Exploring Synergistic Combinations in Extended and Pan-Drug Resistant (XDR and PDR) Whole Genome Sequenced Acinetobacter baumannii
Abstract
1. Introduction
2. Material and Methods
2.1. Whole Genome Sequencing (WGS)
2.2. MIC Determination
2.3. Antimicrobial Synergy Testing
2.4. Checkerboard Assay
2.5. Time-Kill Assay
2.6. Statistical Analysis
3. Results
3.1. Minimal Inhibitory Concentrations (MIC) of Fosfomycin, Meropenem, Tigecycline, Colistin and Amikacin in Acinetobacter baumannii
3.2. Synergy Outcomes
3.3. Outcome of Fosfomycin-Meropenem Combination by Checkerboard Assay
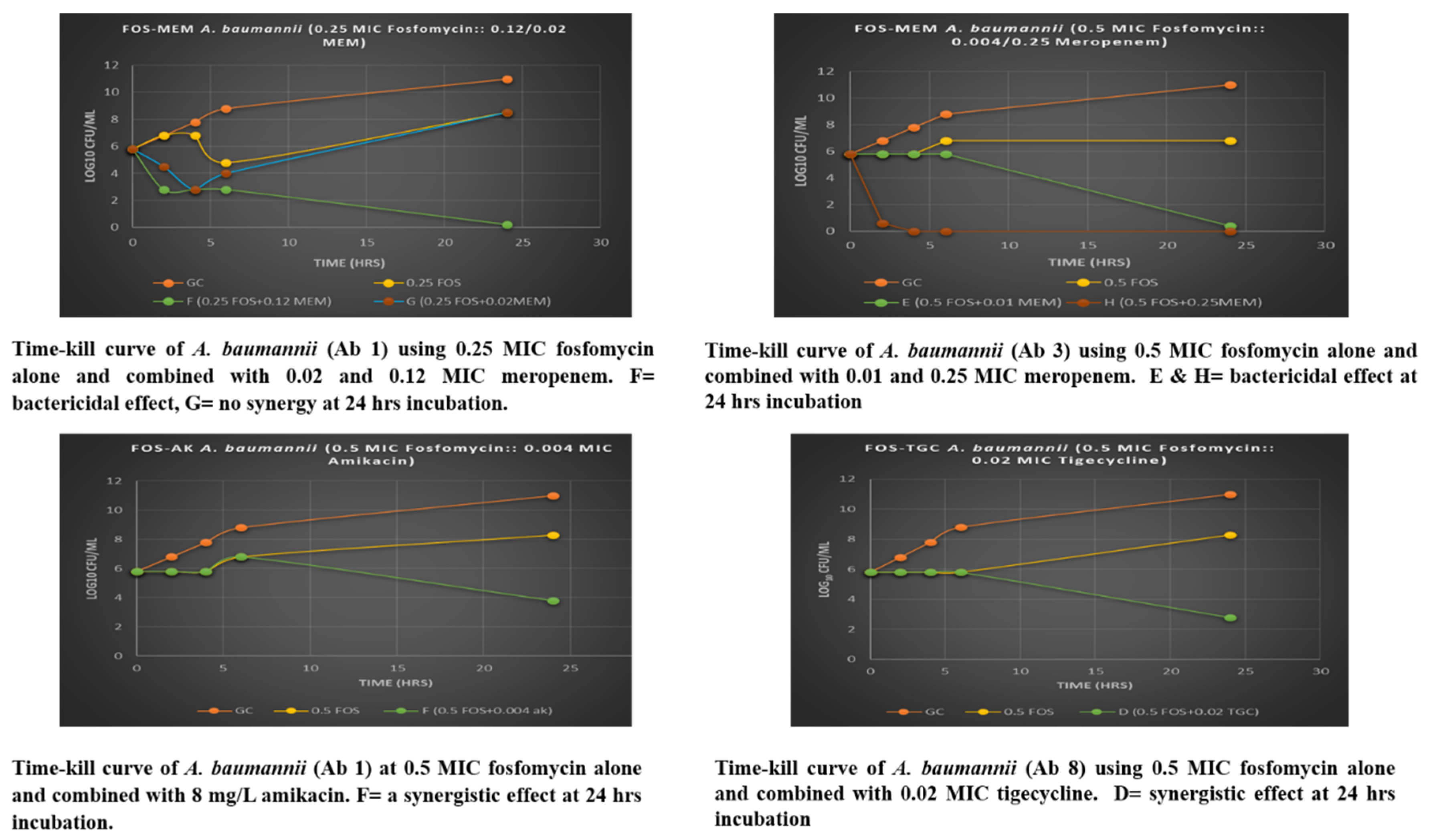
3.4. Assessment of Fosfomycin-Meropenem Interactions of A. baumannii by Time-Kill Assay
3.5. Synergy in PDR Strains
3.6. Identification of STs
3.7. Antibiotic Resistance Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Antunes, L.C.S.; Visca, P.; Towner, K.J. Acinetobacter baumannii: Evolution of a global pathogen. Pathog. Dis. 2014, 71, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Al Samawi, M.S.; Khan, F.Y.; Eldeeb, Y.; Almaslamani, M.; Alkhal, A.; Alsoub, H.; Ghadban, W.; Howady, F.; Hashim, S. Acinetobacter Infections among Adult Patients in Qatar: A 2-Year Hospital-Based Study. Can. J. Infect. Dis. Med. Microbiol. 2016, 2016, e6873689. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Ju, Y.G.; Lee, H.J.; Yim, H.S.; Lee, M.G.; Sohn, J.W.; Yoon, Y.K. In vitro synergistic antimicrobial activity of a combination of meropenem, colistin, tigecycline, rifampin, and ceftolozane/tazobactam against carbapenem-resistant Acinetobacter baumannii. Sci. Rep. 2022, 12, 7541. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Vouloumanou, E.K.; Samonis, G.; Vardakas, K.Z. Fosfomycin. Clin. Microbiol. Rev. 2016, 29, 321–347. [Google Scholar] [CrossRef]
- Sastry, S.; Clarke, L.G.; Alrowais, H.; Querry, A.M.; Shutt, K.A.; Doi, Y. Clinical Appraisal of Fosfomycin in the Era of Antimicrobial Resistance. Antimicrob. Agents Chemother. 2015, 59, 7355–7361. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Test for Bacteria That Grow Aerobically, 10th ed.; CLSI document M07-A10; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Rizvi, M.; Ahmed, J.; Khan, F.; Shukla, I.; Malik, A. Assessment of combination therapy by time kill curve analysis and chequerboard assay for treatment of multi-drug resistant Pseudomonas aeruginosa isolates. J. Glob. Antimicrob. Resist. 2013, 1, 103–108. [Google Scholar] [CrossRef]
- Garcia, L. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Clinical Microbiology Procedures Handbook: Update 2007; American Society for Microbiology: Washington, DC, USA, 2012. [Google Scholar]
- Zaidan, N.; Hornak, J.P.; Reynoso, D. Extensively Drug-Resistant Acinetobacter baumannii Nosocomial Pneumonia Successfully Treated with a Novel Antibiotic Combination. Antimicrob. Agents Chemother. 2021, 65, e00924-21. [Google Scholar] [CrossRef]
- Oo, C.; Sy, S.K.B. Fixed-dose combinations: A potential means to boost drug development for selected drugs. Drug Discov. Today 2018, 23, 457–459. [Google Scholar] [CrossRef]
- AL-Quraini, M.; Rizvi, M.; AL-Jabri, Z.; Sami, H.; AL-Muzahmi, M.; AL-Muharrmi, Z.; Taneja, N.; Al-Busaidi, I.; Soman, R. Assessment of In-Vitro Synergy of Fosfomycin with Meropenem, Amikacin and Tigecycline in Whole Genome Sequenced Extended and Pan Drug Resistant Klebsiella Pneumoniae: Exploring A Colistin Sparing Protocol. Antibiotics 2022, 11, 153. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Ioannou, P.; Kofteridis, D.D. In search for a synergistic combination against pandrug-resistant A. baumannii; methodological considerations. Infection 2022, 50, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Mohd Sazlly Lim, S.; Heffernan, A.J.; Roberts, J.A.; Sime, F.B. Pharmacodynamic Analysis of Meropenem and Fosfomycin Combination Against Carbapenem-Resistant Acinetobacter baumannii in Patients with Normal Renal Clearance: Can It Be a Treatment Option? Microb. Drug Resist. 2021, 27, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Singkham-In, U.; Chatsuwan, T. In vitro activities of carbapenems in combination with amikacin, colistin, or fosfomycin against carbapenem-resistant Acinetobacter baumannii clinical isolates. Diagn. Microbiol. Infect. Dis. 2018, 91, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Lv, Y.; Yuan, M.; Hu, X.; Nie, T.; Yang, X.; Li, G.; Pang, J.; Zhang, J.; Li, C.; et al. Genetic basis of high level aminoglycoside resistance in Acinetobacter baumannii from Beijing, China. Acta Pharm. Sin. B 2014, 4, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Kishk, R.; Soliman, N.; Nemr, N.; Eldesouki, R.; Mahrous, N.; Gobouri, A.; Azab, E.; Anani, M. Prevalence of Aminoglycoside Resistance and Aminoglycoside Modifying Enzymes in Acinetobacter baumannii Among Intensive Care Unit Patients, Ismailia, Egypt. Infect. Drug Resist. 2021, 14, 143–150. [Google Scholar] [CrossRef]
- Xu, C.; Bilya, S.R.; Xu, W. adeABC efflux gene in Acinetobacter baumannii. New Microbes New Infect. 2019, 30, 100549. [Google Scholar] [CrossRef] [PubMed]
- Leite, G.C.; Oliveira, M.S.; Perdigão-Neto, L.V.; Rocha, C.K.D.; Guimarães, T.; Rizek, C.; Levin, A.S.; Costa, S.F. Antimicrobial Combinations against Pan-Resistant Acinetobacter baumannii Isolates with Different Resistance Mechanisms. PLoS ONE 2016, 11, e0151270. [Google Scholar] [CrossRef]
- Montgomery, A.B.; Rhomberg, P.R.; Abuan, T.; Walters, K.A.; Flamm, R.K. Potentiation Effects of Amikacin and Fosfomycin against Selected Amikacin-Nonsusceptible Gram-Negative Respiratory Tract Pathogens. Antimicrob. Agents Chemother. 2014, 58, 3714–3719. [Google Scholar] [CrossRef]
- Martinez-Martinez, L.; Rodriguez, G.; Pascual, A.; Suárez, A.I.; Perea, E.J. In-vitro activity of antimicrobial agent combinations against multiresistant Acinetobacter baumannii. J. Antimicrob. Chemother. 1996, 38, 1107–1108. [Google Scholar] [CrossRef]
- Fan, B.; Guan, J.; Wang, X.; Cong, Y. Activity of Colistin in Combination with Meropenem, Tigecycline, Fosfomycin, Fusidic Acid, Rifampin or Sulbactam against Extensively Drug-Resistant Acinetobacter baumannii in a Murine Thigh-Infection Model. PLoS ONE 2016, 11, e0157757. [Google Scholar] [CrossRef]
- Bian, X.; Liu, X.; Chen, Y.; Chen, D.; Li, J.; Zhang, J. Dose Optimization of Colistin Combinations against Carbapenem-Resistant Acinetobacter baumannii from Patients with Hospital-Acquired Pneumonia in China by Using an In Vitro Pharmacokinetic/Pharmacodynamic Model. Antimicrob. Agents Chemother. 2019, 63, e01989-18. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, G.R.; Lehár, J.; Keith, C.T. Multi-target therapeutics: When the whole is greater than the sum of the parts. Drug Discov. Today 2007, 12, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Maifiah, M.H.M.; Creek, D.J.; Nation, R.L.; Forrest, A.; Tsuji, B.T.; Velkov, T.; Li, J. Untargeted metabolomics analysis reveals key pathways responsible for the synergistic killing of colistin and doripenem combination against Acinetobacter baumannii. Sci. Rep. 2017, 7, 45527. [Google Scholar] [CrossRef] [PubMed]
- Gaiarsa, S.; Batisti Biffignandi, G.; Esposito, E.P.; Castelli, M.; Jolley, K.A.; Brisse, S.; Sassera, D.; Zarrilli, R. Comparative Analysis of the Two Acinetobacter baumanniiMultilocus Sequence Typing (MLST) Schemes. Front. Microbiol. 2019, 10, 930. [Google Scholar] [CrossRef] [PubMed]
| Isolate | MIC Value (mg/L) | |||||
|---|---|---|---|---|---|---|
| Fosfomycin | Meropenem | Tigecycline | Amikacin | Colistin | ||
| AD * | BMD # | BMD # | BMD # | BMD # | BMD # | |
| Ab1 (PDR) | 128 | 128 | 16 | 2 | >512 | 16 |
| Ab 2 | 128 | 64 | 32 | 2 | >512 | 0.5 |
| Ab 3 | 128 | ≤128 | 32 | 4 | >512 | 1 |
| Ab 4 | 32 | 64 | 32 | 4 | >512 | 1 |
| Ab 5 | 32 | 64 | 32 | 2 | >512 | 1 |
| Ab 6 | ≤128 | 128 | 32 | 4 | >512 | 1 |
| Ab 7 | 64 | 32 | 32 | 4 | >512 | 0.25 |
| Ab 8 | 64 | 64 | 64 | ≤0.5 | ≤8 | ≤1 |
| Isolate | Fos MIC (mg/L) | Fold Decline | MEM MIC (mg/L) | Fold Decline | FICI (x̄) | ||
|---|---|---|---|---|---|---|---|
| Alone | Combined with MEM | Alone | Combined with FOS | ||||
| Ab1 | 128 | 32 | 4 | 16 | 4 | 4 | 0.50 (S) |
| Ab2 | 64 | 1 | ≥64 | 32 | 16 | 2 | 0.52 (PS) |
| Ab3 | 64 | 16 | 4 | 32 | 8 | 4 | 0.50 (S) |
| Ab4 | 64 | 16 | 4 | 32 | 8 | 4 | 0.50 (S) |
| Ab5 | 64 | 16 | 4 | 32 | 8 | 4 | 0.50 (S) |
| Ab6 | 128 | 32 | 4 | 32 | 8 | 4 | 0.50 (S) |
| Ab7 | 32 | 8 | 4 | 32 | 8 | 4 | 0.50 (S) |
| Ab8 | 64 | 16 | 4 | 64 | 16 | 4 | 0.50 (S) |
| FOS MIC (mg/L) | AK MIC (mg/L) | ||||||
| alone | Combined with AK | alone | Combined with FOS | ||||
| Ab1 | 128 | 128 | 0 | >1024 | ≤8 | ≥256 | 1.00 (IN) |
| Ab2 | 128 | 128 | 0 | >1024 | ≤8 | ≥256 | 1.00 (IN) |
| Ab3 | 128 | 128 | 0 | >1024 | ≤8 | ≥256 | 1.00 (IN) |
| Ab4 | 64 | 64 | 0 | 1024 | ≤4 | ≥256 | 1.00 (IN) |
| Ab5 | 64 | 64 | 0 | 1024 | ≤4 | ≥256 | 1.00 (IN) |
| Ab6 | 128 | 128 | 0 | 1024 | ≤4 | ≥256 | 1.00 (IN) |
| Ab7 | 32 | 32 | 0 | >1024 | ≤4 | ≥512 | 1.00 (IN) |
| MEM MIC (mg/L) | CL MIC (mg/L) | ||||||
| alone | Combined with COL | alone | Combined with MEM | ||||
| Ab 1 | 16 | 0.5 | 32 | 8 | ≤1 | ≥8 | 0.16 (S) |
| Ab 2 | 32 | 4 | 8 | ≤1 | 0.25 | 4 | 0.37 (S) |
| Ab 3 | 32 | 8 | 4 | 1 | 0.25 | 4 | 0.50 (S) |
| FOS MIC (mg/L) | TGC MIC (mg/L) | ||||||
| alone | Combined with TGC | alone | Combined with FOS | ||||
| Ab 1 | 128 | ≤2 | ≥64 | 2 | 1 | 2 | 0.52 |
| Ab 2 | 128 | ≤4 | ≥32 | 2 | 1 | 2 | 0.53 |
| Ab 3 | 128 | ≤4 | ≥32 | 4 | 2 | 2 | 0.53 |
| Ab 4 | 64 64 | ≤1 32 | ≥64 2 | 4 4 | 2 0.25 | 2 16 | 0.52 0.56 |
| Ab 5 | 64 64 | ≤1 32 | ≥64 2 | 2 2 | 1 ≤0.06 | 2 ≥32 | 0.52 0.53 |
| Ab 6 | 128 128 | ≤1 64 | ≥128 2 | 4 4 | 2 ≤0.06 | 2 ≥64 | 0.51 0.52 |
| Ab 7 | 32 | ≤1 | ≥32 | 4 | 2 | 2 | 0.53 |
| Combination | [(FOS) + (MEM)] mg/L | 6-h Effect | 24-h Effect | 24-h log10 Killing ∆ 24 h | Checkerboard |
|---|---|---|---|---|---|
| Fosfomycin + Meropenem | Isolate Ab 3 | ||||
| 0.25 FOS + 0.02 MEM | (32, 2) | Synergy | None | >+2 | Growth |
| 0.25 FOS + 0.12 MEM | (32, 8) | Bactericidal | Bactericidal | >−5 | Synergy |
| 0.5 FOS + 0.01 MEM | (64, 0.5) | None | Bactericidal | >−5 | Growth |
| 0.5 FOS + 0.25 MEM | (64, 16) | Bactericidal | Bactericidal | −6 | Partial synergy |
| Fosfomycin + Amikacin | Isolate Ab 1 | ||||
| 0.5 FOS + 0.004 AK | (64, 8) | None | Synergy | −2 | Growth |
| Fosfomycin + Tigecycline | Isolate Ab 4 | ||||
| 0.5 FOS + 0.02 TGC | (64, 0.06) | None | Bactericidal | −3 | Partial synergy |
| Sample | ST | gltA | gyrB | gdhB | recA | cpn60 | gpi | rpoD | Mismatches | Uncertainty | Depth | maxMAF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ab1 | 2062 | 1 | 17 | 189 | 2 | 2 | 108 | 3 | 0 | - | 197.8256 | 0.016667 |
| Ab2 | 2063 | 1 | 17 | 3 | 2 | 2 | 108 | 3 | 0 | - | 187.7057 | 0.013793 |
| Ab3 | 1816 | 1 | 3 | 189 | 2 | 2 | 96 | 3 | 0 | - | 161.3086 | 0.015625 |
| Ab4 | 1962 | 1 | 3 | 189 | 2 | 2 | 140 | 3 | 0 | - | 234.2391 | 0.00885 |
| Ab5 | 1962 | 1 | 3 | 189 | 2 | 2 | 140 | 3 | 0 | - | 210.5916 | 0.011364 |
| Ab6 | 1806 | 1 | 3 | 189 | 2 | 2 | 97 | 3 | 0 | - | 179.503 | 0.012422 |
| Ab7 | 234 | 21 | 48 | 58 | 42 | 36 | 109 | 4 | 0 | - | 258.423 | 0.013263 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quraini, M.A.; Jabri, Z.A.; Sami, H.; Mahindroo, J.; Taneja, N.; Muharrmi, Z.A.; Busaidi, I.A.; Rizvi, M. Exploring Synergistic Combinations in Extended and Pan-Drug Resistant (XDR and PDR) Whole Genome Sequenced Acinetobacter baumannii. Microorganisms 2023, 11, 1409. https://doi.org/10.3390/microorganisms11061409
Quraini MA, Jabri ZA, Sami H, Mahindroo J, Taneja N, Muharrmi ZA, Busaidi IA, Rizvi M. Exploring Synergistic Combinations in Extended and Pan-Drug Resistant (XDR and PDR) Whole Genome Sequenced Acinetobacter baumannii. Microorganisms. 2023; 11(6):1409. https://doi.org/10.3390/microorganisms11061409
Chicago/Turabian StyleQuraini, Munawr AL, Zaaema AL Jabri, Hiba Sami, Jaspreet Mahindroo, Neelam Taneja, Zakariya AL Muharrmi, Ibrahim AL Busaidi, and Meher Rizvi. 2023. "Exploring Synergistic Combinations in Extended and Pan-Drug Resistant (XDR and PDR) Whole Genome Sequenced Acinetobacter baumannii" Microorganisms 11, no. 6: 1409. https://doi.org/10.3390/microorganisms11061409
APA StyleQuraini, M. A., Jabri, Z. A., Sami, H., Mahindroo, J., Taneja, N., Muharrmi, Z. A., Busaidi, I. A., & Rizvi, M. (2023). Exploring Synergistic Combinations in Extended and Pan-Drug Resistant (XDR and PDR) Whole Genome Sequenced Acinetobacter baumannii. Microorganisms, 11(6), 1409. https://doi.org/10.3390/microorganisms11061409






