Abstract
An increase in the carbapenem-hydrolyzing capacity of class D β-lactamase has been observed in strains of multiple species, posing a significant challenge to the control of antibiotic resistance. In this study, we aimed to investigate the genetic diversity and phylogenetic characteristics of new blaOXA-48-like variants derived from Shewanella xiamenensis. Three ertapenem-non-susceptible S. xiamenensis strains were identified, one isolated from the blood sample of an inpatient, the other two isolated from the aquatic environment. Phenotypic characterization confirmed that the strains were carbapenemase producers and exhibited antimicrobial resistance patterns to ertapenem, with some showing lower susceptibility to imipenem, chloramphenicol, ciprofloxacin, and tetracycline. No significant resistance to cephalosporins was observed. Sequence analysis revealed that one strain harbored blaOXA-181 and the other two strains harbored blaOXA-48-like genes, with open reading frame (ORF) similarities with blaOXA-48 ranging from 98.49% to 99.62%. The two novel blaOXA-48-like genes, named blaOXA-1038 and blaOXA-1039, respectively, were cloned and expressed in E. coli. The three OXA-48-like enzymes demonstrated significant hydrolysis activity against meropenem, and the classical β-lactamase inhibitor had no significant inhibitory effect. In conclusion, this study demonstrated the diversity of the blaOXA gene and highlighted the emergence of novel OXA carbapenemases in S. xiamenensis. Further attention to S. xiamenensis and OXA carbapenemases is recommended for the effective prevention and control of antibiotic-resistant bacteria.
1. Introduction
The escalation of antimicrobial resistance is an evolutionary response to the selective pressure imposed by antimicrobial agents, and the surge of carbapenem resistance genes in Enterobacterales has become an urgent concern [1,2]. According to the Ambler classification system, clinical β-lactamases are commonly classified into four groups (A, B, C, and D), with oxacillinases (OXA) being categorized as class D-type serine β-lactamases [3,4]. To date, there are 1237 identified class D β-lactamases [5]. The substrate spectrum of carbapenem-hydrolyzing OXA subfamilies is broadened by amino acid motif substitutions [6]. The OXA-48 family is one of the most notorious subgroups of carbapenemase-producing enzymes, presenting a significant threat to public health. Since the discovery of the first blaOXA-48 gene in Klebsiella pneumoniae in Istanbul in 2001, there has been a growing number of carbapenem-hydrolyzing class D β-lactamases (CHDLs) found globally [7,8,9,10,11]. Furthermore, an increasing number of OXA-48-like variants capable of carbapenem hydrolysis have been identified in both the clinical and community settings [12,12,13,14,15,16]. As carbapenems are considered last-resort antimicrobials in the treatment of Gram-negative bacterial infections, the emergence and spread of OXA variants has garnered significant attention because of the potential risks they present in clinical infection management.
The class D β-lactamases of Shewanella spp., encoded by chromosomes, are considered the progenitors of carbapenem-hydrolyzing OXA enzymes, such as blaOXA-48-like and blaOXA-55-like enzymes. Shewanella spp. are widely distributed in marine environments, including extreme ecosystems. Reports indicate that an increasing number of blaOXA-harboring Shewanella spp. strains are closely associated with human diseases [7,8,9,10]. The common pathogenic species of Shewanella, including Shewanella xiamenensis, Shewanella algae, and Shewanella putrefaciens [11,12], are derived from various environments such as saltmarsh plants, freshwater, estuarine water, sewage, river water and livestock wastewater [12,13,14,15,16,17]. According to the latest clinical reports, skin and soft tissue infections, intestinal colonization, respiratory diseases, bacteremia, intra-abdominal infection, and otitis media are the common complications caused by Shewanella spp. pathogens [18,19,20,21,22]. Although cases of clinical infection caused by Shewanella spp. have been reported in many countries [23,24,25], the actual incidence of these infections in China remains unclear. S. xiamenensis was discovered in coastal sea sediment in Xiamen, China, in 2010 [26]. To date, 26 OXAs have been reported in Shewanella spp. strains according to the latest β-lactamase database [5]. Shewanella spp. have been considered important carriers and reservoirs of carbapenem-resistant genes in the natural environment since the first report of chromosome-encoded OXA of Shewanella oneidensis in 2004 [27]. Additionally, there is a high risk of horizontal transfer of the chromosome-based blaOXA variants to other Enterobacterales by various mobile genetic elements, such as plasmids and transposons, which dramatically reduce susceptibility to β-lactam antibiotics [28,29]. In this article, we investigated three carbapenem-resistant S. xiamenensis strains that harbored blaOXA genes, including blaOXA-181 and two novel blaOXA-48-like genes, namely blaOXA-1038 and blaOXA-1039. This study aimed to characterize the carbapenem-hydrolyzing activity of the two new members of OXA carbapenemase and contribute to the known diversity of the OXA-type class D β-lactamase family identified in Shewanella spp. strains.
2. Materials and Methods
2.1. Strain Collection and Identification
Three carbapenem-non-susceptible strains of S. xiamenensis were isolated from aquatic environments (n = 2) and a clinical patient (n = 1) in China between 2019 and 2020. Species identification of the strains was confirmed by culture morphology and the VITEK 2 compact system (bioMérieux, Craponne, France). To ensure consistency, all isolates were sub-cultured at least twice prior to further investigation.
2.2. Antimicrobial Susceptibility Testing
The resistance profiles of S. xiamenensis strains were determined with the BD Phoenix™ M50 system (Becton, Dickinson and Company, Franklin Lakes, NJ, USA); the following 13 agents were included: ampicillin, ampicillin-sulbactam, aztreonam, cefotaxime, cefoxitin, ceftazidime, ceftazidime-avibactam, chloramphenicol, ciprofloxacin, ertapenem, imipenem, meropenem, and tetracycline. The minimal inhibitory concentrations (MICs) of 9 β-lactam antibiotics of cloned strains were obtained via broth microdilution methods.
2.3. Phenotypic Profiles of Isolates
Phenotypic screening for carbapenem non-susceptible strains was performed using the Carba NP test [30] and modified carbapenem inactivation method (mCIM) assay [31], with Escherichia coli ATCC 25922 serving as the quality control strain. All tests were performed and interpreted according to the Clinical and Laboratory Standards Institute (CLSI) M100 guidelines for Enterobacterales (2021).
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) (Bruker Daltonics GmbH, Bremen, Germany) was used to analyze the hydrolysis spectrum of carbapenemase-carrying E. coli cloning strains [32]. The positive cloned strains were induced at 0.5 mM IPTG concentration at 35 ± 2 °C for 3~4 h, and the centrifuged bacteria were incubated with meropenem solutions. A positive result for carbapenemase production was determined if the peak for meropenem at 384.5 m/z and 406.5 m/z disappeared or declined significantly, while the peak at 358.5 m/z appeared during the incubation time.
2.4. Whole-Genome Sequencing and Phylogenetic Analyses
The genomic DNA was extracted following the protocol of the Genomic DNA Purification Kit (Promega, Madison, WI, USA). Sequencing was performed using Illumina Miniseq and PacBio platforms. The probable antibiotic resistance genes of each sequence were detected with ResFinder 4.1 in the Center for Genomic Epidemiology database (https://cge.food.dtu.dk/services/ResFinder/) [accessed on 1 February 2023]. A phylogenetic tree of blaOXA-48-like genes of Shewanella spp. was constructed using MEGA 7. Based on the information, linear comparisons of sequences were made with BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) [accessed on 1 February 2023] and retrieved genes were aligned with the online multiple sequence alignment tool Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/) [accessed on 5 February 2023]. The hypothetic draft genome sequences were annotated using the RAST server (https://rast.nmpdr.org/) [accessed on 5 February 2023].
2.5. Cloning and Functional Verification of BlaOXA Genes
The genomic DNA of the S. xiamenensis strains was extracted as previously described. Specific primers were used to amplify the DNA fragments via polymerase chain reaction (PCR) with 2×EasyTaq® PCR SuperMix (+dye). The PCR products were double-digested with Nde I and Xhol I and ligated into a pET30a (+) vector using T4 DNA Ligase (Takara Biomedical Technology, Co., Ltd., Beijing, China). The resulting ligation products were transformed into chemically competent E. coli DH5α cells via heat shock. The recombinant plasmids were then introduced into E. coli BL21 (DE3) chemically competent cells, which were selected on Luria–Bertani (LB) agar plates containing 50 μg/mL of kanamycin to incubate the specific blaOXA genes. The identity of the inserts was confirmed via sequencing to ensure the integrity of encoding genes.
2.6. β–Lactamase Activity Assay
The OXA enzymes extracted from successfully cloned carbapenemase-producing strains were purified using the His·Bind Purification Kit (Millipore, Burlington, MA, USA). Meropenem hydrolysis activity was directly detected using the BioTek Synergy NEO system, which measures the change in absorption light (Abs) at the wavelength indicated for meropenem (310 nm). Hydrolysis rates were calculated by comparing the change in optical density (OD) using Microsoft Excel. Additionally, an in vitro inhibitory test was performed to demonstrate the hydrolysis ability of the new OXA-type carbapenemase.
3. Results
3.1. Species Isolation and Identification
Three strains of S. xiamenensis were identified using the VITEK 2 compact system. Among them, strain A5468 was isolated from the blood of an inpatient in Beijing, while CW86-2 and CW86-3 were derived from the aquatic environment of Lake Taihu, Jiangsu Province.
3.2. Antimicrobial Profile of Isolates
The broth microdilution test was used to determine the MICs of the S. xiamenensis strains, and the results are presented in Table 1 to display their resistance profiles. The MIC results demonstrated that all three strains were susceptible to expanded-spectrum cephalosporins, resistant to ertapenem (with MICs ranging from 2 to 4 μg/mL) and ciprofloxacin (with MIC >2 μg/mL), and showed reduced susceptibility to carbapenems, especially imipenem (with MICs ranging from 1 to 2 μg/mL). Moreover, strain A5468 showed an intermediary result to chloramphenicol (16 μg/mL), while strain CW86-3 exhibited a high level of resistance to tetracycline (with MIC >16 μg/mL).

Table 1.
MICs of 13 antimicrobial agents for the three S. xiamenensis isolates.
3.3. Phenotypic Detection of Carbapenemase-Producing S. xiamenensis
The molecular phenotypic analysis employed the Carba NP test and mCIM assay for the detection of carbapenemase-producing strains, and all instructions and interpretations followed CLSI guidelines. The mCIM results of the three S. xiamenensis isolates are shown in Figure 1. The specificity of mCIM for detecting carbapenemase activity in the three isolates showed that they have significant carbapenemase-hydrolyzing activity compared with the negative control E. coli ATCC 25922.
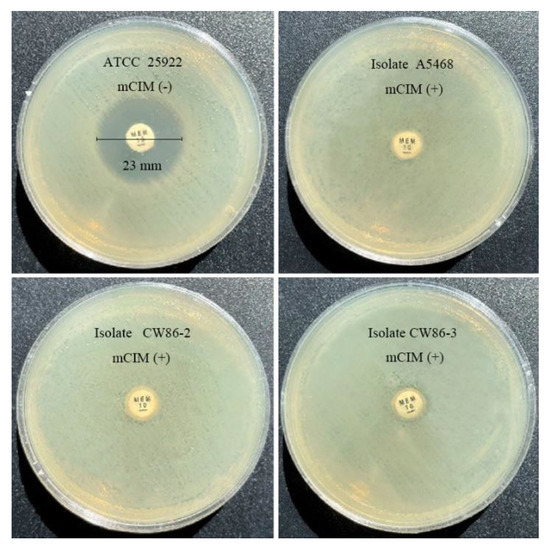
Figure 1.
Identification of carbapenemase-producing strains via mCIM test. The control strain E. coli ATCC 25922 was carbapenemase negative, having a zone size of 23 mm > 19 mm. Three target isolates carried carbapenemase, which can break down 10 μg meropenem after incubation for 4 h ± 15 min at 35 °C.
3.4. Phylogenetic Analysis and Genetic Characterization of Strains
Strains A5468 and CW86-2 were subjected to sequencing with the PacBio platform, while isolate CW86-3 was sequenced with the Illumina Miniseq(MIGIGENE Company, Beijing China,). Sequence annotation showed that these strains carried blaOXA-48-like genes, and all three blaOXA variants were located on chromosomes, as determined by comparison with conserved motifs of genes in Shewanella spp. Antibiotic resistance gene (ARG) screening using CGE ResFinder 4.1 revealed that strain A5468 carried β-lactam, sulfamethoxazole, chloramphenicol, and trimethoprim resistance genes, namely, blaOXA-181, sul1, catA2, and dfrA16, respectively. Strain CW86-2 carried a novel class D blaOXA-48-like gene, while strain CW86-3 harbored another novel blaOXA-48-like gene and the tetracycline resistance gene tet(B). Notably, two novel blaOXA-48-like genes with >99%, but not 100%, identity to blaOXA-48 were present on their chromosomes. To confirm the presence of these two novel genes, their sequences were submitted to the NCBI BankIt database and they were designated as blaOXA-1038 (OK180617) and blaOXA-1039 (OK180618), carried by CW86-2 and CW86-3, respectively in 2021. In 2023, when the manuscript was submitted, we found that the S. xiamenensis strain BC01 (JGVI01000025), reported in 2014 in Xiamen, China, shares a 100% identical amino acid sequence with the OXA-1039, while differing by six nucleotides in nucleotide sequences with blaOXA-1039 mentioned in this study. Because there was no information about blaOXA or oxacillin enzyme mentioned in the context of S. xiamenensis strain BC01 when it was reported in 2014 [33], on NCBI BankIt database, the oxacillin enzyme carried by the isolate CW86-3 was widely regarded as a novel oxacillin enzyme and designated as OXA-1039 in 2021. Now, the time of first report and accession number of blaOXA-1039 are can be as 2014 and JGVI01000025 in Beta-Lactamase DataBase (http://www.bldb.eu/) [accessed on 12 September 2021].
Based on the ORFs of all blaOXA genes in Shewanella spp., the three genes mentioned in this study belong to a cluster of blaOXA-48 genes, as shown in the dashed box in Figure 2A. The phylogenetic tree of 16 amino acid coding sequences (CDS) of the OXA-48-like cluster is depicted in Figure 2B, and some of the OXA variants in this cluster were found to possess carbapenem hydrolysis activity. Phylogenetic analysis based on CDS indicated that OXA-181, OXA-1038, and OXA-1039 had high similarity with other OXA-type carbapenemases in this cluster. The 265 amino acid sequence alignment of the cluster of OXA-48 subgroup variants is shown in Figure 2C. Partial blaOXA genes displayed amino acid motif mutations in consensus regions. Sequencing analysis revealed that blaOXA-181 has 98.49% (261/265) identity to blaOXA-48 and its genome characteristics have been previously reported [12,34,35]. The blaOXA-1038 gene of strain CW86-2 shared 99.62% and 99.25% amino acid identity with blaOXA-894 (S155T) and blaOXA-48 (S155T, T167I), respectively. The blaOXA-1039 gene shared 99.62% with blaOXA-48 (S171T), 99.25% with blaOXA-894 (I167T, S171T), and 99.25% with blaOXA-252 (A45V, S171T).
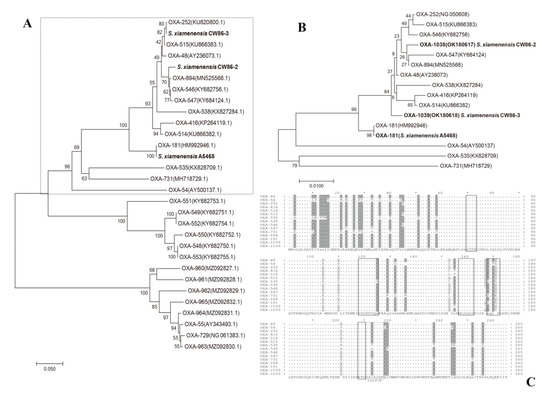
Figure 2.
Molecular phylogeny of the representative class D OXA in Shewanella spp. (A) Phylogenetic tree with 1000 bootstrap replicates generated using the neighbor-joining (NJ) method based on the nucleotides of this study (black bold) and typical blaOXA genes reported in Shewanella spp. strains. The nucleotides corresponding to the OXA-48-like cluster are outlined by the dashed box. The bootstrap values are given at branching points, and the bar represents 0.05 substitutions per nucleotide position. GenBank accession numbers are given in parentheses. (B) Phylogenetic analysis with an NJ tree based on the amino acids of OXAs. Comparison of 16 CDS of the blaOXA-48-like cluster mentioned in (A) to show the genotypic characteristics. The OXA mentioned in this study are in black bold. Bar represents 0.01 substitutions per amino acid position. Numbering is according to NCBI. (C) Alignment of amino acid sequences of the three OXA-type enzymes in this study with the amino acid sequences of OXA-48-like variants reported in Shewanella spp. from NCBI including OXA-48 (AY236073), OXA-54 (AY500137), OXA-252 (NG_050608), OXA-416 (KP264119), OXA-514 (KU866382), OXA-515 (KU866383), OXA-535 (KX828709), OXA-538 (KX827284), OXA-546 (KY682756), OXA-547 (KY684124), OXA-731 (MH718729), OXA-894 (MN525568), OXA-181 (this study), OXA-1038 (OK180617, this study) and OXA-1039 (OK180618, this study). The bottom line shows the consensus sequence of all blaOXA genes. Dashes indicate identical residues among all the amino acid sequences. Amino acid motifs that are well-conserved among class D β-lactamases are indicated by black-outlined boxes, and the single gray-outlined box corresponds to the β5-β6 loop [36,37]. Differences in residues within three kinds of amino acids among all sequences are shaded in dark gray, and differences of more than three are shaded in light gray.
3.5. Hydrolytic Spectra of OXA-48-like-Carrying Cloning Strains
The 798 bp PCR target product of the three blaOXA open reading frames (ORFs) was cloned into E. coli BL21 chemically competent cells after being transformed into E. coli DH5α competent cells using E. coli pET30a (+) plasmids as the recipient strains. The colonies were incubated and screened using X-Gal and IPTG in the 50 μg/mL kanamycin-containing LB culture medium, and the integrity of the gene fragment of each recombinant strain was confirmed using Sanger sequencing. The resistance profiles of the recombinant plasmids pET30a-OXA-181, pET30a-OXA-1038, and pET30a-OXA-1039 were compared with those of the E. coli reference strains for nine β-lactam antibiotics, as shown in Table 2. The expression efficiencies of all the OXA variants against imipenem were higher than non-OXA strains, with MICs of 1 μg/mL in E. coli cloning strains. Furthermore, compared with the MICs of the original S. xiamenensis, only the blaOXA-181-gene-harboring strain showed higher hydrolysis to ampicillin, and no cloning strain had significant intrinsic activity towards ertapenem, meropenem, and expanded-spectrum cephalosporins.

Table 2.
The β-lactam MICs of E. coli harboring blaOXA recombinant plasmid pET30a strains and BL21 (DE3) reference strain.
The MALDI-TOF MS assay was implemented to detect hydrolysis of recombinant cloning strains to five β-lactam antibiotics, including ampicillin, cefotaxime, ceftriaxone, meropenem and imipenem [38,39,40]. After incubation of antibiotics solution as the substrate with different strains for 4 h, the performance of three cloning strains was evaluated. Figure 3 shows the hydrolysis mass spectrum of meropenem. The cloning strains were judged as positive OXA-type carbapenemase-producing strains if the specific peak for meropenem at 384.5 m/z and its monosodium salt at 406.5 m/z disappeared or declined significantly, while its decarboxylated degradation product at 358.5 m/z appeared during the incubation time [41]. It seems that blaOXA-181-, blaOXA-1038- and blaOXA-1039-carrying E. coli cloning strains have a low level of carbapenemase hydrolysis activity similar to OXA-48-like variants previously reported in Shewanella spp. isolates [13,22,42].
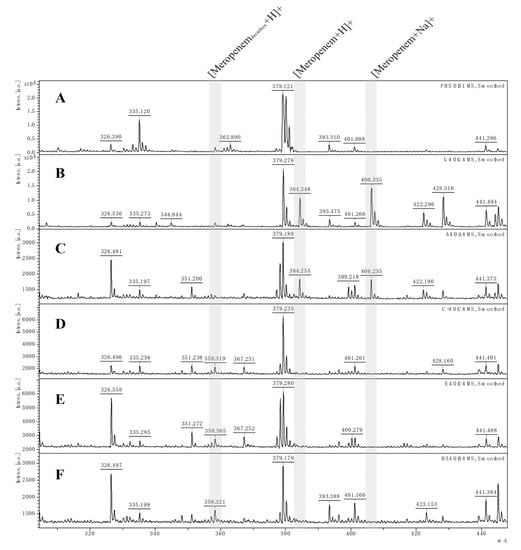
Figure 3.
The MALDI-TOF MS spectra of 0.5 mg/mL meropenem. (A) The spectrum of PBS was included to rule out interference from matrix fluids. (B) Blank control spectrum of pure meropenem solution. (C) Negative control: spectrum of a carbapenemase-non-carrying strain. (D–F) Positive: OXA-181-harboring strain, OXA-1038-harboring strain and OXA-1039-harboring strain, respectively. Shaded area: [Meropenemdecarbox + H]+, decarboxylated degradation product of meropenem after carbapenemase hydrolysis (m/z 358.5); [Meropenem + H]+, meropenem molecule (m/z 384.5); [Meropenem + Na]+, meropenem sodium salt (m/z 406.5).
3.6. Purification and Biochemical Properties of OXA-Type Carbapenemase
The ability of different OXA enzymes to hydrolyze meropenem, imipenem, and classical β-lactam inhibitor solutions was measured via Abs readings continuously monitored at 37 °C for 2 h. The meropenem hydrolysis rates were calculated from the change in absorbances at 310 nm per unit time (Figure 4a), and no OXA enzymes were inhibited by classical enzyme inhibitors (not shown). The results from the enzyme activity test showed that all OXA enzymes had significant meropenem hydrolysis with an efficiency of more than 85% (Figure 4b). In addition, based on the carbapenemase phenotypic test, in vitro enzyme inhibition testing was carried out with antimicrobial solutions to make the hydrolysis results more visible. Meropenem hydrolysis activities were measured with purified protein extracts of the three E. coli cloning strains obtained as previously described, and 100 μg/mL meropenem was used as the substrate for overnight incubation at 37 °C for 2 h. A 5 μL bacterial culture suspension was added on MH agar plates, upon which a carbapenem-susceptible reporter E. coli ATCC 25922 had been freshly applied. The results indicated that the OXA-1038 and OXA-1039 enzymes hydrolyzed meropenem dramatically (Figure 4c).
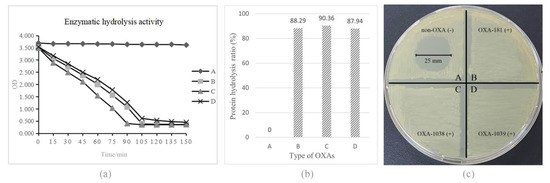
Figure 4.
Results of the evaluation of the meropenem hydrolysis ability of the three OXA enzymes. (a) Verification of the hydrolysis ability of the three new enzymes (A: PBS, negative control; B: OXA-181; C: OXA-1038; D: OXA-1039). (b) Comparison of the hydrolysis rates of PBS, OXA-181, OXA-1038 and OXA-1039. All OXA enzymes showed a significant ability to hydrolyze meropenem. (c) Results of the in vitro enzyme inhibition test, which further confirmed the hydrolysis ability of the OXA enzymes. All OXA-enzyme-carrying cloning strains showed a significantly higher ability to hydrolyze meropenem compared to the non-OXA-carrying negative control (A = 25 mm).
4. Discussion
Chromosome-mediated class D β-lactamases identified in Shewanella spp. were previously thought to be the progenitors of carbapenem-hydrolyzing OXA-48-like enzymes. Furthermore, it was recently discovered that an expanding number of members within the OXA-48-like subfamily possess the ability to hydrolyze β-lactam antibiotics, including carbapenems. Shewanella spp., such as S. xiamenensis, S. algae, and S. putrefaciens, which are commonly found in marine sediments and ecosystems, have been increasingly recognized as opportunistic pathogens of humans and animals, particularly those exposed to livestock wastewater and aquaculture environments [43,44,45,46]. Although the antibiotic resistance statuses of Shewanella spp. are relatively low compared to Enterobacteriaceae, blaNDM, blaSHV, blaCTX-M, qnrA, catA, dfrA, and tet cluster genes have been reported in Shewanella spp. [47,48,49,50,51]. It is clear that the emergence of multiple drug-resistant Shewanella spp., especially those containing blaOXA-48-like genes, poses a significant threat to clinical treatment [50,52].
The phylogenetic analysis revealed that the OXA family could be classified into three clusters, including the OXA-48-like, OXA-55-like and OXA-548-like clusters [5]. Carbapenem-hydrolyzing activity has been reported for almost all OXA-48-like and OXA-55-like enzymes in Shewanella spp. but the details of the carbapenem-hydrolyzing activity of the OXA-548-like enzymes have not yet been determined. The three OXA involved in this study all belonged to the OXA-48-like cluster. The coding sequences of the three blaOXA genes had >99% identity with blaOXA-48 and some of its variants. OXA-1038 had two amino acid differences from OXA-48 (S155T, T167I) in non-conserved regions, and OXA-1039 had only one mutation from OXA-48 in the conserved region (S171T). Partial amino acid residues of new OXA variants, such as OXA-535 and OXA-731, showed more favorable interaction between carbapenem antibiotics and residues, and we raised rational doubt that the substitution residues of OXA-1038 and OXA-1039 may also play a vital role in the evolution of carbapenemases.
The MICs of the blaOXA gene recombinant plasmids pOXA-181, pOXA-1038 and pOXA-1039 in BL21 (DE3) strains showed minor differences in antimicrobial susceptibility compared to the original BL21 (DE3) strains; except for a slight decrease in the sensitivity of pOXA-181 to ampicillin, the MICs of all strains to the antibiotics used did not reach the resistant level. Hydrolysis examinations showed that all recombinant strains demonstrated various sensitivity profiles against ampicillin, cefotaxime, ceftriaxone, meropenem, and imipenem, and the expression of all alleles exhibited hydrolysis activity to meropenem, with decarboxylated (m/z 358.5) and degradation (m/z 384.5, 406.6) products of meropenem degradation identified after co-incubation. All enzyme activity tests confirmed that the OXA enzyme variant OXA-181 has the same resistance profile as previously reported, while OXA-1038 and OXA-1039 possess resistance patterns comparable to OXA-181 carbapenemase. Overall, these findings indicate that all OXA enzymes in this study have a significant ability to resist meropenem, as demonstrated by their hydrolysis rates and strong in vitro enzyme inhibition test results.
The three class D-type blaOXA genes were all found on the chromosome of S. xiamenensis, and the surrounding regions both upstream and downstream of these genes did not contain any mobile genetic elements, such as phages, insertion sequences, transposons, or integrons. This indicates that the transfer ability of the two novel OXA variants from S. xiamenensis to other species is relatively low at present. However, considering the increasing reports of S. xiamenensis as a clinical pathogen [28,29,30,31,32], it is necessary to strengthen the monitoring of OXA in Shewanella spp. strains.
5. Conclusions
In this study, OXA-1038 and OXA-1039, two novel OXA variants with carbapenem hydrolysis activity, were identified in S. xiamenensis strains isolated from aquaculture samples. The discovery expanded the known diversity of the CHDL family identified in Shewanella spp. In addition, an OXA-181-carrying S. xiamenensis strain from a blood sample of an inpatient was detected, which increased the clinical treatment burden because of β-lactam and carbapenem resistance. Furthermore, increased attention should be given to the emergence and evolution of OXA carbapenems in order to prevent transmission between the environment, animals, and humans.
Author Contributions
Conceptualization, X.Z. and X.B.; methodology, B.M., M.Y., Z.L. and X.H.; software, Z.L. and J.L. (Jie Li); validation, B.M., X.C.; formal analysis, X.J., B.M., X.G. and X.H.; investigation, X.J.; resources, X.B. and X.C.; data curation, M.Y. and X.G.; writing—original draft preparation, X.J.; writing—review and editing, J.L. (Juan Li); visualization, J.L. (Jie Li) and S.M.; supervision, X.J. and J.L. (Juan Li); project administration, J.L. (Juan Li); funding acquisition, S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (81861138053).
Data Availability Statement
The two novel OXA varieties sequence OXA-1038 (https://www.ncbi.nlm.nih.gov/nuccore/OK180617), and OXA-1039 (https://www.ncbi.nlm.nih.gov/nuccore/OK180618). All the detail information have submitted to National Microbiology Data Center (NMDC) (https://nmdc.cn/submit/dashboard).
Acknowledgments
The authors thank Rong Zhang (The Second Affiliated Hospital of Zhejiang University, Zhejiang University, Hangzhou, China) and Duochun Wang (Chinese Center for Disease Control and Prevention) for supporting Shewanella spp. strains.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nordmann, P.; Dortet, L.; Poirel, L. Carbapenem resistance in Enterobacteriaceae: Here is the storm! Trends Mol. Med. 2012, 18, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Yoon, E.; Jeong, S.H. Class D β-lactamases. J. Antimicrob. Chemother. 2021, 76, 836–864. [Google Scholar] [CrossRef] [PubMed]
- Bush, K. Past and Present Perspectives on β-Lactamases. Antimicrob. Agents Chemother. 2018, 62, e01076-18. [Google Scholar] [CrossRef] [PubMed]
- Ambler, R.P. The structure of beta-lactamases. Philos. Trans. R. Soc. B Biol. Sci. 1980, 289, 321–331. [Google Scholar] [CrossRef]
- Naas, T.; Oueslati, S.; Bonnin, R.A.; Dabos, M.L.; Zavala, A.; Dortet, L.; Retailleau, P.; Iorga, B.I. Beta-lactamase database (BLDB)—Structure and function. J. Enzym. Inhib. Med. Chem. 2017, 32, 917–919. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Poirel, L. Epidemiology and Diagnostics of Carbapenem Resistance in Gram-negative Bacteria. Clin. Infect. Dis. 2019, 69, S521–S528. [Google Scholar] [CrossRef]
- Muñoz-Gallego, I.; Chaves, F.; Orellana, M.A. Epidemiological and clinical characteristics of Shewanella spp. infections in a tertiary hospital in Madrid. Infect. Dis. 2016, 48, 760–762. [Google Scholar] [CrossRef]
- Wang, J.; Tseng, S.; Tung, K. Genomic investigation of emerging zoonotic pathogen Shewanella xiamenensis. Tzu Chi Med. J. 2020, 32, 162. [Google Scholar] [CrossRef]
- Yousfi, K.; Bekal, S.; Usongo, V.; Touati, A. Current trends of human infections and antibiotic resistance of the genus Shewanella. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1353–1362. [Google Scholar] [CrossRef]
- Yu, K.; Huang, Z.; Xiao, Y.; Wang, D. Shewanella infection in humans: Epidemiology, clinical features and pathogenicity. Virulence 2022, 13, 1515–1532. [Google Scholar] [CrossRef]
- Ohama, Y.; Aoki, K.; Harada, S.; Nagasawa, T.; Sawabe, T.; Nonaka, L.; Moriya, K.; Ishii, Y.; Tateda, K. Genetic Environment Surrounding blaOXA-55-like in Clinical Isolates of Shewanella algae Clade and Enhanced Expression of blaOXA-55-like in a Carbapenem-Resistant Isolate. Msphere 2021, 6, e00593-21. [Google Scholar] [CrossRef] [PubMed]
- Tacão, M.; Araújo, S.; Vendas, M.; Alves, A.; Henriques, I. Shewanella species as the origin of blaOXA-48 genes: Insights into gene diversity, associated phenotypes and possible transfer mechanisms. Int. J. Antimicrob. Agents 2018, 51, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, D.; van Essen-Zandbergen, A.; Veldman, K.T.; Tafro, N.; Haenen, O.; Mevius, D.J. Chromosome-Based blaOXA-48-Like Variants in Shewanella Species Isolates from Food-Producing Animals, Fish, and the Aquatic Environment. Antimicrob. Agents Chemother. 2017, 61, e01013-16. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.N.N.; Nasir, N.M.; Sahrani, F.K.; Ahmad, A.; Sairi, F. Characterization of putative pathogenic Shewanella algae isolated from ballast water. Vet. World 2021, 14, 678–688. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Takemura, T.; Pham, A.H.Q.; Tran, H.T.; Vu, K.C.T.; Tu, N.D.; Huong, L.T.; Cuong, N.T.; Kasuga, I.; Hasebe, F.; et al. Whole-genome sequencing and comparative genomic analysis of Shewanella xiamenensis strains carrying blaOXA-48-like genes isolated from water environment in Vietnam. J. Glob. Antimicrob. Resist. 2020, 21, 272–274. [Google Scholar] [CrossRef]
- Tacão, M.; Correia, A.; Henriques, I. Environmental Shewanella xiamenensis strains that carry blaOXA-48 or blaOXA-204 genes: Additional proof for blaOXA-48-like gene origin. Antimicrob. Agents Chemother. 2013, 57, 6399–6400. [Google Scholar] [CrossRef]
- Zou, H.; Zhou, Z.; Xia, H.; Zhao, Q.; Li, X. Characterization of Chromosome-Mediated BlaOXA-894 in Shewanella xiamenensis Isolated from Pig Wastewater. Int. J. Environ. Res. Public Health 2019, 16, 3768. [Google Scholar] [CrossRef]
- Antonelli, A.; Di Palo, D.M.; Galano, A.; Becciani, S.; Montagnani, C.; Pecile, P.; Galli, L.; Rossolini, G.M. Intestinal carriage of Shewanella xiamenensis simulating carriage of OXA-48–producing Enterobacteriaceae. Diagn. Microbiol. Infect. Dis. 2015, 82, 1–3. [Google Scholar] [CrossRef]
- Benaissa, E.; Abassor, T.; Oucharqui, S.; Maleb, A.; Elouennass, M. Shewanella putrefaciens: A cause of bacteremia not to neglect. Idcases 2021, 26, e1294. [Google Scholar] [CrossRef]
- Cimmino, T.; Olaitan, A.O.; Rolain, J.M. Whole genome sequence to decipher the resistome of Shewanella algae, a multidrug-resistant bacterium responsible for pneumonia, Marseille, France. Expert Rev. Anti-Infect. Ther. 2016, 14, 269–275. [Google Scholar] [CrossRef]
- Lemaire, O.N.; Mejean, V.; Iobbi-Nivol, C. The Shewanella genus: Ubiquitous organisms sustaining and preserving aquatic ecosystems. FEMS Microbiol. Rev. 2020, 44, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Ho, S.; Cheng, J.; Tung, K.; Hong, Y.; Chen, S.; Menzer, A.; Chen, Y.; Huang, Y.; Liu, P. Whole-genome characterization of Shewanella algae strain SYT3 isolated from seawater reveals insight into hemolysis. Future Microbiol. 2018, 13, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Cheng, J.F.; Wu, Z.Y.; Tung, K.C.; Chen, Y.J.; Hong, Y.K.; Chen, S.Y.; Liu, P.Y. Genomic and phylogenetic characterization of Shewanella xiamenensis isolated from giant grouper (Epinephelus lanceolatus) in Taiwan. Zoonoses Public Health 2019, 66, 679–685. [Google Scholar] [CrossRef]
- Ainoda, Y.; Tanaka, E.; Wajima, T.; Nakaminami, H.; Hirota, Y.; Matsushita, T.; Hirai, Y. A case of Shewanella algae-induced bacteremia in Japan: Case report and literature review. J. Infect. Chemother. 2022, 28, 1430–1432. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.; von Bonin, S.; Schneider, R.; Kruger, M.; Quick, S.; Schrottner, P. Shewanella putrefaciens, a rare human pathogen: A review from a clinical perspective. Front. Cell. Infect. Microbiol. 2022, 12, 1033639. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Sun, B.; Zhang, X. Shewanella xiamenensis sp. nov., isolated from coastal sea sediment. Int. J. Syst. Evol. Microbiol. 2010, 60, 1585–1589. [Google Scholar] [CrossRef]
- Laurent, P.; Claire, H.; Patrice, N. Chromosome-Encoded Ambler Class D β-Lactamase of Shewanella oneidensis as a Progenitor of Carbapenem-Hydrolyzing Oxacillinase. Antimicrob. Agents Chemother. 2004, 48, 348–351. [Google Scholar] [CrossRef]
- Ramsamy, Y.; Mlisana, K.P.; Amoako, D.G.; Abia, A.; Ismail, A.; Allam, M.; Mbanga, J.; Singh, R.; Essack, S.Y. Mobile genetic elements-mediated Enterobacterales-associated carbapenemase antibiotic resistance genes propagation between the environment and humans: A One Health South African study. Sci. Total Environ. 2022, 806, 150641. [Google Scholar] [CrossRef]
- Van Duin, D.; Doi, Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence 2017, 8, 460–469. [Google Scholar] [CrossRef]
- Cunningham, S.A.; Limbago, B.; Traczewski, M.; Anderson, K.; Hackel, M.; Hindler, J.; Sahm, D.; Alyanak, E.; Lawsin, A.; Gulvik, C.A.; et al. Multicenter Performance Assessment of Carba NP Test. J. Clin. Microbiol. 2017, 55, 1954–1960. [Google Scholar] [CrossRef]
- Pierce, V.M.; Simner, P.J.; Lonsway, D.R.; Roe-Carpenter, D.E.; Johnson, J.K.; Brasso, W.B.; Bobenchik, A.M.; Lockett, Z.C.; Charnot-Katsikas, A.; Ferraro, M.J.; et al. Modified Carbapenem Inactivation Method for Phenotypic Detection of Carbapenemase Production among Enterobacteriaceae. J. Clin. Microbiol. 2017, 55, 2321–2333. [Google Scholar] [CrossRef] [PubMed]
- Iovleva, A.; Doi, Y. Carbapenem-Resistant Enterobacteriaceae. Clin. Lab. Med. 2017, 37, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ng, I.S.; Zhang, X.; Wang, N. Draft Genome Sequence of the Dye-Decolorizing and Nanowire-Producing Bacterium Shewanella xiamenensis BC01. Genome Announc. 2014, 2, e00721-14. [Google Scholar] [CrossRef] [PubMed]
- Samuelsen, Ø.; Hansen, F.; Aasnæs, B.; Hasman, H.; Lund, B.A.; Leiros, H.S.; Lilje, B.; Janice, J.; Jakobsen, L.; Littauer, P.; et al. Dissemination and Characteristics of a Novel Plasmid-Encoded Carbapenem-Hydrolyzing Class D β-Lactamase, OXA-436, Found in Isolates from Four Patients at Six Different Hospitals in Denmark. Antimicrob. Agents Chemother. 2018, 62, e01260-17. [Google Scholar] [CrossRef] [PubMed]
- Shanmugakani, R.K.; Sugawara, Y.; Akeda, Y.; Hagiya, H.; Sakamoto, N.; Aye, M.M.; Myint, T.; Hamada, S.; Tomono, K. blaOXA-731, a new chromosome-encoded blaOXA-48-like variant in Shewanella sp. from the aquatic environment in Myanmar. Environ. Microbiol. Rep. 2020, 12, 548–554. [Google Scholar] [CrossRef]
- Poirel, L.; Potron, A.; Nordmann, P. OXA-48-like carbapenemases: The phantom menace. J. Antimicrob. Chemother. 2012, 67, 1597–1606. [Google Scholar] [CrossRef]
- Dabos, L.; Jousset, A.B.; Bonnin, R.A.; Fortineau, N.; Zavala, A.; Retailleau, P.; Iorga, B.I.; Naas, T. Genetic and Biochemical Characterization of OXA-535, a Distantly Related OXA-48-Like β-Lactamase. Antimicrob. Agents Chemother. 2018, 62, e01198-18. [Google Scholar] [CrossRef]
- Wang, L.J.; Fan, Y.Y.; Wang, M.; Lu, X.X. Detection of carbapenem-producing Enterobacteriaceae by MALDI-TOF MS. Zhonghua Yi Xue Za Zhi 2013, 93, 2079–2081. [Google Scholar]
- Rotova, V.; Papagiannitsis, C.C.; Skalova, A.; Chudejova, K.; Hrabak, J. Comparison of imipenem and meropenem antibiotics for the MALDI-TOF MS detection of carbapenemase activity. J. Microbiol. Methods 2017, 137, 30–33. [Google Scholar] [CrossRef]
- Wilhelm, C.M.; Forni, G.D.R.; Carneiro, M.D.S.; Barth, A.L. Establishing a quantitative index of meropenem hydrolysis for the detection of KPC- and NDM-producing bacteria by MALDI-TOF MS. J. Microbiol. Methods 2021, 187, 106268. [Google Scholar] [CrossRef]
- Hrabak, J.; Studentova, V.; Bergerova, T.; Walkova, R.; Zemlickova, H.; Jakubu, V.; Chudackova, E.; Gniadkowski, M.; Pfeifer, Y.; Perry, J.D.; et al. Detection of NDM-1, VIM-1, KPC, OXA-48, and OXA-162 Carbapenemases by Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry. J. Clin. Microbiol. 2012, 50, 2441–2443. [Google Scholar] [CrossRef] [PubMed]
- Tafoukt, R.; Leangapichart, T.; Hadjadj, L.; Bakour, S.; Diene, S.M.; Rolain, J.; Touati, A. Characterisation of blaOXA-538, a new variant of blaOXA-48, in Shewanella xiamenensis isolated from river water in Algeria. J. Glob. Antimicrob. Resist. 2018, 13, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Brilhante, M.; Menezes, J.; Belas, A.; Feudi, C.; Schwarz, S.; Pomba, C.; Perreten, V. OXA-181-Producing Extraintestinal Pathogenic Escherichia coli Sequence Type 410 Isolated from a Dog in Portugal. Antimicrob. Agents Chemother. 2020, 64, e02298-19. [Google Scholar] [CrossRef] [PubMed]
- Janda, J.M.; Abbott, S.L. The genus Shewanella: From the briny depths below to human pathogen. Crit. Rev. Microbiol. 2014, 40, 293–312. [Google Scholar] [CrossRef]
- Yousfi, K.; Touati, A.; Lefebvre, B.; Fournier, É.; Côté, J.; Soualhine, H.; Walker, M.; Bougdour, D.; Tremblay, C.; Bekal, S. A Novel Plasmid, pSx1, Harboring a New Tn1696 Derivative from Extensively Drug-Resistant Shewanella xiamenensis Encoding OXA-416. Microb. Drug Resist. 2017, 23, 429–436. [Google Scholar] [CrossRef]
- Zago, V.; Veschetti, L.; Patuzzo, C.; Malerba, G.; Lleo, M.M. Resistome, Mobilome and Virulome Analysis of Shewanella algae and Vibrio spp. Strains Isolated in Italian Aquaculture Centers. Microorganisms 2020, 8, 572. [Google Scholar] [CrossRef]
- Wen, Y.; Xie, X.; Xu, P.; Yang, C.; Zhu, Z.; Zhu, J.; Lv, J.; Zhang, H.; Chen, L.; Du, H. NDM-1 and OXA-48-Like Carbapenemases (OXA-48, OXA-181 and OXA-252) Co-Producing Shewanella xiamenensis from Hospital Wastewater, China. Infect. Drug Resist. 2022, 15, 6927–6938. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, Y.; Liu, Z.; Lu, B.; Dai, H.; Kan, B.; Wang, D. Shewanella carassii sp. nov., isolated from surface swabs of crucian carp and faeces of a diarrhoea patient. Int. J. Syst. Evol. Microbiol. 2017, 67, 5284–5289. [Google Scholar] [CrossRef]
- Araujo, S.; Azenha, S.R.; Henriques, I.; Tacao, M. qnrA gene diversity in Shewanella spp. Microbiology 2021, 167, 001118. [Google Scholar] [CrossRef]
- Da, C.W.; Giambiagi-Demarval, M.; Laport, M.S. Shewanella harboring antimicrobial and copper resistance genes in sea urchins (Paracentrotus lividus) from the Crozon peninsula (Brittany, France). Infect. Genet. Evol. 2020, 85, 104437. [Google Scholar] [CrossRef]
- Jousset, A.B.; Dabos, L.; Bonnin, R.A.; Girlich, D.; Potron, A.; Cabanel, N.; Dortet, L.; Glaser, P.; Naas, T. CTX-M-15-Producing Shewanella Species Clinical Isolate Expressing OXA-535, a Chromosome-Encoded OXA-48 Variant, Putative Progenitor of the Plasmid-Encoded OXA-436. Antimicrob. Agents Chemother. 2018, 62, e01879-17. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Izquierdo, M.; Láinez-Ramos-Bossini, A.J.; Rivera-Izquierdo, C.; López-Gómez, J.; Fernández-Martínez, N.F.; Redruello-Guerrero, P.; Martín-Delosreyes, L.M.; Martínez-Ruiz, V.; Moreno-Roldán, E.; Jiménez-Mejías, E. OXA-48 Carbapenemase-Producing Enterobacterales in Spanish Hospitals: An Updated Comprehensive Review on a Rising Antimicrobial Resistance. Antibiotics 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).