Shifts of Leaf Litter-Induced Plant-Soil Feedback from Negative to Positive Driven by Ectomycorrhizal Symbiosis between Quercus ilex and Pisolithus arrhizus
Abstract
1. Introduction
- Assess the effect of litter-induced plant-soil feedback across different decomposition stages in seedlings with and without ECM symbiosis of P. arrhizus.
- Connect the effect of litter-induced plant-soil feedback response with changes in the root system growth and structure upon addition of ECM inoculum.
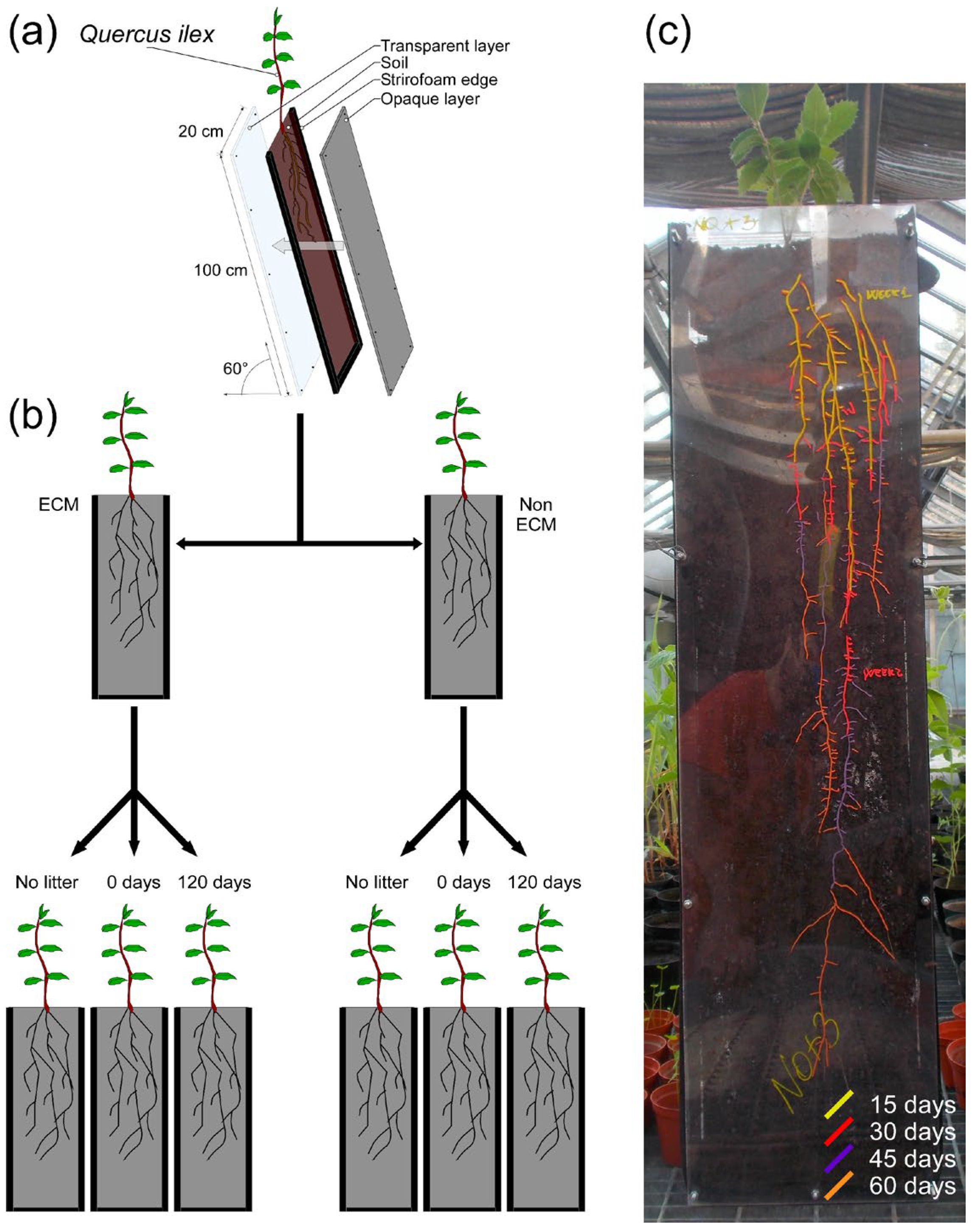
2. Materials and Methods
2.1. Production of Mycorrhized and Non-Mycorrhized Quercus ilex Seedlings
2.2. Rhizotron Experiment
2.3. Statistical Analysis
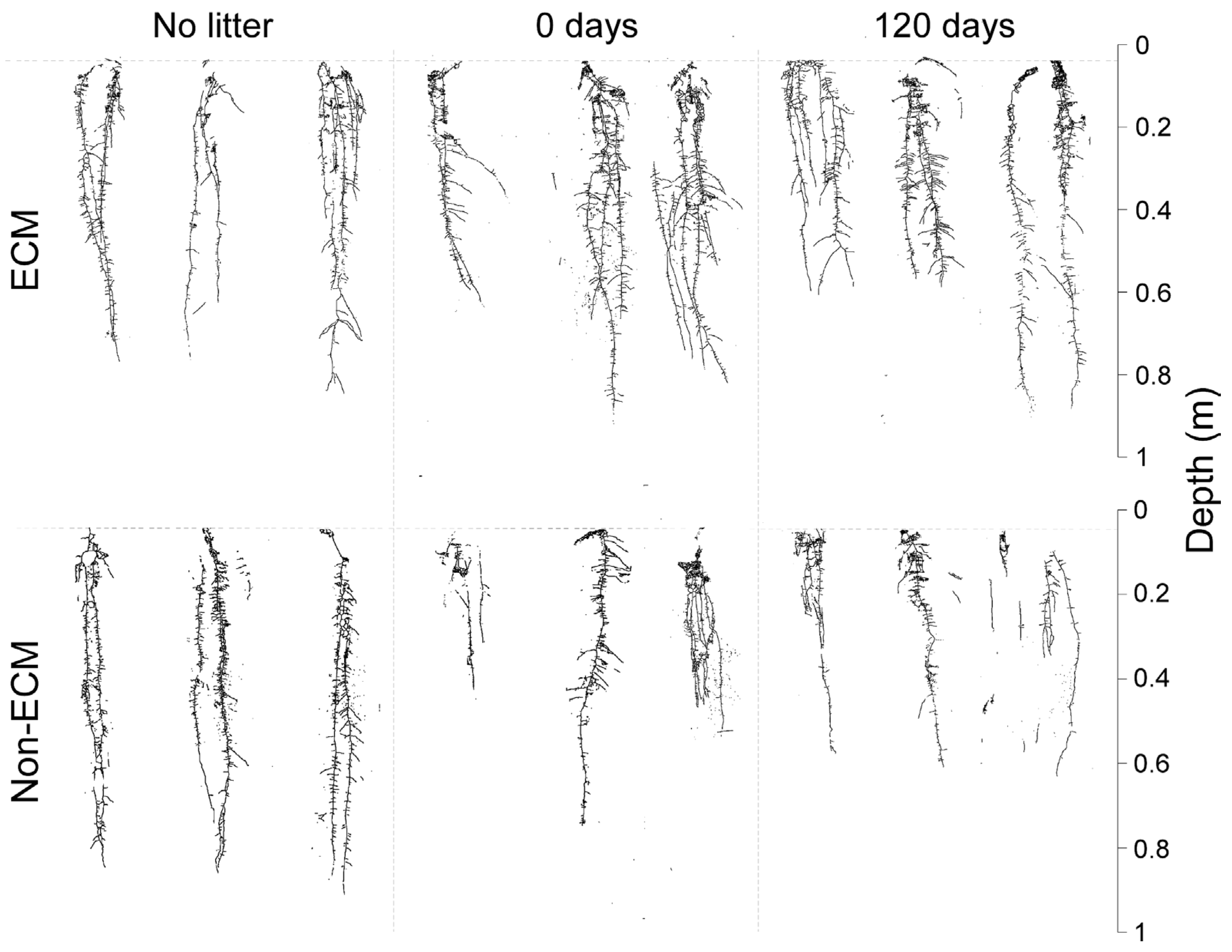
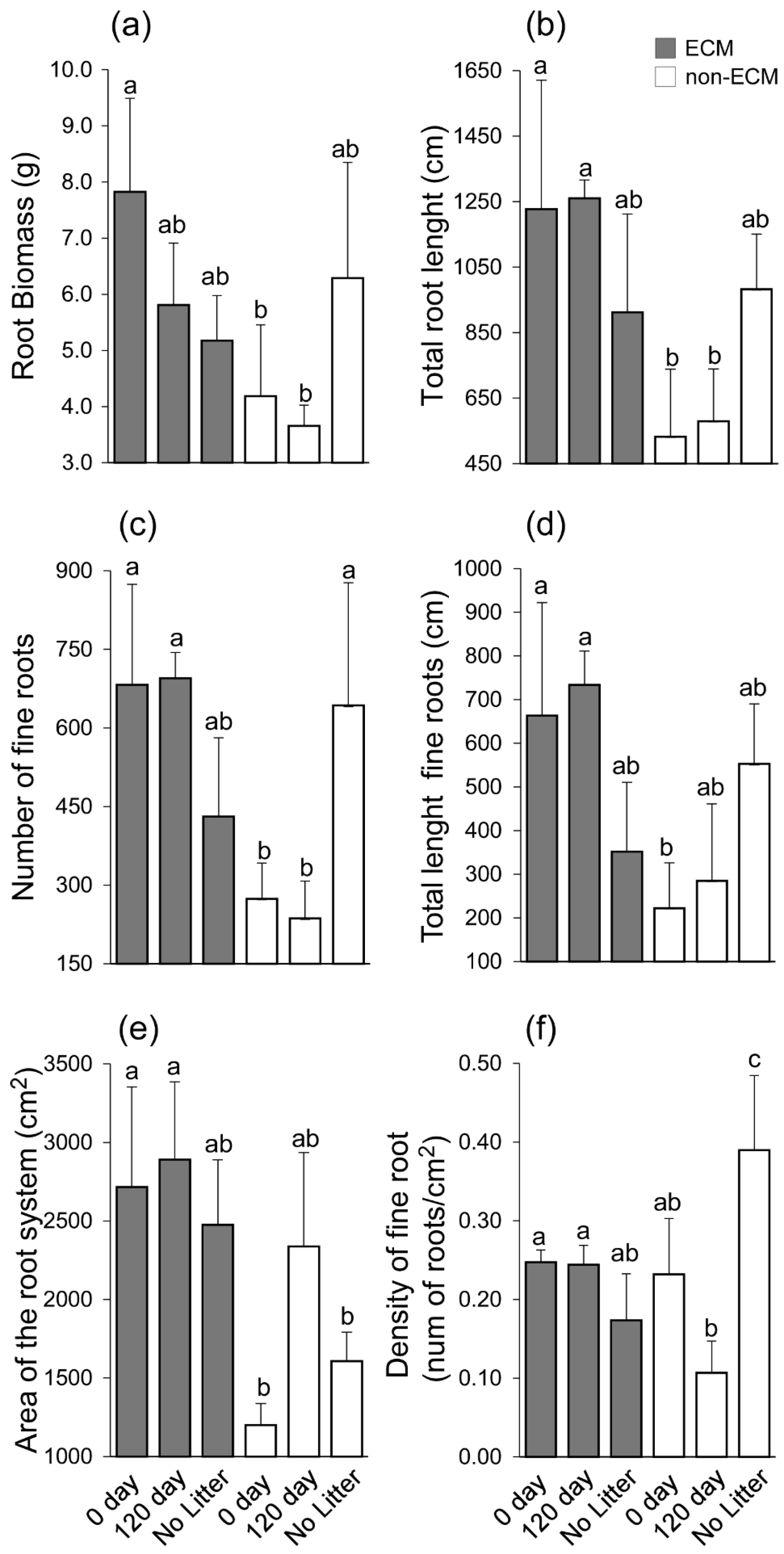
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brundrett, M. Mycorrhizas in natural ecosystems. Adv. Ecol. Res. 1991, 21, 171–313. [Google Scholar]
- Brundrett, M. Diversity and classification of mycorrhizal associations. Biol. Rev. 2004, 79, 473–495. [Google Scholar] [CrossRef] [PubMed]
- Corrales, A.; Henkel, T.W.; Smith, M.E. Ectomycorrhizal associations in the tropics–biogeography, diversity patterns and ecosystem roles. New Phytol. 2018, 220, 1076–1091. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.; Boller, T.; Wiemken, A.; Sanders, I.R. Different arbuscular mycorrhizal fungal species are potential determinants of plant community structure. Ecology 1998, 79, 2082–2091. [Google Scholar] [CrossRef]
- Klironomos, J.; Zobel, M.; Tibbett, M.; Stock, W.D.; Rillig, M.C.; Parrent, J.L.; Moora, M.; Koch, A.M.; Facelli, J.M.; Facelli, E. Forces that structure plant communities: Quantifying the importance of the mycorrhizal symbiosis. New Phytol. 2011, 189, 366–370. [Google Scholar] [CrossRef] [PubMed]
- McGuire, K.L. Common ectomycorrhizal networks may maintain monodominance in a tropical rain forest. Ecology 2007, 88, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.A.; Maherali, H.; Reinhart, K.O.; Lekberg, Y.; Hart, M.M.; Klironomos, J. Plant-soil feedbacks and mycorrhizal type influence temperate forest population dynamics. Science 2017, 355, 181–184. [Google Scholar] [CrossRef]
- Van der Putten, W.H.; Bardgett, R.D.; Bever, J.D.; Bezemer, T.M.; Casper, B.B.; Fukami, T.; Kardol, P.; Klironomos, J.N.; Kulmatiski, A.; Schweitzer, J.A. Plant–soil feedbacks: The past, the present and future challenges. J. Ecol. 2013, 101, 265–276. [Google Scholar] [CrossRef]
- Kulmatiski, A.; Beard, K.H.; Stevens, J.R.; Cobbold, S.M. Plant–soil feedbacks: A meta-analytical review. Ecol. Lett. 2008, 11, 980–992. [Google Scholar] [CrossRef]
- Cesarano, G.; Zotti, M.; Antignani, V.; Marra, R.; Scala, F.; Bonanomi, G. Soil sickness and negative plant-soil feedback: A reappraisal of hypotheses. J. Plant Pathol. 2017, 99, 545–570. [Google Scholar]
- Bonanomi, G.; Incerti, G.; Stinca, A.; Cartenì, F.; Giannino, F.; Mazzoleni, S. Ring formation in clonal plants. Commun. Ecol. 2014, 15, 77–86. [Google Scholar] [CrossRef]
- Cartenì, F.; Marasco, A.; Bonanomi, G.; Mazzoleni, S.; Rietkerk, M.; Giannino, F. Negative plant soil feedback explaining ring formation in clonal plants. J. Theor. Biol. 2012, 313, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, G.; Rietkerk, M.; Dekker, S.C.; Mazzoleni, S. Negative plant–soil feedback and positive species interaction in a herbaceous plant community. Plant Ecol. 2005, 181, 269. [Google Scholar] [CrossRef]
- Kardol, P.; Cornips, N.J.; van Kempen, M.M.; Bakx-Schotman, J.T.; van der Putten, W.H. Microbe-mediated plant–soil feedback causes historical contingency effects in plant community assembly. Ecol. Monogr. 2007, 77, 147–162. [Google Scholar] [CrossRef]
- Kardol, P.; Martijn Bezemer, T.; Van Der Putten, W.H. Temporal variation in plant–soil feedback controls succession. Ecol. Lett. 2006, 9, 1080–1088. [Google Scholar] [CrossRef]
- Reinhart, K.O. The organization of plant communities: Negative plant–soil feedbacks and semiarid grasslands. Ecology 2012, 93, 2377–2385. [Google Scholar] [CrossRef]
- Bonanomi, G.; Giannino, F.; Mazzoleni, S. Negative plant–soil feedback and species coexistence. Oikos 2005, 111, 311–321. [Google Scholar] [CrossRef]
- Packer, A.; Clay, K. Soil pathogens and spatial patterns of seedling mortality in a temperate tree. Nature 2000, 404, 278. [Google Scholar] [CrossRef]
- Smith-Ramesh, L.M.; Reynolds, H.L. The next frontier of plant–soil feedback research: Unraveling context dependence across biotic and abiotic gradients. J. Veg. Sci. 2017, 28, 484–494. [Google Scholar] [CrossRef]
- Bonanomi, G.; Rietkerk, M.; Dekker, S.C.; Mazzoleni, S. Islands of fertility induce co-occurring negative and positive plant-soil feedbacks promoting coexistence. Plant Ecol. 2008, 197, 207–218. [Google Scholar] [CrossRef]
- Bonanomi, G.; Sicurezza, M.G.; Caporaso, S.; Esposito, A.; Mazzoleni, S. Phytotoxicity dynamics of decaying plant materials. New Phytol. 2006, 169, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Mazzoleni, S.; Bonanomi, G.; Incerti, G.; Chiusano, M.L.; Termolino, P.; Mingo, A.; Senatore, M.; Giannino, F.; Cartenì, F.; Rietkerk, M. Inhibitory and toxic effects of extracellular self-DNA in litter: A mechanism for negative plant–soil feedbacks? New Phytol. 2015, 205, 1195–1210. [Google Scholar] [CrossRef] [PubMed]
- Mazzoleni, S.; Bonanomi, G.; Giannino, F.; Rietkerk, M.; Dekker, S.; Zucconi, F. Is plant biodiversity driven by decomposition processes? An emerging new theory on plant diversity. Commun. Ecol. 2007, 8, 103–109. [Google Scholar] [CrossRef]
- Brundrett, M.C. Coevolution of roots and mycorrhizas of land plants. New Phytol. 2002, 154, 275–304. [Google Scholar] [CrossRef]
- ZengPu, L.; JunRan, J.; Changwen, W. Antagonism between Ectomycorrhizal fungi and plant pathogens. In Mycorrhizas for Plantation Forestry in Asia; Brundett, M., Dell, B., Malajczuk, N., Mingqin, G., Eds.; Australian Centre for International Agricultural Research (ACIAR): Canberra, Australia, 1994; pp. 77–81. [Google Scholar]
- Van der Heijden, M.G.; Sanders, I.R. Mycorrhizal ecology: Synthesis and perspectives. In Mycorrhizal Ecology; Springer: Berlin/Heidelberg, Germany, 2002; pp. 441–456. [Google Scholar]
- Tuomi, J.; Kytöviita, M.M.; Härdling, R. Cost efficiency of nutrient acquisition and the advantage of mycorrhizal symbiosis for the host plant. Oikos 2001, 92, 62–70. [Google Scholar] [CrossRef]
- Lindahl, B.D.; Tunlid, A. Ectomycorrhizal fungi–potential organic matter decomposers, yet not saprotrophs. New Phytol. 2015, 205, 1443–1447. [Google Scholar] [CrossRef]
- Bödeker, I.T.; Clemmensen, K.E.; de Boer, W.; Martin, F.; Olson, Å.; Lindahl, B.D. Ectomycorrhizal Cortinarius species participate in enzymatic oxidation of humus in northern forest ecosystems. New Phytol. 2014, 203, 245–256. [Google Scholar] [CrossRef]
- Booth, M.G.; Hoeksema, J.D. Mycorrhizal networks counteract competitive effects of canopy trees on seedling survival. Ecology 2010, 91, 2294–2302. [Google Scholar] [CrossRef]
- Singh, H.; Batish, D.R.; Kohli, R. Autotoxicity: Concept, organisms, and ecological significance. Crit. Rev. Plant Sci. 1999, 18, 757–772. [Google Scholar] [CrossRef]
- An, M.; Pratley, J.; Haig, T. Phytotoxicity of Vulpia residues: IV. Dynamics of allelochemicals during decomposition of Vulpia residues and their corresponding phytotoxicity. J. Chem. Ecol. 2001, 27, 395–409. [Google Scholar] [CrossRef]
- Trifonova, R.; Postma, J.; Verstappen, F.W.; Bouwmeester, H.J.; Ketelaars, J.J.; van Elsas, J.-D. Removal of phytotoxic compounds from torrefied grass fibres by plant-beneficial microorganisms. FEMS Microbiol. Ecol. 2008, 66, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Mazzoleni, S.; Bonanomi, G.; Giannino, F.; Incerti, G.; Dekker, S.C.; Rietkerk, M. Modelling the effects of litter decomposition on tree diversity patterns. Ecol. Model. 2010, 221, 2784–2792. [Google Scholar] [CrossRef]
- Cartenì, F.; Bonanomi, G.; Giannino, F.; Incerti, G.; Vincenot, C.E.; Chiusano, M.L.; Mazzoleni, S. Self-DNA inhibitory effects: Underlying mechanisms and ecological implications. Plant Signal. Behav. 2016, 11, e1158381. [Google Scholar] [CrossRef] [PubMed]
- Vincenot, C.E.; Cartenì, F.; Bonanomi, G.; Mazzoleni, S.; Giannino, F. Plant–soil negative feedback explains vegetation dynamics and patterns at multiple scales. Oikos 2017, 126, 1319–1328. [Google Scholar] [CrossRef]
- Bonanomi, G.; Zotti, M.; Idbella, M.; Termolino, P.; De Micco, V.; Mazzoleni, S. Field evidence for litter and self-DNA inhibitory effects on Alnus glutinosa roots. New Phytol. 2022, 236, 399–412. [Google Scholar] [CrossRef]
- Wurst, S.; Kaiser, N.; Nitzsche, S.; Haase, J.; Auge, H.; Rillig, M.C.; Powell, J.R. Tree diversity modifies distance-dependent effects on seedling emergence but not plant–soil feedbacks of temperate trees. Ecology 2015, 96, 1529–1539. [Google Scholar] [CrossRef]
- Read, D.; Perez-Moreno, J. Mycorrhizas and nutrient cycling in ecosystems—A journey towards relevance? New Phytol. 2003, 157, 475–492. [Google Scholar] [CrossRef]
- Perez-Moreno, J.; Read, D. Mobilization and transfer of nutrients from litter to tree seedlings via the vegetative mycelium of ectomycorrhizal plants. New Phytol. 2000, 145, 301–309. [Google Scholar] [CrossRef]
- Mazzoleni, S.; Cartenì, F.; Bonanomi, G.; Senatore, M.; Termolino, P.; Giannino, F.; Incerti, G.; Rietkerk, M.; Lanzotti, V.; Chiusano, M.L. Inhibitory effects of extracellular self-DNA: A general biological process? New Phytol. 2015, 206, 127–132. [Google Scholar] [CrossRef]
- Molter, D.J. On mycorrhizal individuality. Biol. Philos. 2019, 34, 52. [Google Scholar] [CrossRef]
- Calogne, F. Les gastéromycètes d’Espagne. Bull Soc. Mycol. Fr. 1975, 91, 247–292. [Google Scholar]
- Marx, D.H. Tree host range and world distribution of the ectomycorrhizal fungus Pisolithus tinctorius. Can. J. Microbiol. 1977, 23, 217–223. [Google Scholar] [CrossRef]
- Cairney, J.; Chambers, S. Interactions between Pisolithus tinctorius and its hosts: A review of current knowledge. Mycorrhiza 1997, 7, 117–131. [Google Scholar] [CrossRef]
- Li, J.; Romane, F.J. Effects of germination inhibition on the dynamics of Quercus ilex stands. J. Veg. Sci. 1997, 8, 287–294. [Google Scholar]
- Teobaldelli, M.; Cona, F.; Stinca, A.; Saulino, L.; Anzano, E.; Giordano, D.; Migliozzi, A.; Bonanomi, G.; D’Urso, G.; Mazzoleni, S. Improving resilience of an old-growth urban forest in Southern Italy: Lesson(s) from a stand-replacing windstorm. Urban For. Urban Green. 2020, 47, 126521. [Google Scholar] [CrossRef]
- Bonanomi, G.; Incerti, G.; Abd El-Gawad, A.M.; Sarker, T.C.; Stinca, A.; Motti, R.; Cesarano, G.; Teobaldelli, M.; Saulino, L.; Cona, F. Windstorm disturbance triggers multiple species invasion in an urban Mediterranean forest. Iforest-Biogeosci. For. 2018, 11, 64. [Google Scholar] [CrossRef]
- Bonanomi, G.; Sarker, T.C.; Zotti, M.; Cesarano, G.; Allevato, E.; Mazzoleni, S. Predicting nitrogen mineralization from organic amendments: Beyond C/N ratio by 13C-CPMAS NMR approach. Plant Soil 2019, 441, 129–146. [Google Scholar] [CrossRef]
- Cohen, J. The effect size index: D. Stat. Power Anal. Behav. Sci. 1988, 2. [Google Scholar]
- Teste, F.P.; Kardol, P.; Turner, B.L.; Wardle, D.A.; Zemunik, G.; Renton, M.; Laliberté, E. Plant-soil feedback and the maintenance of diversity in Mediterranean-climate shrublands. Science 2017, 355, 173–176. [Google Scholar] [CrossRef]
- Ditengou, F.A.; Müller, A.; Rosenkranz, M.; Felten, J.; Lasok, H.; Van Doorn, M.M.; Legué, V.; Palme, K.; Schnitzler, J.-P.; Polle, A. Volatile signalling by sesquiterpenes from ectomycorrhizal fungi reprogrammes root architecture. Nat. Commun. 2015, 6, 6279. [Google Scholar] [CrossRef]
- Diaz, E.C.; Martin, F.; Tagu, D. Eucalypt α-tubulin: cDNA cloning and increased level of transcripts in ectomycorrhizal root system. Plant Mol. Biol. 1996, 31, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.T.; Sylvia, D.M.; Shilling, D.G. Pisolithus arhizus ectomycorrhiza affects plant competition for phosphorus between Pinus elliottii and Panicum chamaelonche. Mycorrhiza 1999, 9, 199–204. [Google Scholar] [CrossRef]
- Carteron, A.; Beigas, M.; Joly, S.; Turner, B.L.; Laliberté, E. Temperate forests dominated by arbuscular or ectomycorrhizal fungi are characterized by strong shifts from saprotrophic to mycorrhizal fungi with increasing soil depth. Microb. Ecol. 2021, 82, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, R.; Sotome, K.; Maekawa, N.; Nakagiri, A.; Endo, N. Mycorrhizal synthesis, morpho-anatomical characterization of mycorrhizae, and evaluation of mycorrhiza-forming ability of Hydnum albidum–like species using monokaryotic and dikaryotic cultures. Mycorrhiza 2021, 31, 349–359. [Google Scholar] [CrossRef]
- Koide, R.T.; Fernandez, C.; Petprakob, K. General principles in the community ecology of ectomycorrhizal fungi. Ann. For. Sci. 2011, 68, 45–55. [Google Scholar] [CrossRef]
- Lindahl, B.D.; Ihrmark, K.; Boberg, J.; Trumbore, S.E.; Högberg, P.; Stenlid, J.; Finlay, R.D. Spatial separation of litter decomposition and mycorrhizal nitrogen uptake in a boreal forest. New Phytol. 2007, 173, 611–620. [Google Scholar] [CrossRef]
- DeBell, D.S. Phytotoxic effects of cherrybark oak. For. Sci. 1971, 17, 180–185. [Google Scholar]
- Dabral, A.; Butola, B.; Khanduri, V.; Sharma, C.; Dhanush, C. Autotoxicity against seed germination, seedling emergence of Quercus leucotrichophora A. Camus Ex. Bahadur. J. Pharmacogn. Phytochem. 2018, 7, 1813–1816. [Google Scholar]
- Rytioja, J.; Hildén, K.; Yuzon, J.; Hatakka, A.; de Vries, R.P.; Mäkelä, M.R. Plant-polysaccharide-degrading enzymes from basidiomycetes. Microbiol. Mol. Biol. Rev. 2014, 78, 614–649. [Google Scholar] [CrossRef]
- Baldrian, P. Enzymes of saprotrophic basidiomycetes. In Proceedings of the British Mycological Society Symposia Series; Academic Press: Cambridge, MA, USA, 2008; pp. 19–41. [Google Scholar]
- Op De Beeck, M.; Troein, C.; Peterson, C.; Persson, P.; Tunlid, A. Fenton reaction facilitates organic nitrogen acquisition by an ectomycorrhizal fungus. New Phytol. 2018, 218, 335–343. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zotti, M.; Bonanomi, G.; Saulino, L.; Allevato, E.; Saracino, A.; Mazzoleni, S.; Idbella, M. Shifts of Leaf Litter-Induced Plant-Soil Feedback from Negative to Positive Driven by Ectomycorrhizal Symbiosis between Quercus ilex and Pisolithus arrhizus. Microorganisms 2023, 11, 1394. https://doi.org/10.3390/microorganisms11061394
Zotti M, Bonanomi G, Saulino L, Allevato E, Saracino A, Mazzoleni S, Idbella M. Shifts of Leaf Litter-Induced Plant-Soil Feedback from Negative to Positive Driven by Ectomycorrhizal Symbiosis between Quercus ilex and Pisolithus arrhizus. Microorganisms. 2023; 11(6):1394. https://doi.org/10.3390/microorganisms11061394
Chicago/Turabian StyleZotti, Maurizio, Giuliano Bonanomi, Luigi Saulino, Emilia Allevato, Antonio Saracino, Stefano Mazzoleni, and Mohamed Idbella. 2023. "Shifts of Leaf Litter-Induced Plant-Soil Feedback from Negative to Positive Driven by Ectomycorrhizal Symbiosis between Quercus ilex and Pisolithus arrhizus" Microorganisms 11, no. 6: 1394. https://doi.org/10.3390/microorganisms11061394
APA StyleZotti, M., Bonanomi, G., Saulino, L., Allevato, E., Saracino, A., Mazzoleni, S., & Idbella, M. (2023). Shifts of Leaf Litter-Induced Plant-Soil Feedback from Negative to Positive Driven by Ectomycorrhizal Symbiosis between Quercus ilex and Pisolithus arrhizus. Microorganisms, 11(6), 1394. https://doi.org/10.3390/microorganisms11061394






