Abstract
Soil microbial and enzyme activities are closely related to the spatial variability of soil environmental conditions at the microscale (μm-mm). The origin and localization of the enzymes are somewhat neglected when the measured activity is used to evaluate specific soil functions. The activity of four hydrolytic enzymes (β-glucosidase, Cellobiohydrolase, Chitinase, Xylanase) and microbial diversity based on community-level physiological profiling were determined in samples of arable and native Phaeozems with increasing physical impact to soil solids. The level of impact on the soil solids had a significant effect on enzyme activity and depended on both the enzyme type and soil land use. The highest proportion of the activity of Xylanase and Cellobiohydrolase of arable Phaeozem was determined at the dispersion energy in the range of 450–650 J·mL−1 and was associated with the primary soil particles’ hierarchy level. The highest proportions of β-glucosidase and Chitinase activities were determined for forest Phaeozem after applying energies lower than 150 J·mL−1 and characterizing the level of soil microaggregates. The increased activity of Xylanase and Cellobiohydrolase in primary soil particles of arable soil compared to those in forest soil might be a reflection of the substrates being unavailable to decomposition, leading to enzyme accumulation on the solid surface. For the Phaeozems, the lower the level of soil microstructure organization, the greater the differences observed between soils of different land use type, i.e., microbial communities, associated with lower microstructure levels, were more specific to land use type.
1. Introduction
Soil microbial activity and subsequently enzyme activity (EA) are closely related to the spatial variability of soil environmental conditions at the microscale (μm-mm) [1]. The physical availability of organic compounds to microorganisms strongly influences the ability of microbial communities to feed and function [2,3]. Moreover, the lower the hierarchy level of soil structure organization, the more mineralized soil organic matter (SOM) is involved in its formation [4,5]. Furthermore, the higher the soil aggregation and saturation of SOM, the more protected from degradation the enzymes are [6]. It is assumed that the number of extracellular enzymes stabilized by their interaction with soil solids is much greater than the number of intracellular or extracellular enzymes associated with active microbial cells [7].
“Heterogeneity is a fundamental property of soil that is often overlooked in microbial ecology” [8]. The physical conditions of soil microhabitats, called microenvironments, which determine nutrient availability and competitive conditions, are thought to influence enzyme production by microorganisms, given the cost–benefit relationship of this process [9]. Soil microaggregates have pore sizes ranging from a few units to hundreds of µm [10]; the range of sizes also corresponds to water storage and nutrient supply [5,11], and therefore they are considered as the main habitat for soil microbiota. Smaller pores corresponding to elementary (or composite building units, [12]) and primary soil particles [5,13] contain substrate that is inaccessible to the microbiota, which is reflected in the concept of physically protected organic matter [14,15,16]. It is considered that pores 30–90 µm in size contribute primarily to the decomposition of C in soils [17]. It may seem paradoxical from an energetic perspective, but limitations in substrate availability lead to greater production of extracellular enzymes by microorganisms [18,19]. Thus, the microstructural organization of soils is closely related to the distribution of different types of organic substrates and, accordingly, the EA of soils, and vice versa.
The origin and localization of enzymes is somewhat neglected when EA is used to evaluate the specific functions of soil [20]. The allocation of enzymes according to their localization in the solid phase of soils has been called one of the most important tasks of modern enzymology [20]. “However, in this aspect, soil enzymology still badly needs new methodological approaches to the study of enzymatic activity. Until methodology has reached such perfection that these activities can be defined and investigated separately, the overall picture of soil enzymatic activity will remain incompletely understood” [21] (p. 12). Data on the dynamics of the storage and conservation of extracellular enzymes in soils depending on soil properties and, even more so, on the microstructural organization of soils is limited. Experimental data are predominantly represented by the activity of enzymes in different particle-size fractions (e.g., [22,23,24,25,26]), i.e., immobilized on the surface of the solid phase. Particle-size fractionation schemes vary considerably in terms of applied energy and used particle size limits, which are rarely justified in terms of the microstructural organization of soils. Biogeochemical models of C are rapidly seeking to incorporate metabolic and physiological parameters as well as microbial life strategies to account for microbial regulation of decomposition processes (e.g., [27,28]). To understand the mechanisms responsible for the persistence of SOM, it is necessary to understand how the organization of soil structure at the micro- and nano-scale interacts with biotic processes [29].
This paper aims to identify the redistribution of C-cycle-related enzymes and associated microbial cells within hierarchy levels of soil microstructure in two Silt Loam Phaeozems contrasting in land use type—native forest soil and arable land. Phaeozems are one of the main soil types of the Far East, Siberia, and European Russia, occupying 1.8% of the area, and formed within humid and sub-humid forest and steppe–forest zones [30].
2. Materials and Methods
2.1. Experimental Design
This experiment is based on a sequentially increasing physical impact on soil solids (Figure 1). According to the energy of impact, enzymes and cells were detached from the surface of the solids, and soil microstructural units of different soil hierarchy levels were dispersed [5].
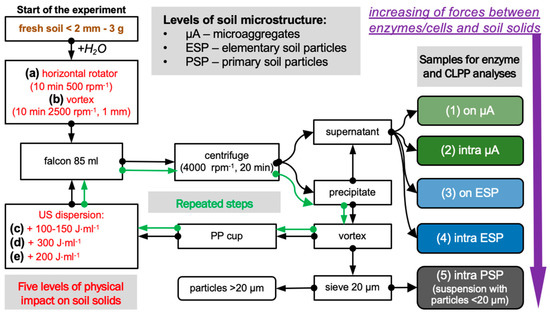
Figure 1.
Experimental design for the isolation of enzymes and microbial cells localized on the surface and within the soil microstructural units (primary soil particles, PSP; elementary soil particles, ESP; microaggregates, µA). In total, isolation according to five levels of physical impact ((a–e), red text) on soil solids was performed from soil samples in four replicates, resulting in subsamples corresponding to surface and intra-microaggregate (on µA and intra µA), and to surface and intra-elementary soil particles (on µA and intra µA). The applied ultrasonic energy was equal to 26 J·s−1.
In total, five levels of dispersion energy were used:
1st level: separation from the surface of microaggregates (µA) was accomplished by gently shaking the soil–water suspension (1:30 by mass) on a horizontal rotatory shaker (Multi Bio RS-24 (Biosan, Rīga, Latvia) for 10 min at 500 rpm;
2nd level: dispersion of µA and separation of intra-microaggregate enzymes and cells was performed by intensive mechanical shaking of the precipitate from the first step on vortex Reax-top (Heidolph Instruments, Schwabach, Germany for 10 min at a frequency of 2500 rpm and an amplitude of 1 mm for 10 min [31];
3d level: separation from the surface of elementary soil particles (ESP) by ultrasonic action on soil–water suspension including precipitate from the second step equal to 100–150 J·mL−1;
4th level: dispersion of ESP and separation of intra-ESP enzymes and cells was carried out using additional ultrasonic dispersion on soil solids equal to 300 J·mL−1; thus, the total energy of ultrasound exposure was equal to ~450 J·mL−1 [32,33,34];
5th level: separation of enzymes and cells associated with primary soil particles (PSP) was performed with excessive ultrasonic action on soil solids separated from the former step equal to 200 J·mL−1, and a suspension with particles less than <20 µm in size was taken for analyses. In total, the energy of soil samples was equal to 650 J·mL−1.
For each soil, four separate samples were used. After each step of dispersion, the soil suspension was centrifuged at 4000 rpm for 20 min, the supernatant was taken for enzyme analyses and community-level physiological profiling (CLPP), and the precipitate was subjected to the next level of dispersion energy. At the 5th level of soil dispersion, soil suspensions sieved through the mesh size equal to 20 µm were used for further analyses.
We used a horn-type ultrasonic disruptor Sonifier S-250D (Branson, MO, USA) with a ½” solid step horn with a threaded body. Calibration of the ultrasonic energy output was carried out according to a common calorimetry method (North, 1976). We used ultrasonic power equal to 26 J·mL−1·s−1 to prevent the destruction of enzymes and microbial cells.
2.2. Study Site, Soil Sampling, and Basic Properties
Samples (four for each soil) from two A horizons of Phaeozems were collected in August 2021: Greyic Phaeozems (Albic) under a temperate mixed forest (>70 years; dominant species were Querqus Robur and Tilia Cordata) from the V.V. Dokuchaev Soil Science Institute Ivanovsky Field Station (Russia, Tula region, 54°78′25.67″ N, 38°03′52.99″ E; additional information about the aggregate composition and mechanical properties, [35]; microbial community, [36]); and soil from the nearby arable field—four-field crop rotation by conventional tillage practice (54°45′56.1″ N, 38°01′28.9″ E) (SOM fractions, for both arable and forest soils, [37]). Since we took soil samples in August, at the end of the growing season, the harvest had already been taken and the field had not yet been ploughed. Thus, both soils were in equilibrium conditions. The mean annual precipitation is equal to 595 mm, the mean annual temperature is +6.4 °C. The parent material is cover loam. The soil textural class is Silt Loam according to USDA classification [38]. The total C contents were 39.99 ± 11.05 and 22.0 ± 0.3 g·kg−1, and the bulk density was 0.92 ± 0.04 and n/a g·cm−3, respectively. The pHH2O was equal to 5.49, pHKCl—4.49 for the forest soil. Before analyses, samples were stored in a laboratory fridge at 4 °C for a month, then homogenized through a sieve with a mesh size of 2 mm.
2.3. Enzyme Activity (EA) Analyses
EA in soil suspensions was measured as hydrolytic enzyme activity of β-D-glucosidase (E.C. 3.2.1.21), cellobiosidase (E.C. 3.2.1.91), β-D-xylosidase (E.C. 3.2.1.37), and N-acetyl-β-D-glucosaminidase (E.C. 3.2.1.52) using modified fluorescent-linked substrates (4-methylumbelliferone, MUF) according to a modified Marx method [39,40]. Activity of hydrolytic enzymes were measured in black polystyrene 96-well, 300-µL microplates (Costar, Corning, New York, NY, USA). The microplates were incubated in the dark at room temperature, 24 °C, for 120 min. A CLARIOstar Plus Microplate Reader (BMG LABTECH, Ortenberg, Germany) with excitation of 360 nm and emission of 465 nm was used to determine the fluorescence of the MUF. Concentrations of the MUF were calculated using the calibration curve, and EAs were expressed as micromoles (μmol) of MUF released per g (g−1) of soil, per hour (h−1):
where CMUF is the concentration of released MUF (µL·L−1), vs. is the volume of soil suspension (50,000 µL), Vwell is the total volume of liquid in each well of the microplate (200 µL), Val is the volume of aliquot of soil suspension (50 µL), mad is the dry weight of soil (g), and tinc is time of incubation (h). The total enzyme activity was calculated for each sample as a sum of activities, determined in soil fractions with increased physical impact (Figure 1a–e).
EA = (CMUF × vs. × Vwell)/(mad × tinc × Val),
2.4. Community-Level Physiological Profiling (CLPP)
Functional diversity and potential metabolic activity of microbial communities of soil suspensions separated according to the experimental scheme (Figure 1) were assessed using community-level physiological profiling (CLPP) [41,42], also known as the multisubstrate testing (MST) method [43,44].
The 96-well microplate included wells containing a set of 47 test substrates and mineral salts and one well containing distilled water with mineral salts (Table A1); the 48th well containing mineral salts without substrate was used as a control of SOM utilization [44]. Microplates were incubated in the dark in a thermostatically controlled chamber (Espec SH-241 Temperature Humidity Chamber, Osaka, Japan) at 24 °C and 99.8 air humidity for 72 h. After incubation, the optical density of the wells was measured with a CLARIOstar Plus Microplate Reader (BMG LABTECH, Ortenberg, Germany) at 450 nm wavelength.
Average well colour development (AWCD) was calculated as AWCD = Σ(Ci − R)/47, where R is the absorbance of the control well (containing distilled water), and Ci is the absorbance of the plate well inoculated with C source i. Richness was calculated as the number of oxidized substrates. The Shannon index (H) was calculated using an optical density value of 0.25 as the threshold for positive response: H = −Σpi (lnpi), where pi = (Ci − R)/Σ(Ci − R). The Jaccard index (J) was calculated as J = c/(a + b − c), where a is the number of substrates consumed by the first microbial community, b is the number of substrates consumed by the second microbial community, and c is the number of intersected substrates consumed by the 1st and 2nd communities.
2.5. Statistical Analyses
Data processing and visualization were carried out in R 3.6.3. The comparison of the variables was performed with ANOVA and Tukey test in the agricolae package (p = 0.05). A significance level of p < 0.05 was applied. Plots were drawn in the ggplot2 package.
3. Results
The total enzyme activity calculated as the sum of activities determined in all fractionated subsamples 1–5 (Figure 1) differed between land use types for β-glucosidase and Cellobiohydrolase, and did not differ for Chitinase or Xylanase (Table 1).

Table 1.
Total enzyme activities (μM MUF (g−1 soil hour−1)) of β-glucosidase, Cellobiohydrolase, Chitinase, and Xylanase determined in soil suspensions, separated from A horizons of arable and forest Phaeozems. Values are mean ± SE (n = 4). Values followed by a different lowercase letter show significant difference among land use types.
Among the four studied enzymes, the activities of β-glucosidase and Chitinase (Figure 2a,c) had a similar distribution within the hierarchy levels of the soil microstructure, opposite those the other two enzymes—Cellobiohydrolase and Xylanase, which were also characterized by similar distributions (Figure 2b,d). General to all four enzymes isolated from the arable soil was a low EA after high physical impact characterizing the level of elementary soil particles (intra-ESP) between the suspension isolated with minimal impact on the solids (enzymes on the microaggregates (µA) surface) and the suspension sample containing primary soil particles (PSP), obtained after maximum physical impact. For soil suspensions separated from the forest soil, no activities tended towards zero value.
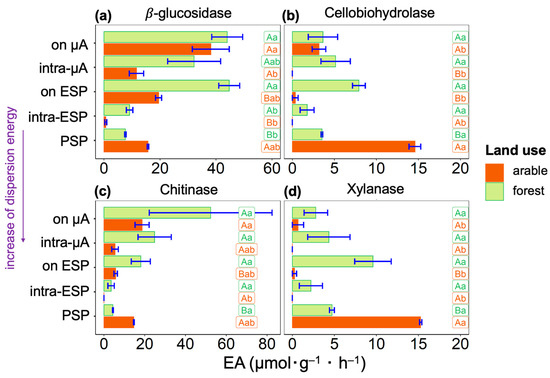
Figure 2.
Soil enzyme activities (X axes, μM MUF (g−1 soil hour−1)) of (a) β-glucosidase, (b) Cellobiohydrolase, (c) Chitinase, and (d) Xylanase determined in soil suspensions, separated from A horizons of arable and forest Phaeozems with increasing dispersion energy (Y axes): on µA—from the surface of microaggregates, intra-µA—within microaggregates, on ESP—from the surface of elementary soil particles, intra-ESP—within elementary soil particles, and PSP—associated with primary soil particles. Values are mean ± SE (n = 4). Values followed by a different lowercase letter represent a significant difference between types of suspension. Values followed by a different capital letter represent a significant difference among land use types.
The activities of β-glucosidase and Chitinase in both arable and forest soils were higher on the surface and within the microaggregates and elementary soil particles than their activities associated with primary soil particles (Figure 2a,c). In arable soil, for Cellobiohydrolase and Xylanase, the highest values of activities were determined in suspension with primary soil particles (Figure 2b,d). However, in the forest soil, we see the opposite for these enzymes; Cellobiohydrolase and Xylanase activities were lower in the sample with primary soil particles than in other suspensions. Therefore, in the arable soil, Cellobiohydrolase and Xylanase are associated with the surface of soil solids < 20 µm or are tightly bound to the solid surface since they are contained in a sample after being exposed to a very high (~650 J·mL−1) ultrasonic action. However, in the forest soil, activities of Cellobiohydrolase and Xylanase are distributed uniformly within different sites of soil microstructural units.
The activities of β-glucosidase and Chitinase in arable soil sample were lower in suspensions, and were associated with elementary soil particles (Figure 2a,c). In forest soil, these enzymes had the lowest activities in suspension associated with the surface of primary soil particles. The maximal activities of β-glucosidase and Chitinase were associated with soil microaggregates.
In forest soil, enzyme activity associated with soil solids (<20 µm) resistant to high total physical impact (~650 J·mL−1) was lower for β-glucosidase, Cellobiohydrolase, and Xylanase, and was higher for Chitinase, than the sum of activities obtained after lower physical impact (Table A2). In arable soil, enzyme activity associated with soil solids (<20 µm) resistant to high total physical impact (~650 J·mL−1) was lower for β-glucosidase and Chitinase, and was higher for Cellobiohydrolase and Xylanase, than the sum of activities obtained after lower physical impact (Table A2). Agricultural use of Phaeozem led to an increase in Cellobiohydrolase, Chitinase, and Xylanase activity tightly bound to the soil solids (<20 µm), and increased activity in Chitinase in suspensions separated with lower (<650 J·mL−1) ultrasonic energies.
After complete disruption of soil microaggregates to primary soil particles and the excessive impact needed to detach cells and enzymes from solids surfaces (total dispersion energy 650·mL−1), the total enzyme activity still associated with soil solids surface ranged from 4 to 20% for the forest soil and from 18 to 94% for the arable soil (Table A2). The proportion of activity associated with primary soil particles increased linearly for Chitinase (4%), β-glucosidase (6%), Cellobiohydrolase (16%), and Xylanase (20%) in forest soil, and β-glucosidase (18%), Chitinase (33%), Cellobiohydrolase (81%), and Xylanase (94%) in arable soil.
In the studied soils, potential microbial metabolic activity estimated based on AWCD was higher in soil suspensions containing cells after low mechanical impact by rotary shaker and vortex on soil solids in contrast to samples resulting from ultrasonic action (Figure 3a). We see a gradual decrease in richness with increased physical impact on soil solids (Figure 3c), and in soil samples of primary soil particles more than 20 of the 47 substrates were consumed by microorganisms. Therefore, we explain the difference in AWCD by a decrease in the microbial abundance in elementary and primary soil particles compared to soil microaggregates. With increasing physical impact, the microbial functional diversity in both forest and arable Phaeozems decreases from microaggregates to the primary soil particle level according to the Shannon index (Figure 3b) and richness (Figure 3c).
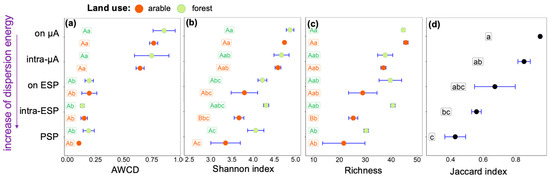
Figure 3.
Microbial metabolic activity and diversity characterized by (a) Average well colour development (AWCD), (b) Shannon diversity index (X axis), (c) Richness (X axis), (d) Jaccard index (X axis), determined in in soil suspensions separated from A horizons of arable and forest Phaeozems with increasing dispersion energy (Y axes): on µA—from the surface of microaggregates, intra-µA—within microaggregates, on ESP—from the surface of elementary soil particles, intra-ESP—within elementary soil particles, and PSP—associated with primary soil particles. Values are mean ± SE (n = 4). Values followed by a different lowercase letter represent a significant difference between types of suspension. Values followed by a different capital letter represent a significant difference among land use types.
Among land use types, differences in microbial diversity were found in soil suspensions isolated after the destruction of the elementary soil particles. The lowest microbial diversity was in the arable sample, associated with intra-ESP microenvironments. The Jaccard index value increased from 0.43 in suspension with primary soil particles to 0.95 in suspension after gently shaking (Figure 3d), i.e., with increasing level of microstructural organization, the similarity of communities between land uses increases.
4. Discussion
Comparison of experiments using ultrasonic soil dispersion is possible when the authors specify the power of dispersion energy in W (J·s1). A comprehensive comparison also requires the known volume of the suspension and its concentration (soil to liquid ratio), as well as the time of ultrasound exposure, which are necessary to calculate the total energy of dispersion [45,46]. We used low-energy ultrasonic action (26 J·s−1) to prevent damage of microbial cells and enzymes, which is close to the physical impact used by [47,48,49]—70 J·s−1 power and total energy 30 J·mL−1—and [23,24] —50 J·s−1 power and total energy ~53 J·mL−1. Due to the gradual decrease in microbial diversity and metabolic activity with increased impact on soil samples (Figure 3b,c), we concluded that the energy of ultrasonic dispersion did not destroy microbial cells.
Soil management considerably influences the microbial activity and diversity at the microscale of the studied Phaeozems. The maximum proportion of enzyme activity in primary soil particles was found for Xylanase compared to other studied enzymes (Table A2). Moreover, for arable Phaeozem, this enzyme accounted for almost all the activity (94% of the total activity). Xylanase is a mainly extracellular enzyme associated with fungi [50], and its increased activity was previously found in coarse fractions of Cambisols, Calcic Chernozems, Luvisols [23,24], and Eutric Cambisol [51]. High activity of Xylanase in the fine fraction of arable Phaeozem could be associated with the redistribution of particulate organic matter into finer fractions. However, we suppose this to be unlikely, because (i) we used low-power ultrasonic energy; (ii) arable soil usually contains small amounts of particulate organic matter [52], whereas in the forest soil we obtained a maximum of Xylanase activity in the sample associated with the surface of elementary soil particles (Figure 2d). The increased activity of Xylanase and Cellobiohydrolase in primary soil particles of arable soil compared to those in forest soil (Table A2) might be a reflection of the substrates unavailable for decomposition, leading to enzyme accumulation on the solid surface. This is because land use results in the destruction of the soil microstructure and, consequently, an increase in the specific surface area [53].
An increased Chitinase activity at the level of microaggregates in both of the studied soils (Figure 2c) is in agreement with the predominant distribution of fungi within large, well-aerated habitats, such as the surface of microaggregates [12,50,54].
The lower the level of soil microstructure organization [5], the more specific the associated microbial community was to land use type (Figure 3d). This is opposite to the results of [47], showing the higher buffering capacity of clay fractions (<2 µm) against long-term (100 years) fertilization. In our case, the microbial diversity was reduced by agricultural activity at the level of elementary soil particles and does not differ at the microaggregate level (Figure 3b,c). At the level of primary particles, a difference was found between land use types in the activity of all four enzymes studied, and was lower in the forest soil (Figure 2).
5. Conclusions
We present here an experimental design based on increasing physical impact on soil solids, allowing us to characterize the microbial activity and diversity within levels of soil microstructure. The level of impact on the soil solids had a significant effect on enzyme activity and depended on the enzyme type and soil land use type. The highest proportion of activity of Xylanase and Cellobiohydrolase in arable Phaeozem was determined when dispersion energy was in the range of 450–650 J·mL−1, and was 94 and 81%, respectively. The highest proportions of β-glucosidase and Chitinase activities were determined for forest Phaeozem within the range of energies lower than 150 J·mL−1. Thus, the indication of soil quality based on the activity of enzymes is problematic without considering the energy of the impact on the solid phase of the soil.
Soil management considerably influenced the microbial activity and diversity at the microscale of the studied Phaeozems. The increased activity of Xylanase and Cellobiohydrolase in primary soil particles of arable soil compared to those in forest soil might be a reflection of substrates being unavailable for decomposition, leading to enzyme accumulation on the solid surface. For the Phaeozems, the lower the level of soil microstructure organization, the greater the differences between soil of different land use types, i.e., microbial communities associated with lower microstructure levels were more specific to land use type.
Author Contributions
Conceptualization, A.Y.; methodology, A.Y., D.F. and V.C.; validation, A.Y., D.F. and V.C.; formal analysis, D.F. and O.O.; investigation, O.O.; resources, A.Y.; data curation, D.F., A.Y., V.C. and O.O.; writing—original draft preparation, A.Y.; writing—review and editing, V.C., D.F. and O.O.; visualization, D.F. and A.Y.; supervision, A.Y.; project administration, A.Y.; funding acquisition, A.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Science Foundation grant №21-74-00090, https://rscf.ru/project/21-74-00090/, accessed on 12 May 2023 (“Lifespan, activity and localization of C cycle enzymes in soil structure on micro-scale”).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A

Table A1.
Sole carbon sources used for CLPP.
Table A1.
Sole carbon sources used for CLPP.
| Pentoses | Arabinose, ribose, xylose |
| Hexoses | Glucose, fructose, rhamnose |
| Oligoses | Cellobiose, lactose, maltose, sucrose |
| Salts of carboxylic acids | Acetate, aspartate, citrate, succinate, maleinate, pyruvate, octanoate, lactate |
| Amino acids | Glycine, proline, leucine, methionine, histidine, alanine, asparagine, valine, serine, phenylalanine, glutamine, arginine, lysine |
| Alcohols | Dulcitol, glycerol, inositol, sorbitol, mannitol |
| Polymers | Soluble starch, corn starch, Dextran 500, Tween 20, Tween 80, gelatin, pullulan |
| Miscellaneous (amides, amines phosphorylated carbons) | Creatinine, carbamide, β-glycerophosphate, glucosamine sulfate |

Table A2.
Enzyme activities (μM MUF (g−1 soil hour−1)) of β-glucosidase, Cellobiohydrolase, Chitinase, and Xylanase determined in samples from A horizons of arable and forest Phaeozems, and associated with particles <20 µm, separated after applying ultrasonic energy equal to 650 J·mL, and the sum of activities determined in soil suspensions obtained after applying lower energies. Values are mean ± SE (n = 4). Values followed by a different capital letter show significant difference among land use types. Values followed by a different lowercase letter represent significant difference between types of suspensions.
Table A2.
Enzyme activities (μM MUF (g−1 soil hour−1)) of β-glucosidase, Cellobiohydrolase, Chitinase, and Xylanase determined in samples from A horizons of arable and forest Phaeozems, and associated with particles <20 µm, separated after applying ultrasonic energy equal to 650 J·mL, and the sum of activities determined in soil suspensions obtained after applying lower energies. Values are mean ± SE (n = 4). Values followed by a different capital letter show significant difference among land use types. Values followed by a different lowercase letter represent significant difference between types of suspensions.
| Enzymes Tested | Associated with Soil Solids < 20 µm | Sum of Activities, Determined in Supernatants | ||
|---|---|---|---|---|
| Forest | Arable | Forest | Arable | |
| β-glucosidase | 7.63 ± 0.54 Ab | 15.74 ± 0.55 Bb | 130.07 ± 36.48 Aa | 69.87 ± 19.01 Ba |
| Cellobiohydrolase | 3.52 ± 0.22 Bb | 14.58 ± 1.39 Aa | 18.46 ± 2.21 Aa | 3.51 ± 0.95 Bb |
| Chitinase | 4.39 ± 0.42 Ba | 14.66 ± 0.33 Ab | 98.74 ± 88.11 Aa | 29.83 ± 8.82 Aa |
| Xylanase | 4.71 ± 0.57 Bb | 15.27 ± 0.28 Aa | 18.92 ± 10.63 Aa | 0.91 ± 1.26 Bb |
References
- Young, I.M.; Crawford, J.W. Interactions and self-organization in the soil-microbe complex. Science 2004, 304, 1634–1637. [Google Scholar] [CrossRef] [PubMed]
- Nunan, N.; Leloup, J.; Ruamps, L.S.; Pouteau, V.; Chenu, C. Effects of habitat constraints on soil microbial community function. Sci. Rep. 2007, 7, 4280. [Google Scholar] [CrossRef]
- Schimel, J.P. Life in dry soils: Effects of drought on soil microbial communities and processes. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 409–432. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J.M. Organic matter and water-stable aggregates in soils. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Yudina, A.; Kuzyakov, Y. Dual nature of soil structure: The unity of aggregates and pores. Geoderma 2023, 434, 116478. [Google Scholar] [CrossRef]
- Alkorta, I.; Aizpurua, A.; Riga, P.; Albizu, I.; Amézaga, I.; Garbisu, C. Soil enzyme activities as biological indicators of soil health. Rev. Environ. Health 2003, 18, 65–73. [Google Scholar] [CrossRef]
- Nannipieri, P. Role of stabilised enzymes in microbial ecology and enzyme extraction from soil with potential applications in soil proteomics. In Nucleic Acids and Proteins in Soil; Nannipieri, P., Smalla, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 75–94. [Google Scholar]
- Nunan, N.; Schmidt, H.; Raynaud, X. The ecology of heterogeneity: Soil bacterial communities and C dynamics. Philos. Trans. R. Soc. B 2020, 375, 20190249. [Google Scholar] [CrossRef]
- Allison, S.D.; Weintraub, M.N.; Gartner, T.B.; Waldrop, M.P. Evolutionary-economic principles as regulators of soil enzyme production and ecosystem function. In Soil Enzymology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 229–243. [Google Scholar]
- McCarthy, J.F.; Ilavsky, J.; Jastrow, J.D.; Mayer, L.M.; Perfect, E.; Zhuang, J. Protection of organic carbon in soil microaggregates via restructuring of aggregate porosity and filling of pores with accumulating organic matter. Geochim. Cosmochim. Acta 2008, 72, 4725–4744. [Google Scholar] [CrossRef]
- Rabot, E.; Wiesmeier, M.; Schlüter, S.; Vogel, H.J. Soil structure as an indicator of soil functions: A review. Geoderma 2018, 314, 122–137. [Google Scholar] [CrossRef]
- Totsche, K.U.; Amelung, W.; Gerzabek, M.H.; Guggenberger, G.; Klumpp, E.; Knief, C.; Lehndorff, E.; Mikutta, R.; Peth, S.; Prechtel, A.; et al. Microaggregates in soils. J. Plant Nutr. Soil Sci. 2018, 181, 104–136. [Google Scholar] [CrossRef]
- Yudina, A.V.; Fomin, D.S.; Kotelnikova, A.D.; Milanovskii, E.Y. From the notion of elementary soil particle to the particle-size and microaggregate-size distribution analyses: A review. Eurasian Soil Sci. 2018, 51, 1326–1347. [Google Scholar] [CrossRef]
- Powlson, D.S. The effects of grinding on microbial and non-microbial organic matter in soil. J. Soil Sci. 1980, 31, 77–85. [Google Scholar] [CrossRef]
- Sollins, P.; Homann, P.; Caldwell, B.A. Stabilization and destabilization of soil organic matter: Mechanisms and controls. Geoderma 1996, 74, 65–105. [Google Scholar] [CrossRef]
- Dungait, J.A.; Hopkins, D.W.; Gregory, A.S.; Whitmore, A.P. Soil organic matter turnover is governed by accessibility not recalcitrance. Glob. Change Biol. 2012, 18, 1781–1796. [Google Scholar] [CrossRef]
- Kravchenko, A.N.; Guber, A.K. Soil pores and their contributions to soil carbon processes. Geoderma 2017, 287, 31–39. [Google Scholar] [CrossRef]
- Schimel, J.P.; Weintraub, M.N. The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: A theoretical model. Soil Biol. Biochem. 2003, 35, 549–563. [Google Scholar] [CrossRef]
- Malik, A.A.; Puissant, J.; Goodall, T.; Allison, S.D.; Griffiths, R.I. Soil microbial communities with greater investment in resource acquisition have lower growth yield. Soil Biol. Biochem. 2019, 132, 36–39. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Renella, G.; Puglisi, E.; Ceccanti, B.; Masciandaro, G.; Fornasier, F.; Moscatelli, M.C.; Marinari, S. Soil enzymology: Classical and molecular approaches. Biol. Fertil. Soils. 2012, 48, 743–762. [Google Scholar] [CrossRef]
- Khaziev, F.K. Methods of Soil Enzymology; Nauka: Moscow, Russia, 2005; 252p. [Google Scholar]
- Perez Mateos, M.; Gonzalez Carcedo, S. Effect of fractionation on location of enzyme activities in soil structural units. Biol. Fertil. Soils 1985, 1, 153–159. [Google Scholar] [CrossRef]
- Stemmer, M.; Gerzabek, M.H.; Kandeler, E. Organic matter and enzyme activity in particle-size fractions of soils obtained after low-energy sonication. Soil Biol. Biochem. 1998, 30, 9–17. [Google Scholar] [CrossRef]
- Stemmer, M.; Gerzabek, M.H.; Kandeler, E. Invertase and xylanase activity of bulk soil and particle-size fractions during maize straw decomposition. Soil Biol. Biochem. 1998, 31, 9–18. [Google Scholar] [CrossRef]
- Kandeler, E.; Stemmer, M.; Klimanek, E.M. Response of soil microbial biomass, urease and xylanase within particle size fractions to long-term soil management. Soil Biol. Biochem. 1999, 31, 261–273. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, W.; Liang, G.; Sun, J.; Wang, X.; He, P. Distribution of soil nutrients, extracellular enzyme activities and microbial communities across particle-size fractions in a long-term fertilizer experiment. Appl. Soil Ecol. 2015, 94, 59–71. [Google Scholar] [CrossRef]
- Le Roux, X.; Bouskill, N.J.; Niboyet, A.; Barthes, L.; Dijkstra, P.; Field, C.B.; Hungate, B.A.; Lerondelle, C.; Pommier, T.; Tang, J.; et al. Predicting the responses of soil nitrite-oxidizers to multi-factorial global change: A trait-based approach. Front. Microbiol. 2016, 7, 628. [Google Scholar] [CrossRef] [PubMed]
- Ingwersen, J.; Poll, C.; Streck, T.; Kandeler, E. Micro-scale modelling of carbon turnover driven by microbial succession at a biogeochemical interface. Soil Biol. Biochem. 2008, 40, 864–878. [Google Scholar] [CrossRef]
- Schweizer, S.A. Perspectives from the Fritz-Scheffer Awardee 2021: Soil organic matter storage and functions determined by patchy and piled-up arrangements at the microscale. J. Plant Nutr. Soil Sci. 2022, 185, 694–706. [Google Scholar] [CrossRef]
- Shoba, S.A.; Dobrovolsky, G.V.; Alyabina, I.O. National Atlas of Soils of the Russian Federation; Astrel: Moscow, Russia, 2006; pp. 130–131. [Google Scholar]
- Yudina, A.V.; Milanovskiy, Y.Y. The microaggregate analysis of soils by the method of laser diffraction: The specificities of sample preparation and result interpretation. Dokuchaev Soil Bull. 2017, 89, 3–20. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Rumpel, C.; Kögel-Knabner, I. Evaluation of an ultrasonic dispersion procedure to isolate primary organomineral complexes from soils. Eur. J. Soil Sci. 1999, 50, 87–94. [Google Scholar] [CrossRef]
- Amelung, W.; Zech, W. Minimisation of organic matter disruption during particle-size fractionation of grassland epipedons. Geoderma 1999, 92, 73–85. [Google Scholar] [CrossRef]
- Graf-Rosenfellner, M.; Kayser, G.; Guggenberger, G.; Kaiser, K.; Büks, F.; Kaiser, M.; Mueller, C.W.; Schrumpf, M.; Rennert, T.; Welp, G.; et al. Replicability of aggregate disruption by sonication—An inter-laboratory test using three different soils from Germany. J. Plant Nutr. Soil Sci. 2018, 181, 894–904. [Google Scholar] [CrossRef]
- Fomin, D.; Timofeeva, M.; Ovchinnikova, O.; Valdes-Korovkin, I.; Holub, A.; Yudina, A. Energy-based indicators of soil structure by automatic dry sieving. Soil Tillage Res. 2021, 214, 105183. [Google Scholar] [CrossRef]
- Zhelezova, A.D.; Kutovaya, O.V.; Dmitrenko, V.N.; Tkhakhahova, A.K.; Khohlov, S.F. Estimation of DNA quantity in different groups of microorganisms within genetic horizons of the dark-gray soil. Dokuchaev Soil Bull. 2015, 78, 87–98. [Google Scholar] [CrossRef]
- Artemyeva, Z.S.; Kogut, B.M. The effect of tillage on organic carbon stabilization in microaggregates in different climatic zones of European Russia. Agriculture 2016, 6, 63. [Google Scholar] [CrossRef]
- Soil Survey Staff. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 2nd ed.; Natural Resources Conservation Service; U.S. Department of Agriculture Handbook; United States Deptartment of Agriculture, Naturel Resources Conservation Service: Portland, OR, USA, 1999.
- Marx, M.C.; Wood, M.; Jarvis, S.C. A microplate fluorimetric assay for the study of enzyme diversity in soils. Soil Biol. Biochem. 2001, 33, 1633–1640. [Google Scholar] [CrossRef]
- Razavi, B.S.; Blagodatskaya, E.; Kuzyakov, Y. Nonlinear temperature sensitivity of enzyme kinetics explains canceling effect—A case study on loamy haplic Luvisol. Front. Microbiol. 2015, 6, 1126. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, J.; Dzantor, E.K.; Momen, B. Soil microbial community profiles and functional diversity in limestone cedar glades. Catena 2016, 147, 216–224. [Google Scholar] [CrossRef]
- Wu, Z.; Li, J.; Zheng, J.; Liu, J.; Liu, S.; Lin, W.; Wu, C. Soil microbial community structure and catabolic activity are significantly degenerated in successive rotations of Chinese fir plantations. Sci. Rep. 2017, 7, 6691. [Google Scholar] [CrossRef]
- Vorobyova, E.; Soina, V.; Gorlenko, M.; Minkovskaya, N.; Zalinova, N.; Mamukelashvili, A.; Gilichinsky, D.; Rivkina, E.; Vishnivetskaya, T. The deep cold biosphere: Facts and hypothesis. FEMS Microbiol. Rev. 1997, 20, 277–290. [Google Scholar] [CrossRef]
- Cheptsov, V.S.; Vorobyova, E.A.; Manucharova, N.A.; Gorlenko, M.V.; Pavlov, A.K.; Vdovina, M.A.; Lomasov, V.N.; Bulat, S.A. 100 kGy gamma-affected microbial communities within the ancient Arctic permafrost under simulated Martian conditions. Extremophiles 2017, 21, 1057–1067. [Google Scholar] [CrossRef]
- Christensen, B.T. Carbon and nitrogen in particle size fractions isolated from Danish arable soils by ultrasonic dispersion and gravity-sedimentation. Acta Agric. Scand. 1985, 35, 175–187. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Voroney, R.P.; Kachanoski, R.G. Ultrasonic dispersion of aggregates: Distribution of organic matter in size fractions. Can. J. Soil Sci. 1988, 68, 395–403. [Google Scholar]
- Neumann, D.; Heuer, A.; Hemkemeyer, M.; Martens, R.; Tebbe, C.C. Response of microbial communities to long-term fertilization depends on their microhabitat. FEMS Microbiol. Ecol. 2013, 86, 71–84. [Google Scholar] [CrossRef]
- Hemkemeyer, M.; Christensen, B.T.; Martens, R.; Tebbe, C.C. Soil particle size fractions harbour distinct microbial communities and differ in potential for microbial mineralisation of organic pollutants. Soil Biol. Biochem. 2015, 90, 255–265. [Google Scholar] [CrossRef]
- Hemkemeyer, M.; Dohrmann, A.B.; Christensen, B.T.; Tebbe, C.C. Bacterial preferences for specific soil particle size fractions revealed by community analyses. Front. Microbiol. 2018, 9, 149. [Google Scholar] [CrossRef]
- Kandeler, E.; Tscherko, D.; Bruce, K.D.; Stemmer, M.; Hobbs, P.J.; Bardgett, R.D.; Amelung, W. Structure and function of the soil microbial community in microhabitats of a heavy metal polluted soil. Biol. Fertil. Soils 2000, 32, 390–400. [Google Scholar] [CrossRef]
- Gerzabek, M.H.; Haberhauer, G.; Kandeler, E.; Sessitsch, A.; Kirchmann, H. Response of organic matter pools and enzyme activities in particle size fractions to organic amendments in a long-term field experiment. In Developments in Soil Science; Elsevier: Amsterdam, The Netherlands, 2002; Volume 28, pp. 329–344. [Google Scholar]
- Semenov, V.M.; Lebedeva, T.N.; Pautova, N.B. Particulate organic matter in noncultivated and arable soils. Eurasian Soil Sci. 2019, 52, 396–404. [Google Scholar] [CrossRef]
- Trasar-Cepeda, C.; Leirós, M.C.; Gil-Sotres, F. Hydrolytic enzyme activities in agricultural and forest soils. Some implications for their use as indicators of soil quality. Soil Quality. Soil Biol. Biochem. 2008, 40, 2146–2155. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro) aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).