Belgian Anopheles plumbeus Mosquitoes Are Competent for Japanese Encephalitis Virus and Readily Feed on Pigs, Suggesting a High Vectorial Capacity
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquito Collection
2.2. Virus Production and Titration
2.3. Oral Infection and Incubation of Mosquitoes
2.4. Mosquito Salivation and Dissection
2.5. JEV Detection
2.5.1. RT-qPCR Analysis
2.5.2. Virus Isolation
2.6. Anopheles plumbeus Blood Feeding on Pigs
2.7. Statistical Analysis
3. Results
3.1. Infection, Dissemination and Transmission Rates
3.1.1. Incubation at a Constant 25 °C Temperature
3.1.2. Incubation at a 25/15 °C Day/Night Temperature Gradient
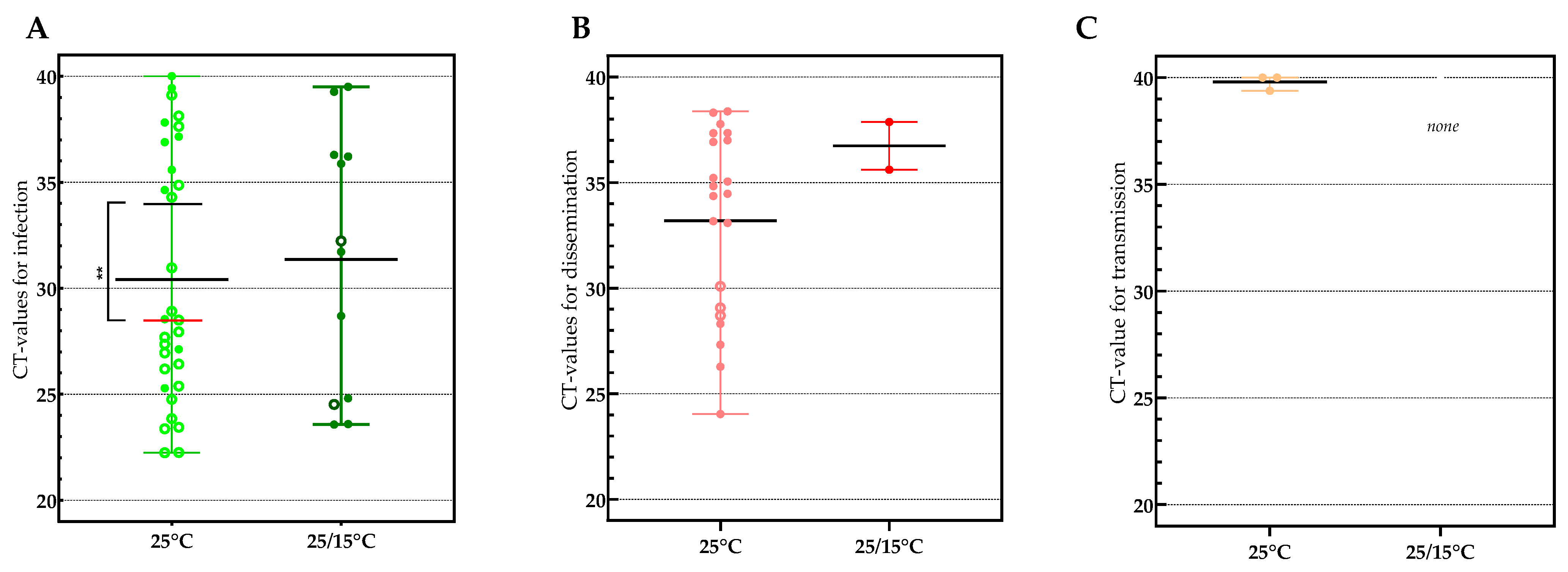
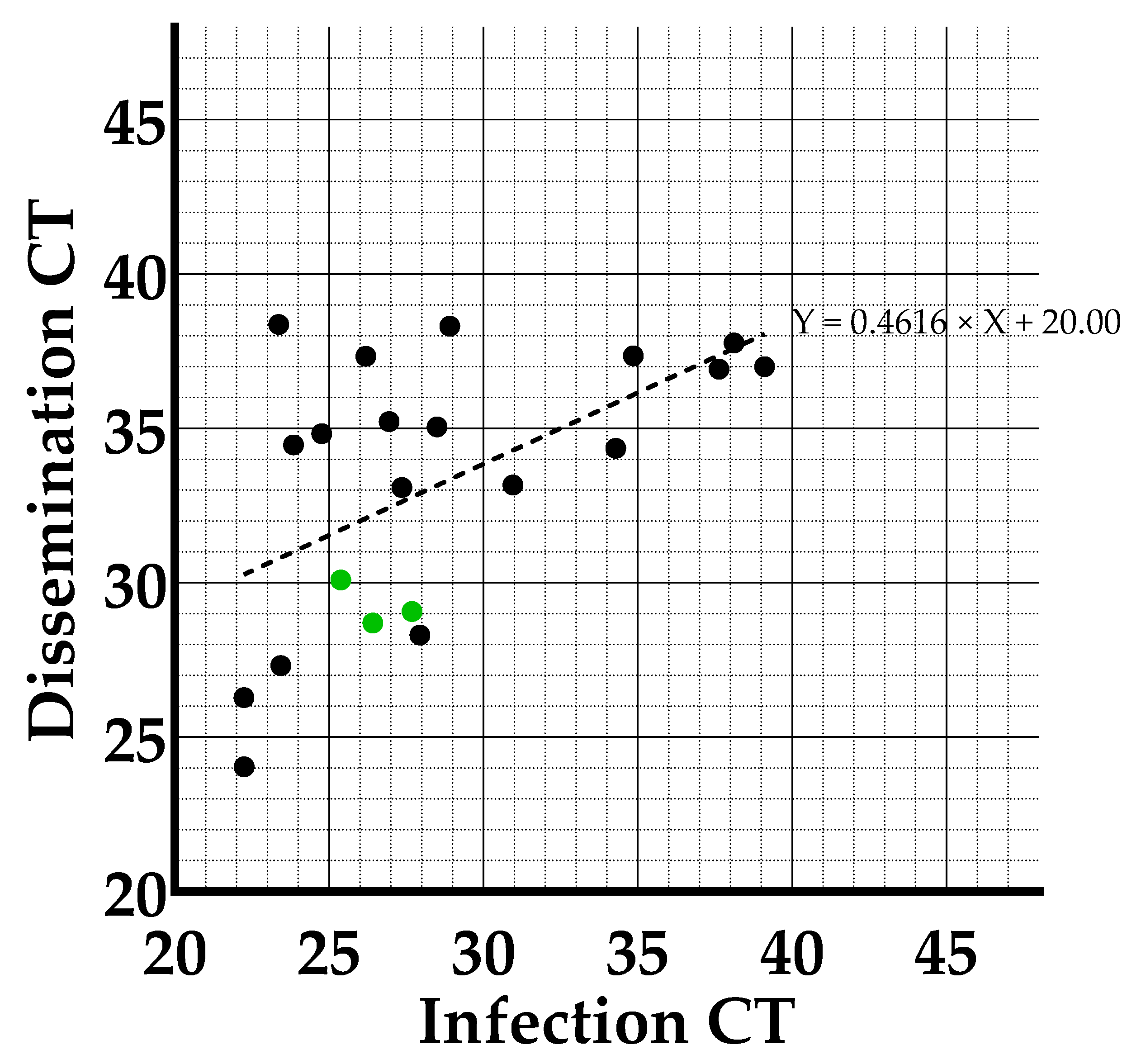
3.2. Viral Loads Found in Infection, Dissemination and Transmission
3.3. Anopheles plumbeus Blood Feeding on Pigs
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bueno-Marí, R.; Jiménez-Peydró, R. Anopheles plumbeus Stephens, 1828: A neglected malaria vector in Europe. Malar. Rep. 2011, 1, 2. [Google Scholar] [CrossRef]
- Becker, N.; Petric, D.; Zgomba, M.; Boase, C.; Madon, M.; Dahl, C.; Kaiser, A. Mosquitoes and Their Control; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Schaffner, F.; Thiéry, I.; Kaufmann, C.; Zettor, A.; Lengeler, C.; Mathis, A.; Bourgouin, C. Anopheles plumbeus (Diptera: Culicidae) in Europe: A mere nuisance mosquito or potential malaria vector? Malar. J. 2012, 11, 393. [Google Scholar] [CrossRef]
- Dekoninck, W.; Hendrickx, F.; Bortel WVan Versteirt, V.; Coosemans, M.; Damiens, D.; Hance, T.; De Clercq, E.M.; Hendrickx, G.; Schaffner, F.; Grootaert, P. Human-Induced Expanded Distribution of Anopheles plumbeus, Experimental Vector of West Nile Virus and a Potential Vector of Human Malaria in Belgium. J. Med. Entomol. 2011, 48, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Kruger, A.; Rech, A.; Su, X.-Z.; Tannich, E. Two cases of autochthonous Plasmodium falciparum malaria in Germany with evidence for local transmission by indigenous Anopheles plumbeus. Trop. Med. Int. Health 2001, 6, 983–985. [Google Scholar] [CrossRef] [PubMed]
- Bertola, M.; Mazzucato, M.; Pombi, M.; Montarsi, F. Updated occurrence and bionomics of potential malaria vectors in Europe: A systematic review (2000–2021). Parasites Vectors 2022, 15, 88. [Google Scholar] [CrossRef]
- Vermeil, C.; Lavillaureix, J.; Reeb, E. The conservation and transmission of West Nile virus by some arthropods. Bull. Société Pathol. Exot. 1960, 53, 273–279. [Google Scholar]
- Martinet, J.-P.; Ferté, H.; Failloux, A.-B.; Schaffner, F.; Depaquit, J. Mosquitoes of North-Western Europe as Potential Vectors of Arboviruses: A Review. Viruses 2019, 11, 1059. [Google Scholar] [CrossRef]
- Heym, E.C.; Schröder, J.; Kampen, H.; Walther, D. The Nuisance Mosquito Anopheles plumbeus (Stephens, 1828) in Germany—A Questionnaire Survey May Help Support Surveillance and Control. Front. Public Health 2017, 5, 278. [Google Scholar] [CrossRef]
- Medlock, J.M.; Snow, K.R.; Leach, S. Potential transmission of West Nile virus in the British Isles: An ecological review of candidate mosquito bridge vectors. Med. Vet. Entomol. 2005, 19, 2–21. [Google Scholar] [CrossRef]
- Service, M.W. Study of the Natural Predators of Aedes Cantans (Meigen) Using the Precipitin Test. J. Med. Entomol. 1973, 10, 503–510. [Google Scholar] [CrossRef]
- Van den Eynde, C.; Sohier, C.; Matthijs, S.; De Regge, N. Japanese Encephalitis Virus Interaction with Mosquitoes: A Review of Vector Competence, Vector Capacity and Mosquito Immunity. Pathogens 2022, 11, 317. [Google Scholar] [CrossRef]
- Calisher, C.H.; Gould, E.A. Taxonomy of the virus family Flaviviridae. Adv. Virus Res. 2003, 59, 1–19. [Google Scholar] [CrossRef]
- Thapaliya, D.; Hanson, B.M.; Kates, A.; Klostermann, C.A.; Nair, R.; Wardyn, S.E.; Smith, T.C. Zoonotic Diseases of Swine: Food-borne and Occupational Aspects of Infection. In Zoonoses—Infections Affecting Humans and Animals; Springer: Dordrecht, The Netherlands, 2015; pp. 23–68. [Google Scholar] [CrossRef]
- Hubálek, Z.; Rudolf, I.; Nowotny, N. Arboviruses pathogenic for domestic and wild animals. Adv. Virus Res. 2014, 89, 201–275. [Google Scholar] [CrossRef]
- Simon, L.; Sandhu, D.; Goyal, A.; Kruse, B. Japanese Encephalitis; StatPearls: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470423/ (accessed on 15 January 2023).
- Spickler, A.R. Japanese Encephalitis; Center for Food Security and Public Health: Ames, IA, USA, 2016.
- European Center of Disease Control. Facts about Japanese Encephalitis. Factsheet 2017. Available online: https://www.ecdc.europa.eu/en/japanese-encephalitis/facts (accessed on 9 February 2021).
- Fang, Y.; Zhang, Y.; Zhou, Z.-B.; Xia, S.; Shi, W.-Q.; Xue, J.-B.; Li, Y.-Y.; Wu, J.-T. New strains of Japanese encephalitis virus circulating in Shanghai, China after a ten-year hiatus in local mosquito surveillance. Parasites Vectors 2019, 12, 22. [Google Scholar] [CrossRef]
- Furuya-Kanamori, L.; Gyawali, N.; Mills, D.J.; Hugo, L.E.; Devine, G.J.; Lau, C.L. The Emergence of Japanese Encephalitis in Australia and the Implications for a Vaccination Strategy. Trop. Med. Infect. Dis. 2022, 7, 85. [Google Scholar] [CrossRef]
- Mackenzie, J.S.; Gubler, D.J.; Petersen, L.R. Emerging flaviviruses: The spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat. Med. 2004, 10, S98–S109. [Google Scholar] [CrossRef]
- van den Hurk, A.F.; Skinner, E.; Ritchie, S.A.; Mackenzie, J.S. The Emergence of Japanese Encephalitis Virus in Australia in 2022: Existing Knowledge of Mosquito Vectors. Viruses 2022, 14, 1208. [Google Scholar] [CrossRef]
- Ravanini, P.; Huhtamo, E.; Ilaria, V.; Crobu, M.G.; Nicosia, A.M.; Servino, L.; Rivasi, F.; Allegrini, S.; Miglio, U.; Magri, A.; et al. Japanese encephalitis virus RNA detected in Culex pipiens mosquitoes in Italy. Eurosurveillance 2012, 17, 20221. [Google Scholar] [CrossRef]
- Simon-Loriere, E.; Faye, O.; Prot, M.; Casademont, I.; Fall, G.; Fernandez-Garcia, M.D.; Diagne, M.M.; Kipela, J.M.; Fall, I.S.; Holmes, E.C.; et al. Autochthonous Japanese Encephalitis with Yellow Fever Coinfection in Africa. N. Engl. J. Med. 2017, 376, 1483–1485. [Google Scholar] [CrossRef]
- Rückert, C.; Ebel, G.D. How do virus–mosquito interactions lead to viral emergence? Trends Parasitol. 2018, 34, 310–321. [Google Scholar] [CrossRef]
- Schulz, C.; Becker, S.C. Mosquitoes as arbovirus vectors: From species identification to vector competence. In Mosquito-Borne Diseases; Nature Publishing Group: Berlin, Germany, 2018; Volume 10, pp. 163–212. [Google Scholar] [CrossRef]
- Souza-Neto, J.A.; Powell, J.R.; Bonizzoni, M. Aedes aegypti vector competence studies: A review. Infect. Genet. Evol. 2018, 67, 191–209. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, E.B.; Kramer, L.D. Zika virus mosquito vectors: Competence, biology, and vector control. J. Infect. Dis. 2017, 216, S976–S990. [Google Scholar] [CrossRef] [PubMed]
- WeatherOnline. WeatherOnline Ltd.—Meteorological Services 2022. Available online: https://www.weatheronline.co.uk/weather/maps/current?LANG=en&DATE=1659265200&CONT=euro&LAND=__&KEY=__&SORT=1&UD=0&INT=06&TYP=tmin&ART=karte&RUBRIK=akt&R=310&CEL=C&SI=mph (accessed on 7 April 2023).
- Van den Eynde, C.; Sohier, C.; Matthijs, S.; De Regge, N. Relevant Day/Night Temperatures Simulating Belgian Summer Conditions Reduce Japanese Encephalitis Virus Dissemination and Transmission in Belgian Field-Collected Culex pipiens Mosquitoes. Viruses 2023, 15, 764. [Google Scholar] [CrossRef] [PubMed]
- Heitmann, A.; Jansen, S.; Lühken, R.; Leggewie, M.; Schmidt-Chanasit, J.; Tannich, E. Forced salivation as a method to analyze vector competence of mosquitoes. J. Vis. Exp. 2018, 2018, 3–7. [Google Scholar] [CrossRef]
- Hernández-Triana, L.M.; de Marco, M.F.; Mansfield, K.L.; Thorne, L.; Lumley, S.; Marston, D.; Fooks, A.A.; Johnson, N. Assessment of vector competence of UK mosquitoes for Usutu virus of African origin. Parasites Vectors 2018, 11, 381. [Google Scholar] [CrossRef]
- Bharucha, T.; Sengvilaipaseuth, O.; Vongsouvath, M.; Vongsouvath, M.; Davong, V.; Panyanouvong, P.; Piorkowski, G.; Garson, J.; Newton, P.; De Lamballerie, X.; et al. Development of an improved RT-qPCR Assay for detection of Japanese encephalitis virus (JEV) RNA including a systematic review and comprehensive comparison with published methods. PLoS ONE 2018, 13, e0194412. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control and European Food Safety Authority. Mosquito Maps; ECDC: Stockholm, Sweden, 2022; Available online: https://ecdc.europa.eu/en/disease-vectors/surveillance-and-disease-data/mosquito-maps (accessed on 22 February 2022).
- de Wispelaere, M.; Desprès, P.; Choumet, V. European Aedes albopictus and Culex pipiens are competent vectors for Japanese encephalitis virus. PLoS Neglected Trop. Dis. 2017, 11, e0005294. [Google Scholar] [CrossRef]
- Folly, A.J.; Dorey-Robinson, D.; Hernández-Triana, L.M.; Ackroyd, S.; Vidana, B.; Lean, F.Z.X.X.; Hicks, D.; Nuñez, A.; Johnson, N. Temperate conditions restrict Japanese encephalitis virus infection to the mid-gut and prevents systemic dissemination in Culex pipiens mosquitoes. Sci. Rep. 2021, 11, 6133. [Google Scholar] [CrossRef]
- Takken, W.; Verhulst, N.O. Host preferences of blood-feeding mosquitoes. Annu. Rev. Èntomol. 2013, 58, 433–453. [Google Scholar] [CrossRef]
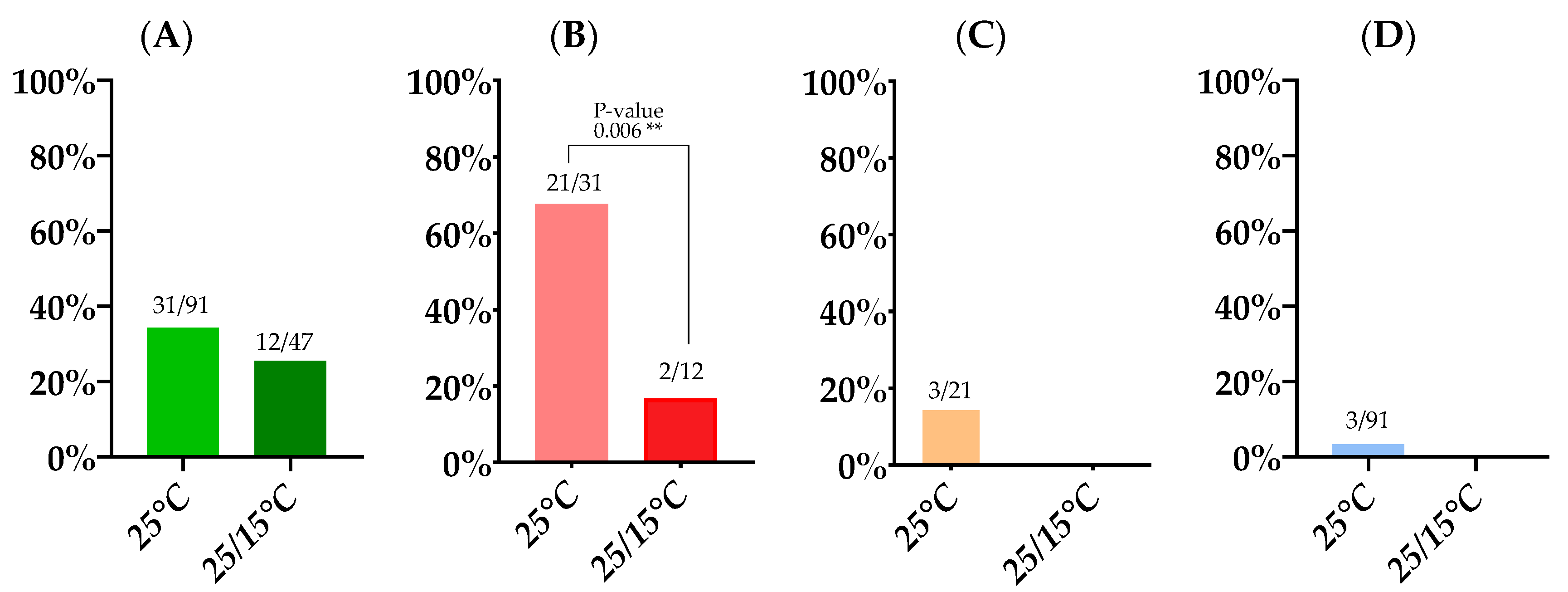
| 14 Day Survival Rate | Infection Rate | Dissemination Rate | Transmission Rate | Transmission Efficiency | |
|---|---|---|---|---|---|
| 25 °C | 30.7% (91/293) | 34.1% (31/91) | 67.7% (21/31) | 14.3% (3/21) | 3.3% (3/91) |
| 25/15 °C | 29.7% (47/158) | 25.5% (12/47) | 16.7% (2/12) | 0% (0/2) | 0% (0/47) |
| Infection RT-qPCR Positive Samples Confirmed by Isolation | Dissemination RT-qPCR Positive Samples Confirmed by Isolation | Transmission RT-qPCR Positive Samples Confirmed by Isolation | |
|---|---|---|---|
| 25 °C | 96.7% (30/31) | 33.3% (7/21) | 0% (0/3) |
| 25/15 °C | 91.7% (11/12) | 100% (2/2) | No RT-qPCR positives |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van den Eynde, C.; Sohier, C.; Matthijs, S.; De Regge, N. Belgian Anopheles plumbeus Mosquitoes Are Competent for Japanese Encephalitis Virus and Readily Feed on Pigs, Suggesting a High Vectorial Capacity. Microorganisms 2023, 11, 1386. https://doi.org/10.3390/microorganisms11061386
Van den Eynde C, Sohier C, Matthijs S, De Regge N. Belgian Anopheles plumbeus Mosquitoes Are Competent for Japanese Encephalitis Virus and Readily Feed on Pigs, Suggesting a High Vectorial Capacity. Microorganisms. 2023; 11(6):1386. https://doi.org/10.3390/microorganisms11061386
Chicago/Turabian StyleVan den Eynde, Claudia, Charlotte Sohier, Severine Matthijs, and Nick De Regge. 2023. "Belgian Anopheles plumbeus Mosquitoes Are Competent for Japanese Encephalitis Virus and Readily Feed on Pigs, Suggesting a High Vectorial Capacity" Microorganisms 11, no. 6: 1386. https://doi.org/10.3390/microorganisms11061386
APA StyleVan den Eynde, C., Sohier, C., Matthijs, S., & De Regge, N. (2023). Belgian Anopheles plumbeus Mosquitoes Are Competent for Japanese Encephalitis Virus and Readily Feed on Pigs, Suggesting a High Vectorial Capacity. Microorganisms, 11(6), 1386. https://doi.org/10.3390/microorganisms11061386






