Combinatory Library of Microorganisms in the Selection of Reductive Activity Applied to a Ketone Mixture: Unexpected Highlighting of an Enantioselective Oxidative Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Analytical Methods
2.2. Screening of Microorganisms
2.3. Biotransformation by Rhodotorula buffoni
3. Results
3.1. Screening of Active Strains
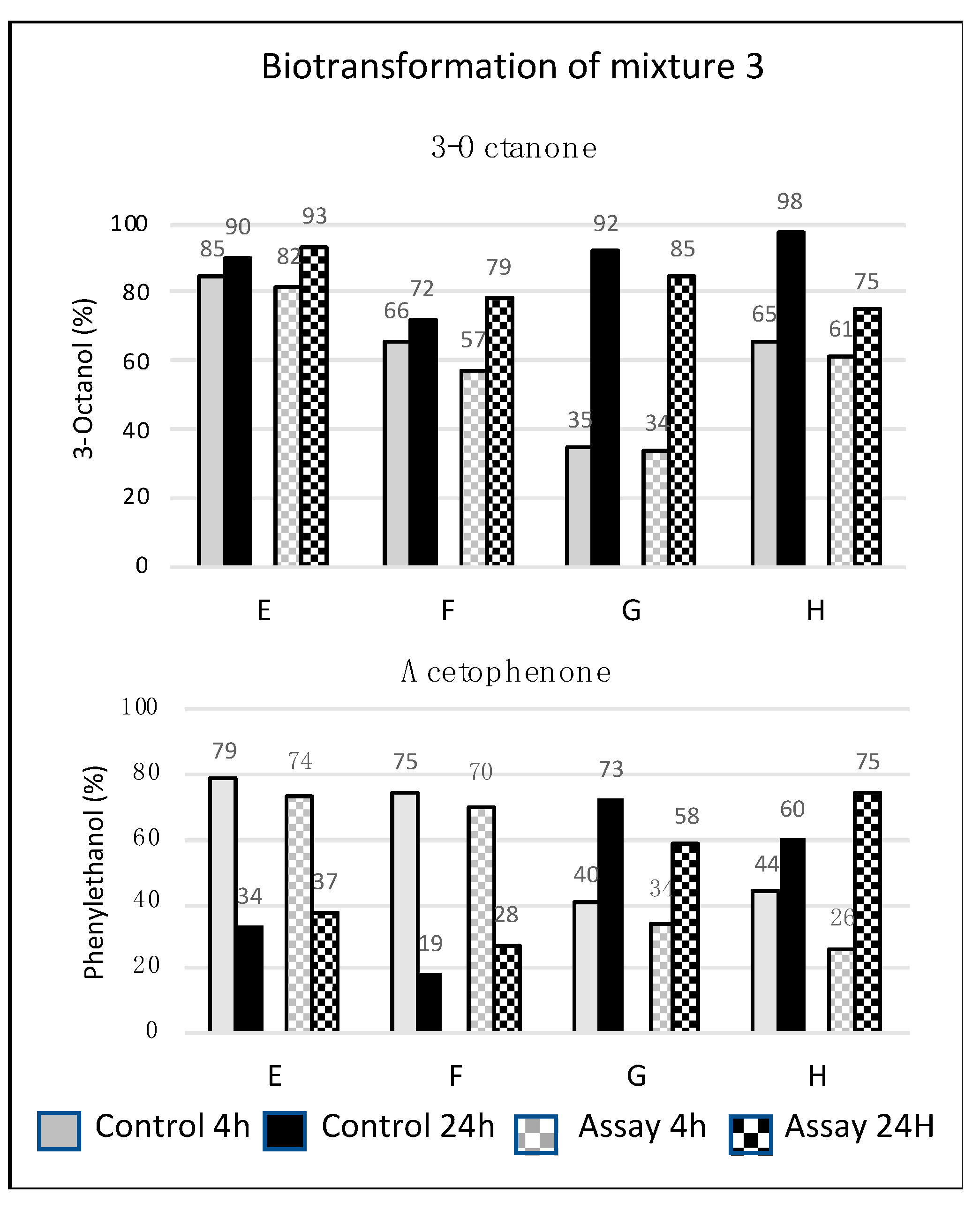
3.1.1. Biotransformation with CLM-1
3.1.2. Biotransformation with CLM-2
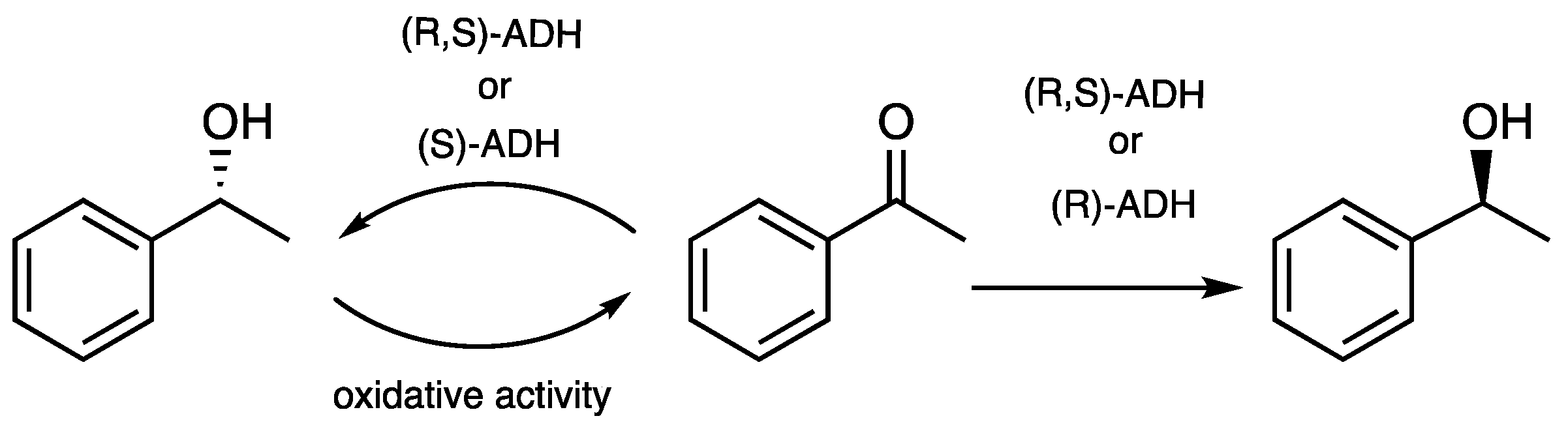
3.2. Biotransformation by R. buffoni
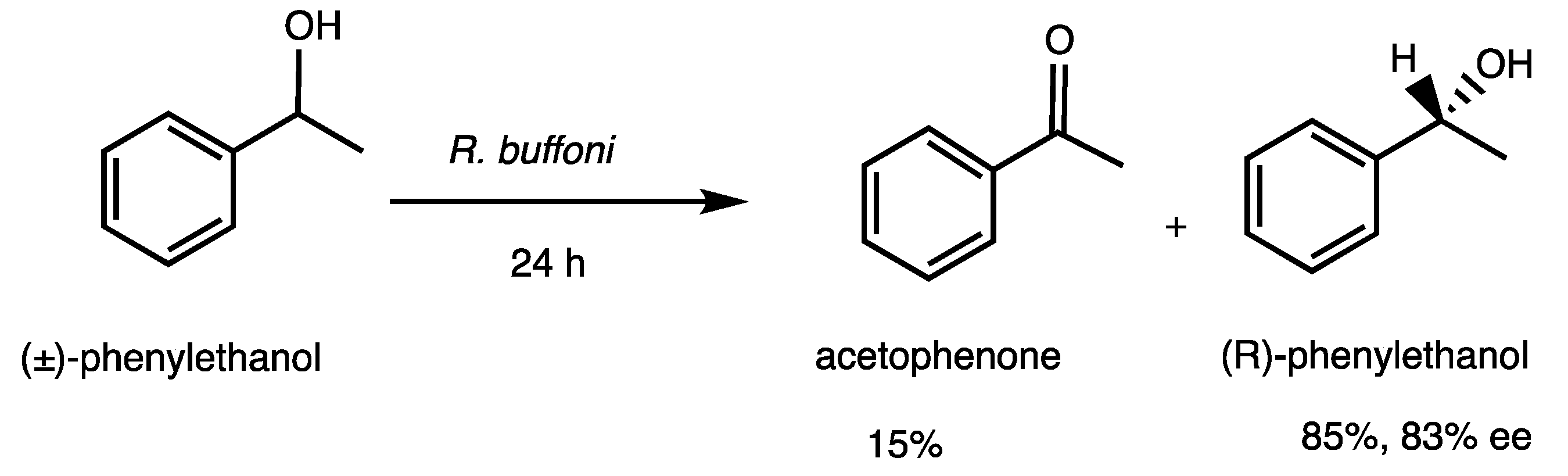
3.2.1. Evidence of Oxidation of Phenylethanol by R. buffoni
3.2.2. Biotransformation in Anaerobic Conditions
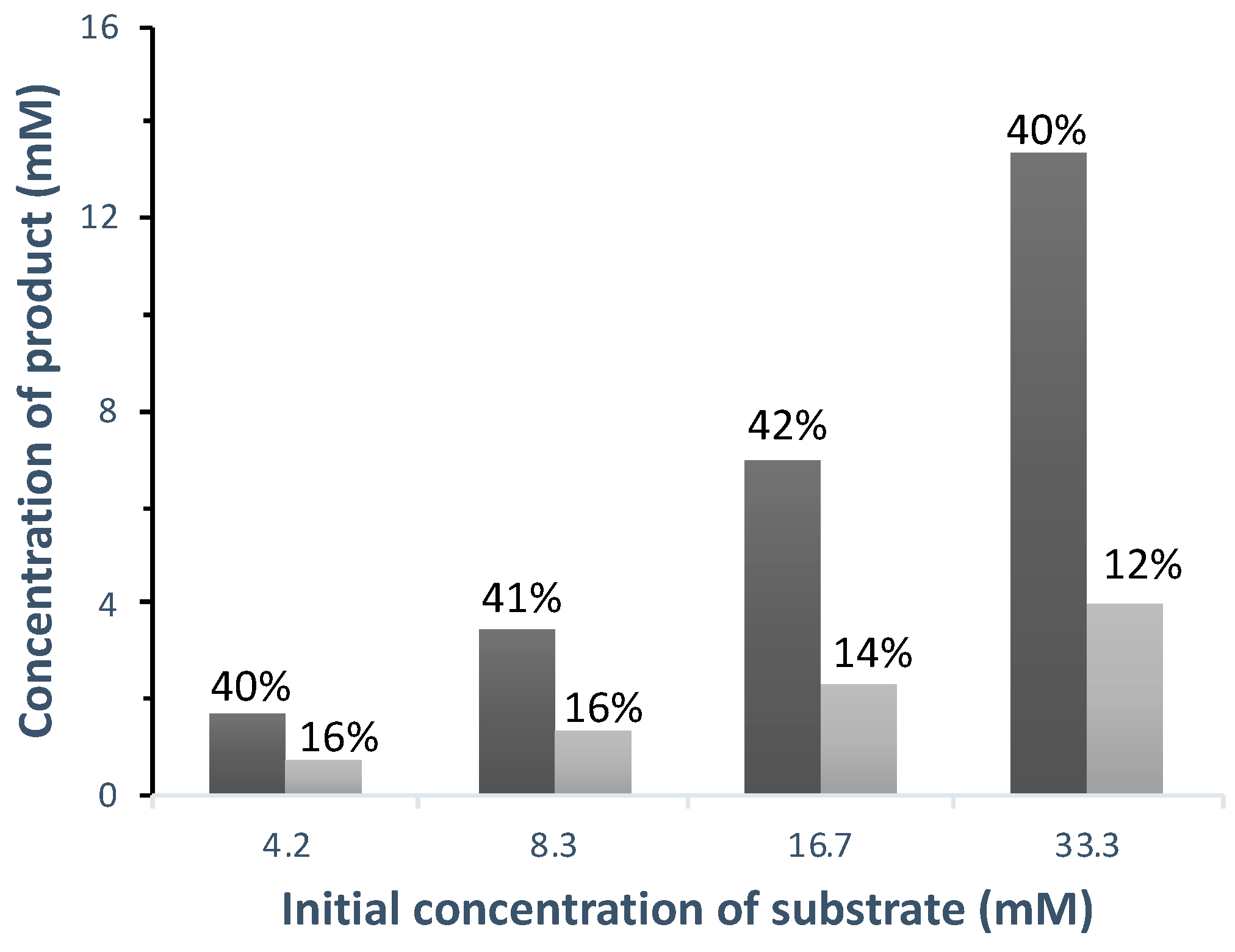
3.2.3. Biotransformation in Aerobic Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marshall, J.R.; Mangas-Sanchez, J.; Turner, N.J. Expanding the synthetic scope of biocatalysis by enzyme discovery and protein engineering. Tetrahedron 2021, 82, 131926. [Google Scholar] [CrossRef]
- Homann, M.J.; Vail, R.B.; Previte, E.; Tamarez, M.; Morgan, B.; Dodds, D.R.; Zaks, A. Rapid identification of enantioselective ketone reductions using targeted microbial libraries. Tetrahedron 2004, 60, 789–797. [Google Scholar] [CrossRef]
- Marvalin, C.; Azerad, R. Microbial production of phase I and phase II metabolites of propranolol. Xenobiotica 2011, 41, 175–186. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, P.; Zhou, Z.; Hu, Y.; Sha, Q.; Zhang, H.; Liu, X.; Du, W.; Feng, X.; Liu, B.-F. Agarose-based microwell array chip for high-throughput screening of functional microorganisms. Talanta 2019, 191, 342–349. [Google Scholar] [CrossRef]
- Beneyton, T.; Wijaya, I.P.M.; Postros, P.; Najah, M.; Leblond, P.; Couvent, A.; Mayot, E.; Griffiths, A.D.; Drevelle, A. High-throughput screening of filamentous fungi using nanoliter-range droplet-based microfluidics. Sci. Rep. 2016, 6, 27223. [Google Scholar] [CrossRef]
- Trower, M.K.; Buckland, R.M.; Higgins, R.; Griffin, M. Isolation and Characterization of a Cyclohexane-Metabolizing Xanthobacter sp. Appl. Environ. Microbiol. 1985, 49, 1282–1289. [Google Scholar] [CrossRef]
- Zheng, G.W.; Yu, H.L.; Zhang, J.D.; Xu, J.H. Enzymatic Production of l-Menthol by a High Substrate Concentration Tolerable Esterase from Newly Isolated Bacillus subtilis ECU0554. Adv. Synth. Catal. 2009, 351, 405–414. [Google Scholar] [CrossRef]
- Asano, Y.; Yasuda, T.; Tani, Y.; Yamada, H. A New Enzymatic Method of Acrylamide Production. Agric. Biol. Chem. 1982, 46, 1183–1189. [Google Scholar] [CrossRef]
- Eichhorn, E.; Roduit, J.P.; Shaw, N.; Heinzmann, K.; Kiener, A. Preparation of (S)-piperazine-2-carboxylic acid, (R)-piperazine-2-carboxylic acid, and (S)-piperidine-2-carboxylic acid by kinetic resolution of the corresponding racemic carboxamides with stereoselective amidases in whole bacterial cells. Tetrahedron Asymmetry 1997, 8, 2533–2536. [Google Scholar] [CrossRef]
- Bicalho, B.; Chen, L.S.; Grognux, J.; Reymond, J.-L.; Marsaioli, A.J. Studies on whole cell fluorescence-based screening for epoxide hydrolases and Baeyer-Villiger monoxygenases. J. Braz. Chem. Soc. 2004, 15, 911–916. [Google Scholar] [CrossRef]
- Angelini, L.M.L.; Silva, A.R.M.d.; Rocco, L.d.F.C.; Milagre, C.D.d.F. A high-throughput screening assay for distinguishing nitrile hydratases from nitrilases. Braz. J. Microbiol. 2015, 46, 113–116. [Google Scholar] [CrossRef]
- Fromentin, Y.; Grellier, P.; Wansi, J.D.; Lallemand, M.C.; Buisson, D. Yeast-Mediated Xanthone Synthesis through Oxidative Intramolecular Cyclization. Org. Lett. 2012, 14, 5054–5057. [Google Scholar] [CrossRef] [PubMed]
- Joyeau, R.; Planchon, M.; Abessolo, J.; Aissa, K.; Bance, C.; Buisson, D. Combinatorial approach to the selection of active microorganisms in biotransformation: Application to sinomenine. J. Mol. Catal. B-Enz. 2013, 85–86, 65–70. [Google Scholar] [CrossRef]
- Laurence, C.; Rivard, M.; Martens, T.; Morin, C.; Buisson, D.; Bourcier, S.; Sablier, M.; Oturan, M.A. Anticipating the fate and impact of organic environmental contaminants: A new approach applied to the pharmaceutical furosemide. Chemosphere 2014, 113, 193–199. [Google Scholar] [CrossRef]
- Boufridi, A.; Petek, S.; Evanno, L.; Beniddir, M.A.; Debitus, C.; Buisson, D.; Poupon, E. Biotransformations versus chemical modifications: New cytotoxic analogs of marine sesquiterpene ilimaquinone. Tetrahedron Lett. 2016, 57, 4922–4925. [Google Scholar] [CrossRef]
- Pinheiro, L.; Buisson, D.; Cortial, S.; Delaforge, M.; Ouazzani, J. Microbial decyanation of 1-benzylpyrrolidine-2,5-dicarbonitrile. Mechanistic investigations. J. Mol. Catal. B-Enz. 2011, 68, 211–215. [Google Scholar] [CrossRef]
- Kozłowska, J.; Potaniec, B.; Anioł, M. Biotransformation of Hydroxychalcones as a Method of Obtaining Novel and Unpredictable Products Using Whole Cells of Bacteria. Catalysts 2020, 10, 1167. [Google Scholar] [CrossRef]
- Liu, R.; Li, X.; Lam, K.S. Combinatorial chemistry in drug discovery. Curr. Opin. Chem. Biol. 2017, 38, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Sicard, R.; Goddard, J.-P.; Mazel, M.; Audiffrin, C.; Fourage, L.; Ravot, G.; Wahler, D.; Lefèvre, F.; Reymond, J.-L. Multienzyme Profiling of Thermophilic Microorganisms with a Substrate Cocktail Assay. Adv. Synth. Catal. 2005, 347, 987–996. [Google Scholar] [CrossRef]
- Azerad, R.; Buisson, D. Dynamic resolution and stereoinversion of secondary alcohols by chemo-enzymatic processes. Curr. Opin. Biotechnol. 2000, 11, 565–571. [Google Scholar] [CrossRef]
- Hall, M.; Bommarius, A.S. Enantioenriched Compounds via Enzyme-Catalyzed Redox Reactions. Chem. Rev. 2011, 111, 4088–4110. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Inoue, Y.; Matsuda, T.; Ohno, A. Microbial Deracemization of 1-Arylethanol. Tetrahedron Lett. 1995, 36, 6263–6266. [Google Scholar] [CrossRef]
- Comasseto, J.V.; Omori, A.T.; Andrade, L.H.; Porto, A.L.M. Bioreduction of fluoroacetophenones by the fungi Aspergillus terreus and Rhizopus oryzae. Tetrahedron Asymmetry 2003, 14, 711–715. [Google Scholar] [CrossRef]
- Li, Y.L.; Xu, J.H.; Xu, Y. Deracemization of aryl secondary alcohols via enantioselective oxidation and stereoselective reduction with tandem whole-cell biocatalysts. J. Mol. Catal. B-Enz. 2010, 64, 48–52. [Google Scholar] [CrossRef]
- Nasário, F.D.; Cazetta, T.; Moran, P.J.S.; Rodrigues, J.A.R. Deracemization of 1-phenylethanol via tandem biocatalytic oxidation and reduction. Tetrahedron Asymmetry 2016, 27, 404–409. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Ruan, G.; Yu, Y.; Liu, X. Asymmetric reduction of acetophenone into R-(+)-1-phenylethanol by endophytic fungus Neofusicoccum parvum BYEF07 isolated from Illicium verum. Biochem. Biophys. Res. Commun. 2016, 473, 874–878. [Google Scholar] [CrossRef]
- Araujo, L.S.; Kagohara, E.; Garcia, T.P.; Pellizari, V.H.; Andrade, L.H. Screening of microorganisms producing cold-active oxidoreductases to be applied in enantioselective alcohol oxidation. An Antarctic survey. Mar. Drugs 2011, 9, 889–905. [Google Scholar] [CrossRef]
- Janeczko, T.; Panek, A.; Świzdor, A.; Dmochowska-Gładysz, J.; Kostrzewa-Susłow, E. Enantioselective Dynamic Process Reduction of α- and β-Tetralone and Stereoinversion of Resulting Alcohols in a Selected Strain Culture. Curr. Microbiol. 2012, 65, 189–194. [Google Scholar] [CrossRef]
- Hilker, I.; Baldwin, C.; Alphand, V.; Furstoss, R.; Woodley, J.; Wohlgemuth, R. On the influence of oxygen and cell concentration in an SFPR whole cell biocatalytic Baeyer–Villiger oxidation process. Biotechnol. Bioeng. 2006, 93, 1138–1144. [Google Scholar] [CrossRef]
- Buisson, D.; Sanner, C.; Larchevêque, M.; Azerad, R. A study of the stereocontrolled reduction of aliphatic b-ketoesters by Geotrichum candidum. Biocatalysis 1992, 5, 249–265. [Google Scholar] [CrossRef]
- Buisson, D.; Elbaba, S.; Azerad, R. Yeast-Catalyzed Asymmetric Reduction of Benzil and Benzoin to Hydrobenzoin. Tetrahedron Lett. 1986, 27, 4453–4454. [Google Scholar] [CrossRef]
- Michels, P.C.; Khmelnitsky, Y.L.; Dordick, J.S.; Clark, D.S. Combinatorial biocatalysis: A natural approach to drug discovery. Trends Biotechnol. 1998, 16, 210–215. [Google Scholar] [CrossRef] [PubMed]





| CLM-1 | CLM-2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yeasts | Batch | A | B | C | D | E | F | G | H | |
| Pichia minuta MUCL 27758 | x | x | ||||||||
| Rhodosporidium toruloides MUCL 30010 | x | x | ||||||||
| Pichia anomala NRRL Y40 | x | |||||||||
| Kloekera magna NRRL Y1611 | x | x | ||||||||
| Torulaspora delbruckii MUCL 27816 | x | x | ||||||||
| Candida pinus MUCL 27856 | x | |||||||||
| Saccharomyces montanus CBS 6772 | x | x | ||||||||
| Saccharomyces sp. | x | x | ||||||||
| Rhodotorula rubra ATCC 4055 | x | |||||||||
| Rhodotorula pilimanae MUCL 27811 | x | x | ||||||||
| Rhodotorula glutinis NRRL 41091 | x | x | ||||||||
| Yarrowia lipolytica MUCL 29853 | x | |||||||||
| Candida magnolia MUCL27871 | x | x | ||||||||
| Cryptococcus humicola MUCL 30305 | x | x | ||||||||
| Kluyveromyces dobzanskii | x | |||||||||
| Cryptococcus macerans MUCL 30616 | x | x | ||||||||
| Rhodotorula buffonii MUCL 29812 | x | x | ||||||||
| Pichia guilliermondii MUCL 27758 | x | |||||||||
| Rhodosporidium toruloides MUCL 30328 | x | x | ||||||||
| Saccharomyces uvarum NRRL Y969 | x | x | ||||||||
| Sporidiobolus johnsonii ATCC 20490 | x | |||||||||
| Zygosaccharomyces fermentati MUCL 31281 | x | x | ||||||||
| Rhodotorula minuta MUCL 30637 | x | x | ||||||||
| Rhodotorula mucilaginosa | x | |||||||||
| Biomass g/L | Oxidation of Phenylethanol | Reduction of Acetophenone | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 4 h | 8 h | 24 h | 4 h | 8 h | 24 h | ||||
| % | % | % Abs. Conf. (ee) | % Abs. Conf. (ee) | % | % Abs. Conf. (ee) | ||||
| 50 | 58 | 63 | 69 | R (97) | 14 | S (7) | 21 | 36 | R (95) |
| 100 | 64 | 66 | 80 | R (89) | 24 | S (15) | 31 | 49 | R (89) |
| 150 | 73 | 73 | 88 | - | 35 | - | 37 | 59 | R (71) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali Rachedi, S.; Genest, M.; Mann, S.; Buisson, D. Combinatory Library of Microorganisms in the Selection of Reductive Activity Applied to a Ketone Mixture: Unexpected Highlighting of an Enantioselective Oxidative Activity. Microorganisms 2023, 11, 1415. https://doi.org/10.3390/microorganisms11061415
Ali Rachedi S, Genest M, Mann S, Buisson D. Combinatory Library of Microorganisms in the Selection of Reductive Activity Applied to a Ketone Mixture: Unexpected Highlighting of an Enantioselective Oxidative Activity. Microorganisms. 2023; 11(6):1415. https://doi.org/10.3390/microorganisms11061415
Chicago/Turabian StyleAli Rachedi, Sofiane, Maximillien Genest, Stéphane Mann, and Didier Buisson. 2023. "Combinatory Library of Microorganisms in the Selection of Reductive Activity Applied to a Ketone Mixture: Unexpected Highlighting of an Enantioselective Oxidative Activity" Microorganisms 11, no. 6: 1415. https://doi.org/10.3390/microorganisms11061415
APA StyleAli Rachedi, S., Genest, M., Mann, S., & Buisson, D. (2023). Combinatory Library of Microorganisms in the Selection of Reductive Activity Applied to a Ketone Mixture: Unexpected Highlighting of an Enantioselective Oxidative Activity. Microorganisms, 11(6), 1415. https://doi.org/10.3390/microorganisms11061415





