Safety and Potential Role of Lactobacillus rhamnosus GG Administration as Monotherapy in Ulcerative Colitis Patients with Mild–Moderate Clinical Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
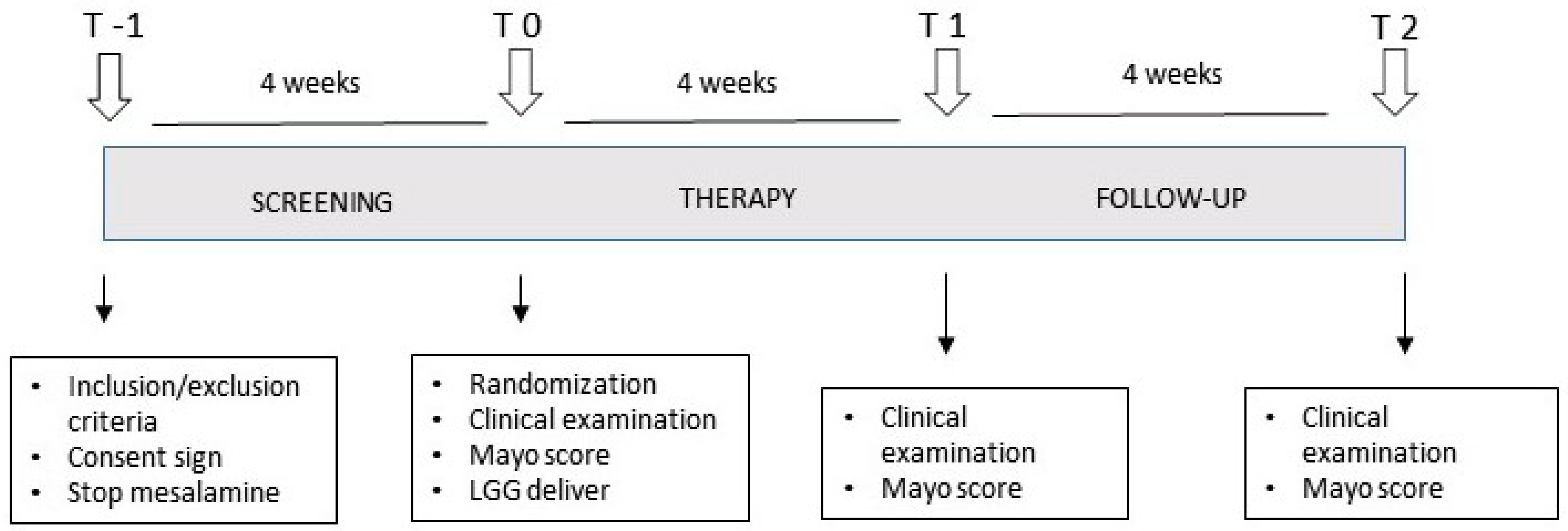
2.2. Study Design
2.3. Statistics
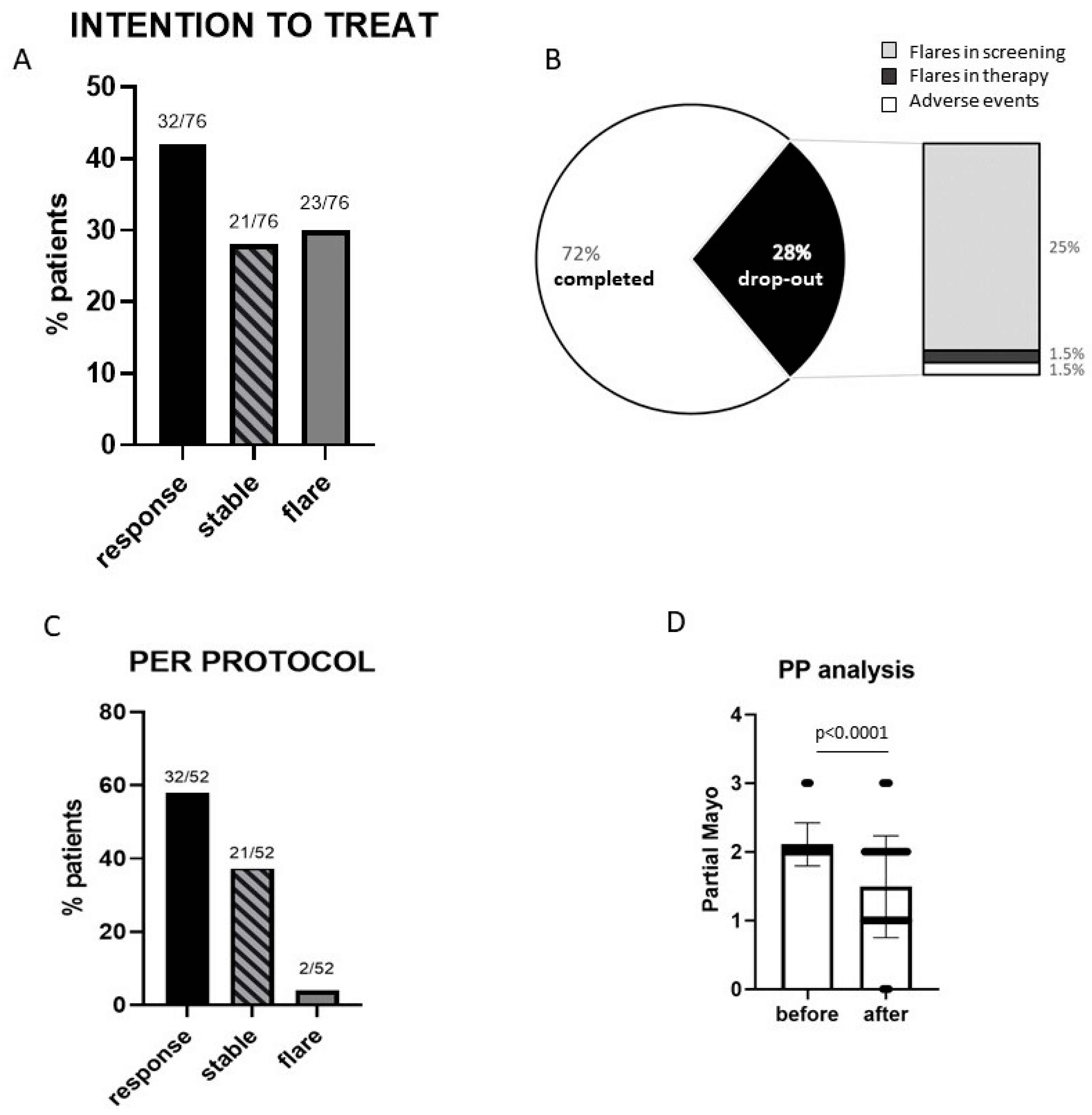
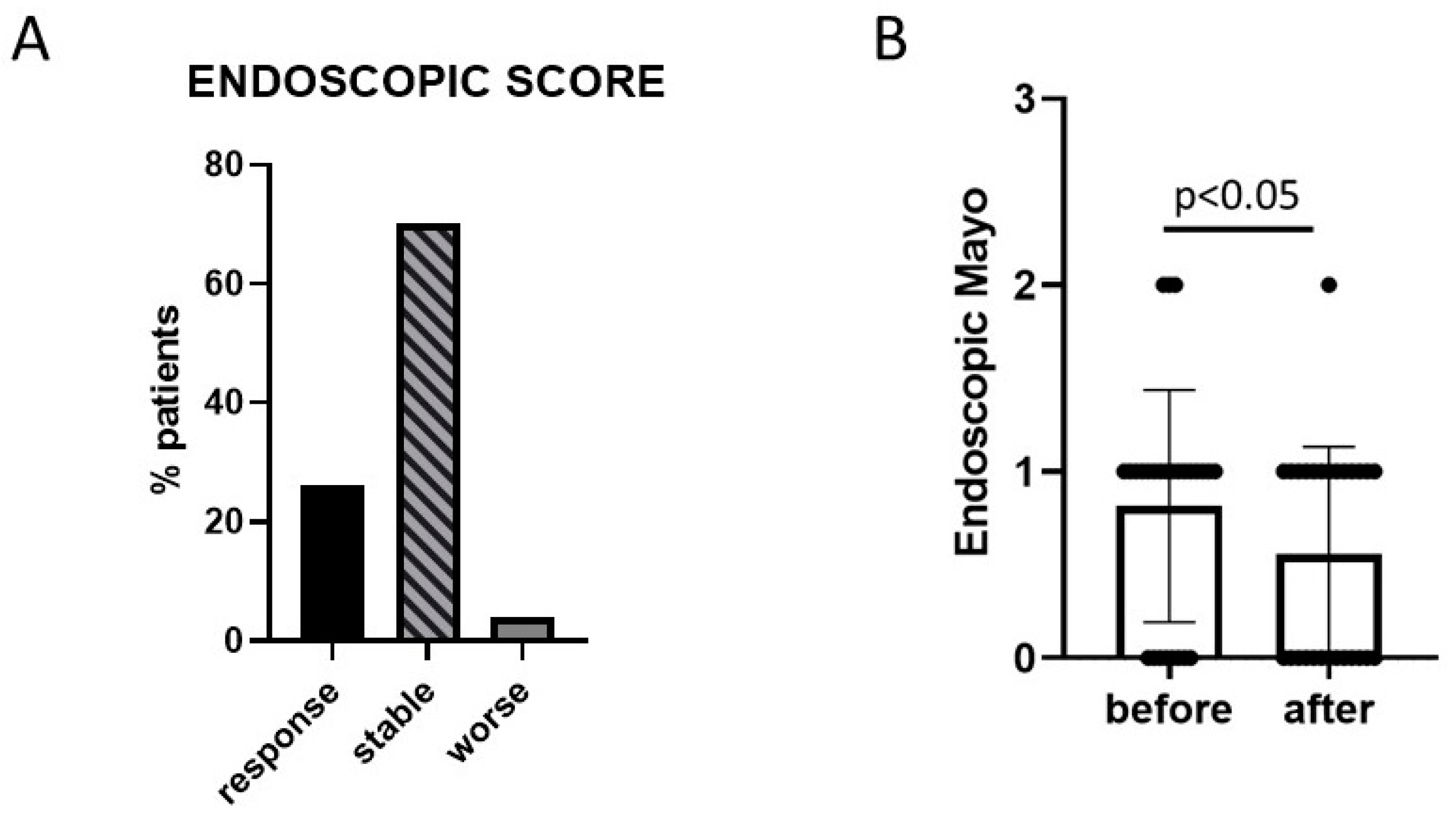
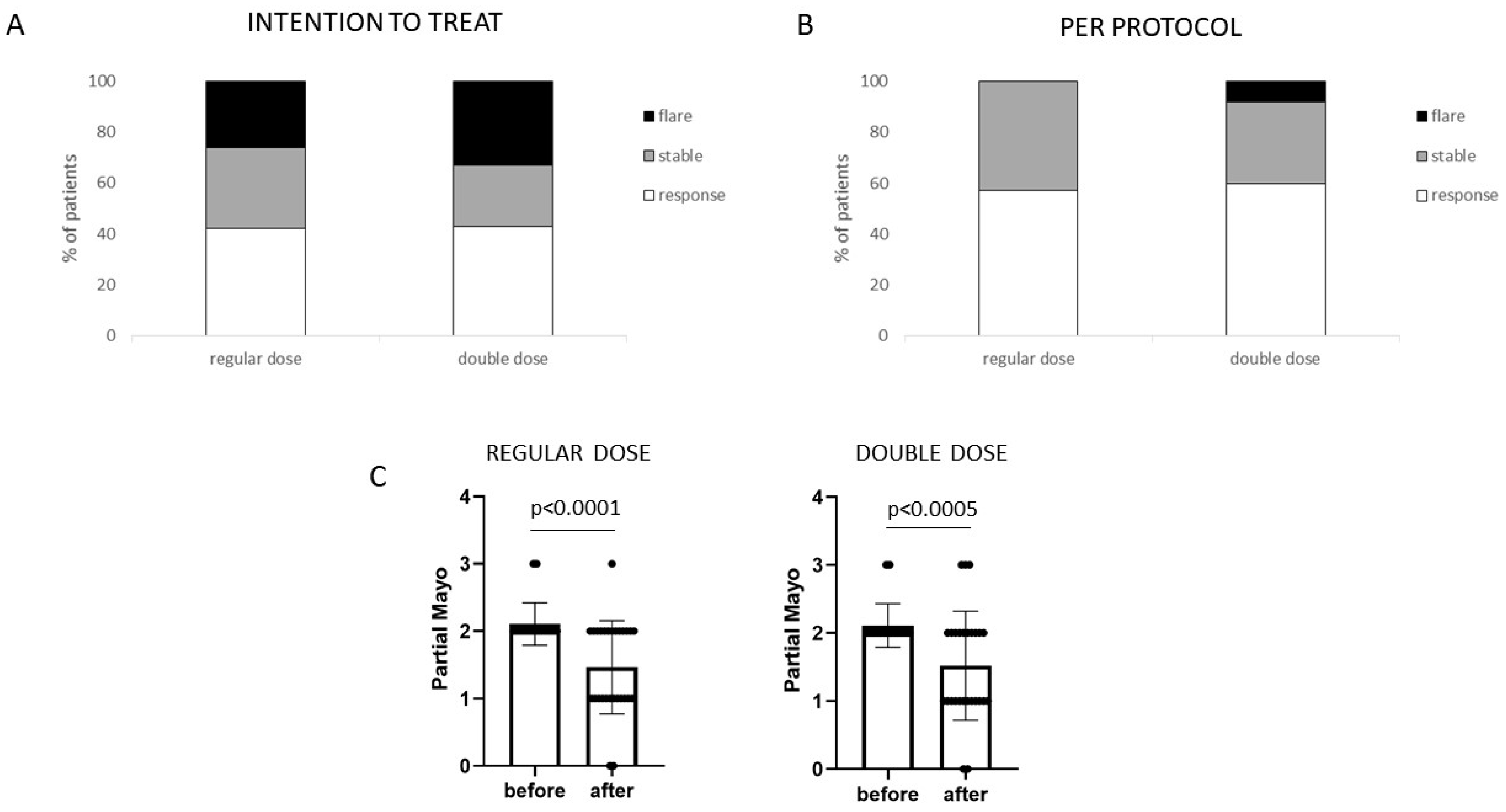
3. Results
3.1. Efficacy
3.2. Safety
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abraham, C.; Cho, J.H. Inflammatory bowel disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68 (Suppl. S3), s1–s106. [Google Scholar] [CrossRef] [PubMed]
- Wong, U.; Cross, R.K. Primary and secondary nonresponse to infliximab: Mechanisms and countermeasures. Expert Opin. Drug Metab. Toxicol. 2017, 13, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Pagnini, C.; Pizarro, T.T.; Cominelli, F. Novel Pharmacological Therapy in Inflammatory Bowel Diseases: Beyond Anti-Tumor Necrosis Factor. Front. Pharmacol. 2019, 10, 671. [Google Scholar] [CrossRef]
- Caruso, R.; Lo, B.C.; Nunez, G. Host-microbiota interactions in inflammatory bowel disease. Nat. Rev. Immunol. 2020, 20, 411–426. [Google Scholar] [CrossRef]
- Walker, A.W.; Sanderson, J.D.; Churcher, C.; Parkes, G.C.; Hudspith, B.N.; Rayment, N.; Brostoff, J.; Parkhill, J.; Dougan, G.; Petrovska, L. High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol. 2011, 11, 7. [Google Scholar] [CrossRef]
- Andoh, A.; Imaeda, H.; Aomatsu, T.; Inatomi, O.; Bamba, S.; Sasaki, M.; Saito, Y.; Tsujikawa, T.; Fujiyama, Y. Comparison of the fecal microbiota profiles between ulcerative colitis and Crohn’s disease using terminal restriction fragment length polymorphism analysis. J. Gastroenterol. 2011, 46, 479–486. [Google Scholar] [CrossRef]
- Darfeuille-Michaud, A.; Boudeau, J.; Bulois, P.; Neut, C.; Glasser, A.L.; Barnich, N.; Bringer, M.A.; Swidsinski, A.; Beaugerie, L.; Colombel, J.F. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology 2004, 127, 412–421. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermudez-Humaran, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef]
- Zou, J.; Liu, C.; Jiang, S.; Qian, D.; Duan, J. Cross Talk between Gut Microbiota and Intestinal Mucosal Immunity in the Development of Ulcerative Colitis. Infect. Immun. 2021, 89, e0001421. [Google Scholar] [CrossRef]
- Shen, Z.H.; Zhu, C.X.; Quan, Y.S.; Yang, Z.Y.; Wu, S.; Luo, W.W.; Tan, B.; Wang, X.Y. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J. Gastroenterol. 2018, 24, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Huang, C.; Xu, J.; Xu, H.; Liu, L.; Zhao, H.; Wang, J.; Huang, W.; Peng, W.; Chen, Y.; et al. Gut Microbiota Is a Potential Biomarker in Inflammatory Bowel Disease. Front. Nutr. 2022, 8, 818902. [Google Scholar] [CrossRef] [PubMed]
- Derwa, Y.; Gracie, D.J.; Hamlin, P.J.; Ford, A.C. Systematic review with meta-analysis: The efficacy of probiotics in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2017, 46, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Markowiak-Kopec, P.; Slizewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Plaza-Diaz, J.; Munoz-Quezada, S.; Gomez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef]
- Ohland, C.L.; Macnaughton, W.K. Probiotic bacteria and intestinal epithelial barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G807–G819. [Google Scholar] [CrossRef]
- Pagnini, C.; Delle Fave, G.; Bamias, G. Probiotics in inflammatory bowel disease: Pathophysiological background and clinical applications. World J. Immunol. 2013, 3, 31–43. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef]
- Capurso, L. Thirty Years of Lactobacillus rhamnosus GG: A Review. J. Clin. Gastroenterol. 2019, 53 (Suppl. S1), S1–S41. [Google Scholar] [CrossRef]
- Pagnini, C.; Corleto, V.D.; Martorelli, M.; Lanini, C.; D’Ambra, G.; Di Giulio, E.; Delle Fave, G. Mucosal adhesion and anti-inflammatory effects of Lactobacillus rhamnosus GG in the human colonic mucosa: A proof-of-concept study. World J. Gastroenterol. 2018, 24, 4652–4662. [Google Scholar] [CrossRef]
- Kaur, L.; Gordon, M.; Baines, P.A.; Iheozor-Ejiofor, Z.; Sinopoulou, V.; Akobeng, A.K. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2020, 3, CD005573. [Google Scholar] [CrossRef] [PubMed]
- Zallot, C.; Peyrin-Biroulet, L. Deep remission in inflammatory bowel disease: Looking beyond symptoms. Curr. Gastroenterol. Rep. 2013, 15, 315. [Google Scholar] [CrossRef] [PubMed]
- Kankainen, M.; Paulin, L.; Tynkkynen, S.; von Ossowski, I.; Reunanen, J.; Partanen, P.; Satokari, R.; Vesterlund, S.; Hendrickx, A.P.; Lebeer, S.; et al. Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human- mucus binding protein. Proc. Natl. Acad. Sci. USA 2009, 106, 17193–17198. [Google Scholar] [CrossRef] [PubMed]
- Velez, M.P.; Petrova, M.I.; Lebeer, S.; Verhoeven, T.L.; Claes, I.; Lambrichts, I.; Tynkkynen, S.; Vanderleyden, J.; De Keersmaecker, S.C. Characterization of MabA, a modulator of Lactobacillus rhamnosus GG adhesion and biofilm formation. FEMS Immunol. Med. Microbiol. 2010, 59, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Donato, K.A.; Gareau, M.G.; Wang, Y.J.J.; Sherman, P.M. Lactobacillus rhamnosus GG attenuates interferon-{gamma} and tumour necrosis factor-alpha-induced barrier dysfunction and pro-inflammatory signaling. Microbiology 2010, 156 Pt 11, 3288–3297. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Polk, D.B. Characterization of a probiotic-derived soluble protein which reveals a mechanism of preventive and treatment effects of probiotics on intestinal inflammatory diseases. Gut Microbes 2012, 3, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Cao, H.; Cover, T.L.; Washington, M.K.; Shi, Y.; Liu, L.; Chaturvedi, R.; Peek, R.M., Jr.; Wilson, K.T.; Polk, D.B. Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism. J. Clin. Investig. 2011, 121, 2242–2253. [Google Scholar] [CrossRef]
- Segers, M.E.; Lebeer, S. Towards a better understanding of Lactobacillus rhamnosus GG—Host interactions. Microb. Cell Factories 2014, 13 (Suppl. S1), S7. [Google Scholar] [CrossRef]
- Pena, J.A.; Versalovic, J. Lactobacillus rhamnosus GG decreases TNF-alpha production in lipopolysaccharide-activated murine macrophages by a contact-independent mechanism. Cell. Microbiol. 2003, 5, 277–285. [Google Scholar] [CrossRef]
- Miettinen, M.; Lehtonen, A.; Julkunen, I.; Matikainen, S. Lactobacilli and Streptococci activate NF-kappa B and STAT signaling pathways in human macrophages. J. Immunol. 2000, 164, 3733–3740. [Google Scholar] [CrossRef]
- Eloe-Fadrosh, E.A.; Brady, A.; Crabtree, J.; Drabek, E.F.; Ma, B.; Mahurkar, A.; Ravel, J.; Haverkamp, M.; Fiorino, A.M.; Botelho, C.; et al. Functional dynamics of the gut microbiome in elderly people during probiotic consumption. mBio 2015, 6, e00231-15. [Google Scholar] [CrossRef] [PubMed]
- Berni Canani, R.; Sangwan, N.; Stefka, A.T.; Nocerino, R.; Paparo, L.; Aitoro, R.; Calignano, A.; Khan, A.A.; Gilbert, J.A.; Nagler, C.R. Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J. 2016, 10, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Skorka, A.; Ruszczynski, M.; Gieruszczak-Bialek, D. Meta-analysis: Lactobacillus GG for treating acute gastroenteritis in children--updated analysis of randomised controlled trials. Aliment. Pharmacol. Ther. 2013, 38, 467–476. [Google Scholar] [CrossRef]
- Szajewska, H.; Kolodziej, M. Systematic review with meta-analysis: Lactobacillus rhamnosus GG in the prevention of antibiotic-associated diarrhoea in children and adults. Aliment. Pharmacol. Ther. 2015, 42, 1149–1157. [Google Scholar] [CrossRef]
- Hao, Q.; Dong, B.R.; Wu, T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst. Rev. 2015, 2, CD006895. [Google Scholar] [CrossRef]
- Kirjavainen, P.V.; Salminen, S.J.; Isolauri, E. Probiotic bacteria in the management of atopic disease: Underscoring the importance of viability. J. Pediatr. Gastroenterol. Nutr. 2003, 36, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Zocco, M.A.; dal Verme, L.Z.; Cremonini, F.; Piscaglia, A.C.; Nista, E.C.; Candelli, M.; Novi, M.; Rigante, D.; Cazzato, I.A.; Ojetti, V.; et al. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Aliment. Pharmacol. Ther. 2006, 23, 1567–1574. [Google Scholar] [CrossRef]
- Tong, L.; Zhang, X.; Hao, H.; Liu, Q.; Zhou, Z.; Liang, X.; Liu, T.; Gong, P.; Zhang, L.; Zhai, Z.; et al. Lactobacillus rhamnosus GG Derived Extracellular Vesicles Modulate Gut Microbiota and Attenuate Inflammatory in DSS-Induced Colitis Mice. Nutrients 2021, 13, 3319. [Google Scholar] [CrossRef]
- Zmora, N.; Zilberman-Schapira, G.; Suez, J.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Kotler, E.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.; et al. Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell 2018, 174, 1388–1405.e21. [Google Scholar] [CrossRef]
| Characteristics | Regular Dose (n = 38) | Double Dose (n = 37) | Total (n = 76) |
|---|---|---|---|
| Age (years) | 54 ± 14 | 60 ± 14 * | 57 ± 15 |
| Gender (M/F) | 15/23 | 21/16 | 37/39 |
| Disease duration (years) | 9.5 ± 8 | 9.8 ± 6.2 | 9.6 ± 7.1 |
| Extension: | |||
| Proctitis | 6 (16%) | 6 (17%) | 12 (16%) |
| Proctosigmoiditis | 21 (55%) | 19 (51%) | 41 (54%) |
| Pancolitis | 11 (29%) | 12 (32%) | 23 (30%) |
| Partial Mayo | |||
| 2 | 31 (82%) | 30 (81%) | 62 (82%) |
| 3 | 4 (10%) | 6 (16%) | 10 (13%) |
| 4 | 3 (6%) | 1 (3%) | 4 (5%) |
| Adverse Event (AE) | Frequency |
|---|---|
| Disease flare | 24/76 (32%) |
| Bloating | 9/76 (12%) |
| Constipation | 4/76 (5%) |
| Abdominal pain | 2/76 (3%) |
| Headache | 1/76 (1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagnini, C.; Di Paolo, M.C.; Urgesi, R.; Pallotta, L.; Fanello, G.; Graziani, M.G.; Delle Fave, G. Safety and Potential Role of Lactobacillus rhamnosus GG Administration as Monotherapy in Ulcerative Colitis Patients with Mild–Moderate Clinical Activity. Microorganisms 2023, 11, 1381. https://doi.org/10.3390/microorganisms11061381
Pagnini C, Di Paolo MC, Urgesi R, Pallotta L, Fanello G, Graziani MG, Delle Fave G. Safety and Potential Role of Lactobacillus rhamnosus GG Administration as Monotherapy in Ulcerative Colitis Patients with Mild–Moderate Clinical Activity. Microorganisms. 2023; 11(6):1381. https://doi.org/10.3390/microorganisms11061381
Chicago/Turabian StylePagnini, Cristiano, Maria Carla Di Paolo, Riccardo Urgesi, Lorella Pallotta, Gianfranco Fanello, Maria Giovanna Graziani, and Gianfranco Delle Fave. 2023. "Safety and Potential Role of Lactobacillus rhamnosus GG Administration as Monotherapy in Ulcerative Colitis Patients with Mild–Moderate Clinical Activity" Microorganisms 11, no. 6: 1381. https://doi.org/10.3390/microorganisms11061381
APA StylePagnini, C., Di Paolo, M. C., Urgesi, R., Pallotta, L., Fanello, G., Graziani, M. G., & Delle Fave, G. (2023). Safety and Potential Role of Lactobacillus rhamnosus GG Administration as Monotherapy in Ulcerative Colitis Patients with Mild–Moderate Clinical Activity. Microorganisms, 11(6), 1381. https://doi.org/10.3390/microorganisms11061381






