Abstract
Candida albicans and Streptococcus mutans are known to synergistically interact with each other in the oral cavity. For example, glucosyltransferase B (GtfB), secreted by S. mutans, can bind to the C. albicans cell surface, promoting dual-species biofilm formation. However, the fungal factors mediating interactions with S. mutans are unknown. The C. albicans adhesins Als1, Als3, and Hwp1 are key players in C. albicans single-species biofilm formation, but their roles, if any, in interacting with S. mutans have not been assessed. Here, we investigated the roles of the C. albicans cell wall adhesins Als1, Als3, and Hwp1 on forming dual-species biofilms with S. mutans. We assessed the abilities of the C. albicans wild-type als1Δ/Δ, als3Δ/Δ, als1Δ/Δ/als3Δ/Δ, and hwp1Δ/Δ strains to form dual-species biofilms with S. mutans by measuring optical density, metabolic activity, cell enumeration, biomass, thickness, and architecture of the biofilms. We observed that the C. albicans wild-type strain formed enhanced dual-species biofilms in the presence of S. mutans in these different biofilm assays, confirming that C. albicans and S. mutans synergistically interact in the context of biofilms. Our results reveal that C. albicans Als1 and Hwp1 are major players in interacting with S. mutans, since dual-species biofilm formation was not enhanced when the als1Δ/Δ or hwp1Δ/Δ strains were cultured with S. mutans in dual-species biofilms. Als3, however, does not seem to play a clear role in interacting with S. mutans in dual-species biofilm formation. Overall, our data suggest that the C. albicans adhesins Als1 and Hwp1 function to modulate interactions with S. mutans and could be potential targets for future therapeutics.
1. Introduction
The opportunistic fungal pathogen Candida albicans is a normal colonizer of the oral cavity, gastrointestinal tract, and genitourinary tract of healthy humans. However, due to host environmental changes or immunocompromisation, C. albicans can become pathogenic and cause superficial and disseminated infections [1,2]. Biofilm formation is a common virulence factor of C. albicans that enhances its persistence and pathogenicity in the host [2]. In the oral cavity, C. albicans alone can form biofilms and cause infections [1], but C. albicans can also form synergistic biofilms with certain bacteria, such as those in the Streptococcus genus [3,4]. These interspecies interactions have been shown to enhance microbial colonization [5,6] and persistence, particularly in the oral cavity [7,8]. The presence of Streptococcus mutans, for example, is known to lead to the upregulation of common C. albicans virulence genes, such as HWP1, SAP4, and SAP6, when S. mutans and C. albicans are co-cultured together in biofilms [9,10,11]. In addition, these dual-species biofilms have been shown to cause enhanced tissue invasion and damage to the oral epithelium compared to single-species biofilms of S. mutans and C. albicans alone [9].
Fungal cell wall proteins are important for mediating cell–cell interactions between C. albicans cells. For example, the C. albicans cell wall adhesins Als1 and Als3 in the agglutinin-like sequence (Als) family are important for C. albicans cell–cell interactions during single-species biofilm formation [12,13,14]. In addition, Hwp1, a protein expressed on the C. albicans hyphal cell surface, is also important for promoting cell–cell interactions and single-species biofilm formation in C. albicans [15,16].
On the bacterial side, S. mutans is known to secrete a glucosyltransferase, GtfB, that has been shown to promote coaggregation between S. mutans and other microorganisms, including C. albicans, enhancing dual-species biofilm formation [7,17,18]. GtfB has been shown to bind to different sites along the C. albicans cell well [7,18], suggesting that specific fungal cell wall components could be involved in mediating its binding.
Here, we investigate the roles of the C. albicans cell wall proteins Als1, Als3, and Hwp1 on dual-species biofilm formation between S. mutans and C. albicans. Our results reveal that C. albicans Als1 and Hwp1 function to modulate interactions with S. mutans in the context of dual-species biofilms.
2. Material and Methods
2.1. Experimental Design
Experiments followed established protocols designed to evaluate aspects of biofilm formation [19]. Single-species and dual-species biofilms were compared to assess the roles of C. albicans Als1, Als3, and Hwp1 proteins in interacting with S. mutans using C. albicans mutant strains deleted for ALS1, ALS3, and HWP1, respectively. Single-species C. albicans wild-type biofilms, single-species S. mutans wild-type biofilms, and dual-species C. albicans-S. mutans wild-type biofilms were used as controls. Biofilms were formed on the bottoms of 6-well or 96-well plates for 24 h to obtain mature biofilms. Subsequently, biofilms were analyzed to assess optical density, metabolic activity, cell enumeration, biomass, thickness, and architecture. Cell–cell interactions between microorganisms were visualized at the cellular level via optical microscopy. Experiments were performed in triplicate unless stated otherwise.
2.2. Strains and Media
All strains used in this study have been previously published and are listed in Table 1. Deletion strains of C. albicans lacking ALS1, ALS3, and HWP1 as well as a double-deletion strain lacking both ALS1 and ALS3 were used for the biofilm assays. In addition, an S. mutans GFP-tagged reference strain was used.

Table 1.
Strains used in this study.
C. albicans strains were grown from −80 °C glycerol stocks at 30 °C on Yeast Extract Peptone Dextrose (YPD) (Thermo Fisher Scientific, Waltham, MA, USA) agar plates. Overnight C. albicans cultures were grown at 30 °C with shaking at 225 rpm in YPD liquid media. S. mutans strains were grown from −80 °C glycerol stocks at 37 °C with 10% CO2 on Brain Heart Infusion (BHI) (Thermo Fisher Scientific) agar plates. Overnight S. mutans cultures were grown on BHI supplemented with 1% glucose (37 °C with 10% CO2). RPMI 1640 medium (Sigma Aldrich, St. Louis, MO, USA) was used for the biofilm assays because it supports biofilm formation of both C. albicans and S. mutans [23].
2.3. Biofilm Growth Conditions
Cell enumeration using a hemocytometer relative to the optical density readings was performed for each microorganism to establish a 1:1 ratio of each species in culture, which was equivalent to an OD600 of 1 × 106 colony forming units per mL (CFUs/mL) for C. albicans, and an OD600 of 0.15 × 106 CFUs/mL for S. mutans. These CFUs/mL were added to 6-well or 96-well plates for single-species biofilm formation and the same CFUs/mL of each species was added to 6-well or 96-well plates for dual-species biofilm formation. Plates were sealed with Breathe-Easy® sealing membranes and incubated at 37 °C for 90 min at 250 rpm shaking with 10% CO2. Cells were washed with phosphate-buffered saline (PBS) to remove non-adhered cells and a fresh RPMI 1640 medium was added to each well. Biofilms were grown for 24 h.
2.4. Standard Optical Density Biofilm Assay
The standard biofilm optical density biofilm assay was performed as previously described [19]. Following biofilm growth on 96-well plates, the media were aspirated from the wells, and the biofilm formed on the bottom of each well was measured according to OD600 readings obtained using a plate reader. An average of 24 readings per well were obtained and normalized by subtracting the OD600 reading of a blank well containing an RPMI 1640 medium only.
2.5. Cell Metabolism Biofilm Assay
The 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) reduction assay was performed to measure the biofilm metabolic activity, as previously described [19]. A mixture of 0.5 mg/mL of XTT (Sigma Aldrich) in PBS and 0.32 mg/mL of phenazine methosulfate (Sigma Aldrich) in water was added to each well and incubated in the dark for 30 min. After incubation, OD492 readings were taken in a plate reader.
2.6. Biofilm Cell Enumeration Assay
The biofilm cell enumeration assay was performed as previously described [19]. After the 24 h period of biofilm formation, the media were aspirated from the 96-well plates, and the biofilms were washed to remove non-adhered cells. The biofilm formed on the bottom of each well was vigorously scraped using a pipette tip and resuspended in PBS. Cell suspensions were homogenized and serially diluted in PBS. Aliquots were plated onto YPD plates for C. albicans (grown at 30 °C for 48 h) and onto Mitis Salivarius Agar (MSA) plates for S. mutans (grown at 37 °C for 72 h) to enumerate colony-forming units (CFUs) for each species. We note that although C. albicans colonies can also grow on MSA plates, they are clearly distinct from the S. mutans colonies, and supplementation of the medium with antibiotics is not necessary. Additionally, with MSA plates, under these growth conditions, S. mutans colonies come up first and are easily counted before C. albicans colonies begin to appear on the plates.
2.7. Biofilm Biomass Determination
The biofilm biomass assay (i.e., dry weight assay) was performed as previously described [19]. Biofilms were grown on the bottoms of 6-well plates. Media was aspirated from the wells and PBS was added to each well. Biofilms were vigorously scraped and resuspended from the bottoms of each well using a pipette tip. Biofilm cell suspensions were aspirated and filtered onto mixed cellulose esters membranes (Millipore, Burlington, MA, USA) using a filtration device (Millipore). Membranes were subsequently dried for 24 h at 37 °C, and the weights of the membranes (in mg) were measured to obtain the dry weights of the biofilms. Data were normalized by subtracting the average weight of the control (media only) membrane.
2.8. Confocal Scanning Laser Microscopy (CSLM) Biofilm Assay
The confocal scanning laser microscopy (CSLM) biofilm assay was performed as previously described [19], with slight modifications (detailed below). Representative images of biofilms (n = 3 per group) were obtained via CSLM using a Zeiss LSM 880 upright confocal microscope. Biofilms were grown on the bottoms of silicone squares for 24 h using the same biofilm growth conditions described above. Biofilms were fixed with a formaldehyde solution (38% formaldehyde in water). C. albicans biofilms were stained with Concanavalin A-Alexa Fluor 594 (Sigma Aldrich) and visualized using a 555 nm diode (red) laser. The S. mutans GFP-tagged strain was visualized via excitation at 488 nm (green). Z-Stacks were obtained at 652 × 652 pixels, imaging every 0.5 μm intervals using a water-dipping 40X objective lens. The .czi files were analyzed using the project stacks function in ImageJ to generate side views. Biofilm thickness was measured in µm by taking the median thicknesses of each strain/condition (n = 3) using the Zeiss ZEN software version 3.6. Three .czi files of each sample (containing a combined total of at least 100 cells in a 0.5 μm interval) were used to quantify the average number of potential physical interactions between C. albicans and S. mutans cells.
2.9. Optical Microscopy
Planktonic cultures of C. albicans and S. mutans were grown separately overnight in YPD (for C. albicans), and BHI supplemented with 1% glucose (for S. mutans). Cultures were diluted to an OD600 of 0.5 in an RPMI 1640 medium and co-cultured at 37 °C with 10% CO2 for 4 h, with shaking at 250 rpm. An aliquot of the co-cultures was visualized using an EVOS FL microscope with a 60× oil immersion objective. Representative images of potential physical interactions were obtained for three independent experiments. Three images of each experiment (containing a combined total of at least 100 cells) were used to quantify the average number of potential physical interactions between C. albicans and S. mutans cells.
2.10. Statistical Analyses
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) software (version 22.0). Means and standard deviations were calculated. Based on the results of Levene’s test for equality of variances, Student’s unpaired two-tailed t-tests for unequal variance or one-way ANOVAs were performed. GraphPad Prism software (version 9.4) was used to generate the graphs.
3. Results
To test our hypothesis that Als1, Als3, and Hwp1 play roles in dual-species C. albicans-S. mutans biofilm formation, we first compared the biofilms formed by the single-species and dual-species biofilms of each strain using the standard optical density biofilm assay [19], which correlates with biofilm thickness. Using this assay, we found a synergistic interaction between C. albicans and S. mutans, where an increase in optical density was observed for the dual-species wild-type biofilms, compared to the single-species C. albicans wild-type biofilms and the single-species S. mutans wild-type biofilms (Figure 1). We found that the presence of S. mutans had no effect on the biofilm formation capacity of the C. albicans als1Δ/Δ or hwp1Δ/Δ strains compared to the single-species als1Δ/Δ or hwp1Δ/Δ strains, respectively; however, the presence of S. mutans strikingly increased the biofilm formation capacity of the C. albicans als3Δ/Δ strain compared to the single-species als3Δ/Δ strain (Figure 1). We note that complementation (addback) strains als1Δ/Δ + ALS1, hwp1Δ/Δ + HWP1, and als3Δ/Δ + ALS3 restored the mutant phenotypes for dual-species biofilms back to near wild-type levels (als1Δ/Δ + ALS1, OD600 = 0.18 ± 0.01; hwp1Δ/Δ + HWP1, OD600 = 0.16 ± 0.02; als3Δ/Δ + ALS3, OD600 = 0.15 ± 0.02). Interestingly, the presence of S. mutans impaired the biofilm formation capacity of the C. albicans als1Δ/Δ/als3Δ/Δ double-deletion strain compared to the single-species als1Δ/Δ/als3Δ/Δ strain (Figure 1).
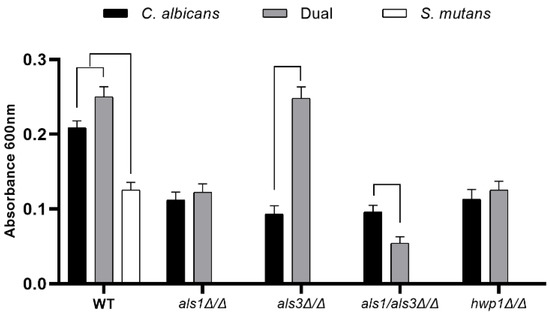
Figure 1.
Standard optical density biofilm assay of single-species and dual-species biofilms. Data shown include n = 6 per group. A line connecting two bars indicates p < 0.05 as determined using Student’s unpaired two-tailed t-tests assuming unequal variance. Error bars represent standard deviations.
Similar results to the optical density biofilm assay were obtained for the XTT cell metabolism biofilm assay and the dry weight biofilm biomass assay [19], where an increase in metabolic activity (Figure 2) and biomass (Table 2) was observed for the dual-species wild-type biofilms, compared to the single-species C. albicans wild-type biofilms and the single-species S. mutans wild-type biofilms. In addition, the presence of S. mutans increased the metabolic activity (Figure 2) and biomass (Table 2) of the C. albicans als3Δ/Δ strain compared to the single-species als3Δ/Δ strain, but had no effect on the metabolic activity (Figure 2) or biomass (Table 2) of the C. albicans als1Δ/Δ or hwp1Δ/Δ strains compared to the single-species als1Δ/Δ or hwp1Δ/Δ strains, respectively. Likewise, the presence of S. mutans reduced the metabolic activity (Figure 2) and biomass (Table 2) of the C. albicans als1Δ/Δ/als3Δ/Δ double-deletion strain compared to the single-species als1Δ/Δ/als3Δ/Δ strain.
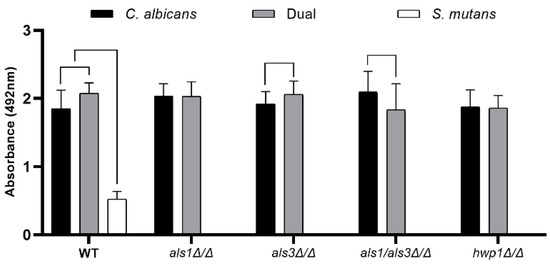
Figure 2.
XTT metabolic activity assay of single-species and dual-species biofilms. Data shown include n = 6 per group. A line connecting two bars indicates p < 0.05 as determined using Student’s unpaired two-tailed t-tests assuming unequal variance. Error bars represent standard deviations.

Table 2.
Biofilm dry weights.
To determine the number of cells of each species present in the biofilms, we next measured the CFUs for each biofilm sample [19]. Overall, S. mutans CFUs were higher in all dual-species biofilms compared to S. mutans CFUs in all single-species biofilms (Figure 3), indicating that S. mutans benefits from C. albicans by increasing its cell population. Furthermore, C. albicans CFUs were lower in dual-species biofilms of the C. albicans als1Δ/Δ/als3Δ/Δ double-deletion strain compared to the single-species als1Δ/Δ/als3Δ/Δ strain (Figure 3). C. albicans CFUs were also lower in the single-species biofilms of the als3Δ/Δ strain compared to the dual-species biofilms of the als3Δ/Δ strain (Figure 3).
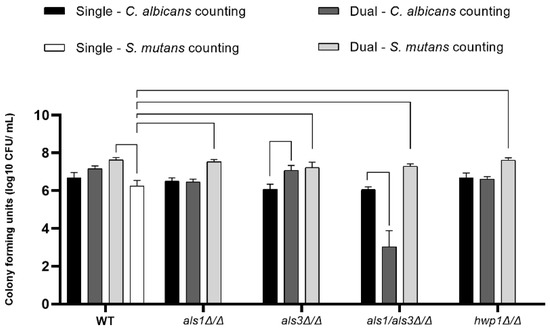
Figure 3.
Cell enumeration in biofilms. Data shown are CFUs on a log10 scale (n = 4 per group). A line connecting two bars indicates p < 0.05 according to one-way ANOVA tests. Error bars represent standard deviations.
To assess biofilm architecture and thickness, we next performed confocal scanning laser microscopy (CSLM) assays on the biofilms [19]. Thickness measurements of biofilms were determined based on the CSLM medians of all images taken for a given strain and condition and are reported in Table 3. We observed that wild-type dual-species biofilms were overall thicker than C. albicans single-species biofilms as measured in the CSLM side-view images (Figure 4; Table 3). In addition, as expected, all single-species C. albicans mutant strains produced defective biofilms (Figure 4; Table 3). Consistent with our results reported above for the other biofilm assays, the thicknesses of the biofilms of the als1Δ/Δ and hwp1Δ/Δ strains were similar between the single-species and dual-species biofilms (Table 3). In addition, the presence of S. mutans in the dual-species biofilms increased the biofilm thickness of the C. albicans als3Δ/Δ strain compared to the single-species als3Δ/Δ strain (Table 3). Overall, the CSLM results indicate that S. mutans benefits from the presence of C. albicans in all dual-species biofilms. We note that single-species S. mutans biofilms formed thin cell aggregates restricted to the bottoms of the substrates, while in dual-species, the S. mutans cells were observed throughout the biofilms, often appearing along C. albicans hyphal cells. Specifically, the CSLM images of the dual-species biofilms showed predominance of S. mutans cells in close physical proximity to C. albicans-elongated hyphal cells in the wild-type als1Δ/Δ, als3Δ/Δ, and als1Δ/Δ/als3Δ/Δ strains (Figure 4; Table 4). In dual-species biofilms with the hwp1Δ/Δ strain, however, there appeared to be a predominance of S mutans cells in close physical proximity to C. albicans around yeast-form cells (Figure 4; Table 4).

Table 3.
Median biofilm thicknesses from CSLM images.
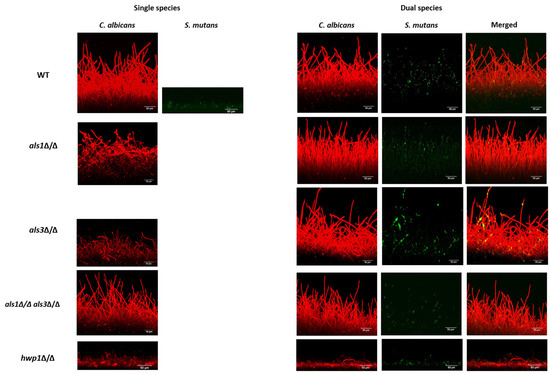
Figure 4.
CSLM biofilm assay. Biofilms were imaged using a Zeiss LSM 880 upright confocal microscope. C. albicans biofilms were stained with Concanavalin A-Alexa Fluor 594 (red) and visualized using a 555-nm diode laser. The S. mutans GFP strain (green) was detected via excitation at 488 nm. Representative images are shown. All Z-Stacks were obtained at 652 × 652 pixels, with imaging undertaken at 0.5 μm intervals using a water-dipping 40X objective. The .czi files were analyzed using the project stacks function in ImageJ to generate side-views. Scales bars = 50 μm.

Table 4.
Average number of potential binding interactions observed using CSLM between C. albicans and S. mutans cells under dual-species biofilm conditions.
Given our observation that S. mutans cells appeared generally to be in close physical proximity to C. albicans hyphal cells in the context of dual-species biofilms (Figure 4; Table 4), we also performed optical microscopy of planktonic cultures to visualize in more detail the physical proximity and/or potential cellular interactions occurring between C. albicans and S. mutans under non-biofilm co-culture conditions. Co-cultures of the wild-type C. albicans and S. mutans strains showed on average increased physical proximity of S. mutans cells to C. albicans hyphal cells compared to yeast-form cells (Figure 5; Table 5). Interestingly, co-cultures of the C. albicans als1Δ/Δ, hwp1Δ/Δ, and als1Δ/Δ/als3Δ/Δ strains with S. mutans showed strikingly fewer potential binding events on average compared to co-cultures of the C. albicans wild-type strain with S. mutans (Figure 5; Table 5). Co-cultures of the C. albicans als3Δ/Δ strain with S. mutans showed similar potential binding events of S. mutans, specifically to C. albicans hyphal cells, compared to co-cultures of the C. albicans wild-type strain with S. mutans (Figure 5; Table 5).
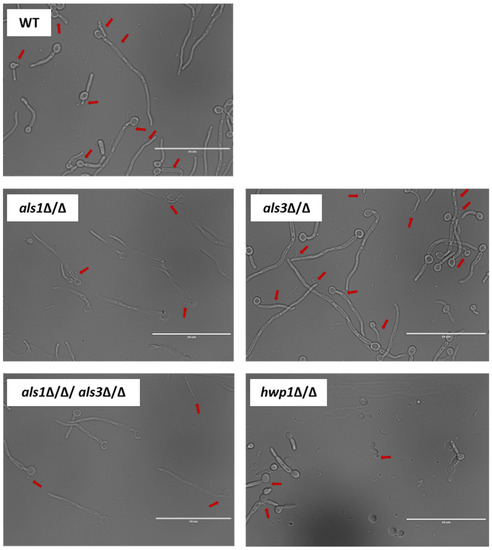
Figure 5.
Optical microscopy cellular images of planktonic co-cultures of C. albicans and S. mutans. Representative images are shown that were taken using an EVOS FL microscope with a 60X oil immersion objective. Red arrows indicate potential binding events of S. mutans along C. albicans cells. Scales bars = 50 μm.

Table 5.
Average number of potential binding interactions observed via optical microscopy between C. albicans and S. mutans cells under planktonic conditions.
4. Discussion
The oral cavity is home to thousands of microorganisms existing in biofilm microbial communities and is an important niche in the study of interspecies interactions [24,25]. The opportunistic fungal pathogen C. albicans and the opportunistic bacterial pathogen S. mutans are prevalent microorganisms in the oral cavity [1,26], where they are known to benefit from one another’s presence [7,8]. When C. albicans and S. mutans are grown together in biofilms under oral cavity conditions, the presence of S. mutans has been shown to enhance fungal virulence by increasing the yeast–hyphal transition in C. albicans [8,9]. In addition, under oral cavity conditions, C. albicans has been shown to acidify its environment, thereby supporting the growth and metabolism of S. mutans [27]. Together, C. albicans and S. mutans can form pathogenic dual-species biofilms in the oral cavity that can lead to the development of caries and other oral diseases [9].
Cell–cell interactions between C. albicans and S. mutans are thought to occur through physical binding events between proteins that are either already present on the cell surface and/or that are secreted by one microorganism to mediate binding to the other microorganism [28,29]. For example, glucosyltransferase B (GtfB), secreted by S. mutans, can bind to the C. albicans cell surface, promoting dual-species biofilm formation between the two species [17]. However, the C. albicans factors mediating interactions with S. mutans are unknown. Here, we investigated the roles of the fungal cell surface adhesin proteins Als1, Als3, and Hwp1 in interacting with S. mutans.
Als1 is a GPI-anchored adhesin that is involved in C. albicans single-species biofilm formation, cell–cell and cell-surface interactions, as well as interactions with the host epithelium [14,15,30]. Als1 is known to be required for physically interacting with the oral bacterium Streptococcus gordonii [31], and thus it is feasible that Als1 could be a key player in mediating interactions with other oral bacterial species, such as S. mutans. Als3 is a GPI-anchored adhesin with 88% amino acid sequence similarity to Als1 [32] that is involved in C. albicans single-species biofilm formation and is known to play functionally redundant roles with Als1 [13,15]. Given its functional redundancy with Als1, we reasoned that Als3 could also be a key player in mediating interactions with S. mutans. Hwp1 is a well-known C. albicans adhesin that is expressed only on hyphal cells and is covalently linked to the fungal cell wall via a remnant of its GPI anchor [33,34]. Like Als1 and Als3, Hwp1 is also important for C. albicans single-species biofilm formation [16,35], and we reasoned that it could also be a candidate cell-surface protein for mediating interactions with S. mutans. Based on this information, we hypothesized that Als1, Als3, and Hwp1 play roles in interacting with S. mutans during dual-species biofilm formation.
To test our hypothesis, we assessed the abilities of the C. albicans wild-type als1Δ/Δ, als3Δ/Δ, als1Δ/Δ/als3Δ/Δ, and hwp1Δ/Δ strains to form dual-species biofilms with S. mutans by measuring the optical density, metabolic activity, cell enumeration, biomass, thickness, and architecture of the biofilms. We also performed confocal and optical microscopy assays under biofilm and planktonic conditions to quantify potential binding interactions occurring between C. albicans and S. mutans cells, where we observed that S. mutans cells were generally found to be in close proximity with C. albicans hyphal cells rather than yeast-form cells. We observed that the C. albicans wild-type strain formed enhanced dual-species biofilms in the presence of S. mutans in all of the different biofilm assays, confirming that C. albicans and S. mutans synergistically interact in the context of dual-species biofilms. Our results revealed that C. albicans Als1 and Hwp1 are major players in interacting with S. mutans since dual-species biofilm formation was not enhanced when the C. albicans als1Δ/Δ or hwp1Δ/Δ strains were cultured with S. mutans in dual-species biofilms. The role of Als3, however, in interacting with S. mutans is not as straightforward. In general, we observed that when the C. albicans als3Δ/Δ strain was cultured with S. mutans in dual-species biofilms, biofilm formation was rescued to varying degrees depending on the biofilm assay used. In all of the assays, the rescue was within or close to wild-type levels, indicating that Als3 does not seem to play a clear role in interacting with S. mutans in dual-species biofilms. Finally, in all of the assays used, the combined absence of Als1 and Als3 in the als1Δ/Δ/als3Δ/Δ strain showed a detrimental impact on dual-species biofilm formation beyond that of either of the single als1Δ/Δ or als3Δ/Δ strains alone. Although there is not a clear-cut explanation for this finding, one possibility is that when both C. albicans Als1 and Als3 are absent, and with the additional presence of S. mutans cells, the overall structural integrity of the dual-species biofilm is disrupted. We note that our dual-species biofilm experiments were performed using a 1:1 ratio of S. mutans-to-C. albicans cells to seed the biofilms. We chose this ratio so that each species could be equally represented irrespective of their cell size differences and because we obtained robust dual-species biofilms under these seeding conditions. Nonetheless, it is possible that the dual-species biofilm architectures and interactions that we observed here could be population-dependent and would change if different seeding ratios were used.
Given our findings that C. albicans Als1 and Hwp1 function to modulate interactions with S. mutans, and that these two proteins are located on the C. albicans cell surface, Als1 and Hwp1 could be promising therapeutic targets to consider in the future development of novel therapeutics to treat polymicrobial biofilm infections.
Author Contributions
Conceptualization, L.M.-F., A.P.R.-F., C.J.N. and A.A.D.B.C.; Methodology, L.M.-F., J.S.G., A.P.R.-F. and A.A.D.B.C.; Validation, L.M.-F. and C.J.N.; Formal analysis, L.M.-F.; Investigation, L.M.-F., J.S.G. and A.A.D.B.C.; Resources, C.J.N. and A.A.D.B.C.; Data curation, L.M.-F.; Writing—original draft, L.M.-F.; Writing—review & editing, L.M.-F., C.J.N. and A.A.D.B.C.; Visualization, L.M.-F.; Supervision, C.J.N. and A.A.D.B.C.; Funding acquisition, C.J.N. and A.A.D.B.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Institutes of Health (NIH) National Institute of General Medical Sciences (NIGMS) award number R35GM124594, and by the Kamangar family in the form of an endowed chair to C.J.N. We are grateful to the Coordenação de Pessoal de Ensino Superior (CAPES) foundation of Brazil for providing a scholarship (001/88887.508855/2020-00) to L.M.-F. The CSLM data were obtained using a confocal microscope acquired through the National Science Foundation (NSF) MRI Award Number DMR-1625733. The funders had no role in the study design, data collection and interpretation, or the decision to submit the work for publication.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We thank all members of the Nobile lab for insightful discussions on the topic of this manuscript. We are especially grateful to Pegah Mosharaf Ghahfarokhy for her assistance with the analysis of the .czi files from the CSLM experiments. We thank Robert Shields at Arkansas State University and Jonathon Baker at the J. Craig Venter Institute for providing S. mutans strains.
Conflicts of Interest
C.J.N. is a cofounder of BioSynesis, Inc., a company developing diagnostics and therapeutics for biofilm infections.
References
- Millsop, J.W.; Fazel, N. Oral candidiasis. Clin. Dermatol. 2016, 34, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Nobile, C.J.; Johnson, A.D. Candida albicans Biofilms and Human Disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Ren, B.; Zhou, X.; Zhang, L.; Xu, X. Cross-kingdom interaction between Candida albicans and oral bacteria. Front. Microbiol. 2022, 13, 911623. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jenkinson, H.F.; Dongari-Bagtzoglou, A. Innocent until proven guilty: Mechanisms and roles of Streptococcus-Candida interactions in oral health and disease. Mol. Oral Microbiol. 2014, 29, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Aljaffary, M.; Jang, H.; Alomeir, N.; Zeng, Y.; Alkhars, N.; Vasani, S.; Almulhim, A.; Wu, T.T.; Quataert, S.; Bruno, J.; et al. Effects of nystatin oral rinse on oral Candida species and Streptococcus mutans among healthy adults. Clin. Oral Investig. 2023. [Google Scholar] [CrossRef]
- Alomeir, N.; Zeng, Y.; Fadaak, A.; Wu, T.T.; Malmstrom, H.; Xiao, J. Effect of nystatin on Candida albicans-Streptococcus mutans duo-species biofilms. Arch. Oral Biol. 2023, 145, 105582. [Google Scholar] [CrossRef]
- Falsetta, M.L.; Klein, M.I.; Colonne, P.M.; Scott-Anne, K.; Gregoire, S.; Pai, C.H.; Gonzalez-Begne, M.; Watson, G.; Krysan, D.J.; Bowen, W.H.; et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect. Immun. 2014, 82, 1968–1981. [Google Scholar] [CrossRef]
- Kim, H.E.; Liu, Y.; Dhall, A.; Bawazir, M.; Koo, H.; Hwang, G. Synergism of Streptococcus mutans and Candida albicans Reinforces Biofilm Maturation and Acidogenicity in Saliva: An In Vitro Study. Front. Cell Infect. Microbiol. 2021, 10, 623980. [Google Scholar] [CrossRef]
- Cavalcanti, Y.W.; Morse, D.J.; da Silva, W.J.; Del-Bel-Cury, A.A.; Wei, X.; Wilson, M.; Milward, P.; Lewis, M.; Bradshaw, D.; Williams, D.W. Virulence and pathogenicity of Candida albicans is enhanced in biofilms containing oral bacteria. Biofouling 2014, 31, 27–38. [Google Scholar] [CrossRef]
- Ellepola, K.; Truong, T.; Liu, Y.; Lin, Q.; Lim, T.K.; Lee, Y.M.; Cao, T.; Koo, H.; Seneviratne, C.J. Multi-omics analyses reveal synergistic carbohydrate metabolism in Streptococcus mutans-Candida albicans mixed-species biofilms. Infect. Immun. 2019, 87, e00339-19. [Google Scholar] [CrossRef]
- Xiao, J.; Zeng, Y.; Rustchenko, E.; Huang, X.; Wu, T.T.; Falsetta, M.L. Dual transcriptome of Streptococcus mutans and Candida albicans interplay in biofilms. J. Oral Microbiol. 2022, 15, 2144047. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, L.L.; Cota, E. Candida albicans agglutinin-like sequence (Als) family vignettes: A review of Als protein structure and function. Front. Microbiol. 2016, 7, 280. [Google Scholar] [CrossRef] [PubMed]
- Nobile, C.J.; Schneider, H.A.; Nett, J.E.; Sheppard, D.C.; Filler, S.G.; Andes, D.R.; Mitchell, A.P. Complementary adhesin function in C. albicans biofilm formation. Curr. Biol. 2008, 18, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Oh, S.H.; Coleman, D.A.; Hoyer, L.L. ALS1 Deletion increases the proportion of small cells in a Candida albicans culture population: Hypothesizing a novel role for Als1. Front. Cell Infect. Microbiol. 2022, 12, 895068. [Google Scholar] [CrossRef] [PubMed]
- Nobile, C.J.; Andes, D.R.; Nett, J.E.; Smith, F.J.; Yue, F.; Phan, Q.T.; Edwards, J.E.; Filler, S.G.; Mitchell, A.P. Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo. PLoS Pathog. 2006, 2, e63. [Google Scholar] [CrossRef]
- Nobile, C.J.; Nett, J.E.; Andes, D.R.; Mitchell, A.P. Function of Candida albicans adhesin Hwp1 in biofilm formation. Eukaryot. Cell 2006, 5, 1604–1610. [Google Scholar] [CrossRef]
- Gregoire, S.; Xiao, J.; Silva, B.B.; Gonzalez, I.; Agidi, P.S.; Klein, M.I.; Ambatipudi, K.S.; Rosalen, P.L.; Bauserman, R.; Waugh, R.E.; et al. Role of glucosyltransferase B in interactions of Candida albicans with Streptococcus mutans and with an experimental pellicle on hydroxyapatite surfaces. Appl. Environ. Microbiol. 2011, 77, 6357–6367. [Google Scholar] [CrossRef]
- Hwang, G.; Liu, Y.; Kim, D.; Li, Y.; Krysan, D.J.; Koo, H. Candida albicans mannans mediate Streptococcus mutans exoenzyme GtfB binding to modulate cross-kingdom biofilm development in vivo. PLoS Pathog. 2017, 13, e1006407. [Google Scholar] [CrossRef]
- Gulati, M.; Lohse, M.B.; Ennis, C.L.; Gonzalez, R.E.; Perry, A.M.; Bapat, P.; Arevalo, A.V.; Rodriguez, D.L.; Nobile, C.J. In Vitro culturing and screening of Candida albicans biofilms. Curr. Protoc. Microbiol. 2018, 50, e60. [Google Scholar] [CrossRef]
- Gillum, A.M.; Tsay, E.Y.; Kirsch, D.R. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 1984, 198, 179–182. [Google Scholar] [CrossRef]
- Ajdić, D.; McShan, W.M.; McLaughlin, R.E.; Savić, G.; Chang, J.; Carson, M.B.; Primeaux, C.; Tian, R.; Kenton, S.; Jia, H.; et al. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. USA 2002, 99, 14434–14439. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.C.; Kaspar, J.R.; Lee, K.; Underhill, S.A.M.; Burne, R.A. Fluorescence tools adapted for real-time monitoring of the behaviors of Streptococcus species. Appl. Environ. Microbiol. 2019, 85, e00620-19. [Google Scholar] [CrossRef] [PubMed]
- Heersema, L.A.; Smyth, H.D.C. A multispecies biofilm in vitro screening model of dental caries for high-throughput susceptibility testing. High Throughput 2019, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Diaz, P.I.; Valm, A.M. Microbial interactions in oral communities mediate emergent biofilm properties. J. Dent. Res. 2019, 99, 18–25. [Google Scholar] [CrossRef]
- Lohse, M.B.; Gulati, M.; Johnson, A.D.; Nobile, C.J. Development and regulation of single- and multi-species Candida albicans biofilms. Nat. Rev. Microbiol. 2018, 16, 19–31. [Google Scholar] [CrossRef]
- Keijser, B.J.; Zaura, E.; Huse, S.M.; van der Vossen, J.M.; Schuren, F.H.; Montijn, R.C.; ten Cate, J.M.; Crielaard, W. Pyrosequencing analysis of the oral microflora of healthy adults. J. Dent. Res. 2008, 87, 1016–1020. [Google Scholar] [CrossRef]
- Klinke, T.; Kneist, S.; de Soet, J.J.; Kuhlisch, E.; Mauersberger, S.; Forster, A.; Klimm, W. Acid production by oral strains of Candida albicans and lactobacilli. Caries. Res. 2009, 43, 83–91. [Google Scholar] [CrossRef]
- Hwang, G.; Marsh, G.; Gao, L.; Waugh, R.; Koo, H. Binding force dynamics of Streptococcus mutans glucosyltransferase B to Candida albicans. J. Dent. Res. 2015, 94, 1310–1317. [Google Scholar] [CrossRef]
- Wan, S.X.; Tian, J.; Liu, Y.; Dhall, A.; Koo, H.; Hwang, G. Cross-kingdom cell-to-cell interactions in cariogenic biofilm initiation. J. Dent. Res. 2020, 100, 74–81. [Google Scholar] [CrossRef]
- Kamai, Y.; Kubota, M.; Kamai, Y.; Hosokawa, T.; Fukuoka, T.; Filler, S.G. Contribution of Candida albicans ALS1 to the pathogenesis of experimental oropharyngeal candidiasis. Infect. Immun. 2002, 70, 5256–5258. [Google Scholar] [CrossRef]
- Klotz, S.A.; Gaur, N.K.; De Armond, R.; Sheppard, D.; Khardori, N.; Edwards, J.E., Jr.; Lipke, P.N.; El-Azizi, M. Candida albicans Als proteins mediate aggregation with bacteria and yeasts. Med. Mycol. 2007, 45, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, D.C.; Yeaman, M.R.; Welch, W.H.; Phan, Q.T.; Fu, Y.; Ibrahim, A.S.; Filler, S.G.; Zhang, M.; Waring, A.J.; Edwards, J.E., Jr. Functional and structural diversity in the Als protein family of Candida albicans. J. Biol. Chem. 2004, 279, 30480–30489. [Google Scholar] [CrossRef] [PubMed]
- Staab, J.F.; Bahn, Y.S.; Tai, C.H.; Cook, P.F.; Sundstrom, P. Expression of transglutaminase substrate activity on Candida albicans germ tubes through a coiled, disulfide-bonded N-terminal domain of Hwp1 requires C-terminal glycosylphosphatidylinositol modification. J. Biol. Chem. 2004, 279, 40737–40747. [Google Scholar] [CrossRef] [PubMed]
- Sundstrom, P. Adhesion in Candida spp. Cell Microbiol. 2002, 4, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Daniels, K.J.; Oh, S.H.; Green, C.B.; Yeater, K.M.; Soll, D.R.; Hoyer, L.L. Candida albicans Als3p is required for wild-type biofilm formation on silicone elastomer surfaces. Microbiology 2006, 152 Pt 8, 2287–2299. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).