Healthy Diet and Lifestyle Improve the Gut Microbiota and Help Combat Fungal Infection
Abstract
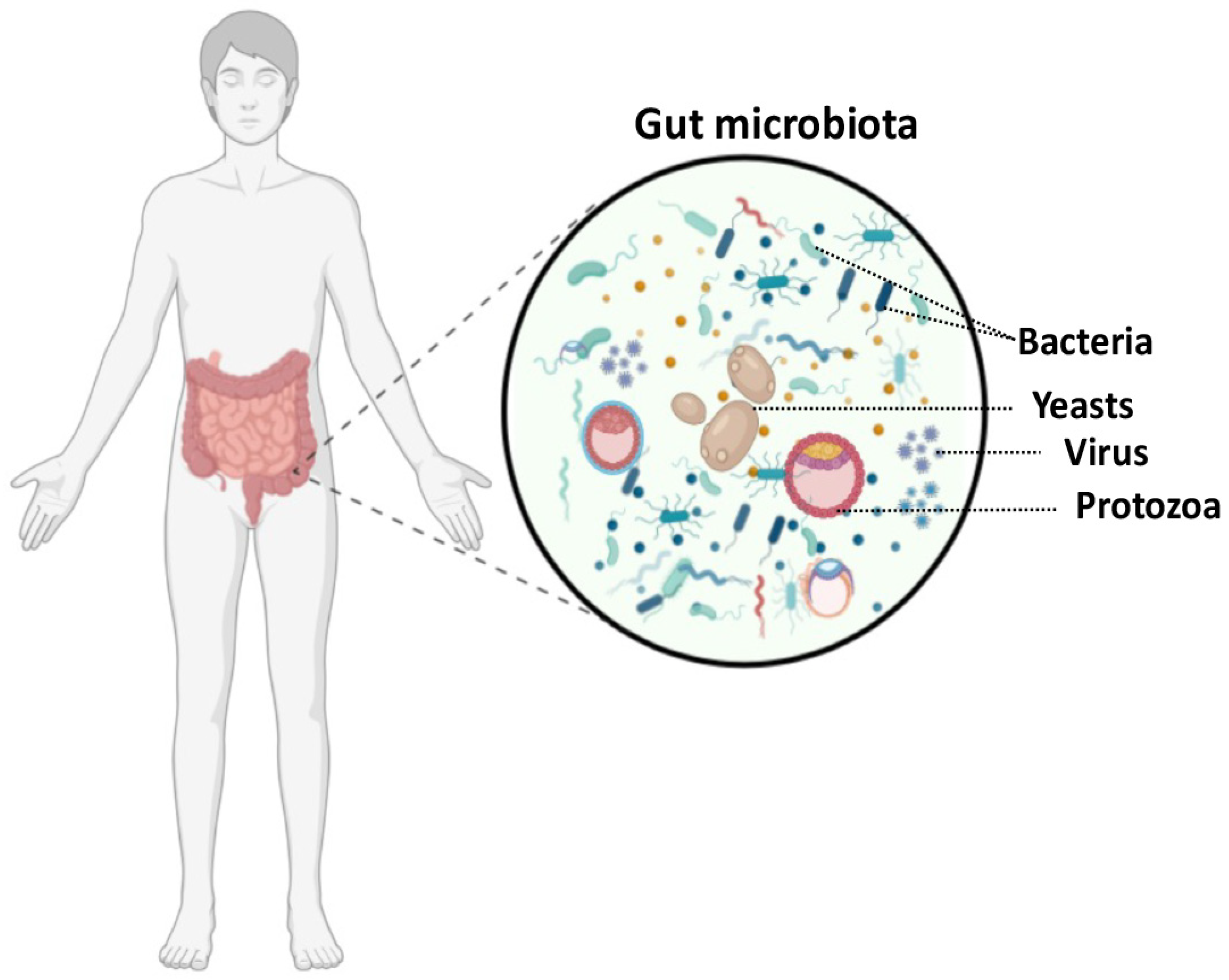
1. Introduction
2. Is Intestinal Candida albicans Colonization Linked to Inflammatory Diseases?
3. Unhealthy Diets That Are High in Fat and Sugar with a Special Focus on Processed Food
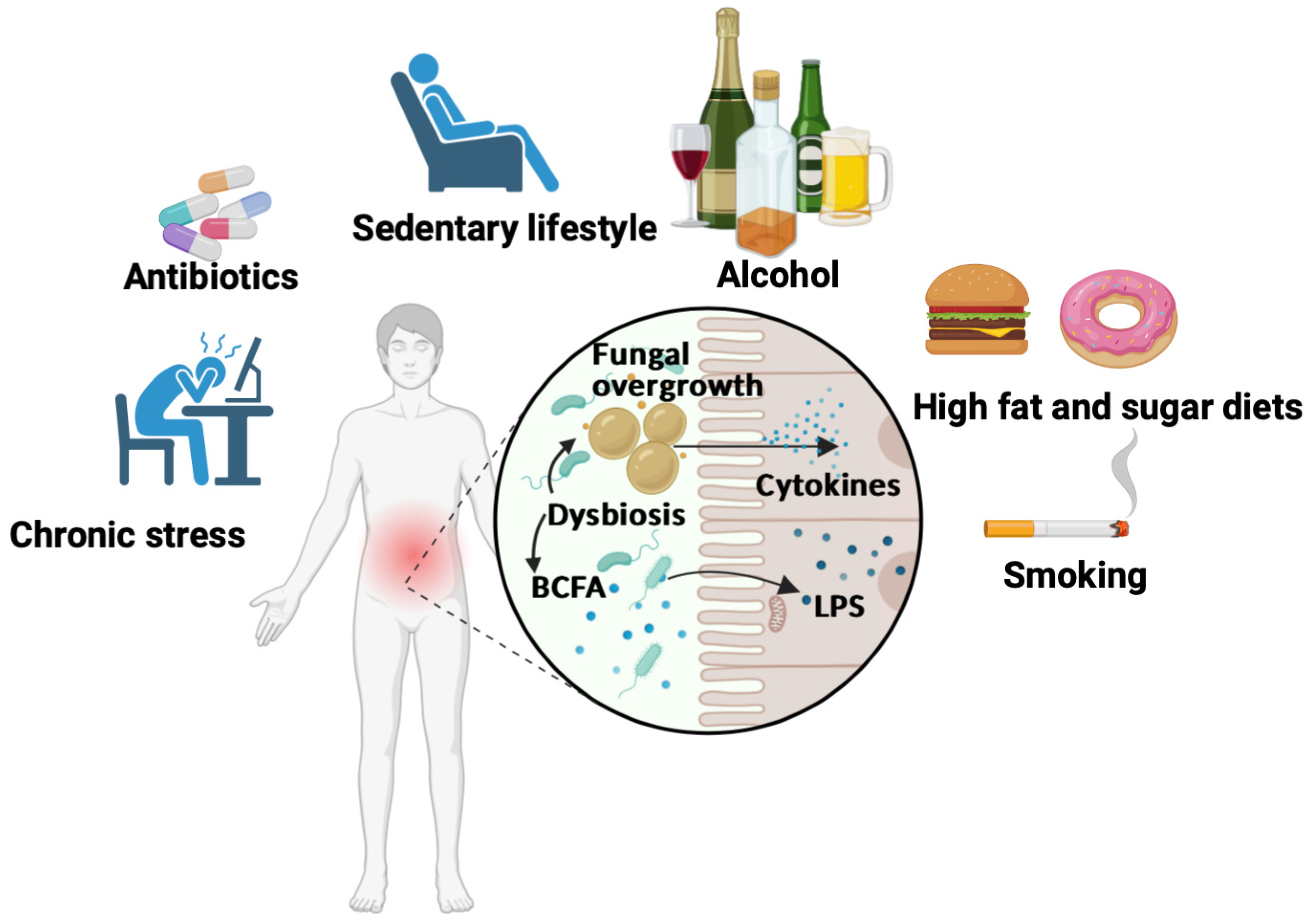
4. Factors Contributing to Inflammatory Pathogenesis and Gut Dysbiosis
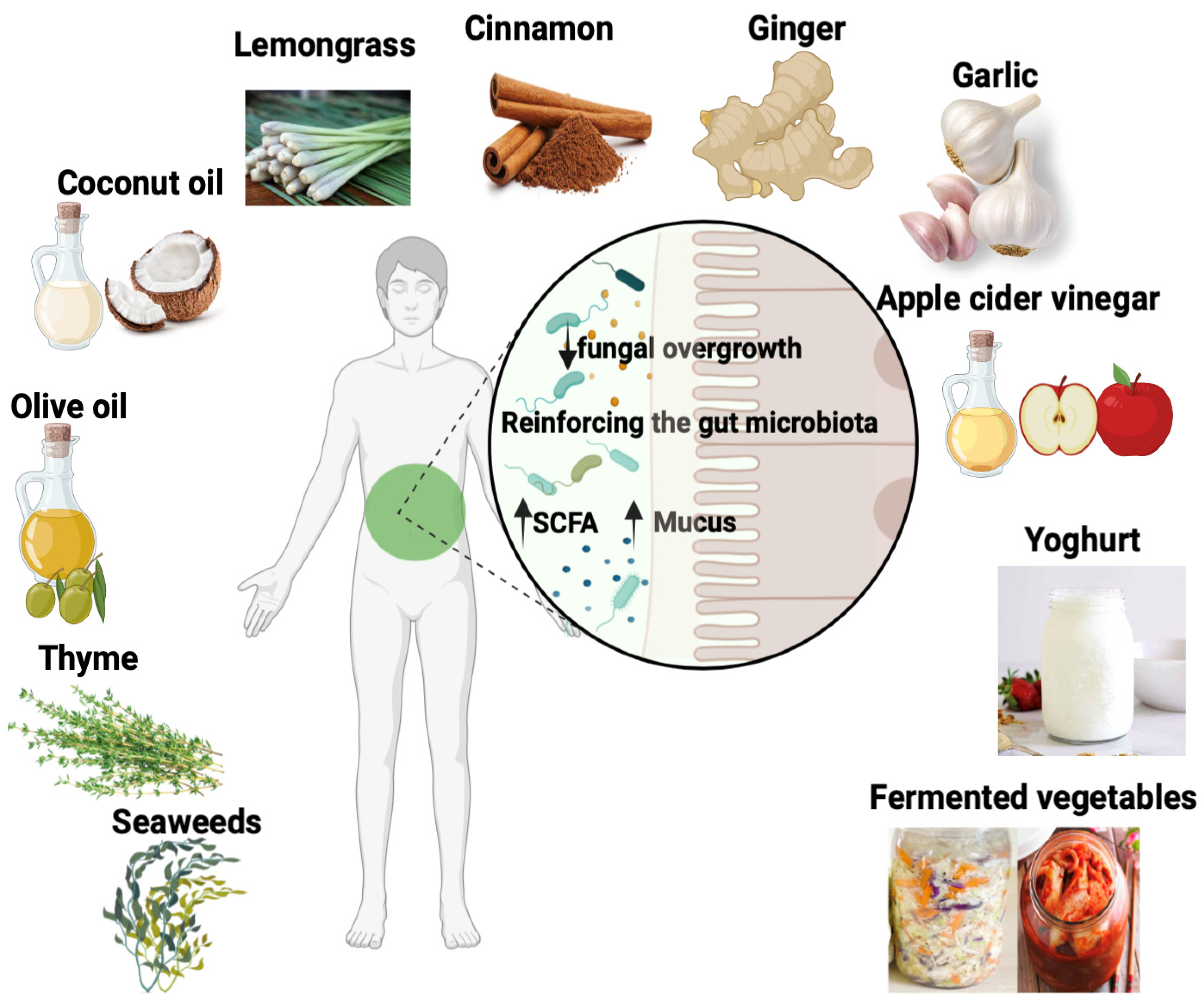
5. Strategies for Improving the Gut Microbiota and Reducing Fungal Load in the Gut
6. Plant and Food Compounds with Antifungal Properties
7. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Honda, K.; Littman, D.R. The microbiome in infectious disease and inflammation. Annu. Rev. Immunol. 2012, 30, 759–795. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, D.; Moran, C.; Shanahan, F. The microbiota in inflammatory bowel disease. J. Gastroenterol. 2015, 50, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Sartor, R.B. Microbial influences in inflammatory bowel diseases. Gastroenterology 2008, 134, 577–594. [Google Scholar] [CrossRef]
- Andoh, A. Physiological Role of Gut Microbiota for Maintaining Human Health. Digestion 2016, 93, 176–181. [Google Scholar] [CrossRef]
- Poulain, D.; Sendid, B.; Standaert-Vitse, A.; Fradin, C.; Jouault, T.; Jawhara, S.; Colombel, J.F. Yeasts: Neglected pathogens. Dig. Dis. 2009, 27 (Suppl. 1), 104–110. [Google Scholar] [CrossRef]
- Jawhara, S. How Gut Bacterial Dysbiosis Can Promote Candida albicans Overgrowth during Colonic Inflammation. Microorganisms 2022, 10, 1014. [Google Scholar] [CrossRef]
- Chaffin, W.L. Candida albicans cell wall proteins. Microbiol. Mol. Biol. Rev. 2008, 72, 495–544. [Google Scholar] [CrossRef]
- Jawhara, S. How Fungal Glycans Modulate Platelet Activation via Toll-Like Receptors Contributing to the Escape of Candida albicans from the Immune Response. Antibiotics 2020, 9, 385. [Google Scholar] [CrossRef]
- Poulain, D. Candida albicans, plasticity and pathogenesis. Crit. Rev. Microbiol. 2015, 41, 208–217. [Google Scholar] [CrossRef]
- Lenardon, M.D.; Munro, C.A.; Gow, N.A. Chitin synthesis and fungal pathogenesis. Curr. Opin. Microbiol. 2010, 13, 416–423. [Google Scholar] [CrossRef]
- Trinel, P.A.; Borg-von-Zepelin, M.; Lepage, G.; Jouault, T.; Mackenzie, D.; Poulain, D. Isolation and preliminary characterization of the 14- to 18-kilodalton Candida albicans antigen as a phospholipomannan containing beta-1,2-linked oligomannosides. Infect. Immun. 1993, 61, 4398–4405. [Google Scholar] [CrossRef]
- Hofs, S.; Mogavero, S.; Hube, B. Interaction of Candida albicans with host cells: Virulence factors, host defense, escape strategies, and the microbiota. J. Microbiol. 2016, 54, 149–169. [Google Scholar] [CrossRef]
- Konig, A.; Hube, B.; Kasper, L. The Dual Function of the Fungal Toxin Candidalysin during Candida albicans-Macrophage Interaction and Virulence. Toxins 2020, 12, 469. [Google Scholar] [CrossRef]
- Leonardi, I.; Gao, I.H.; Lin, W.Y.; Allen, M.; Li, X.V.; Fiers, W.D.; De Celie, M.B.; Putzel, G.G.; Yantiss, R.K.; Johncilla, M.; et al. Mucosal fungi promote gut barrier function and social behavior via Type 17 immunity. Cell 2022, 185, 831–846.E814. [Google Scholar] [CrossRef]
- Break, T.J.; Oikonomou, V.; Dutzan, N.; Desai, J.V.; Swidergall, M.; Freiwald, T.; Chauss, D.; Harrison, O.J.; Alejo, J.; Williams, D.W.; et al. Aberrant type 1 immunity drives susceptibility to mucosal fungal infections. Science 2021, 371, eaay5731. [Google Scholar] [CrossRef]
- Swidergall, M.; LeibundGut-Landmann, S. Immunosurveillance of Candida albicans commensalism by the adaptive immune system. Mucosal Immunol. 2022, 15, 829–836. [Google Scholar] [CrossRef]
- Standaert-Vitse, A.; Jouault, T.; Vandewalle, P.; Mille, C.; Seddik, M.; Sendid, B.; Mallet, J.M.; Colombel, J.F.; Poulain, D. Candida albicans is an immunogen for anti-Saccharomyces cerevisiae antibody markers of Crohn’s disease. Gastroenterology 2006, 130, 1764–1775. [Google Scholar] [CrossRef]
- Jawhara, S.; Thuru, X.; Standaert-Vitse, A.; Jouault, T.; Mordon, S.; Sendid, B.; Desreumaux, P.; Poulain, D. Colonization of mice by Candida albicans is promoted by chemically induced colitis and augments inflammatory responses through galectin-3. J. Infect. Dis. 2008, 197, 972–980. [Google Scholar] [CrossRef]
- Sendid, B.; Dotan, N.; Nseir, S.; Savaux, C.; Vandewalle, P.; Standaert, A.; Zerimech, F.; Guery, B.P.; Dukler, A.; Colombel, J.F.; et al. Antibodies against glucan, chitin, and Saccharomyces cerevisiae mannan as new biomarkers of Candida albicans infection that complement tests based on C. albicans mannan. Clin. Vaccine Immunol. 2008, 15, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Sendid, B.; Quinton, J.F.; Charrier, G.; Goulet, O.; Cortot, A.; Grandbastien, B.; Poulain, D.; Colombel, J.F. Anti-Saccharomyces cerevisiae mannan antibodies in familial Crohn’s disease. Am. J. Gastroenterol. 1998, 93, 1306–1310. [Google Scholar] [CrossRef] [PubMed]
- Sendid, B.; Jawhara, S.; Sarter, H.; Maboudou, P.; Thierny, C.; Gower-Rousseau, C.; Colombel, J.F.; Poulain, D. Uric acid levels are independent of anti-Saccharomyces cerevisiae antibodies (ASCA) in Crohn’s disease: A reappraisal of the role of S. cerevisiae in this setting. Virulence 2018, 9, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Jawhara, S.; Mogensen, E.; Maggiotto, F.; Fradin, C.; Sarazin, A.; Dubuquoy, L.; Maes, E.; Guerardel, Y.; Janbon, G.; Poulain, D. Murine model of dextran sulfate sodium-induced colitis reveals Candida glabrata virulence and contribution of beta-mannosyltransferases. J. Biol. Chem. 2012, 287, 11313–11324. [Google Scholar] [CrossRef]
- Jawhara, S.; Poulain, D. Saccharomyces boulardii decreases inflammation and intestinal colonization by Candida albicans in a mouse model of chemically-induced colitis. Med. Mycol. 2007, 45, 691–700. [Google Scholar] [CrossRef]
- Jawhara, S.; Habib, K.; Maggiotto, F.; Pignede, G.; Vandekerckove, P.; Maes, E.; Dubuquoy, L.; Fontaine, T.; Guerardel, Y.; Poulain, D. Modulation of intestinal inflammation by yeasts and cell wall extracts: Strain dependence and unexpected anti-inflammatory role of glucan fractions. PLoS ONE 2012, 7, e40648. [Google Scholar] [CrossRef]
- Jawhara, S. Editorial of Special Issue Human Pathogenic Fungi: Host-Pathogen Interactions and Virulence. Microorganisms 2023, 11, 963. [Google Scholar] [CrossRef]
- Charlet, R.; Pruvost, Y.; Tumba, G.; Istel, F.; Poulain, D.; Kuchler, K.; Sendid, B.; Jawhara, S. Remodeling of the Candida glabrata cell wall in the gastrointestinal tract affects the gut microbiota and the immune response. Sci. Rep. 2018, 8, 3316. [Google Scholar] [CrossRef]
- Charlet, R.; Bortolus, C.; Barbet, M.; Sendid, B.; Jawhara, S. A decrease in anaerobic bacteria promotes Candida glabrata overgrowth while beta-glucan treatment restores the gut microbiota and attenuates colitis. Gut Pathog. 2018, 10, 50. [Google Scholar] [CrossRef]
- Diotallevi, C.; Fava, F.; Gobbetti, M.; Tuohy, K. Healthy dietary patterns to reduce obesity-related metabolic disease: Polyphenol-microbiome interactions unifying health effects across geography. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 437–444. [Google Scholar] [CrossRef]
- Kopp, W. How Western Diet And Lifestyle Drive The Pandemic Of Obesity And Civilization Diseases. Diabetes Metab. Syndr. Obes. 2019, 12, 2221–2236. [Google Scholar] [CrossRef]
- Hildebrandt, M.A.; Hoffmann, C.; Sherrill-Mix, S.A.; Keilbaugh, S.A.; Hamady, M.; Chen, Y.Y.; Knight, R.; Ahima, R.S.; Bushman, F.; Wu, G.D. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 2009, 137, 1716–1724.e1–e2. [Google Scholar] [CrossRef]
- Graham, C.; Mullen, A.; Whelan, K. Obesity and the gastrointestinal microbiota: A review of associations and mechanisms. Nutr. Rev. 2015, 73, 376–385. [Google Scholar] [CrossRef]
- Graf, D.; Di Cagno, R.; Fak, F.; Flint, H.J.; Nyman, M.; Saarela, M.; Watzl, B. Contribution of diet to the composition of the human gut microbiota. Microb. Ecol. Health Dis. 2015, 26, 26164. [Google Scholar] [CrossRef]
- Cani, P.D.; Delzenne, N.M. The gut microbiome as therapeutic target. Pharmacol. Ther. 2011, 130, 202–212. [Google Scholar] [CrossRef]
- Ji, Y.; Sakata, Y.; Tso, P. Nutrient-induced inflammation in the intestine. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 315–321. [Google Scholar] [CrossRef]
- Laugerette, F.; Vors, C.; Geloen, A.; Chauvin, M.A.; Soulage, C.; Lambert-Porcheron, S.; Peretti, N.; Alligier, M.; Burcelin, R.; Laville, M.; et al. Emulsified lipids increase endotoxemia: Possible role in early postprandial low-grade inflammation. J. Nutr. Biochem. 2011, 22, 53–59. [Google Scholar] [CrossRef]
- Tsuzuki, Y.; Miyazaki, J.; Matsuzaki, K.; Okada, Y.; Hokari, R.; Kawaguchi, A.; Nagao, S.; Itoh, K.; Miura, S. Differential modulation in the functions of intestinal dendritic cells by long- and medium-chain fatty acids. J. Gastroenterol. 2006, 41, 209–216. [Google Scholar] [CrossRef]
- Fritsch, J.; Garces, L.; Quintero, M.A.; Pignac-Kobinger, J.; Santander, A.M.; Fernandez, I.; Ban, Y.J.; Kwon, D.; Phillips, M.C.; Knight, K.; et al. Low-Fat, High-Fiber Diet Reduces Markers of Inflammation and Dysbiosis and Improves Quality of Life in Patients With Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2021, 19, 1189–1199.e30. [Google Scholar] [CrossRef]
- Garcia-Gamboa, R.; Kirchmayr, M.R.; Gradilla-Hernandez, M.S.; Perez-Brocal, V.; Moya, A.; Gonzalez-Avila, M. The intestinal mycobiota and its relationship with overweight, obesity and nutritional aspects. J. Hum. Nutr. Diet. 2021, 34, 645–655. [Google Scholar] [CrossRef]
- Laffin, M.; Fedorak, R.; Zalasky, A.; Park, H.; Gill, A.; Agrawal, A.; Keshteli, A.; Hotte, N.; Madsen, K.L. A high-sugar diet rapidly enhances susceptibility to colitis via depletion of luminal short-chain fatty acids in mice. Sci. Rep. 2019, 9, 12294. [Google Scholar] [CrossRef] [PubMed]
- Do, M.H.; Lee, E.; Oh, M.J.; Kim, Y.; Park, H.Y. High-Glucose or -Fructose Diet Cause Changes of the Gut Microbiota and Metabolic Disorders in Mice without Body Weight Change. Nutrients 2018, 10, 761. [Google Scholar] [CrossRef] [PubMed]
- Fajstova, A.; Galanova, N.; Coufal, S.; Malkova, J.; Kostovcik, M.; Cermakova, M.; Pelantova, H.; Kuzma, M.; Sediva, B.; Hudcovic, T.; et al. Diet Rich in Simple Sugars Promotes Pro-Inflammatory Response via Gut Microbiota Alteration and TLR4 Signaling. Cells 2020, 9, 2701. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J. The impact of nutrition on the human microbiome. Nutr. Rev. 2012, 70 (Suppl. S1), S10–S13. [Google Scholar] [CrossRef]
- Clarke, J.M.; Young, G.P.; Topping, D.L.; Bird, A.R.; Cobiac, L.; Scherer, B.L.; Winkler, J.G.; Lockett, T.J. Butyrate delivered by butyrylated starch increases distal colonic epithelial apoptosis in carcinogen-treated rats. Carcinogenesis 2012, 33, 197–202. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.M.; Bast, A.; Vanhoutvin, S.A.; Fischer, M.A.; Kodde, A.; Troost, F.J.; Venema, K.; Brummer, R.J. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin. Nutr. 2009, 28, 88–93. [Google Scholar] [CrossRef]
- Knudsen, K.E.B. Microbial degradation of whole-grain complex carbohydrates and impact on short-chain fatty acids and health. Adv. Nutr. 2015, 6, 206–213. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; Gonzalez, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Lopes, L.C.; Cordero, R.J.; Nosanchuk, J.D. Sodium butyrate inhibits pathogenic yeast growth and enhances the functions of macrophages. J. Antimicrob. Chemother. 2011, 66, 2573–2580. [Google Scholar] [CrossRef]
- Garcia, C.; Tebbji, F.; Daigneault, M.; Liu, N.N.; Kohler, J.R.; Allen-Vercoe, E.; Sellam, A. The Human Gut Microbial Metabolome Modulates Fungal Growth via the TOR Signaling Pathway. mSphere 2017, 2, e00555-17. [Google Scholar] [CrossRef]
- Bailey, M.T.; Dowd, S.E.; Galley, J.D.; Hufnagle, A.R.; Allen, R.G.; Lyte, M. Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain Behav. Immun. 2011, 25, 397–407. [Google Scholar] [CrossRef]
- Rodriguez-Galan, M.C.; Correa, S.G.; Cejas, H.; Sotomayor, C.E. Impaired activity of phagocytic cells in Candida albicans infection after exposure to chronic varied stress. Neuroimmunomodulation 2001, 9, 193–202. [Google Scholar] [CrossRef]
- Yang, A.M.; Inamine, T.; Hochrath, K.; Chen, P.; Wang, L.; Llorente, C.; Bluemel, S.; Hartmann, P.; Xu, J.; Koyama, Y.; et al. Intestinal fungi contribute to development of alcoholic liver disease. J. Clin. Invest. 2017, 127, 2829–2841. [Google Scholar] [CrossRef]
- Lang, S.; Duan, Y.; Liu, J.; Torralba, M.G.; Kuelbs, C.; Ventura-Cots, M.; Abraldes, J.G.; Bosques-Padilla, F.; Verna, E.C.; Brown, R.S., Jr.; et al. Intestinal Fungal Dysbiosis and Systemic Immune Response to Fungi in Patients With Alcoholic Hepatitis. Hepatology 2020, 71, 522–538. [Google Scholar] [CrossRef]
- Palleja, A.; Mikkelsen, K.H.; Forslund, S.K.; Kashani, A.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Liang, S.; Feng, Q.; Zhang, C.; et al. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat. Microbiol. 2018, 3, 1255–1265. [Google Scholar] [CrossRef]
- Spinillo, A.; Capuzzo, E.; Acciano, S.; De Santolo, A.; Zara, F. Effect of antibiotic use on the prevalence of symptomatic vulvovaginal candidiasis. Am. J. Obstet. Gynecol. 1999, 180, 14–17. [Google Scholar] [CrossRef]
- Mason, K.L.; Erb Downward, J.R.; Mason, K.D.; Falkowski, N.R.; Eaton, K.A.; Kao, J.Y.; Young, V.B.; Huffnagle, G.B. Candida albicans and bacterial microbiota interactions in the cecum during recolonization following broad-spectrum antibiotic therapy. Infect. Immun. 2012, 80, 3371–3380. [Google Scholar] [CrossRef]
- Rashid, M.U.; Rosenborg, S.; Panagiotidis, G.; Soderberg-Lofdal, K.; Weintraub, A.; Nord, C.E. Ecological Effect of Ceftaroline-Avibactam on the Normal Human Intestinal Microbiota. Antimicrob. Agents Chemother. 2015, 59, 4504–4509. [Google Scholar] [CrossRef]
- Mokeem, S.A.; Abduljabbar, T.; Al-Kheraif, A.A.; Alasqah, M.N.; Michelogiannakis, D.; Samaranayake, L.P.; Javed, F. Oral Candida carriage among cigarette- and waterpipe-smokers, and electronic cigarette users. Oral. Dis. 2019, 25, 319–326. [Google Scholar] [CrossRef]
- Mun, M.; Yap, T.; Alnuaimi, A.D.; Adams, G.G.; McCullough, M.J. Oral candidal carriage in asymptomatic patients. Aust. Dent. J. 2016, 61, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Macgregor, I.D. Effects of smoking on oral ecology. A review of the literature. Clin. Prev. Dent. 1989, 11, 3–7. [Google Scholar] [PubMed]
- Hise, A.G.; Tomalka, J.; Ganesan, S.; Patel, K.; Hall, B.A.; Brown, G.D.; Fitzgerald, K.A. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe 2009, 5, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Henson, J.; Yates, T.; Edwardson, C.L.; Khunti, K.; Talbot, D.; Gray, L.J.; Leigh, T.M.; Carter, P.; Davies, M.J. Sedentary time and markers of chronic low-grade inflammation in a high risk population. PLoS ONE 2013, 8, e78350. [Google Scholar] [CrossRef] [PubMed]
- Ortega, E.; Collazos, M.E.; Maynar, M.; Barriga, C.; De la Fuente, M. Stimulation of the phagocytic function of neutrophils in sedentary men after acute moderate exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1993, 66, 60–64. [Google Scholar] [CrossRef]
- De la Fuente, M.; Martin, I.; Ortega, E. Effect of physical exercise on the phagocytic function of peritoneal macrophages from Swiss mice. Comp. Immunol. Microbiol. Infect. Dis. 1993, 16, 29–37. [Google Scholar] [CrossRef]
- Nguyen, N.L.; Pilewski, J.M.; Celedon, J.C.; Mandalapu, S.; Blanchard, M.L.; DeRicco, A.; Hartigan, E.; Alcorn, J.F.; Kolls, J.K. Vitamin D supplementation decreases Aspergillus fumigatus specific Th2 responses in CF patients with aspergillus sensitization: A phase one open-label study. Asthma Res. Pract. 2015, 1, 3. [Google Scholar] [CrossRef]
- Khoo, A.L.; Chai, L.Y.; Koenen, H.J.; Kullberg, B.J.; Joosten, I.; van der Ven, A.J.; Netea, M.G. 1,25-dihydroxyvitamin D3 modulates cytokine production induced by Candida albicans: Impact of seasonal variation of immune responses. J. Infect. Dis. 2011, 203, 122–130. [Google Scholar] [CrossRef]
- Bouzid, D.; Merzouki, S.; Bachiri, M.; Ailane, S.E.; Zerroug, M.M. Vitamin D(3) a new drug against Candida albicans. J. Mycol. Med. 2017, 27, 79–82. [Google Scholar] [CrossRef]
- Lei, J.; Xiao, W.; Zhang, J.; Liu, F.; Xin, C.; Zhou, B.; Chen, W.; Song, Z. Antifungal activity of vitamin D(3) against Candida albicans in vitro and in vivo. Microbiol. Res. 2022, 265, 127200. [Google Scholar] [CrossRef]
- Zaaboul, F.; Liu, Y. Vitamin E in foodstuff: Nutritional, analytical, and food technology aspects. Compr. Rev. Food Sci. Food Saf. 2022, 21, 964–998. [Google Scholar] [CrossRef]
- Barros, S.; Ribeiro, A.P.D.; Offenbacher, S.; Loewy, Z.G. Anti-Inflammatory Effects of Vitamin E in Response to Candida albicans. Microorganisms 2020, 8, 804. [Google Scholar] [CrossRef]
- Belhachemi, M.H.; Boucherit, K.; Boucherit-Otmani, Z.; Belmir, S.; Benbekhti, Z. Effects of ascorbic acid and α-tocopherol on the therapeutic index of amphotericin B. J. Mycol. Med. 2014, 24, e137–e142. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Huang, C.B.; Ebersole, J.L. A novel bioactivity of omega-3 polyunsaturated fatty acids and their ester derivatives. Mol. Oral. Microbiol. 2010, 25, 75–80. [Google Scholar] [CrossRef]
- Tran, P.A.; Webster, T.J. Antimicrobial selenium nanoparticle coatings on polymeric medical devices. Nanotechnology 2013, 24, 155101. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Papp, L.V.; Lu, J.; Holmgren, A.; Khanna, K.K. From selenium to selenoproteins: Synthesis, identity, and their role in human health. Antioxid. Redox Signal. 2007, 9, 775–806. [Google Scholar] [CrossRef]
- Guisbiers, G.; Lara, H.H.; Mendoza-Cruz, R.; Naranjo, G.; Vincent, B.A.; Peralta, X.G.; Nash, K.L. Inhibition of Candida albicans biofilm by pure selenium nanoparticles synthesized by pulsed laser ablation in liquids. Nanomedicine 2017, 13, 1095–1103. [Google Scholar] [CrossRef]
- Boyne, R.; Arthur, J.R. The response of selenium-deficient mice to Candida albicans infection. J. Nutr. 1986, 116, 816–822. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Ganji-Arjenaki, M.; Rafieian-Kopaei, M. Probiotics are a good choice in remission of inflammatory bowel diseases: A meta analysis and systematic review. J. Cell. Physiol. 2018, 233, 2091–2103. [Google Scholar] [CrossRef]
- Huang, R.; Wang, K.; Hu, J. Effect of Probiotics on Depression: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2016, 8, 483. [Google Scholar] [CrossRef] [PubMed]
- Judkins, T.C.; Archer, D.L.; Kramer, D.C.; Solch, R.J. Probiotics, Nutrition, and the Small Intestine. Curr. Gastroenterol. Rep. 2020, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, V.H.; Wang, Y.; Bandara, H.; Mayer, M.P.A.; Samaranayake, L.P. Probiotic lactobacilli inhibit early stages of Candida albicans biofilm development by reducing their growth, cell adhesion, and filamentation. Appl. Microbiol. Biotechnol. 2016, 100, 6415–6426. [Google Scholar] [CrossRef]
- Kohler, G.A.; Assefa, S.; Reid, G. Probiotic interference of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 with the opportunistic fungal pathogen Candida albicans. Infect. Dis. Obstet. Gynecol. 2012, 2012, 636474. [Google Scholar] [CrossRef]
- Rios-Covian, D.; Gueimonde, M.; Duncan, S.H.; Flint, H.J.; de los Reyes-Gavilan, C.G. Enhanced butyrate formation by cross-feeding between Faecalibacterium prausnitzii and Bifidobacterium adolescentis. FEMS Microbiol. Lett. 2015, 362, fnv176. [Google Scholar] [CrossRef]
- Sanchez, B.; Noriega, L.; Ruas-Madiedo, P.; de los Reyes-Gavilan, C.G.; Margolles, A. Acquired resistance to bile increases fructose-6-phosphate phosphoketolase activity in Bifidobacterium. FEMS Microbiol. Lett. 2004, 235, 35–41. [Google Scholar] [CrossRef]
- Bagarolli, R.A.; Tobar, N.; Oliveira, A.G.; Araujo, T.G.; Carvalho, B.M.; Rocha, G.Z.; Vecina, J.F.; Calisto, K.; Guadagnini, D.; Prada, P.O.; et al. Probiotics modulate gut microbiota and improve insulin sensitivity in DIO mice. J. Nutr. Biochem. 2017, 50, 16–25. [Google Scholar] [CrossRef]
- Bubnov, R.V.; Babenko, L.P.; Lazarenko, L.M.; Mokrozub, V.V.; Demchenko, O.A.; Nechypurenko, O.V.; Spivak, M.Y. Comparative study of probiotic effects of Lactobacillus and Bifidobacteria strains on cholesterol levels, liver morphology and the gut microbiota in obese mice. EPMA J. 2017, 8, 357–376. [Google Scholar] [CrossRef]
- Alagumoorthi, G.; Jebakani, D.B.; Thirunavukarasu, S.; Ramachandaran, V.; Kumaresan, A. Effectiveness of Wii sports- based strategy training in reducing risk of falling, falls and improving quality of life in adults with idiopathic Parkinson’s disease—A randomized comparative trial. Clin. Rehabil. 2022, 36, 1097–1109. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Guan, X.X.; Tang, Y.J.; Sun, J.F.; Wang, X.K.; Wang, W.D.; Fan, J.M. Clinical effects and gut microbiota changes of using probiotics, prebiotics or synbiotics in inflammatory bowel disease: A systematic review and meta-analysis. Eur. J. Nutr. 2021, 60, 2855–2875. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Zhang, Y.; Qiu, Y.; Wu, Z.; Zhong, Z.; Zeng, X.; Zeng, Y.; Xiong, L.; Wen, Y.; Liu, R. Fructooligosaccharide supplementation alleviated the pathological immune response and prevented the impairment of intestinal barrier in DSS-induced acute colitis mice. Food Funct. 2021, 12, 9844–9854. [Google Scholar] [CrossRef]
- Rousseau, V.; Lepargneur, J.P.; Roques, C.; Remaud-Simeon, M.; Paul, F. Prebiotic effects of oligosaccharides on selected vaginal lactobacilli and pathogenic microorganisms. Anaerobe 2005, 11, 145–153. [Google Scholar] [CrossRef]
- Valeur, J.; Puaschitz, N.G.; Midtvedt, T.; Berstad, A. Oatmeal porridge: Impact on microflora-associated characteristics in healthy subjects. Br. J. Nutr. 2016, 115, 62–67. [Google Scholar] [CrossRef]
- Stull, V.J.; Finer, E.; Bergmans, R.S.; Febvre, H.P.; Longhurst, C.; Manter, D.K.; Patz, J.A.; Weir, T.L. Impact of Edible Cricket Consumption on Gut Microbiota in Healthy Adults, a Double-blind, Randomized Crossover Trial. Sci. Rep. 2018, 8, 10762. [Google Scholar] [CrossRef]
- Alessandri, G.; Milani, C.; Duranti, S.; Mancabelli, L.; Ranjanoro, T.; Modica, S.; Carnevali, L.; Statello, R.; Bottacini, F.; Turroni, F.; et al. Ability of bifidobacteria to metabolize chitin-glucan and its impact on the gut microbiota. Sci. Rep. 2019, 9, 5755. [Google Scholar] [CrossRef]
- Ankri, S.; Mirelman, D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999, 1, 125–129. [Google Scholar] [CrossRef]
- Lemar, K.M.; Turner, M.P.; Lloyd, D. Garlic (Allium sativum) as an anti-Candida agent: A comparison of the efficacy of fresh garlic and freeze-dried extracts. J. Appl. Microbiol. 2002, 93, 398–405. [Google Scholar] [CrossRef]
- Ghannoum, M.A. Inhibition of Candida adhesion to buccal epithelial cells by an aqueous extract of Allium sativum (garlic). J. Appl. Bacteriol. 1990, 68, 163–169. [Google Scholar] [CrossRef]
- Low, C.F.; Chong, P.P.; Yong, P.V.; Lim, C.S.; Ahmad, Z.; Othman, F. Inhibition of hyphae formation and SIR2 expression in Candida albicans treated with fresh Allium sativum (garlic) extract. J. Appl. Microbiol. 2008, 105, 2169–2177. [Google Scholar] [CrossRef]
- San-Blas, G.; Marino, L.; San-Blas, F.; Apitz-Castro, R. Effect of ajoene on dimorphism of Paracoccidioides brasiliensis. J. Med. Vet. Mycol. 1993, 31, 133–141. [Google Scholar] [CrossRef]
- Sharma, S.; Raj, K.; Riyaz, M.; Singh, D.D. Antimicrobial Studies on Garlic Lectin. Probiotics Antimicrob. Proteins 2022. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, D.D. Investigations on the Biological Activity of Allium sativum Agglutinin (ASA) Isolated from Garlic. Protein Pept. Lett. 2022, 29, 555–566. [Google Scholar] [CrossRef]
- Atai, Z.; Ansari, M.; Mousavi, A.; Mirzaei, A. In-vitro study of antifungal effects of selected herbal extracts on standard and wild strains of Candida albicans. J. Iran. Dent. Assoc. 2007, 19, 91–97. [Google Scholar]
- Aneja, R.K.; Joshi, R.; Sharma, C. Antimicrobial activity of Dalchini (Cinnamomum zeylanicum bark) extracts on some dental caries pathogens. J. Pharm. Res. 2009, 2, 1387–1390. [Google Scholar]
- Velluti, A.; Sanchis, V.; Ramos, A.J.; Egido, J.; Marin, S. Inhibitory effect of cinnamon, clove, lemongrass, oregano and palmarose essential oils on growth and fumonisin B1 production by Fusarium proliferatum in maize grain. Int. J. Food Microbiol. 2003, 89, 145–154. [Google Scholar] [CrossRef]
- Carvalho, P.; Sá, N.; Lacerda, I.; Pataro, C.; Rosa, L.; Alves, R.; Lyon, J.; Rosa, C.; Johann, S. Anti-candida activity of cinnamon inhibition of virulence factors of clinical strains of Candida albicans by essential oil of Cinnamomum zeylanicum. PSM Microbiol. 2018, 3, 4–12. [Google Scholar]
- Gruenwald, J.; Freder, J.; Armbruester, N. Cinnamon and health. Crit. Rev. Food Sci. Nutr. 2010, 50, 822–834. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N.H.; Graham, L.; Adukwu, E.C. In vitro antifungal activity of Cinnamomum zeylanicum bark and leaf essential oils against Candida albicans and Candida auris. Appl. Microbiol. Biotechnol. 2020, 104, 8911–8924. [Google Scholar] [CrossRef] [PubMed]
- Shah, G.; Shri, R.; Panchal, V.; Sharma, N.; Singh, B.; Mann, A.S. Scientific basis for the therapeutic use of Cymbopogon citratus, stapf (Lemon grass). J. Adv. Pharm. Technol. Res. 2011, 2, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, M.; Shah, M.; Ranginwala, A.; Agrawal, P.; Acharya, D.; Thakkar, S. Antifungal effects of tulsi, garlic, cinnamon and lemongrass in powder and oil form on Candida albicans: An in vitro study. J. Oral. Maxillofac. Pathol. 2021, 25, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Amornvit, P.; Choonharuangdej, S.; Srithavaj, T. Lemongrass-Incorporated Tissue Conditioner against Candida albicans Culture. J. Clin. Diagn. Res. 2014, 8, ZC50-2. [Google Scholar] [CrossRef]
- Da Silva, C.d.B.; Guterres, S.S.; Weisheimer, V.; Schapoval, E.E. Antifungal activity of the lemongrass oil and citral against Candida spp. Braz. J. Infect. Dis. 2008, 12, 63–66. [Google Scholar] [CrossRef]
- Almeida Lde, F.; Paula, J.F.; Almeida, R.V.; Williams, D.W.; Hebling, J.; Cavalcanti, Y.W. Efficacy of citronella and cinnamon essential oils on Candida albicans biofilms. Acta Odontol. Scand. 2016, 74, 393–398. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Malik, A. Liquid and vapour-phase antifungal activities of selected essential oils against Candida albicans: Microscopic observations and chemical characterization of Cymbopogon citratus. BMC Complement. Altern. Med. 2010, 10, 65. [Google Scholar] [CrossRef]
- Carpo, B.G.; Verallo-Rowell, V.M.; Kabara, J. Novel antibacterial activity of monolaurin compared with conventional antibiotics against organisms from skin infections: An in vitro study. J. Drugs Dermatol. 2007, 6, 991–998. [Google Scholar]
- Seleem, D.; Freitas-Blanco, V.S.; Noguti, J.; Zancope, B.R.; Pardi, V.; Murata, R.M. In Vivo Antifungal Activity of Monolaurin against Candida albicans Biofilms. Biol. Pharm. Bull. 2018, 41, 1299–1302. [Google Scholar] [CrossRef]
- Seleem, D.; Chen, E.; Benso, B.; Pardi, V.; Murata, R.M. In vitro evaluation of antifungal activity of monolaurin against Candida albicans biofilms. PeerJ 2016, 4, e2148. [Google Scholar] [CrossRef]
- Gunsalus, K.T.; Tornberg-Belanger, S.N.; Matthan, N.R.; Lichtenstein, A.H.; Kumamoto, C.A. Manipulation of Host Diet to Reduce Gastrointestinal Colonization by the Opportunistic Pathogen Candida albicans. mSphere 2016, 1, e00020-15. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Shabrmi, F.M.; Aly, S.M. Active ingredients of ginger as potential candidates in the prevention and treatment of diseases via modulation of biological activities. Int. J. Physiol. Pathophysiol. Pharmacol. 2014, 6, 125–136. [Google Scholar]
- Kim, H.S.; Park, H.D. Ginger extract inhibits biofilm formation by Pseudomonas aeruginosa PA14. PLoS ONE 2013, 8, e76106. [Google Scholar] [CrossRef]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H.B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Bialvaei, A.Z.; Aghazadeh, M.; Kabiri, F.; Saliani, N.; Yousefi, M.; Eslami, H.; Kafil, H.S. Survey of the Antibiofilm and Antimicrobial Effects of Zingiber officinale (in Vitro Study). Jundishapur J. Microbiol. 2016, 9, e30167. [Google Scholar] [CrossRef]
- Khan, A.; Azam, M.; Allemailem, K.S.; Alrumaihi, F.; Almatroudi, A.; Alhumaydhi, F.A.; Ahmad, H.I.; Khan, M.U.; Khan, M.A. Coadministration of Ginger Extract and Fluconazole Shows a Synergistic Effect in the Treatment of Drug-Resistant Vulvovaginal Candidiasis. Infect. Drug Resist. 2021, 14, 1585–1599. [Google Scholar] [CrossRef]
- Fathy, S.A.; Mohamed, M.R.; Emam, M.A.; Mohamed, S.S.; Ghareeb, D.A.; Elgohary, S.A.; Abd-El Megeed, D.F. Therapeutic efficacy of seaweed extract (Ulva Fasciata Delile) against invasive candidiasis in mice. Trop. Biomed. 2019, 36, 972–986. [Google Scholar]
- Mubarak, Z.; Humaira, A.; Gani, B.A.; Muchlisin, Z.A. Preliminary study on the inhibitory effect of seaweed Gracilaria verrucosa extract on biofilm formation of Candida albicans cultured from the saliva of a smoker. F1000Resarch 2018, 7, 684. [Google Scholar] [CrossRef]
- Oka, S.; Okabe, M.; Tsubura, S.; Mikami, M.; Imai, A. Properties of fucoidans beneficial to oral healthcare. Odontology 2020, 108, 34–42. [Google Scholar] [CrossRef]
- Lopes, G.; Pinto, E.; Andrade, P.B.; Valentao, P. Antifungal activity of phlorotannins against dermatophytes and yeasts: Approaches to the mechanism of action and influence on Candida albicans virulence factor. PLoS ONE 2013, 8, e72203. [Google Scholar] [CrossRef] [PubMed]
- Jafri, H.; Ahmad, I. Thymus vulgaris essential oil and thymol inhibit biofilms and interact synergistically with antifungal drugs against drug resistant strains of Candida albicans and Candida tropicalis. J. Mycol. Med. 2020, 30, 100911. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.I.; de Lima, A.F.A.; Grilli, D.G.; Cuppari, L. The short-term effects of olive oil and flaxseed oil for the treatment of constipation in hemodialysis patients. J. Ren. Nutr. 2015, 25, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Cariello, M.; Contursi, A.; Gadaleta, R.M.; Piccinin, E.; De Santis, S.; Piglionica, M.; Spaziante, A.F.; Sabba, C.; Villani, G.; Moschetta, A. Extra-Virgin Olive Oil from Apulian Cultivars and Intestinal Inflammation. Nutrients 2020, 12, 1084. [Google Scholar] [CrossRef]
- Charlet, R.; Le Danvic, C.; Sendid, B.; Nagnan-Le Meillour, P.; Jawhara, S. Oleic Acid and Palmitic Acid from Bacteroides thetaiotaomicron and Lactobacillus johnsonii Exhibit Anti-Inflammatory and Antifungal Properties. Microorganisms 2022, 10, 1083. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Garnås, E. Fermented Vegetables as a Potential Treatment for Irritable Bowel Syndrome. Curr. Dev. Nutr. 2023, 7, 100039. [Google Scholar] [CrossRef]
- Shahbazi, R.; Sharifzad, F.; Bagheri, R.; Alsadi, N.; Yasavoli-Sharahi, H.; Matar, C. Anti-Inflammatory and Immunomodulatory Properties of Fermented Plant Foods. Nutrients 2021, 13, 1516. [Google Scholar] [CrossRef]
- Plengvidhya, V.; Breidt, F., Jr.; Lu, Z.; Fleming, H.P. DNA fingerprinting of lactic acid bacteria in sauerkraut fermentations. Appl. Environ. Microbiol. 2007, 73, 7697–7702. [Google Scholar] [CrossRef]
- Lee, H.; Yoon, H.; Ji, Y.; Kim, H.; Park, H.; Lee, J.; Shin, H.; Holzapfel, W. Functional properties of Lactobacillus strains isolated from kimchi. Int. J. Food Microbiol. 2011, 145, 155–161. [Google Scholar] [CrossRef]
- Beck, B.R.; Park, G.S.; Lee, Y.H.; Im, S.; Jeong, D.Y.; Kang, J. Whole Genome Analysis of Lactobacillus plantarum Strains Isolated From Kimchi and Determination of Probiotic Properties to Treat Mucosal Infections by Candida albicans and Gardnerella vaginalis. Front. Microbiol. 2019, 10, 433. [Google Scholar] [CrossRef]
- Ghoneum, M.; Abdulmalek, S. KDP, a Lactobacilli Product from Kimchi, Enhances Mucosal Immunity by Increasing Secretory IgA in Mice and Exhibits Antimicrobial Activity. Nutrients 2021, 13, 3936. [Google Scholar] [CrossRef]
- Yagnik, D.; Serafin, V.; Shah, A.J. Antimicrobial activity of apple cider vinegar against Escherichia coli, Staphylococcus aureus and Candida albicans; downregulating cytokine and microbial protein expression. Sci. Rep. 2018, 8, 1732. [Google Scholar] [CrossRef]
- Fisberg, M.; Machado, R. History of yogurt and current patterns of consumption. Nutr. Rev. 2015, 73 (Suppl. S1), 4–7. [Google Scholar] [CrossRef]
- Shalev, E.; Battino, S.; Weiner, E.; Colodner, R.; Keness, Y. Ingestion of yogurt containing Lactobacillus acidophilus compared with pasteurized yogurt as prophylaxis for recurrent candidal vaginitis and bacterial vaginosis. Arch. Fam. Med. 1996, 5, 593–596. [Google Scholar] [CrossRef]
- Aitzhanova, A.; Oleinikova, Y.; Mounier, J.; Hymery, N.; Salas, M.L.; Amangeldi, A.; Saubenova, M.; Alimzhanova, M.; Ashimuly, K.; Sadanov, A. Dairy associations for the targeted control of opportunistic Candida. World J. Microbiol. Biotechnol. 2021, 37, 143. [Google Scholar] [CrossRef]
- Hu, H.; Merenstein, D.J.; Wang, C.; Hamilton, P.R.; Blackmon, M.L.; Chen, H.; Calderone, R.A.; Li, D. Impact of eating probiotic yogurt on colonization by Candida species of the oral and vaginal mucosa in HIV-infected and HIV-uninfected women. Mycopathologia 2013, 176, 175–181. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jawhara, S. Healthy Diet and Lifestyle Improve the Gut Microbiota and Help Combat Fungal Infection. Microorganisms 2023, 11, 1556. https://doi.org/10.3390/microorganisms11061556
Jawhara S. Healthy Diet and Lifestyle Improve the Gut Microbiota and Help Combat Fungal Infection. Microorganisms. 2023; 11(6):1556. https://doi.org/10.3390/microorganisms11061556
Chicago/Turabian StyleJawhara, Samir. 2023. "Healthy Diet and Lifestyle Improve the Gut Microbiota and Help Combat Fungal Infection" Microorganisms 11, no. 6: 1556. https://doi.org/10.3390/microorganisms11061556
APA StyleJawhara, S. (2023). Healthy Diet and Lifestyle Improve the Gut Microbiota and Help Combat Fungal Infection. Microorganisms, 11(6), 1556. https://doi.org/10.3390/microorganisms11061556





