Microbiome-Related and Infection Control Approaches to Primary and Secondary Prevention of Clostridioides difficile Infections
Abstract
1. Introduction
2. Materials and Methods
3. Results
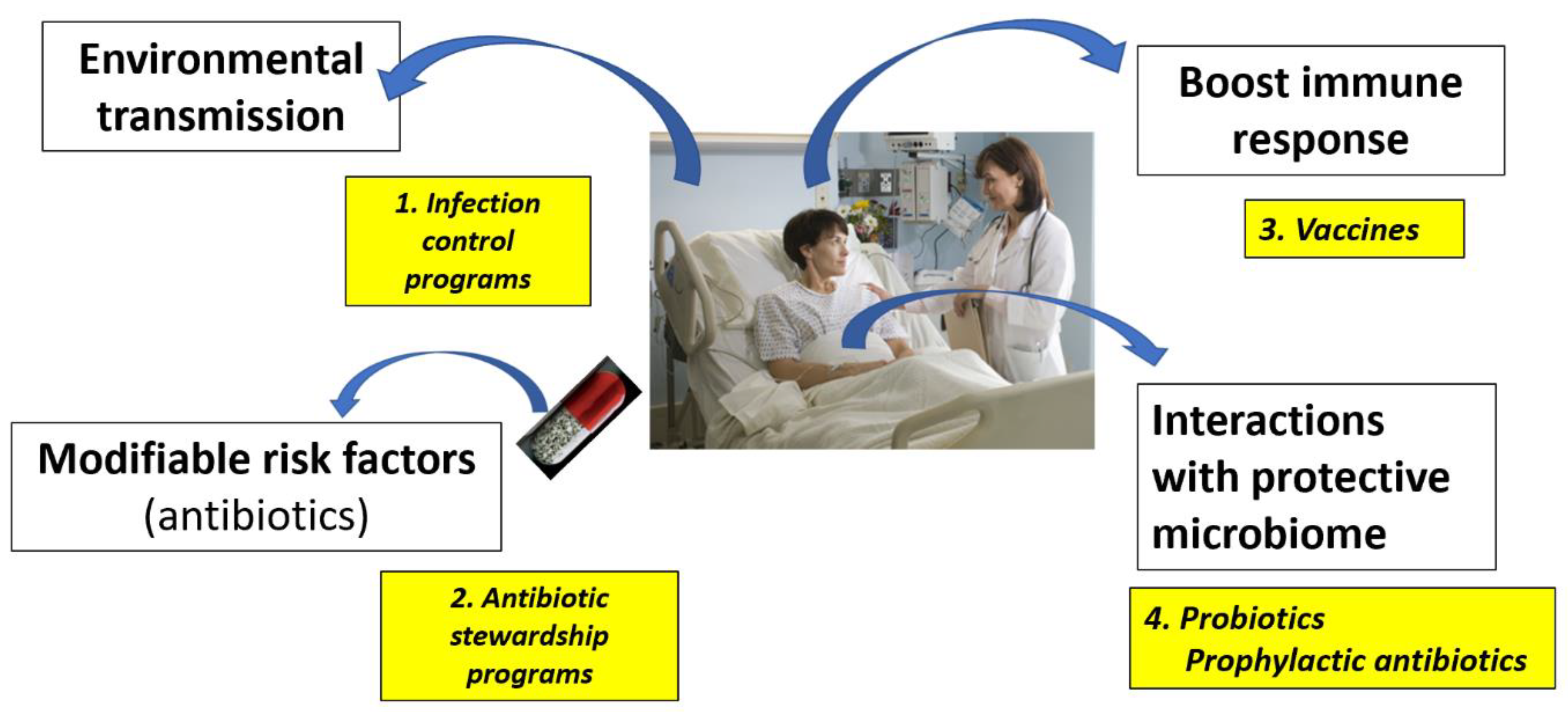
3.1. Strategies for Primary Prevention of CDI
3.1.1. Interruption of C. difficile Transmission
3.1.2. Modifying CDI Risk Factors
3.1.3. Enhancing the Immune Response
3.1.4. Enhancing the Intestinal Microbiome with Probiotics
3.2. Strategies for the Secondary Prevention of CDI
3.2.1. Fecal Microbial Therapy (FMT)
3.2.2. Live Biotherapeutic Products (LBP)
3.2.3. Probiotics
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Centers for Communicable Diseases (CDC). 2020 National and State Healthcare-Associated Infections Progress Report. Available online: https://www.cdc.gov/hai/data/portal/progress-report.html (accessed on 2 February 2023).
- Wiuff, C.; Banks, A.L.; Fitzpatrick, F.; Cottom, L. The need for European surveillance of CDI. In Updates on Clostridium Difficile in Europe; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2018; Volume 1050, pp. 13–25. [Google Scholar]
- Du, T.; Choi, K.B.; Silva, A.; Golding, G.R.; Pelude, L.; Hizon, R.; Al-Rawahi, G.N.; Brooks, J.; Chow, B.; Collet, J.C.; et al. Characterization of healthcare-associated and community-associated Clostridioides difficile infections among adults, Canada, 2015–2019. Emerg. Infect. Dis. 2022, 28, 1128–1136. [Google Scholar] [CrossRef]
- Spigaglia, P. Clostridioides difficile infection (CDI) during the COVID-19 pandemic. Anaerobe 2022, 74, 102518. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.D.; Kelly, C.P.; Garey, K.W.; Gonzales-Luna, A.J.; Williams, D.; Daugherty, K.; Cuddemi, C.; Villafuerte-Gálvez, J.; White, N.C.; Chen, X.; et al. Ultrasensitive and quantitative toxin measurement correlates with baseline severity, severe outcomes, and recurrence among hospitalized patients with Clostridioides difficile infection. Clin. Infect. Dis. 2022, 74, 2142–2149. [Google Scholar] [CrossRef]
- Davies, E.; Jolles, D. Safe prevention of Clostridium difficile using infectious disease guidelines at an urban hospital in North Carolina. BMJ Open Qual. 2022, 11, e000618. [Google Scholar] [CrossRef]
- Feuerstadt, P.; Stong, L.; Dahdal, D.N.; Sacks, N.; Lang, K.; Nelson, W.W. Healthcare resource utilization and direct medical costs associated with index and recurrent Clostridioides difficile infection: A real-world data analysis. J. Med. Econ. 2020, 23, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Feuerstadt, P.; Boules, M.; Strong, L.; Dahdal, D.N.; Sacks, N.C.; Lang, K.; Nelson, W.W. Clinical complications in patients with primary and recurrent Clostridioides difficile infection: A real-world data analysis. SAGE Open Med. 2021, 9, 2050312120986733. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.B.; Lee, M.; Suh, J.W.; Yang, K.S.; Chung, Y.; Kim, J.Y.; Kim, S.B.; Sohn, J.W.; Yoon, Y.K. Clinical prediction rule for identifying older patients with toxigenic Clostridioides difficile at the time of hospital admission. BMC Geriatr. 2023, 23, 127. [Google Scholar] [CrossRef] [PubMed]
- Thandavaram, A.; Channar, A.; Purohit, A.; Shrestha, B.; Patel, D.; Shah, H.; Hanna, K.; Kaur, H.; Alazzeh, M.S.; Mohammed, L. The efficacy of bezlotoxumab in the prevention of recurrent Clostridium difficile: A systematic review. Cureus 2022, 14, e27979. [Google Scholar] [CrossRef]
- Mengoli, M.; Barone, M.; Fabbrini, M.; D’Amico, F.; Brigidi, P.; Turroni, S. Make it less difficile: Understanding genetic evolution and global spread of Clostridioides difficile. Genes 2022, 13, 2200. [Google Scholar] [CrossRef]
- Monaghan, T.M.; Biswas, R.; Satav, A.; Ambalkar, S.; Kashyap, R.S. Mini-review: Clostridioides difficile epidemiology in India. Anaerobe 2022, 74, 102517. [Google Scholar] [CrossRef]
- Gou, H.Z.; Zhang, Y.L.; Ren, L.F.; Li, Z.J.; Zhang, L. How do intestinal probiotics restore the intestinal barrier? Front. Microb. 2022, 13, 929346. [Google Scholar] [CrossRef] [PubMed]
- Haran, J.P.; McCormick, B.A. Aging, frailty, and the microbiome—How dysbiosis influences human aging and disease. Gastroenterology 2021, 160, 507–523. [Google Scholar] [CrossRef]
- Wensel, C.R.; Pluznick, J.L.; Salzberg, S.L.; Sears, C.L. Next-generation sequencing: Insights to advance clinical investigations of the microbiome. J. Clin. Investig. 2022, 132, e154944. [Google Scholar] [CrossRef]
- Kim, J.; Cho, Y.; Seo, M.R.; Bae, M.H.; Kim, B.; Rho, M.; Pai, H. Quantitative characterization of Clostridioides difficile population in the gut microbiome of patients with C. difficile infection and their association with clinical factors. Sci. Rep. 2020, 10, 17608. [Google Scholar] [CrossRef] [PubMed]
- Kachrimanidou, M.; Tsintarakis, E. Insights into the role of human gut microbiota in Clostridioides difficile infection. Microorganisms 2020, 8, 200. [Google Scholar] [CrossRef]
- Kordus, S.L.; Thomas, A.K.; Lacy, D.B. Clostridioides difficile toxins: Mechanisms of action and antitoxin therapeutics. Nat. Rev. Microbiol. 2022, 20, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Vasilescu, I.M.; Chifiriuc, M.C.; Pircalabioru, G.G.; Filip, R.; Bolocan, A.; Lazăr, V.; Diţu, L.M.; Bleotu, C. Gut dysbiosis and Clostridioides difficile infection in neonates and adults. Front. Microbiol. 2022, 12, 651081. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, S.R.; Robinson, J.I.; Hink, T.; Reske, K.A.; Newcomer, E.P.; Burnham, C.A.; Henderson, J.P.; Dubberke, E.R.; Dantas, G. Multiomics investigation of Clostridioides difficile-colonized patients reveals pathogen and commensal correlates of C. difficile pathogenesis. eLife 2022, 11, e72801. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y.; Antonopoulos, D.A.; Kalra, A.; Tonelli, A.; Khalife, W.T.; Schmidt, T.M.; Young, V.B. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile-associated diarrhea. J. Infect. Dis. 2008, 197, 435–438. [Google Scholar] [CrossRef]
- Piccioni, A.; Rosa, F.; Manca, F.; Pignataro, G.; Zanza, C.; Savioli, G.; Covino, M.; Ojetti, V.; Gasbarrini, A.; Franceschi, F.; et al. Gut Microbiota and Clostridium difficile: What We Know and the New Frontiers. Int. J. Mol. Sci. 2022, 23, 13323. [Google Scholar] [CrossRef] [PubMed]
- Lemiech-Mirowska, E.; Michałkiewicz, M.; Sierocka, A.; Gaszyńska, E.; Marczak, M. The hospital environment as a potential source for Clostridioides difficile transmission based on spore detection surveys conducted at paediatric oncology and gastroenterology units. Int. J. Environ. Res. Public Health 2023, 20, 1590. [Google Scholar] [CrossRef]
- McFarland, L.V.; Mulligan, M.E.; Kwok, R.Y.; Stamm, W.E. Nosocomial acquisition of Clostridium difficile infection. NEJM 1989, 320, 204–210. [Google Scholar] [CrossRef]
- Lim, S.C.; Knight, D.R.; Riley, T.V. Clostridium difficile and one health. Clin. Microbiol. Infect. 2020, 26, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.C.; Arakkal, A.T.; Sewel, D.K.; Segre, A.M.; Pemmaraju, S.V.; Polgreen, P.M.; CDC MInD-Healthcare Group. Risk for asymptomatic household transmission of Clostridioides difficile infection associated with recently hospitalized family members. Emerg. Infect. Dis. 2022, 28, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Al Sharaby, A.; Abugoukh, T.M.; Ahmed, W.; Ahmed, S.; Elshaikh, A.O. Do probiotics prevent Clostridium difficile-associated diarrhea? Cureus 2022, 14, e27624. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Li, Z.R.; Qin, P.; Qiang, C.X.; Yang, J.; Niu, Y.N.; Niu, X.R.; Liu, X.X.; Wang, W.G.; Wen, B.J.; et al. Risk factors for Clostridioides difficile infection in children: A systematic review and meta-analysis. J. Hosp. Infect. 2022, 130, 112–121. [Google Scholar] [CrossRef]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, e1–e48. [Google Scholar] [CrossRef]
- Kociolek, L.K.; Gerding, D.N.; Carrico, R.; Carling, P.; Donskey, C.J.; Dumyati, G.; Kuhar, D.T.; Loo, V.G.; Maragakis, L.L.; Pogorzelska-Maziarz, M.; et al. Strategies to prevent Clostridioides difficile infections in acute-care hospitals: 2022 Update. Infect. Control Hosp. Epidemiol. 2023, 44, 527–549. [Google Scholar] [CrossRef]
- Papanikolopoulou, A.; Maltezou, H.C.; Gargalianos-Kakolyris, P.; Pangalis, A.; Pantazis, N.; Pantos, C.; Tountas, Y.; Tsakris, A.; Kantzanou, M. Association between consumption of antibiotics, infection control interventions and Clostridioides difficile infections: Analysis of six-year time-series data in a tertiary-care hospital in Greece. Infect. Dis. Health 2022, 27, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Baur, D.; Gladstone, B.P.; Burkert, F.; Carrara, E.; Foschi, F.; Döbele, S.; Tacconelli, E. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: A systematic review and meta-analysis. Lancet Infect. Dis. 2017, 17, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- Kullar, R.; Johnson, S.; McFarland, L.V.; Goff, D.A.; Goldstein, E.J. Bundling probiotics with antimicrobial stewardship programs for the prevention of Clostridiodes difficile infections in acute care hospitals. Infect. Dis. Clin. Pract. 2020, 28, 123–129. [Google Scholar] [CrossRef]
- Johnson, S.W.; Brown, S.V.; Priest, D.H. Effectiveness of oral vancomycin for prevention of healthcare facility–onset Clostridioides difficile infection in targeted patients during systemic antibiotic exposure. Clin. Infect. Dis. 2020, 71, 1133–1139. [Google Scholar] [CrossRef]
- Maraolo, A.E.; Mazzitelli, M.; Zappulo, E.; Scotto, R.; Granata, G.; Andini, R.; Durante-Mangoni, E.; Petrosillo, N.; .Gentile, I. Oral vancomycin prophylaxis for primary and secondary prevention of Clostridioides difficile infection in patients treated with systemic antibiotic therapy: A systematic review, meta-analysis and trial sequential analysis. Antibiotics 2022, 11, 183. [Google Scholar] [CrossRef]
- Mullane, K.M.; Winston, D.J.; Nooka, A.; Morris, M.I.; Stiff, P.; Dugan, M.J.; Holland, H.; Gregg, K.; Adachi, J.A.; Pergam, S.A.; et al. A randomized, placebo-controlled trial of fidaxomicin for prophylaxis of Clostridium difficile–associated diarrhea in adults undergoing hematopoietic stem cell transplantation. Clin. Infect. Dis. 2019, 68, 196–203. [Google Scholar] [CrossRef] [PubMed]
- van Prehn, J.; Reigadas, E.; Vogelzang, E.H.; Bouza, E.; Hristea, A.; Guery, B.; Krutova, M.; Norén, T.; Allerberger, F.; Coia, J.E.; et al. European Society of Clinical Microbiology and Infectious Diseases: 2021 update on the treatment guidance document for Clostridioides difficile infection in adults. Clin. Microbiol. Infect. 2021, 27, S1–S21. [Google Scholar] [CrossRef] [PubMed]
- De Bruyn, G.; Gordon, D.L.; Steiner, T.; Tambyah, P.; Cosgrove, C.; Martens, M.; Bassily, E.; Chan, E.S.; Patel, D.; Chen, J.; et al. Safety, immunogenicity, and efficacy of a Clostridioides difficile toxoid vaccine candidate: A phase 3 multicentre, observer-blind, randomised, controlled trial. Lancet Infect. Dis. 2021, 21, 252–262. [Google Scholar] [CrossRef]
- Kitchin, N.; Remich, S.A.; Peterson, J.; Peng, Y.; Gruber, W.C.; Jansen, K.U.; Pride, M.W.; Anderson, A.S.; Knirsch, C.; Webber, C. A phase 2 study evaluating the safety, tolerability, and immunogenicity of two 3-dose regimens of a Clostridium difficile vaccine in healthy US adults aged 65 to 85 years. Clin. Infect. Dis. 2020, 70, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bézay, N.; Ayad, A.; Dubischar, K.; Firbas, C.; Hochreiter, R.; Kiermayr, S.; Kiss, I.; Pinl, F.; Jilma, B.; Westritschnig, K. Safety, immunogenicity and dose response of VLA84, a new vaccine candidate against Clostridium difficile, in healthy volunteers. Vaccine 2016, 34, 2585–2592. [Google Scholar] [CrossRef]
- Hong, H.A.; Hitri, K.; Hosseini, S.; Kotowicz, N.; Bryan, D.; Mawas, F.; Wilkinson, A.J.; van Broekhoven, A.; Kearsey, J.; Cutting, S.M. Mucosal antibodies to the C terminus of toxin A prevent colonization of Clostridium difficile. Infect. Immun. 2017, 85, e01060-16. [Google Scholar] [CrossRef]
- Reid, G.; Gadir, A.A.; Dhir, R. Probiotics: Reiterating what they are and what they are not. Front. Microbiol. 2019, 10, 424. [Google Scholar] [CrossRef]
- Gunaratnam, S.; Millette, M.; McFarland, L.V.; DuPont, H.L.; Lacroix, M. Potential role of probiotics in reducing Clostridioides difficile virulence: Interference with quorum sensing systems. Microb. Pathog. 2021, 153, 104798. [Google Scholar] [CrossRef]
- Briand, F.; Sulpice, T.; Giammarinaro, P.; Roux, X. Saccharomyces boulardii CNCM I-745 changes lipidemic profile and gut microbiota in a hamster hypercholesterolemic model. Benef. Microb. 2019, 10, 555–567. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Swidsinski, A.; Loening–Baucke, V.; Verstraelen, H.; Osowska, S.; Doerffel, Y. Biostructure of fecal microbiota in healthy subjects and patients with chronic idiopathic diarrhea. Gastroenterol 2008, 135, 568–579. [Google Scholar] [CrossRef]
- McFarland, L.V.; Evans, C.T.; Goldstein, E.J. Strain-specificity and disease-specificity of probiotic efficacy: A systematic review and meta-analysis. Front. Med. 2018, 5, 124. [Google Scholar] [CrossRef]
- Plummer, S.; Weaver, M.A.; Harris, J.C.; Dee, P.; Hunter, J. Clostridium difficile pilot study: Effects of probiotic supplementation on the incidence of C. difficile diarrhoea. Int. Microbiol. 2004, 7, 59–62. [Google Scholar] [PubMed]
- Rafiq, R.; Randey, D.; Osman, S.M.; Masood, R.; Donepudi, I.; Norkus, E.; Kiyici, N.; Hertan, H.I. Prevention of Clostridium difficile (C. difficile) diarrhea with probiotic in hospitalized patients treated with antibiotics. Gastroenterology 2007, 132, A187. [Google Scholar]
- Stein, G.Y.; Nanim, R.; Karniel, E.; Moskowitz, I.; Zeidman, A. Probiotics as prophylactic agents against antibiotic-associated diarrhea in hospitalized patients. Harefuah 2007, 146, 520–522. [Google Scholar]
- Miller, M.; Florencio, S.; Eastmond, J.; Reynolds, S. Results of 2 prospective randomized studies of Lactobacillus GG to prevent C. difficile infection in hospitalized adults receiving antibiotics. In Abstracts of Interscience Conference on Antimicrobial Agents and Chemotherapy; American Society for Microbiology: Washington, DC, USA, 2008; Volume 48, pp. 578–579. [Google Scholar]
- McFarland, L.V. Primary prevention of Clostridium difficile infections–how difficult can it be? Exp. Rev. Gastroenterol. Hepatol. 2017, 11, 507–521. [Google Scholar] [CrossRef]
- Cárdenas, P.A.; Garcés, D.; Prado-Vivar, B.; Flores, N.; Fornasini, M.; Cohen, H.; Salvador, I.; Cargua, O.; Baldeón, M.E. Effect of Saccharomyces boulardii CNCM I-745 as complementary treatment of Helicobacter pylori infection on gut microbiome. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wang, C.; Qin, X.; Zhou, B.; Liu, X.; Liu, T.; Xie, R.; Liu, J.; Wang, B.; Cao, H. Saccharomyces boulardii, a yeast probiotic, inhibits gut motility through upregulating intestinal serotonin transporter and modulating gut microbiota. Pharmacol. Res. 2022, 181, 106291. [Google Scholar] [CrossRef]
- De Las Casas, V.; Miller, S.; DuPont, H.; Jiang, Z.D. In vitro Effects of probiotics on Clostridium difficile toxin production and sporulation. Int. Arch. Public Health Community Med. 2020, 4, 40. [Google Scholar] [CrossRef]
- Maziade, P.J.; Andriessen, J.A.; Pereira, P.; Currie, B.; Goldstein, E.J.C. Impact of adding prophylactic probiotics to a bundle of standard preventative measures for Clostridium difficile infections: Enhanced and sustained decrease in the incidence and severity of infection at a community hospital. Curr. Med. Res. Opin. 2013, 29, 1341–1347. [Google Scholar] [CrossRef]
- Trick, W.E.; Sokalski, S.J.; Johnson, S.; Bunnell, K.L.; Levato, J.; Ray, M.J.; Weinstein, R.A. Effectiveness of probiotic for primary prevention of Clostridium difficile infection: A single-center before-and-after quality improvement intervention at a tertiary-care medical center. Infect. Control Hosp. Epidemiol. 2018, 39, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Olson, B.; Floyd, R.; Howard, J.; Hassanein, T.; Warm, K.; Oen, R. A multipronged approach to decrease the risk of Clostridium difficile infection at a community hospital and long-term care facility. J. Clin. Outcomes Manag. 2015, 22, 398. [Google Scholar]
- Box, M.J.; Ortwine, K.N.; Goicoechea, M. Scripps Antimicrobial Stewardship Program (SASP). No impact of probiotics to reduce Clostridium difficile infection in hospitalized patients: A real-world experience. Open Forum Infect. Dis. 2018, 5, ofy192. [Google Scholar] [CrossRef]
- Shihadeh, K.; Young, H.; Knepper, B.; Tapia, R.; Jenkins, T.C. 516. Implementation of a Probiotic for the Primary Prevention of Hospital-Onset Clostridium difficile Infection. Open Forum Infect. Dis. 2018, 5 (Suppl. S1), S191. [Google Scholar] [CrossRef]
- Flatley, E.A.; Wilde, A.M.; Nailor, M.D. Saccharomyces boulardii for the prevention of hospital onset Clostridium difficile infection. J. Gastrointest. Liver Dis. 2015, 24, 21–24. [Google Scholar] [CrossRef]
- Slain, D.; Georgulis, A.; Dermitt, R.; Morris, L.; Colodny, S.M. Impact of an automatic hospital probiotic protocol on Clostridioides (Clostridium) difficile infection (CDI) rates and CDI antibiotic usage in a community hospital setting. J. Infect. Prev. 2020, 21, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Wombwell, E.; Patterson, M.E.; Bransteitter, B.; Gillen, L.R. The effect of Saccharomyces boulardii primary prevention on risk of hospital onset Clostridioides difficile infection in hospitalized patients administered antibiotics frequently associated with Clostridioides difficile infection. Clin. Infect. Dis. 2021, 73, e2512–e2518. [Google Scholar] [CrossRef]
- Graul, T.; Cain, A.M.; Karpa, K.D. Lactobacillus and bifidobacteria combinations: A strategy to reduce hospital-acquired Clostridium difficile diarrhea incidence and mortality. Med. Hypotheses 2009, 73, 194–198. [Google Scholar] [CrossRef]
- Lewis, P.O.; Lundberg, T.S.; Tharp, J.L.; Runnels, C.W. Implementation of global strategies to prevent hospital-onset Clostridium difficile infection: Targeting proton pump inhibitors and probiotics. Ann. Pharmacother. 2017, 51, 848–854. [Google Scholar] [CrossRef]
- Maziade, P.J.; Ship, N.; Sniffen, J.C.; Goldstein, E.J. Enhanced Clostridioides difficile infection prevention with a pharmacy-controlled policy that adds a 3-strain lactobacillus probiotic concomitantly to antibiotic therapy. Clin. Infect. Dis. 2021, 73, 1524–1527. [Google Scholar] [CrossRef] [PubMed]
- Maziade, P.J.; Lussier, D.; Dubé, F. Abstract 2417. Feasibility and safety of using a probiotic comprised of Lactobacillus acidophilus CL1285, L. casei LBC80R and L. rhamnosus CLR2 for C. difficile infection prevention among antibiotic users: 15-years of prospective results from a single-center. Open Forum Infect. Dis. 2019, 6, S834–S835. [Google Scholar]
- McFarland, L.V.; Johnson, S.B.; Evans, C.T. Perils and pitfalls of probiotic quasi-experimental studies for primary prevention of Clostridioides difficile infection: A review of the evidence. Am. J. Infect. Control 2021, 49, 375–384. [Google Scholar] [CrossRef]
- Preidis, G.A.; Weizman, A.V.; Kashyap, P.C.; Morgan, R.L. AGA technical review on the role of probiotics in the management of gastrointestinal disorders. Gastroenterology 2020, 159, 708–738. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.R.; Fischer, M.; Allegretti, J.R.; LaPlante, K.; Stewart, D.B.; Limketkai, B.N.; Stollman, N.H. ACG clinical guidelines: Prevention, diagnosis, and treatment of Clostridioides difficile infections. Am. J. Gastroenterol. 2021, 116, 1124–1147. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V.; Kullar, R.; Johnson, S.; Sniffen, J.C.; Woolard, K.; Goldstein, E.J. Why do ACG and AGA guidelines differ for the use of probiotics and the prevention of CDI? Am. J. Gastroenterol. 2022, 117, 501. [Google Scholar] [CrossRef]
- Allen, S.J.; Wareham, K.; Wang, D.; Bradley, C.; Hutchings, H.; Harris, W.; Dhar, A.; Brown, H.; Foden, A.; Gravenor, M.B.; et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2013, 382, 1249–1257. [Google Scholar] [CrossRef]
- Besselink, M.G.; van Santvoort, H.C.; Buskens, E.; Boermeester, M.A.; van Goor, H.; Timmerman, H.M.; Nieuwenhuijs, V.B.; Bollen, T.L.; van Ramshorst, B.; Witteman, B.J.; et al. Dutch Acute Pancreatitis Study Group. Probiotic prophylaxis in predicted severe acute pancreatitis: A randomised, double-blind, placebo-controlled trial. Lancet 2008, 371, 651–659. [Google Scholar] [CrossRef]
- Kullar, R.; Johnson, S.; Goldstein, E.J.C.; McFarland, L.V. Lactobacillus bacteremia and probiotics: A review. Microorganisms 2023, 11, 896. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V.; Elmer, G.W.; Surawicz, C.M. Breaking the cycle: Treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am. J. Gastroenterol. 2002, 97, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Gerding, D.N.; Sambol, S.P.; Johnson, S. Non-toxigenic Clostridioides (formerly Clostridium) difficile for prevention of C. difficile infection: From bench to bedside back to bench and back to bedside. Front. Microb. 2018, 9, 1700. [Google Scholar] [CrossRef] [PubMed]
- Bainum, T.B.; Reveles, K.R.; Hall, R.G.; Cornell, K.; Alvarez, C.A. Controversies in the prevention and treatment of Clostridioides difficile in adults: A narrative review. Microorganisms 2023, 11, 387. [Google Scholar] [CrossRef]
- Johnson, S.; Lavergne, V.; Skinner, A.M.; Gonzales-Luna, A.J.; Garey, K.W.; Kelly, C.P.; Wilcox, M.H. Clinical practice guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. Clin. Infect. Dis. 2021, 73, e1029–e1044. [Google Scholar] [CrossRef]
- Bishop, E.J.; Tiruvoipati, R. Management of Clostridioides difficile infection in adults and challenges in clinical practice: Review and comparison of current IDSA/SHEA, ESCMID and ASID guidelines. J. Antimicrob. Chemother. 2023, 78, 21–30. [Google Scholar] [CrossRef]
- Clancy, C.J.; Buehrle, D.; Vu, M.; Wagener, M.M.; Nguyen, M.H. Impact of revised Infectious Diseases Society of America and Society for Healthcare Epidemiology of America clinical practice guidelines on the treatment of Clostridium difficile infections in the United States. Clin. Infect. Dis. 2021, 72, 1944–1949. [Google Scholar] [CrossRef]
- Chiu, C.W.; Tsai, P.J.; Lee, C.C.; Ko, W.C.; Hung, Y.P. Application of microbiome management in therapy for Clostridioides difficile infections: From fecal microbiota transplantation to probiotics to microbiota-preserving antimicrobial agents. Pathogens 2021, 10, 649. [Google Scholar] [CrossRef]
- Van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; de Vos, W.M.; Visser, C.E.; Kuijper, E.J.; Bartelsman, J.F.; Tijssen, J.G.; et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. NEJM 2013, 368, 407–415. [Google Scholar] [CrossRef]
- Cammarota, G.; Masucci, L.; Ianiro, G.; Bibbo, S.; Dinoi, G.; Costamagna, G.; Sanguinetti, M.; Gasbarrini, A. Randomised clinical trial: Faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment. Pharmacol. Ther. 2015, 41, 835–843. [Google Scholar] [CrossRef]
- Hota, S.S.; Sales, V.; Tomlinson, G.; Salpeter, M.J.; McGeer, A.; Coburn, B.; Guttman, D.S.; Low, D.E.; Poutanen, S.M. Oral vancomycin followed by fecal transplantation versus tapering oral vancomycin treatment for recurrent Clostridium difficile infection:An open-label, randomized controlled trial. Clin. Infect. Dis. 2017, 64, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Assi, M.; Lee, C.; Yoho, D.; Louie, T.; Knapple, W.; Aguilar, H.; Garcia-Diaz, J.; Wang, G.P.; Berry, S.M.; et al. Efficacy and safety of RBX2660 in PUNCH CD3, a Phase III, randomized, double-blind, placebo-controlled trial with a Bayesian primary analysis for the prevention of recurrent Clostridioides difficile infection. Drugs 2022, 82, 1527–1538. [Google Scholar] [CrossRef] [PubMed]
- Feuerstadt, P.; Louie, T.J.; Lashner, B.; Wang, E.E.; Diao, L.; Bryant, J.A.; Sims, M.; Kraft, C.S.; Cohen, S.H.; Berenson, C.S.; et al. SER-109, an oral microbiome therapy for recurrent Clostridioides difficile infection. N. Eng. J. Med. 2022, 20, 220–229. [Google Scholar] [CrossRef]
- Allegretti, J.R.; Kelly, C.R.; Louie, T.; Fisher, M.; Hota, S.; Misra, B.; Van Hise, N.W.; Yen, E.F.; Bullock, J.S.; Pullman, J.; et al. Abstract S145, Week 24 Efficacy and safety data from PRISM3: A randomized, placebo-controlled trial evaluating CP101, an investigational orally administered microbiome therapeutic for the prevention of recurrent C. difficile infection. Am. J. Gastroenterol. 2021, 116, S63–S64. [Google Scholar]
- Louie, T.; Golan, Y.; Khanna, S.; Bobilev, D.; Erpelding, N.; Fratazzi, C.; Carini, M.; Menon, R.; Ruisi, M.; Norman, J.M.; et al. VE303, a defined bacterial consortium, for prevention of recurrent Clostridioides difficile infection: A randomized clinical trial. JAMA 2023, 329, 1356–1366. [Google Scholar] [CrossRef]
- McFarland, L.V.; Surawicz, C.M.; Greenberg, R.N.; Fekety, R.; Elmer, G.W.; Moyer, K.A.; Melcher, S.A.; Bowen, K.E.; Cox, J.L.; Noorani, Z.; et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA 1994, 271, 1913–1918. [Google Scholar] [CrossRef] [PubMed]
- Surawicz, C.M.; McFarland, L.V.; Greenberg, R.N.; Rubin, M.; Fekety, R.; Mulligan, M.E.; Garcia, R.J.; Brandmarker, S.; Bowen, K.; Borjal, D.; et al. The search for a better treatment for recurrent Clostridium difficile disease: Use of high-dose vancomycin combined with Saccharomyces boulardii. Clin. Infect. Dis. 2000, 31, 1012–1017. [Google Scholar] [CrossRef]
- Pomares Bascuñana, R.Á.; Veses, V.; Sheth, C.C. Effectiveness of fecal microbiota transplant for the treatment of Clostridioides difficile diarrhea: A systematic review and meta-analysis. Lett. Appl. Microbiol. 2021, 73, 149–158. [Google Scholar] [CrossRef]
- Tun, K.M.; Hsu, M.; Batra, K.; Lo, C.H.; Laeeq, T.; Vongsavath, T.; Mohammed, S.; Hong, A.S. Efficacy and safety of fecal microbiota transplantation in treatment of Clostridioides difficile infection among pediatric patients: A systematic review and meta-analysis. Microorganisms 2022, 10, 2450. [Google Scholar] [CrossRef] [PubMed]
- Tariq, R.; Pardi, D.S.; Bartlett, M.G.; Khanna, S. Low cure rates in controlled trials of fecal microbiota transplantation for recurrent Clostridium difficile infection: A systematic review and meta-analysis. Clin. Infect. Dis. 2019, 68, 1351–1358. [Google Scholar] [CrossRef]
- Fuentes, S.; Van Nood, E.; Tims, S.; Heikamp-de Jong, I.; Ter Braak, C.J.; Keller, J.J.; Zoetendal, E.G.; De Vos, W.M. Reset of a critically disturbed microbial ecosystem: Faecal transplant in recurrent Clostridium difficile infection. ISME J. 2014, 8, 1621–1633. [Google Scholar] [CrossRef] [PubMed]
- Terveer, E.M.; van Gool, T.; Ooijevaar, R.E.; Sanders, I.M.; Boeije-Koppenol, E.; Keller, J.J.; Bart, A.; Kuijper, E.J. Human transmission of Blastocystis by fecal microbiota transplantation without development of gastrointestinal symptoms in recipients. Clin. Infect. Dis. 2020, 71, 2630–2636. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Khanna, S. Safety of fecal microbiota transplantation for Clostridioides difficile infection focusing on pathobionts and SARS-CoV-2. Ther. Adv. Gastroenterol. 2021, 14, 17562848211009694. [Google Scholar] [CrossRef] [PubMed]
- Zellmer, C.; Sater, M.R.; Huntley, M.H.; Osman, M.; Olesen, S.W.; Ramakrishna, B. Shiga toxin–producing Escherichia coli transmission via fecal microbiota transplant. Clin. Infect. Dis. 2021, 72, e876–e880. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Wong, S.H.; Lam, K.; Lui, R.; Cheung, K.; Tang, W.; Ching, J.Y.; Chan, P.K.; Chan, M.C.; Wu, J.C.; et al. Bacteriophage transfer during faecal microbiota transplantation in Clostridium difficile infection is associated with treatment outcome. Gut 2018, 67, 634–643. [Google Scholar]
- Chopra, T. A profile of the live biotherapeutic product RBX2660 and its role in preventing recurrent Clostridioides difficile infection. Exp. Rev. Anti-Infect. Ther. 2023, 21, 243–253. [Google Scholar] [CrossRef]
- Orenstein, R.; Dubberke, E.; Hardi, R.; Ray, A.; Mullane, K.; Pardi, D.S.; Ramest, M.S.; for the PUNCH CD Investigators; Dubberke, E.R.; Hardi, R.; et al. Safety and durability of RBX2660 (microbiota suspension) for recurrent Clostridium difficile infection: Results of the PUNCH CD study. Clin. Infect. Dis. 2016, 62, 596–602. [Google Scholar] [CrossRef]
- Orenstein, R.; Dubberke, E.R.; Khanna, S.; Lee, C.H.; Yoho, D.; Johnson, S.; Hecht, G.; DuPont, H.L.; Gerding, D.N.; Blount, K.F.; et al. Durable reduction of Clostridioides difficile infection recurrence and microbiome restoration after treatment with RBX2660: Results from an open-label phase 2 clinical trial. BMC Infect. Dis. 2022, 22, 245. [Google Scholar] [CrossRef]
- Blount, K.; Walsh, D.M.; Gonzalez, C.; Shannon, B. Abstract 1064. Treatment Success in Reducing Recurrent Clostridioides difficile Infection with Investigational Live Biotherapeutic RBX2660 Is Associated with Microbiota Restoration: Consistent Evidence from a Phase 3 Clinical Trial. Open Forum Infect. Dis. 2021, 8 (Suppl. S1), S624–S625. [Google Scholar] [CrossRef]
- Khanna, S.; Sims, M.; Louie, T.J.; Fischer, M.; LaPlante, K.; Allegretti, J.; Hasson, B.R.; Fonte, A.T.; McChalicher, C.; Ege, D.S.; et al. SER-109: An oral investigational microbiome therapeutic for patients with recurrent Clostridioides difficile infection (rCDI). Antibiotics 2022, 11, 1234. [Google Scholar] [CrossRef] [PubMed]
- Sims, M.D.; Khanna, S.; Feuerstadt, P.; Louie, T.J.; Kelly, C.R.; Huang, E.S.; Hohmann, E.L.; Wang, E.E.; Oneto, C.; Cohen, S.H.; et al. Safety and Tolerability of SER-109 as an Investigational Microbiome Therapeutic in Adults with Recurrent Clostridioides difficile Infection: A Phase 3, Open-Label, Single-Arm Trial. JAMA Netw. Open 2023, 6, e2255758. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Kelly, C.R.; Louie, T.; Fisher, M.; Hota, S.; Misra, B.; Van Hise, N.W.; Yen, E.F.; Bullock, J.S.; Pullman, J.; et al. S131 CP101, an investigational orally administered microbiome therapeutic, increases intestinal microbiome diversity and prevents recurrent C. difficile infection: Results from a randomized, placebo-controlled trial. Am. J. Gastroentol. 2021, 116, S57. [Google Scholar] [CrossRef]
- Pochapin, M. The effect of probiotics on Clostridium difficile diarrhea. Am. J. Gastroentol. 2000, 95, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Wullt, M.; Hagslätt, M.L.; Odenholt, I. Lactobacillus plantarum 299v for the treatment of recurrent Clostridium difficile-associated diarrhoea: A double-blind, placebo-controlled trial. Scand. J. Infect. Dis. 2003, 35, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.K.; Duster, M.; Valentine, S.; Hess, T.; Archbald-Pannone, L.; Guerrant, R.; Safdar, N. A randomized controlled trial of probiotics for Clostridium difficile infection in adults (PICO). J. Antimicrob. Chemother. 2017, 72, 3177–3180. [Google Scholar] [CrossRef]
- Lawrence, S.J.; Korzenik, J.R.; Mundy, L.M. Probiotics for recurrent Clostridium difficile disease. J. Med. Microb. 2005, 54 Pt 9, 905–906. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Gnocchi, M.; Gagliardi, M.; Argentiero, A.; Neglia, C.; Esposito, S. Prevention of Clostridium difficile infection and associated diarrhea: An unsolved problem. Microorganisms 2020, 8, 1640. [Google Scholar] [CrossRef]
- Binda, S.; Hill, C.; Johansen, E.; Obis, D.; Pot, B.; Sanders, M.E.; Tremblay, A.; Ouwehand, A.C. Criteria to qualify microorganisms as “probiotic” in foods and dietary supplements. Front. Microbiol. 2020, 11, 1662. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Baldi, A. Regulatory categories of probiotics across the globe: A review representing existing and recommended categorization. Ind. J. Med. Microbiol. 2015, 33, S2–S10. [Google Scholar] [CrossRef]
- Kullar, R.; Johnson, S.; McFarland, L.V.; Goldstein, E.J.C. Potential roles for probiotics in the treatment of COVID-19 patients and prevention of complications associated with increased antibiotic use. Antibiotics 2021, 10, 408. [Google Scholar] [CrossRef]
- Khanna, S.; Pardi, D.S.; Jones, C.; Shannon, W.D.; Gonzalez, C.; Blount, K. RBX7455, a non-frozen, orally administered investigational live biotherapeutic, is safe, effective, and shifts patients’ microbiomes in a phase 1 study for recurrent Clostridioides difficile infections. Clin. Infect. Dis. 2021, 73, e1613–e1620. [Google Scholar] [CrossRef]
- Jones, J.; Pradhan, A.; Pizzuti, M.E.; Bland, C.M.; Bookstaver, P.B. Is three company or a crowd? Comparing and contrasting US and European Clostridioides difficile clinical practice guidelines. Antibiotics 2022, 11, 1247. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Zilberman-Schapira, G.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.Z.; Federici, S.; et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell 2018, 174, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Carneiro, S.; de Freitas, H.F.; do Rosário Esteves Guimarães, C. Can use of probiotics drugs cause fungemia?—A literature review. Res. Soc. Dev. 2022, 11, e45611932030. [Google Scholar] [CrossRef]
| Study Population | Probiotic Intervention | CDI in Probiotic vs. Control | Reference |
|---|---|---|---|
| Randomized controlled trials | |||
| N = 138 elderly inpatients given antibiotics U.K. | 2 strain mix (L. acido. + B. bifidum) 2 × 1010 for 20 days | 2.9% vs. 7.2% ns | Plummer S 2004 [48] |
| N = 100 inpatient adults or oral/IV antibiotics USA | 3 strain mix (L. acido.+ L. bulgaricus + B. bifidum) 1.2 × 1010 for duration antibiotics | 11% * vs. 40% | Rafiq K 2007 [49] |
| N = 42 inpatient adults on antibiotics Israel | 4 strain mix (L. acido. + L. bulgaricus + B. bifidum + Strept. thermophilus) 6 × 109 for 3 w | 14.3% vs. 4.8% ns | Stein GY 2007 [50] |
| N = 189 inpatient adults on antibiotics USA | Lcb. rhamnosus GG 4 × 1010 for 2 w | 4.2% vs. 7.4% ns | Miller M 2008 [51] |
| N = 316 inpatient adults on antibiotics USA | Lcb. rhamnosus GG 1.2 × 1011 for 2 w | 1.3% vs. 0% ns | Miller M 2008 [51] |
| Meta-analysis | |||
| N = 22 RCTs inpatient adults on antibiotics, separate subgroups by strain(s) | S. boulardii CNCM I-745 | RR = 0.52 (CI 0.31, 0.88) * | McFarland LV 2017 [52] |
| Lcb. casei DN114001 | RR = 0.07 (CI 0.01, 0.55) * | ||
| L. acido. + B. bifidum | RR = 0.41 (CI 0.21, 0.80) * | ||
| L. acido. + Lcb. casei LBC80R + Lcb. rhamnosus CLR2 | RR = 0.21 (CI 0.11, 0.40) * | ||
| Lcb. rhamnosus GG | RR = 0.56 (CI 0.29, 1.06) ns | ||
| Probiotic | Dose (cfu/d) | # Inpatients on Antibiotics | HO-CDI (during Probiotic vs. during Baseline/Control) | References |
|---|---|---|---|---|
| L. acidophilus CL1285 + Lbc. casei LBC80R + Lbc. rhamnosus CLR2 | 5–6 × 1010 | 6548 | 5.2 vs. 8.6/10,000 pd * | Maziade PJ 2013 [56] |
| 1 × 1011 | 985 | 5.5 vs. 6.9/10,000 pd *,** | Trick WE 2018 [57] | |
| 1 × 1011 | 8763 | 2.8 vs. 7.6/10,000 pd * | Olson B 2015 [58] | |
| 1 × 1011 | 1576 | 1.7 vs. 0.9/100 ns | Box MJ 2018 [59] | |
| 1 × 1011 | 3291 | 6 vs. 7.5/10,000 pd ns | Shihadeh K 2018 [60] | |
| S. boulardii CNCM I-745 | 1 × 1010 | 358 | 9.9 vs. 10.4/10,000 pd ns | Flatley EA 2015 [61] |
| 2 × 1010 | not reported | 9 vs. 10/10,000 pd ns | Slain D 2020 [62] | |
| 2 × 1010 | 8594 | 0.6 vs. 0.82/100 * | Wombwell 2021 [63] | |
| L. acidophilus + B. longum + B. bifidum Bb12 | 3 × 1010 | 743 | 5.5 vs. 16.8/100 * | Graul T 2009 [64] |
| 3 × 1010 | 43,206 | 3.9 vs. 4.9/10,000 pd * | Lewis PO 2017 [65] |
| Intervention + SoC Antibiotics | Study Population | Dose and Route | Follow-Up (w) | CDI Recurrence in Test vs. Control Group | Reference |
|---|---|---|---|---|---|
| FMT | rCDI (N = 43) | 1X, NG tube | 10 | 19% * vs. 69% | Van Nood E 2013 [82] |
| FMT | rCDI (N = 39) | 1–4X colonoscopy | 10 | 10% * vs. 74% | Cammarota G 2015 [83] |
| FMT | rCDI (N = 38) | 1X enema | 17 | 56% vs. 42% ns | Hota SS 2017 [84] |
| RBX2660 | rCDI (N = 267) | 1.5 × 109 1X enema | 8 | 29.4% * vs. 42.5% | Khanna S 2022 [85] |
| SER-109 | rCDI (N = 182) | 3 × 107 for 7 d oral capsule | 8 | 12% * vs. 40% | Feuerstadt P 2022 [86] |
| CP101 | rCDI (N = 198) | 6 × 1011 1X oral capsule | 24 | 26% * vs. 41% | Allegretti JR 2021 [87] |
| VE303 | rCDI (N = 79) | 2 × 1010 2 w 1 × 1011 2 w oral capsules | 8 | 37% vs. 46% ns 14% * vs. 46% | Louie T 2023 [88] |
| S. boulardii CNCM I-745 | iCDI (N = 64) rCDI (N = 60) | 3 × 1010 4 w oral capsules | 4 | 19.3% vs. 24.2% ns 34.6% * vs. 64.7% | McFarland L 1994 [89] |
| S. boulardii CNCM I-745 | rCDI (N = 32) | 3 × 1010 4 w oral capsules | 4 | 16.7% * vs. 50% ** | Surawicz C 2000 [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McFarland, L.V.; Goldstein, E.J.C.; Kullar, R. Microbiome-Related and Infection Control Approaches to Primary and Secondary Prevention of Clostridioides difficile Infections. Microorganisms 2023, 11, 1534. https://doi.org/10.3390/microorganisms11061534
McFarland LV, Goldstein EJC, Kullar R. Microbiome-Related and Infection Control Approaches to Primary and Secondary Prevention of Clostridioides difficile Infections. Microorganisms. 2023; 11(6):1534. https://doi.org/10.3390/microorganisms11061534
Chicago/Turabian StyleMcFarland, Lynne V., Ellie J. C. Goldstein, and Ravina Kullar. 2023. "Microbiome-Related and Infection Control Approaches to Primary and Secondary Prevention of Clostridioides difficile Infections" Microorganisms 11, no. 6: 1534. https://doi.org/10.3390/microorganisms11061534
APA StyleMcFarland, L. V., Goldstein, E. J. C., & Kullar, R. (2023). Microbiome-Related and Infection Control Approaches to Primary and Secondary Prevention of Clostridioides difficile Infections. Microorganisms, 11(6), 1534. https://doi.org/10.3390/microorganisms11061534