, 21, 101–11421. [Google Scholar]
- Medley, K.A.; Havel, J.E. Hydrology and Local Environmental Factors Influencing Zooplankton Communities in Floodplain Ponds. Wetlands 2007, 27, 864–872. [Google Scholar] [CrossRef]
- Weydmann, A.; Carstensen, J.; Goszczko, I.; Dmoch, K.; Olszewska, A.; Kwasniewski, S. Shift towards the Dominance of Boreal Species in the Arctic: Inter-Annual and Spatial Zooplankton Variability in the West Spitsbergen Current. Mar. Ecol. Prog. Ser. 2014, 501, 41–52. [Google Scholar] [CrossRef]
- de Oliveira Dias, C.; de Araujo, A.V.; Bonecker, S.L.C. Vertical Distribution and Structure of Copepod (Arthropoda: Copepoda) Assemblages in Two Different Seasons down to 1200 m in the Tropical Southwestern Atlantic. Zoologia 2018, 35, 1–11. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, Y.; Liu, G. Characteristics of Zooplankton Community in North Yellow Sea Unveiled an Indicator Species for the Yellow Sea Warm Current in Winter: Euchaeta Plana. J. Ocean. Limnol. 2020, 38, 1762–1770. [Google Scholar] [CrossRef]
- Kang, J.-H.; Seo, M.; Kwon, O.Y.; Kim, W.-S. Diel Vertical Migration of the Copepod Calanus Sinicus before and during Formation of the Yellow Sea Cold Bottom Water in the Yellow Sea. Acta Oceanol. Sin. 2013, 32, 99–106. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, M.; Lin, H.; Gao, P.; Yang, Z.; Wang, D.; Sun, X.; Li, B.; Wang, Q.; Sun, W. Response of Soil Protozoa to Acid Mine Drainage in a Contaminated Terrace. J. Hazard. Mater. 2022, 421, 126790. [Google Scholar] [CrossRef]
- Peng, L.; Lan, C.Q.; Zhang, Z.; Sarch, C.; Laporte, M. Control of Protozoa Contamination and Lipid Accumulation in Neochloris Oleoabundans Culture: Effects of PH and Dissolved Inorganic Carbon. Bioresour. Technol. 2015, 197, 143–151. [Google Scholar] [CrossRef]
- Ferrando, N.S.; Nandini, S.; Claps, M.C.; Sarma, S.S.S. Effect of Salinity and Food Concentration on Competition between Brachionus Plicatilis Müller, 1786 and Brachionus Calyciflorus Pallas, 1776 (Rotifera). Mar. Freshwater Res. 2020, 71, 493. [Google Scholar] [CrossRef]
- Sarma, S.S.S.; Nandini, S.; Morales-Ventura, J.; Delgado-Martínez, I.; González-Valverde, L. Effects of NaCl Salinity on the Population Dynamics of Freshwater Zooplankton (Rotifers and Cladocerans). Aquat. Ecol. 2006, 40, 349–360. [Google Scholar] [CrossRef]
- Ho, T.W.; Hwang, J.-S.; Cheung, M.K.; Kwan, H.S.; Wong, C.K. DNA-Based Study of the Diet of the Marine Calanoid Copepod Calanus Sinicus. J. Exp. Mar. Biol. Ecol. 2017, 494, 1–9. [Google Scholar] [CrossRef]
- Sogawa, S.; Kidachi, T.; Nagayama, M.; Ichikawa, T.; Hidaka, K.; Ono, T.; Shimizu, Y. Short-Term Variation in Copepod Community and Physical Environment in the Waters Adjacent to the Kuroshio Current. J. Oceanogr. 2017, 73, 603–622. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Y.; Yang, G.; Zhang, X. Effects of Diet, Temperature and Salinity on Ingestion and Egestion of Two Species of Marine Copepods. Period. Ocean Univ. China 2012, 42, 45–52. [Google Scholar] [CrossRef]
- Anufriieva, E.V. Do Copepods Inhabit Hypersaline Waters Worldwide? A Short Review and Discussion. Chin. J. Ocean Limnol. 2015, 33, 1354–1361. [Google Scholar] [CrossRef]
- Gao, Q.; Xu, Z.; Zhuang, P. The Relation between Distribution of Zooplankton and Salinity in the Changjiang Estuary. Chin. J. Ocean Limnol. 2008, 26, 178–185. [Google Scholar] [CrossRef]
- Zakaria, H.Y.; Radwan, A.A.; Said, M.A. Influence of Salinity Variations on Zooplankton Community In El-Mex Bay, Alexandria, Egypt. Egypt. J. Aquat. Res. 2007, 33, 52–67. [Google Scholar]
- Silva, A.M.A.; Barbosa, J.E.L.; Medeiros, P.R.; Rocha, R.M.; Lucena-Filho, M.A.; Silva, D.F. Zooplankton (Cladocera and Rotifera) Variations along a Horizontal Salinity Gradient and during Two Seasons (Dry and Rainy) in a Tropical Inverse Estuary (Northeast Brazil). Pan-Am. J. Aquat. Sci. 2009, 4, 226–238. [Google Scholar]
- Araújo, L.R.; Lopes, P.M.; Santangelo, J.M.; de Assis Esteves, F.; Bozelli, R.L. Long-Term Dynamics of the Zooplankton Community during Large Salinity Fluctuations in a Coastal Lagoon. Mar. Freshwater Res. 2015, 66, 352. [Google Scholar] [CrossRef]
- Zadereev, E.; Drobotov, A.; Anishchenko, O.; Kolmakova, A.; Lopatina, T.; Oskina, N.; Tolomeev, A. The Structuring Effects of Salinity and Nutrient Status on Zooplankton Communities and Trophic Structure in Siberian Lakes. Water 2022, 14, 1468. [Google Scholar] [CrossRef]
- Hall, C.A.M.; Lewandowska, A.M. Zooplankton Dominance Shift in Response to Climate-Driven Salinity Change: A Mesocosm Study. Front. Mar. Sci. 2022, 9, 861297. [Google Scholar] [CrossRef]
- Khlebovich, V.V. Aspects of Animal Evolution Related to Critical Salinity and Internal State. Marine Biol. 1969, 2, 338–345. [Google Scholar] [CrossRef]
- Khlebovich, V.V. Applied Aspects of the Concept of Critical Salinity. Biol. Bull. Rev. 2015, 5, 562–567. [Google Scholar] [CrossRef]
- Telesh, I.V.; Khlebovich, V.V. Principal Processes within the Estuarine Salinity Gradient: A Review. Mar. Pollut. Bull. 2010, 61, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Environmental Protection of the People’s Republic of China. Determination Methods for Examination of Water and Wastewater, 4th ed.; China Environmental Science Press: Beijing, China, 2002. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Zhang, H.; Wang, Z.; Huang, T.; Tian, W.; Huang, H. Responses of Zooplankton Community Pattern to Environmental Factors along the Salinity Gradient in a Seagoing River in Tianjin, China. Microorganisms 2023, 11, 1638. https://doi.org/10.3390/microorganisms11071638
Sun X, Zhang H, Wang Z, Huang T, Tian W, Huang H. Responses of Zooplankton Community Pattern to Environmental Factors along the Salinity Gradient in a Seagoing River in Tianjin, China. Microorganisms. 2023; 11(7):1638. https://doi.org/10.3390/microorganisms11071638
Chicago/Turabian StyleSun, Xuewei, Huayong Zhang, Zhongyu Wang, Tousheng Huang, Wang Tian, and Hai Huang. 2023. "Responses of Zooplankton Community Pattern to Environmental Factors along the Salinity Gradient in a Seagoing River in Tianjin, China" Microorganisms 11, no. 7: 1638. https://doi.org/10.3390/microorganisms11071638
APA StyleSun, X., Zhang, H., Wang, Z., Huang, T., Tian, W., & Huang, H. (2023). Responses of Zooplankton Community Pattern to Environmental Factors along the Salinity Gradient in a Seagoing River in Tianjin, China. Microorganisms, 11(7), 1638. https://doi.org/10.3390/microorganisms11071638






