TgKDAC4: A Unique Deacetylase of Toxoplasma’s Apicoplast
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasites Culture
2.2. Transfection and Endogenous Tagging Confirmation
2.3. Immunofluorescence Assays
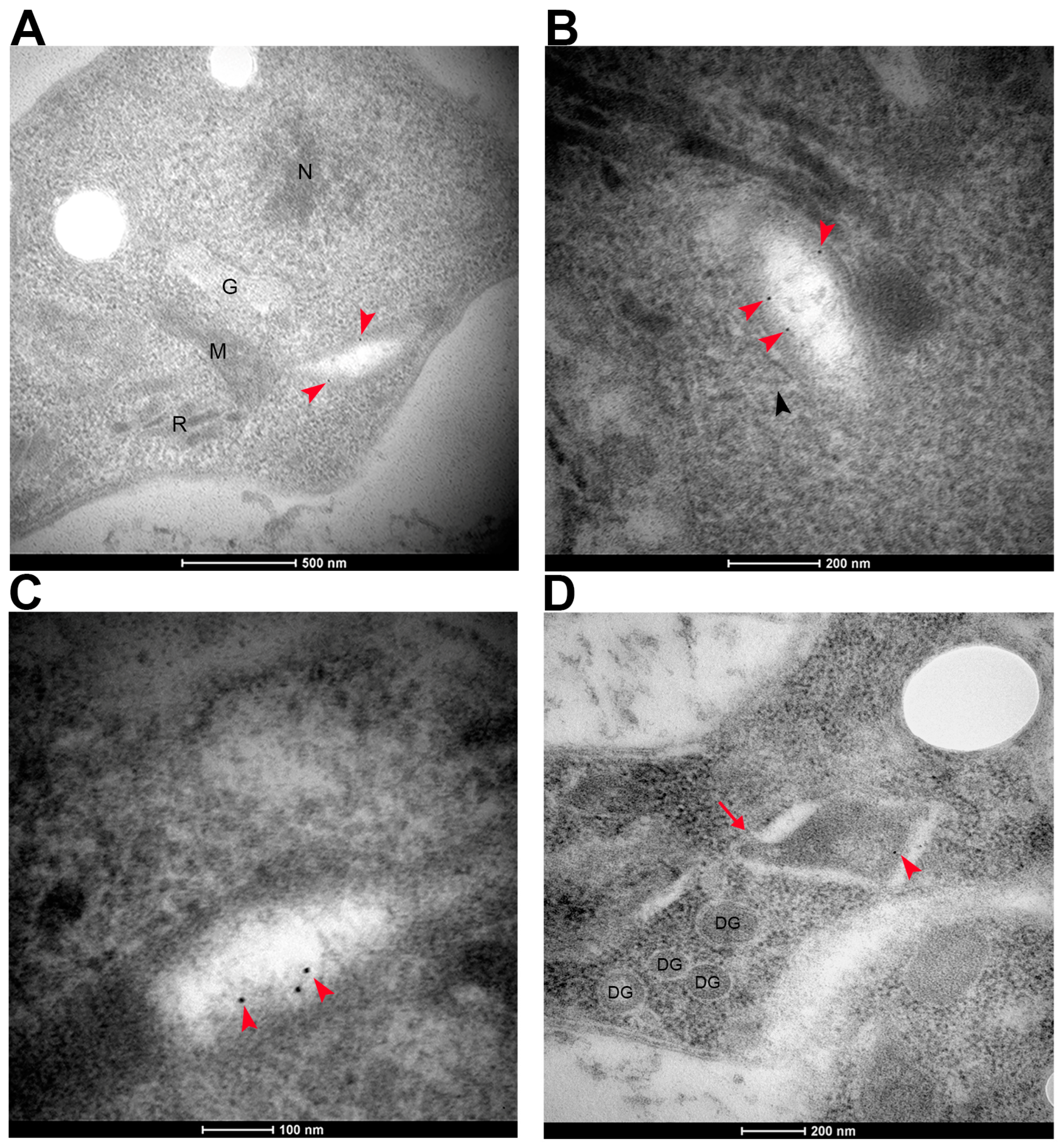
2.4. Transmission Electron Microscopy
2.5. Immunoprecipitation of TgKDAC4 Complex
2.6. Mass Spectrometry and Data Analysis
2.7. Phylogenetic Analysis
3. Results
3.1. TgKDAC4 Has a Putative Prokaryotic Origin
3.2. TgKDAC4 Localizes in the Apicoplast
3.3. TgKDAC4 Interacts with Apicoplast Proteins
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Choudhary, C.; Kumar, C.; Gnad, F.; Nielsen, M.L.; Rehman, M.; Walther, T.C.; Olsen, J.V.; Mann, M. Lysine Acetylation Targets Protein Complexes and Co-Regulates Major Cellular Functions. Science 2009, 325, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Philllips, D.M.P. The Presence of Acetyl Groups in Histones. Biochem. J. 1963, 87, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Poirier, Y. The Protein Acetylome and the Regulation of Metabolism. Trends Plant Sci. 2012, 17, 423–430. [Google Scholar] [CrossRef]
- Allfrey, G.V.; Faulkner, R.; Mirsky, A.E. Acetylation and Methylation Of Histones and Their Possible Role in the Regulation of RNA Synthesis. Biochemistry 1964, 315, 786–794. [Google Scholar] [CrossRef]
- Narita, T.; Weinert, B.T.; Choudhary, C. Functions and Mechanisms of Non-Histone Protein Acetylation. Nat. Rev. Mol. Cell Biol. 2019, 20, 156–174. [Google Scholar] [CrossRef] [PubMed]
- Verdin, E.; Ott, M. 50 Years of Protein Acetylation: From Gene Regulation to Epigenetics, Metabolism and Beyond. Nat. Rev. Mol. Cell Biol. 2014, 16, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Carabetta, V.J.; Cristea, M. Regulation, Function, and Detection of Protein Acetylation in Bacteria. J. Bacteriol. 2017, 199, e00107-17. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.G.; Baumgartner, J.T.; Xie, X.; Jew, K.M.; Basisty, N.; Schilling, B. Mechanisms, Detection, and Relevance of Protein Acetylation in Prokaryotes. MBio 2019, 10, e02708-18. [Google Scholar] [CrossRef]
- Yakhine-diop, S.M.S.; Rodríguez-arribas, M.; Martínez-chacón, G.; Uribe-Carretero, E.; Gómez-Sánchez, R.; Aiastui, A.; de Munain, A.L.; Pedro, J.M.B.-S.; Niso-Santano, M.; González-Polo, R.A.; et al. Acetylome in Human Fibroblasts from Parkinson’s Disease Patients. Front. Cell. Neurosci. 2018, 12, 97. [Google Scholar] [CrossRef]
- Jeffers, V.; Sullivan, W.J. Lysine Acetylation Is Widespread on Proteins of Diverse Function and Localization in the Protozoan Parasite Toxoplasma gondii. Eukaryot. Cell 2012, 11, 735–742. [Google Scholar] [CrossRef]
- Pappas, G.; Roussos, N.; Falagas, M.E. Toxoplasmosis Snapshots: Global Status of Toxoplasma gondii Seroprevalence and Implications for Pregnancy and Congenital Toxoplasmosis. Int. J. Parasitol. 2009, 39, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Saksouk, N.; Bhatti, M.M.; Kieffer, S.; Aaron, T.; Musset, K.; Garin, J.; Sullivan, W.J., Jr.; Hakimi, M.; Smith, A.T. Histone-Modifying Complexes Regulate Gene Expression Pertinent to the Differentiation of the Protozoan Parasite Toxoplasma gondii. Mol. Cell. Biol. 2005, 25, 10301–10314. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, R.; Zhou, C.; He, J.; Elsheikha, H.M. Label-Free Quantitative Acetylome Analysis Reveals Toxoplasma gondii Genotype-Specific Acetylomic Signatures. Microorganisms 2019, 7, 510. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Lindsay, D.S.; Speer, C.A. Structures of Toxoplasma gondii Tachyzoites, Bradyzoites, and Sporozoites and Biology and Development of Tissue Cysts. Clin. Microbiol. Rev. 1998, 11, 267–299. [Google Scholar] [CrossRef] [PubMed]
- Lüder, C.G.K.; Rahman, T. Impact of the Host on Toxoplasma Stage Differentiation. Microb. Cell 2017, 4, 203–211. [Google Scholar] [CrossRef]
- de Monerri, N.C.S.; Yakubu, R.R.; Chen, A.L.; Bradley, P.J.; Nieves, E.; Weiss, L.M.; Kim, K. The Ubiquitin Proteome of Toxoplasma gondii Reveals Roles for Protein Ubiquitination in Cell Cycle Transitions. Cell Host Microbe 2016, 18, 621–633. [Google Scholar] [CrossRef]
- Weiss, L.M.; Fiser, A.; Angeletti, R.H.; Kim, K. Toxoplasma gondii Proteomics. Expert Rev. Proteom. 2009, 6, 303–313. [Google Scholar] [CrossRef]
- Yakubu, R.R.; Weiss, L.M.; Silmon de Monerri, N.C. Post-Translational Modifications as Key Regulators of Apicomplexan Biology: Insights from Proteome-Wide Studies. Mol. Microbiol. 2018, 107, 1–23. [Google Scholar] [CrossRef]
- Hakimi, M.-A.; Deitsch, K.W. Epigenetics in Apicomplexa: Control of Gene Expression during Cell Cycle Progression, Differentiation and Antigenic Variation. Curr. Opin. Microbiol. 2007, 10, 357–362. [Google Scholar] [CrossRef]
- Vanagas, L.; Jeffers, V.; Bogado, S.S.; Dalmasso, M.C.; Sullivan, W.J., Jr.; Angel, S.O. Toxoplasma Histone Acetylation Remodelers as Novel Drug Targets. Expert Rev. Anti-Infect. Ther. 2012, 2, 1189–1201. [Google Scholar] [CrossRef]
- Kutil, Z.; Novakova, Z.; Meleshin, M.; Mikesova, J.; Schutkowski, M.; Barinka, C. Histone Deacetylase 11 Is a Fatty-Acid Deacylase. ACS Chem. Biol. 2018, 13, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Bisson, W.H.; Lohr, C.V.; Williams, D.E.; Ho, E.; Dashwood, R.H.; Rajendran, P. Histone and Non-Histone Targets of Dietary Deacetylase Inhibitors. Curr. Top. Med. Chem. 2016, 16, 714–731. [Google Scholar] [CrossRef] [PubMed]
- Gregoretti, I.V.; Lee, Y.M.; Goodson, H.V. Molecular Evolution of the Histone Deacetylase Family: Functional Implications of Phylogenetic Analysis. J. Mol. Biol. 2004, 338, 17–31. [Google Scholar] [CrossRef] [PubMed]
- de Ruijter, A.J.M.; van Gennip, A.H.; Caron, H.N.; Kemp, S.; van Kuilenburg, A.B.P. Histone Deacetylases (HDACs): Characterization of the Classical HDAC Family. Biochem. J. 2003, 370, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Micelli, C.; Rastelli, G. Histone Deacetylases: Structural Determinants of Inhibitor Selectivity. Drug Discov. Today 2015, 20, 718–735. [Google Scholar] [CrossRef] [PubMed]
- Bougdour, A.; Maubon, D.; Baldacci, P.; Ortet, P.; Bastien, O.; Bouillon, A.; Barale, J.; Pelloux, H.; Ménard, R.; Hakimi, M. Drug Inhibition of HDAC3 and Epigenetic Control of Differentiation in Apicomplexa Parasites. J. Exp. Med. 2009, 206, 953–966. [Google Scholar] [CrossRef]
- Ho, T.C.S.; Chan, A.H.Y.; Ganesan, A. Thirty Years of HDAC Inhibitors: 2020 Insight and Hindsight. J. Med. Chem. 2020, 63, 12460–12484. [Google Scholar] [CrossRef]
- Singh, N.B.; Zhang, G.; Hwa, Y.L.; Li, J.; Dowdy, S.C.; Jiang, S.-W. Nonhistone Protein Acetylation as Cancer Therapy Targets. Expert Rev. Anticancer. Ther. 2010, 10, 935–954. [Google Scholar] [CrossRef]
- Xu, W.S.; Parmigiani, R.B.; Marks, P.A. Histone Deacetylase Inhibitors: Molecular Mechanisms of Action. Oncogene 2007, 26, 5541–5552. [Google Scholar] [CrossRef]
- Araujo-Silva, C.A.; De Souza, W.; Martins-Duarte, E.S.; Vommaro, R.C. HDAC Inhibitors Tubastatin A and SAHA Affect Parasite Cell Division and Are Potential Anti-Toxoplasma gondii Chemotherapeutics. Int. J. Parasitol. Drugs Drug Resist. 2021, 15, 25–35. [Google Scholar] [CrossRef]
- Fleige, T.; Fischer, K.; Ferguson, D.J.P.; Gross, U.; Bohne, W. Carbohydrate Metabolism in the Toxoplasma gondii Apicoplast: Localization of Three Glycolytic Isoenzymes, the Single Pyruvate Dehydrogenase Complex, and a Plastid Phosphate Translocator. Eukaryot. Cell 2007, 6, 984–996. [Google Scholar] [CrossRef] [PubMed]
- Kloehn, J.; Oppenheim, R.D.; Siddiqui, G.; De Bock, P.; Dogga, S.K.; Coute, Y.; Hakimi, M.; Creek, D.J.; Soldati-favre, D. Multi-Omics Analysis Delineates the Distinct Functions of Sub-Cellular Acetyl-CoA Pools in Toxoplasma gondii. BMC Biol. 2020, 18, 67. [Google Scholar] [CrossRef] [PubMed]
- McFadden, G.I.; Waller, R.F. Plastids in Parasites of Humans. Bioessays 1997, 19, 1033–1040. [Google Scholar] [CrossRef]
- De Souza, W.; Martins-duarte, É.S.; Lemgruber, L. Organização Estrutural Do Taquizoíto de Toxoplasma gondii. Sci. Med. 2010, 20, 131–143. [Google Scholar]
- Striepen, B. The Apicoplast: A Red Alga in Human Parasites. Essays Biochem. Mol. Parasitol. 2011, 51, 111–125. [Google Scholar] [CrossRef]
- Wilson, R.J.M.; Gardner, M.J.; Feagin, J.E.; Williamson, D.H. Have Malaria Parasites Three Genomes? Parasitol. Today 1991, 7, 134–136. [Google Scholar] [CrossRef]
- Martins-Duarte, E.; Sheiner, L.; Reiff, S.; de Souza, W.; Striepen, B. Replication and Partitioning of the Apicoplast Genome of Toxoplasma gondii Is Linked to the Cell Cycle and Requires DNA Polymerase and Gyrase. Int. J. Parasitol. 2021, 51, 493–504. [Google Scholar] [CrossRef]
- Reiff, S.B.; Vaishnava, S.; Striepen, B. The HU Protein Is Important for Apicoplast Genome Maintenance and Inheritance in Toxoplasma gondii. Eukaryot. Cell 2012, 11, 905–915. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Chen, C.Y.; Doerig, C.; Henriquez, F.L.; Roberts, C.W.; Barrett, M.P. The Toxoplasma gondii Plastid Replication and Repair Enzyme Complex, PREX. Parasitology 2009, 136, 747–755. [Google Scholar] [CrossRef]
- Fleige, T.; Limenitakis, J.; Soldati-Favre, D. Apicoplast: Keep It or Leave It. Microbes Infect. 2010, 12, 253–262. [Google Scholar] [CrossRef]
- Mazumdar, J.; Wilson, E.H.; Masek, K.; Hunter, C.A.; Striepen, B. Apicoplast Fatty Acid Synthesis Is Essential for Organelle Biogenesis and Parasite Survival in Toxoplasma gondii. Proc. Natl. Acad. Sci. USA 2006, 103, 13192–13197. [Google Scholar] [CrossRef] [PubMed]
- Mcfadden, G.I.; Yeh, E. The Apicoplast: Now You See It, Now You Don’t. Int. J. Parasitol. 2017, 47, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Huynh, M.H.; Carruthers, V.B. Tagging of Endogenous Genes in a Toxoplasma gondii Strain Lacking Ku80. Eukaryot. Cell 2009, 8, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Nishi, M.; Hu, K.; Murray, J.M.; Roos, D.S. Organellar Dynamics during the Cell Cycle of Toxoplasma gondii. J. Cell Sci. 2008, 121, 1559–1568. [Google Scholar] [CrossRef]
- He, C.Y.; Striepen, B.; Pletcher, C.H.; Murray, J.M.; Roos, D.S. Targeting and Processing of Nuclear-Encoded Apicoplast Proteins in Plastid Segregation Mutants of Toxoplasma gondii. J. Biol. Chem. 2001, 276, 28436–28442. [Google Scholar] [CrossRef]
- Obado, S.O.; Brillantes, M.; Uryu, K.; Zhang, W.; Ketaren, N.E.; Chait, B.T.; Field, M.C.; Rout, M.P. Interactome Mapping Reveals the Evolutionary History of the Nuclear Pore Complex. PLoS Biol. 2016, 14, e1002365. [Google Scholar] [CrossRef]
- van Dooren, G.G.; Tomova, C.; Agrawal, S.; Humbel, B.M.; Striepen, B. Toxoplasma gondii Tic20 Is Essential for Apicoplast Protein Import. Proc. Natl. Acad. Sci. USA 2008, 105, 13574–13579. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus Computational Platform for Comprehensive Analysis of (Prote) Omics Data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Ramaprasad, A.; Mourier, T.; Naeem, R.; Malas, T.B.; Moussa, E. Comprehensive Evaluation of Toxoplasma gondii VEG and Neospora caninum LIV Genomes with Tachyzoite Stage Transcriptome and Proteome Defines Novel Transcript Features. PLoS ONE 2015, 10, e0124473. [Google Scholar] [CrossRef]
- Agrawal, S.; Van Dooren, G.G.; Beatty, W.L.; Striepen, B. Genetic Evidence That an Endosymbiont-Derived Endoplasmic Reticulum-Associated Protein Degradation (ERAD) System Functions in Import of Apicoplast Proteins. J. Biol. Chem. 2009, 284, 33683–33691. [Google Scholar] [CrossRef]
- Pino, P.; Foth, B.J.; Pino, P.; Foth, B.J.; Kwok, L.; Sheiner, L.; Schepers, R.; Soldati, T.; Soldati-favre, D. Dual Targeting of Antioxidant and Metabolic Enzymes to the Mitochondrion and the Apicoplast of Toxoplasma gondii. PLoS Pathog. 2007, 3, e115. [Google Scholar] [CrossRef] [PubMed]
- Oborník, M.; Van de Peer, Y.; Hypsa, V.; Frickey, T.; Slapeta, J.R.; Meyer, A.; Lukes, J. Phylogenetic Analyses Suggest Lateral Gene Transfer from the Mitochondrion to the Apicoplast. Gene 2002, 285, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Voelter-Mahlknecht, S.; Ho, A.D.; Mahlknecht, U. Chromosomal Organization and Localization of the Novel Class IV Human Histone Deacetylase 11 Gene. Int. J. Mol. Med. 2005, 16, 589–598. [Google Scholar] [PubMed]
- Villagra, A.; Cheng, F.; Wang, H.; Suarez, I.; Maurin, M.; Nguyen, D.; Wright, K.L.; Atadja, P.W.; Bhalla, K.; Pinilla-Ibarz, J.; et al. The Histone Deacetylase HDAC11 Regulates the Expression of Interleukin 10 and Immune Tolerance. Nat. Immunol. 2009, 10, 92–100. [Google Scholar] [CrossRef]
- Cheng, F.; Lienlaf, M.; Perez-Villarroel, P.; Wang, H.W.; Lee, C.; Woan, K.; Woods, D.; Knox, T.; Bergman, J.; Pinilla-Ibarz, J.; et al. Divergent Roles of Histone Deacetylase 6 (HDAC6) and Histone Deacetylase 11 (HDAC11) on the Transcriptional Regulation of IL10 in Antigen Presenting Cells. Mol. Immunol. 2014, 60, 44–53. [Google Scholar] [CrossRef]
- McClure, J.J.; Inks, E.S.; Zhang, C.; Peterson, Y.K.; Li, J.; Chundru, K.; Lee, B.; Buchanan, A.; Miao, S.; Chou, C.J. Comparison of the Deacylase and Deacetylase Activity of Zinc- Dependent HDACs. ACS Chem. Biol. 2017, 12, 39–46. [Google Scholar] [CrossRef]
- Gao, L.; Cueto, M.A.; Asselbergs, F.; Atadja, P. Cloning and Functional Characterization of HDAC11, a Novel Member of the Human Histone Deacetylase Family. J. Biol. Chem. 2002, 277, 25748–25755. [Google Scholar] [CrossRef]
- Grozinger, C.M.; Hassig, C.A.; Schreiber, S.L. Three Proteins Define a Class of Human Histone Deacetylases Related to Yeast Hda1p. Proc. Natl. Acad. Sci. USA 1999, 96, 4868–4873. [Google Scholar] [CrossRef]
- Chung, P.J.; Kim, Y.S.; Park, S.H.; Nahm, B.H.; Kim, J.K. Subcellular Localization of Rice Histone Deacetylases in Organelles. FEBS Lett. 2009, 583, 2249–2254. [Google Scholar] [CrossRef]
- Ledent, V.; Vervoort, M. Comparative Genomics of the Class 4 Histone Deacetylase Family Indicates a Complex Evolutionary History. BMC Biol. 2006, 4, 24. [Google Scholar] [CrossRef]
- Schulz, F.; Roux, S.; Paez-espino, D.; Jungbluth, S.; Walsh, D.A.; Denef, V.J.; Mcmahon, K.D.; Konstantinidis, K.T.; Eloe-fadrosh, E.A.; Kyrpides, N.C.; et al. Giant Virus Diversity and Host Interactions through Global Metagenomics. Nature 2020, 578, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Glaser, S.; Van Dooren, G.G.; Agrawal, S.; Brooks, C.F.; McFadden, G.I.; Striepen, B.; Higgins, M.K. Tic22 Is an Essential Chaperone Required for Protein Import into the Apicoplast. J. Biol. Chem. 2012, 287, 39505–39512. [Google Scholar] [CrossRef] [PubMed]
- Sheiner, L.; Fellows, J.D.; Ovciarikova, J.; Brooks, C.F.; Agrawal, S.; Holmes, Z.C.; Bietz, I.; Flinner, N.; Heiny, S.; Mirus, O.; et al. Toxoplasma gondii Toc75 Functions in Import of Stromal but Not Peripheral Apicoplast Proteins. Traffic 2015, 16, 1254–1269. [Google Scholar] [CrossRef] [PubMed]
- Tonkin, C.J.; Roos, D.S.; McFadden, G.I. N-Terminal Positively Charged Amino Acids, but Not Their Exact Position, Are Important for Apicoplast Transit Peptide Fidelity in Toxoplasma gondii. Mol. Biochem. Parasitol. 2006, 150, 192–200. [Google Scholar] [CrossRef]
- Hartl, M.; Füßl, M.; Boersema, P.J.; Jost, J.; Kramer, K.; Bakirbas, A.; Sindlinger, J.; Plöchinger, M.; Leister, D.; Uhrig, G.; et al. Lysine Acetylome Profiling Uncovers Novel Histone Deacetylase Substrate Proteins in Arabidopsis. Mol. Syst. Biol. 2017, 13, 949. [Google Scholar] [CrossRef]
- Sirover, M.A. Structural Analysis of Glyceraldehyde-3-Phosphate Dehydrogenase Functional Diversity. Int. J. Biochem. Cell Biol. 2014, 57, 20–26. [Google Scholar] [CrossRef]
- Tisdale, E.J. Glyceraldehyde-3-Phosphate Dehydrogenase Is Required for Vesicular Transport in the Early Secretory Pathway. J. Biol. Chem. 2001, 276, 2480–2486. [Google Scholar] [CrossRef]
- Chakravarti, R.; Aulak, K.S.; Fox, P.L.; Stuehr, D.J. GAPDH Regulates Cellular Heme Insertion into Inducible Nitric Oxide Synthase. Proc. Natl. Acad. Sci. USA 2010, 107, 18004–18009. [Google Scholar] [CrossRef]
- Li, T.; Liu, M.; Feng, X.; Wang, Z.; Das, I.; Xu, Y.; Zhou, X.; Sun, Y.; Guan, K.; Xiong, Y.; et al. Glyceraldehyde-3-Phosphate Dehydrogenase Is Activated by Lysine 254 Acetylation in Response to Glucose Signal. J. Biol. Chem. 2014, 289, 3775–3785. [Google Scholar] [CrossRef]
- Sato, S. The Apicomplexan Plastid and Its Evolution. Cell. Mol. Life Sci. 2011, 68, 1285–1296. [Google Scholar] [CrossRef]
- Miao, J.; Lawrence, M.; Jeffers, V.; Zhao, F.; Parker, D.; Ge, Y.; Sullivan, W.J., Jr.; Cui, L. Extensive Lysine Acetylation Occurs in Evolutionarily Conserved Metabolic Pathways and Parasite-Specific Functions during Plasmodium falciparum Intraerythrocytic Development. Mol. Microbiol. 2013, 89, 660–675. [Google Scholar] [CrossRef] [PubMed]
- Ouidir, T.; Kentache, T.; Hardouin, J. Protein Lysine Acetylation in Bacteria: Current State of the Art. Proteomics 2016, 16, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Torchy, M.P.; Hamiche, A.; Klaholz, B.P. Structure and Function Insights into the NuRD Chromatin Remodeling Complex. Cell. Mol. Life Sci. 2015, 72, 2491–2507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Aubert, A.; De Segura, J.M.G.; Karuppasamy, M.; Basu, S. The Nucleosome Remodeling and Deacetylase Complex NuRD Is Built from Preformed Catalytically Active Sub-Modules. J. Mol. Biol. 2016, 428, 2931–2942. [Google Scholar] [CrossRef]
- Matsuzaki, M.; Kikuchi, T.; Kita, K.; Kojima, S.; Kuroiwa, T. Large Amounts of Apicoplast Nucleoid DNA and Its Segregation in Toxoplasma gondii. Protoplasma 2001, 218, 180–191. [Google Scholar] [CrossRef]
- Ghosh, S.; Padmanabhan, B.; Anand, C.; Nagaraja, V. Lysine Acetylation of the Mycobacterium tuberculosis HU Protein Modulates Its DNA Binding and Genome Organization. Mol. Microbiol. 2016, 100, 577–588. [Google Scholar] [CrossRef]
- Chaal, B.K.; Gupta, A.P.; Wastuwidyaningtyas, B.D.; Luah, Y.H.; Bozdech, Z. Histone Deacetylases Play a Major Role in the Transcriptional Regulation of the Plasmodium falciparum Life Cycle. PLoS Pathog. 2010, 6, e1000737. [Google Scholar] [CrossRef]
- Darkin-Rattray, S.J.; Gurnett, A.M.; Myers, R.W.; Dulski, P.M.; Crumley, T.M.; Allocco, J.J.; Cannova, C.; Meinke, P.T.; Colletti, S.L.; Bednarek, M.A.; et al. Apicidin: A Novel Antiprotozoal Agent That Inhibits Parasite Histone Deacetylase. Med. Sci. 1996, 93, 13143–13147. [Google Scholar] [CrossRef]
- Mukherjee, P.; Pradhan, A.; Shah, F.; Tekwani, B.L.; Avery, M.A. Structural Insights into the Plasmodium falciparum Histone Deacetylase 1 (PfHDAC-1): A Novel Target for the Development of Antimalarial Therapy. Bioorganic Med. Chem. 2008, 16, 5254–5265. [Google Scholar] [CrossRef]
- Maubon, D.; Bougdour, A.; Wong, Y.; Brenier-Pinchart, M.-P.; Curt, A.; Hakimi, M.; Pelloux, H. Activity of the Histone Deacetylase Inhibitor FR235222 on Toxoplasma gondii: Inhibition of Stage Conversion of the Parasite Cyst Form and Study of New Derivative Compounds. Antimicrob. Agents Chemother. 2010, 54, 4843–4850. [Google Scholar] [CrossRef]
- Dahl, E.L.; Rosenthal, P.J. Apicoplast Translation, Transcription and Genome Replication: Targets for Antimalarial Antibiotics. Trends Parasitol. 2008, 24, 279–284. [Google Scholar] [CrossRef] [PubMed]
- McConkeyc, G.; Rogers, M.J.; Mccutchan, T.F. Inhibition of Plasmodium falciparum Protein Synthesis. J. Biol. Chem. 1997, 272, 2046–2049. [Google Scholar] [CrossRef] [PubMed]
- McFadden, G.I.; Roos, D.S. Apicomplexan Plastids as Drug Targets. Trends Microbiol. 1999, 7, 328–333. [Google Scholar] [CrossRef] [PubMed]



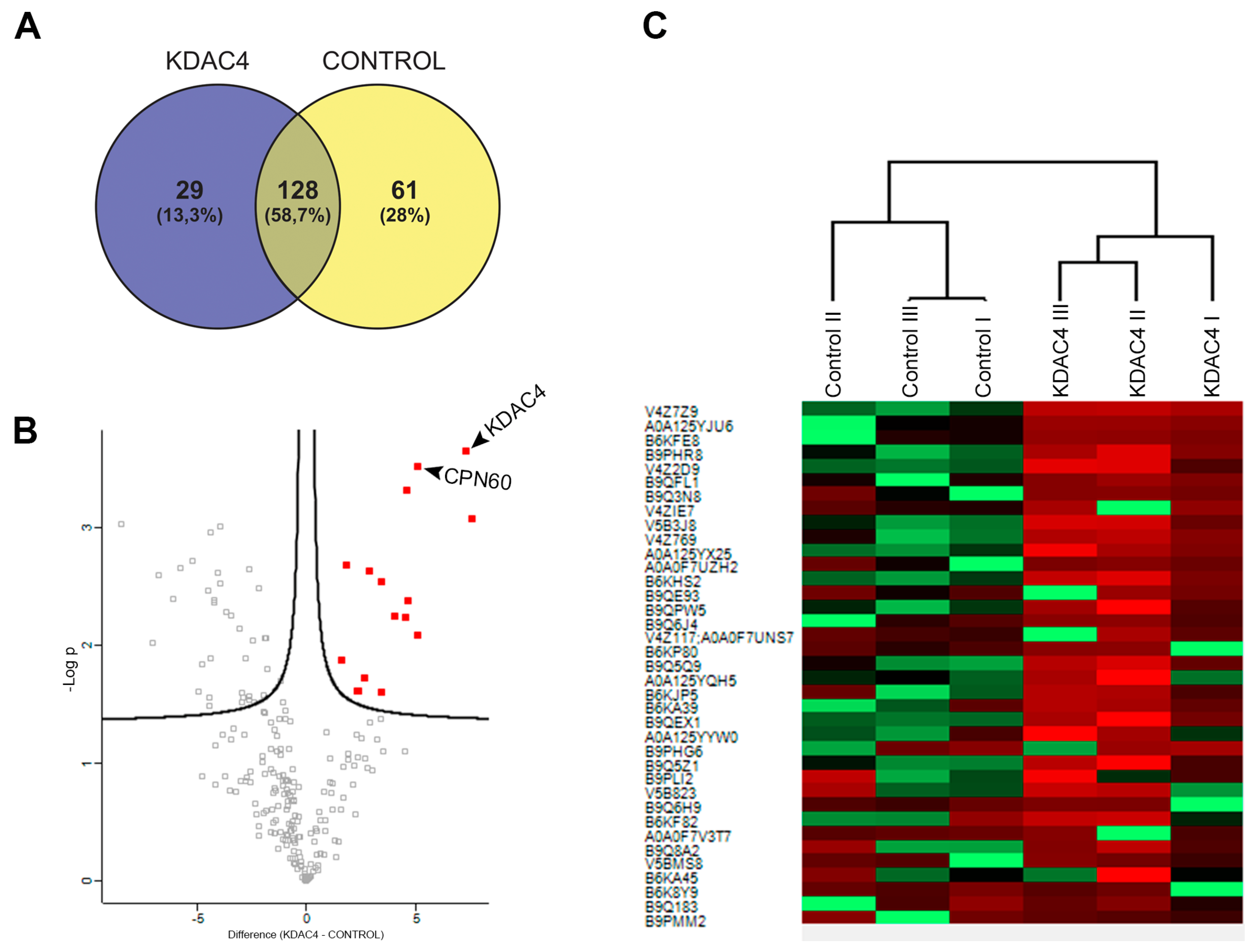
| ID | Protein Name | Difference (KDAC4-WT) | (−LOG (p-Value)) |
|---|---|---|---|
| V4Z7Z9 | Uncharacterized protein (KDAC4 domain Fragment) | 7.557988803 | 3.07964558 |
| A0A125YJU6 | Histone deacetylase KDAC4 | 7.262962341 | 3.654127752 |
| B6KFE8 | Putative chaperonin cpn60 | 5.038188934 | 3.522729209 |
| B9PHR8 | Protein phosphatase 2C domain-containing protein | 5.025075277 | 2.092972874 |
| V4Z2D9 | Uncharacterized protein (Fragment) | 4.627913793 | 2.386059714 |
| B9QFL1 | Putative transmembrane protein | 4.563500086 | 3.319557656 |
| V4ZIE7 | Uncharacterized protein | 4.480522792 | 2.244625048 |
| V5B3J8 | Putative transmembrane protein | 4.005473455 | 2.253793632 |
| V4Z769 | Kelch motif domain-containing protein | 3.407096227 | 1.610467411 |
| A0A125YX25 | Microneme protein putative | 3.376639048 | 2.540889206 |
| B6KHS2 | Dense granule protein GRA9 | 2.832564672 | 2.637648916 |
| B9QPW5 | Proline-rich protein | 2.602699916 | 1.727458536 |
| B6KP80 | Uncharacterized protein | 2.340368907 | 1.618390219 |
| B9Q5Q9 | Putative transmembrane protein | 2.286419551 | 1.619608828 |
| B9QEX1 | SAG-related sequence SRS44 | 1.815659205 | 2.686913967 |
| B9Q5Z1 | Cyst matrix protein | 1.570529302 | 1.878802921 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fragoso, M.S.I.; de Siqueira, C.M.; Vitorino, F.N.L.; Vieira, A.Z.; Martins-Duarte, É.S.; Faoro, H.; da Cunha, J.P.C.; Ávila, A.R.; Nardelli, S.C. TgKDAC4: A Unique Deacetylase of Toxoplasma’s Apicoplast. Microorganisms 2023, 11, 1558. https://doi.org/10.3390/microorganisms11061558
Fragoso MSI, de Siqueira CM, Vitorino FNL, Vieira AZ, Martins-Duarte ÉS, Faoro H, da Cunha JPC, Ávila AR, Nardelli SC. TgKDAC4: A Unique Deacetylase of Toxoplasma’s Apicoplast. Microorganisms. 2023; 11(6):1558. https://doi.org/10.3390/microorganisms11061558
Chicago/Turabian StyleFragoso, Mariana Sayuri Ishikawa, Caroline Moraes de Siqueira, Francisca Nathália Luna Vitorino, Alexandre Zanatta Vieira, Érica Santos Martins-Duarte, Helisson Faoro, Júlia Pinheiro Chagas da Cunha, Andréa Rodrigues Ávila, and Sheila Cristina Nardelli. 2023. "TgKDAC4: A Unique Deacetylase of Toxoplasma’s Apicoplast" Microorganisms 11, no. 6: 1558. https://doi.org/10.3390/microorganisms11061558
APA StyleFragoso, M. S. I., de Siqueira, C. M., Vitorino, F. N. L., Vieira, A. Z., Martins-Duarte, É. S., Faoro, H., da Cunha, J. P. C., Ávila, A. R., & Nardelli, S. C. (2023). TgKDAC4: A Unique Deacetylase of Toxoplasma’s Apicoplast. Microorganisms, 11(6), 1558. https://doi.org/10.3390/microorganisms11061558






