Abstract
Endophytic fungi are a remarkably diverse group of microorganisms that have imperceptible associations with their hosts for at least a part of their life cycle. The enormous biological diversity and the capability of producing bioactive secondary metabolites such as alkaloids, terpenoids, and polyketides have attracted the attention of different scientific communities, resulting in numerous investigations on these fungal endophytes. During our surveys of plant-root-based fungi in the mountain areas of Qingzhen, Guizhou Province, several isolates of endophytic fungi were identified. In this study, a novel endophytic fungus was discovered in the roots of a medicinal plant (Orixa japonica) in Southern China and introduced as a new species (Amphisphaeria orixae) based on morphological evidence and molecular phylogenetic analysis (combined ITS and LSU sequence data). To the best of our knowledge, A. orixae is the first reported endophyte as well as the first hyphomycetous asexual morph in Amphisphaeria. A new isocoumarin, (R)-4,6,8-trihydroxy-5-methylisochroman-1-one (1), and 12 known compounds (2–13) were isolated from the rice fermentation products of this fungus. Using 1D- and 2D-NMR, mass spectrometry, and ECD studies, their structures were identified. The antitumor activity of these compounds was tested. Unfortunately, none of the compounds tested showed significant antitumor activity.
1. Introduction
Endophytic fungi are usually found in the internal tissues or organs of plants, but they do not cause noticeable tissue damage or symptoms. These fungi are often found in a wide array of plant species. At times, endophytic fungi are able to convert to a saprotrophic lifestyle when the host plant undergoes senescence [1]. Even though endophytic fungi can be found in a broad range of hosts, some fungi may be specific to certain hosts and have been extensively researched to gain an understanding of their biological properties. These fungi have also been studied as a source of novel and natural bioactive compounds. Each plant contains one or more endophytic microorganisms that have the potential to produce compounds similar to those of the host plant, and the secondary metabolites they produce are often characterized by novel structures that make them a hot research topic in the field of natural products [2,3]. As new microbial resources, endophytic fungi are an important source of natural bioactive products. The exploitation of endophytic fungi can help alleviate the problems of plant resource shortages and ecological imbalances. Additionally, the use of endophytic fungi can aid in the conservation of rare and endangered plant resources, making this research significant [4]. For these reasons, it is important to explore the secondary metabolites produced by endophytic fungi and to understand their potential applications.
Orixa japonica is a plant of the family Rutaceae and mainly distributed in China, Korea, and Japan [5]. It is a traditional folk medicine that is frequently used to cure diseases or ease symptoms, such as clearing heat and dampness, relieving cough, and acting as an analgesic for stomach pain and rheumatic joint pain [6]. Its extract has pharmacological and biological activities [7]. Liu et al. [8] obtained a new pyrrolizidine alkaloid from the ethanol extract of the root bark of O. japonica. The compound exhibited larvicidal activity against the fourth-instar larvae of Aedes aegypti, Anopheles sinensis, and Culex pipiens pallens, and it also possessed nematocidal activity against Bursaphelenchus xylophilus and Meloidogynein congnita. Choe et al. [9] reported that the leaf extract of O. japonica showed significant antimicrobial activity against Bacillus cereus, Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococcus mutans. Huang et al. [10] isolated eighteen quinoline alkaloids from the roots of O. japonica, and some of the compounds showed significant anti-pathogenic fungal activity, with one compound demonstrating superior efficacy to that of the positive control hymexazol. Moreover, O. japonica can be used in conjunction with antibiotics such as florfenicol or amoxicillin to eliminate persistent cells. This may be attributable to the synergistic action of one or more compounds within the plant [11]. Domestic and international research on O. japonica has focused on its chemical composition, and a variety of chemicals have been isolated and identified from the O. japonica plant in previous studies [8]. In contrast, studies on endophytic fungi of O. japonica are relatively scarce or neglected. From the stem of O. japonica, Lu et al. [6] isolated the novel endophytic fungus Diaporthe orixae and discovered a new host record for Diaporthe caryae. At the same time, Japanese scholars similarly obtained an endophytic fungus belonging to Diaporthe from the leaves of O. japonica and inoculated it on MEA medium to obtain six polyketide derivatives as secondary metabolites from large-scale fermentation [12]. Polyketides, alkaloids, anthraquinones, and other types of novel metabolic products have been found in this genus, and many of them have significant antitumor, antibacterial, antioxidant, and other biological activities [13].
Since 2020, our research group has been focusing on the diversity of endophytic fungi of Orixa japonica [6]. In this study, a new endophytic fungus, Amphisphaeria orixae, was isolated from O. japonica and is introduced based on multi-gene phylogenetic analyses and morphological evidence. A. orixae is the first endophytic fungus of Amphisphaeria that forms a hyphomycetous asexual morph and is unique in undergoing thallic or blastic conidiogenesis and having polymorphic conidia. Thirteen secondary metabolites were identified from the rice fermentation product of A. orixae, including a new isocoumarin, (R)-4,6,8-trihydroxy-5-methylisochroman-1-one (1), and 12 known compounds (2–13). Their chemical structures are depicted in Figure 4 and were established using 1D- and 2D-NMR, mass spectrometry, and chemical computations.
2. Materials and Methods
2.1. Plant Material
The fresh and healthy whole plant of Orixa japonica was collected from Jiulong Mountain, Qingzhen City, Guizhou Province, China (106°30′7″ E 26°40′36″ N). Jiulong Mountain is located in the middle of the Yunnan–Guizhou Plateau, with a hilly landscape and a subtropical monsoonal humid climate, with an average annual temperature of 20 °C. For ease of transport to the laboratory and labeling with relevant metadata (including the date, habitat, location, and host), the materials were enclosed in sealed bags. Fungal isolation was carried out within 24 h of collection.
2.2. Isolation of Fungal Endophytes
The fresh and healthy materials were washed with running tap water for at least 10 min. The materials were surface-sterilized to eliminate epiphytic microorganisms in a benchtop by immersing them in 75% (v/v) ethanol for 3 min, then rinsed with sterilized distilled water for 2 min, then soaked with 10% (v/v) NaClO for 2 min, and finally rinsed with sterile distilled water three times continuously. The materials were dried on sterilized filter papers and then cut into small cubes (ca. 3 mm long segments) and placed on fresh potato dextrose agar (PDA) containing an antibiotic (50 μg/mL penicillin). Samples were incubated in a constant-temperature incubator (28 °C). The plates were observed daily, and mycelia from the edges of fungal colonies were transferred to fresh PDA plates to obtain pure cultures. To induce spore production, polypropylene (PP) was mixed with PDA medium and inoculated with the fungal mycelium. The mycelium was gently swept with a sterilized brush, and variable-temperature incubation was applied alternatively between 24 h at 28 °C and 24 h at 4 °C [14]. Specimens of the dried cultures were deposited at the Herbarium of Guizhou Academy of Agricultural Sciences (Herb. GZAAS). Living cultures were deposited at the Guizhou Culture Collection (GZCC).
2.3. Morphological Study
Micro-morphological characters were photographed using an ECLIPSE Ni-U compound microscope (Nikon, Tokyo, Japan) fitted with an EOS 90D digital camera (Canon, Tokyo, Japan). Tarosoft (R) Image Frame Work was used to measure different morphological features (including conidiogenous cells, mycelia, conidia, and conidiophores), while Adobe Illustrator CS6 (Adobe Systems, San Jose, CA, USA) was used for the processing of figures and pictures.
2.4. DNA Extraction, PCR Amplification, and Sequencing
Using sterile scalpels, fresh mycelia of fungi were scraped. Genomic DNA was extracted from the scraped mycelium by using the Biospin Fungus Genomic DNA Extraction Kit (BioFlux, Shanghai, China) following the manufacturer’s instructions. Two gene regions were amplified with universal primers, viz., the internal transcribed spacer region of ribosomal DNA (ITS: ITS5/ITS4) [15] and the partial large subunit nuclear ribosomal DNA (LSU: LR0R/LR5) [16]. The method used for PCR amplification of ITS and LSU by polymerase chain reaction is described by Lu et al. [17]. The quality of PCR products was observed by using ethidium bromide staining in 1% agarose gel electrophoresis. Successfully amplified PCR products were sent to Sangon Biotech (Shanghai, China) for purification and sequencing (with the same primers).
2.5. Sequence Alignment and Phylogenetic Analyses
The raw forward and reverse reads were edited for ambiguous bases at both ends and assembled using DNASTAR Lasergene SeqMan Pro v.7.1.0 (44.1). In order to compare species and build databases, newly acquired sequences were utilized as queries in BLAST searches of the NCBI GenBank nucleotide database. Each dataset was aligned using MAFFT v.7 [18]. Trimal was used to trim each alignment [19]. Alignment was checked manually using BioEdit [20].
For phylogenetic inferences, two genetic markers, ITS and LSU, were applied (Table 1). The phylogenetic tree was extrapolated using 58 taxa according to recent publications [21,22,23,24]. Maximum likelihood trees (ML) were constructed using IQ-Tree v.2 [25]. The combined datasets were analyzed and divided into groups based on genetic markers. One thousand ultrafast bootstrap replicates were used to estimate branch support. MP analysis was performed with the CIPRES Science Gateway platform using the PAUP on XSEDE (4.a168) tool. ModelTest, implemented in MrMTgui [26], was used to identify the most suitable evolutionary model for Bayesian inference analysis using the Akaike Information Criterion (AIC). One thousand rapid bootstrap replicates were used to estimate bootstrap support. Posterior probabilities (PPs) were evaluated in MrBayes v.3.1.2 [27] with Markov chain Monte Carlo sampling (MCMC). The number of generations for each dataset was determined independently and is explicitly stated in the legend of each tree. The top 25% of the trees represent the aging stage of the analysis and were therefore discarded, while the rest of the trees were used to compute PPs for the majority-rule consensus tree. Convergence was declared for all Bayesian inference trees when the average standard deviation was 0.01. The FigTree v1.4.0 program was used to draw the figures of the trees [28]. The new family’s placement was evaluated using the approximately unbiased (AU) test implemented in CONSEL [29]. Topologies with p values less than 0.05 in the AU test were regarded as being rejected.

Table 1.
GenBank accession numbers of the DNA sequences in this study and the taxa used.
2.6. Fermentation, Extraction, and Separation
The strain was incubated on PDA medium at a constant temperature of 28 °C for 7 days, then cut into small pieces (about 3 × 3 mm) with a scalpel and transferred to a 250 mL conical flask containing 100 mL of liquid medium (20 g of maltose, 10 g of sodium glutamate, 0.5 g of KH2PO4, 20 g of mannitol, 0.3 g of MgSO4-7H2O, 3 g of yeast paste, 10 g of glucose, and 1 L of tap water), and incubated on a 28 °C shaker (150 rpm) for about 10 days. Then, 5 mL of the seed liquid that had been made with 50 g of rice and 55 mL of distilled water was transferred to a sterilized plastic container with a capacity of 200 mL. The sterilized bags were then cultured for 65 days at a constant 28 °C temperature. A total of 1000 bags were cultured.
The crude extract was obtained by extracting the fermented product in a 10:1 ratio of ethyl acetate to methanol and then removing the solvent under reduced pressure. The crude extract was first dissolved in a methanol solution, and then an equal amount of a petroleum ether solution was added, and the petroleum ether extract (212 g) and methanol extract (380 g) were obtained after partitioning. In order to enrich the alkaloids, the methanol layer of the extract was dissolved with an appropriate amount of 2% HCl solution, then the acidic water was extracted three times with an equal volume of ethyl acetate, the ethyl acetate layer was discarded, and the pH was adjusted to about 10 with 25% aqueous ammonia. The mixture was extracted three times with an equal volume of chloroform, and the chloroform layer was combined and concentrated to obtain 2 g of extract (Fr.1). The remaining solution was adjusted to neutral pH, concentrated under reduced pressure, and then separated by silica gel column chromatography (Qingdao Marine Chemistry Co. Ltd., Qingdao, China) with dichloromethane/methanol (100:0→0:100, v/v) to obtain six fractions (Fr.2–Fr.7). Fr.1 (2 g) was further separated using Sephadex LH-20 (Amersham Pharmacia Biotech AB) (MeOH) into three fractions (Fr.1.1–Fr.1.3). Fr.1.1 (500 mg) was further separated by semi-preparative HPLC (SHIMADZU Essentia LC-16P, GH0525010C18 column 10 × 250 mm, 5 µm) using CH3OH/H2O (55:45) to obtain compound 5 (4.5 mg, 3 mL/min, tR = 18.1 min) and compound 13 (6 mg, 3 mL/min, tR = 30.1 min), Fr.1.2 (320 mg) was further separated by semi-preparative HPLC using CH3OH/H2O (40:60) to obtain compound 10 (4.4 mg, 3 mL/min, tR = 6.2 min) and compound 11 (3.2 mg, 3 mL/min, tR = 7.19 min), and Fr.1.3 (70 mg) was further separated by semi-preparative HPLC using CH3CN/H2O (40:60) to obtain compound 12 (9.5 mg, 3 mL/min, tR = 8.5 min). Fr.2 (20 g) was separated by silica gel column chromatography (200–300 mesh) to obtain three fractions (Fr.2.1–Fr.2.3). Fr.2.2 (4 g) was further separated by Sephadex LH-20 and semi-preparative HPLC using CH3OH/H2O (65:35) to obtain compound 6 (16 mg, 3 mL/min, tR = 19.6 min), and Fr.2.3 (600 mg) was further separated by semi-preparative HPLC using CH3CN/H2O (38:62) to obtain compound 7 (150 mg, 3 mL/min, tR = 8.6 min) and compound 8 (4.4 mg, 3 mL/min, tR = 11.3 min). Fr.3 (20 g) was separated by repeated silica gel column chromatography (200–300 mesh) to obtain 5 fractions (Fr.3.1–Fr.3.5). Fr.3.2 (3 g) was separated by Sephadex LH-20 (MeOH) to obtain 4 fractions (Fr.3.2.1–Fr.3.2.4), and Fr.3.2.4 (510 mg) was further separated by semi-preparative HPLC using CH3OH/H2O (45:55) to obtain compound 2 (18 mg, 3 mL/min, tR = 17 min), compound 3 (29 mg, 3 mL/min, tR = 12.3 min), and compound 4 (18 mg, 3 mL/min, tR = 8.5 min). Fr.5 (20 g) was separated by repeated silica gel column chromatography (200–300 mesh) to obtain 6 fractions (Fr.4.1–Fr.4.6), and Fr.4.2 (3 g) was further separated by Sephadex LH-20 (methanol elution) to obtain 4 fractions (Fr.4.2.1–Fr.4.2.4). Fr.4.2.4 (162 mg) was further separated by semi-preparative HPLC using CH3OH/H2O (55:45) to obtain compound 9 (11 mg, 3 mL/min, tR = 19.2 min), Fr.4.4 (1.7 g) was further separated by Sephadex LH-20 (dichloromethane/methanol 1:1 ratio elution) to obtain 3 fractions (Fr.4.4.1–Fr.4.3), and Fr.4.4.2 (69 mg) was further separated by semi-preparative HPLC using CH3OH/H2O (30:70) to obtain compound 1 (36.6 mg, 3 mL/min, tR = 11.6 min).
2.7. Antitumor Bioassay
The following established in vitro human cancer cell lines were used: human breast cancer cells (MCF-7), human hepatocellular carcinoma cells (HepG2), and pancreatic cancer cells (PANC-1). Adriamycin was used as a positive control, and antitumor activity was measured for compounds 1, 3, 4, 7, 9, 12, and 13. Cell counting kit-8 was obtained from GLPBIO (USA). MCF-7 and PANC-1 cells in the logarithmic growth phase were digested and added to 96-well plates at a density of 0.6 × 104 cells per well and incubated at 37 °C in a 5% CO2 incubator for 12 h. After the cells were plastered, culture solutions containing 0, 50, 100, and 200 µM of the drug at different concentrations were prepared by performing DMSO gradient dilution in a volume of 100 µL. Each group of five replicate wells was incubated in the cell culture incubator for 24 h; the plate was washed twice with 100 µL of PBS, and then 100 µL of culture solution containing 10% CCK-8 was added. The OD value was measured at a 450 nm wavelength. It was calculated by the following equation.
Cell survival rate (%) = [(As − Ab)/(Ac − Ab)] × 100.
As = absorbance of experimental wells (containing cells, medium, CCK-8, and compound to be measured).
Ab = absorbance of blank wells (containing medium, and CCK-8).
Ac = absorbance of control wells (with cells, medium, and CCK-8).
3. Results
3.1. Phylogenetic Analysis of ITS and LSU Sequences
The aligned sequence matrix comprised ITS (617 bp) and LSU (850 bp) sequence data, including 56 ingroup taxa and two outgroup taxa. The dataset has 1467 characters, of which 884 characters are constant, 160 variable characters are quasi-informative, and 423 characters are quasi-informative. ML, MP, and Bayesian analyses of the combined dataset resulted in the reconstruction of plant genetics with essentially similar topologies, and the ML tree is shown in Figure 1. The tree is rooted with Achaetominum macrosporum (CBS 532.94) and Chaetomium elatum (CBS 374.66). The genus Amphisphaeria contained 25 taxa retrieved from GenBank and two new strains generated in this study. The two new isolates are recognized as one new species, viz., Amphisphaeria orixae, and it formed a sister clade with A. camelliae (HKAS 107021 and MFLUCC 20-0122) and A. uniseptata (CBS 114967).
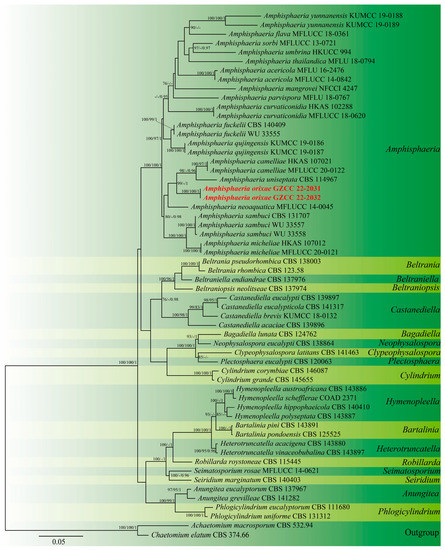
Figure 1.
Maximum likelihood analysis of the phylogenetic tree constructed from the concatenated alignment of the ITS and LSU sequence data. Maximum likelihood (MLBS), maximum parsimony (MPBS), and Bayesian posterior probability (PP) bootstrap support values equal to or greater than 75% and 0.95, respectively, are provided close to the nodes. Bold and red are sequences that were recently generated.
3.2. Taxonomy
Amphisphaeria Ces. & De Not., Comm. Soc. crittog. Ital. 1(fasc. 4): 223 (1863).
Index Fungorum number: IF 173; Facesoffungi number: FoF 02099.
Type species: Amphisphaeria umbrina (Fr.) De Not, Sfer. Ital: 69 (1863).
Notes: Amphisphaeria is the type genus of Amphisphaeriaceae, with A. umbrina as the type species [22,23]. Most of the species of Amphisphaeria are found on grasses, woody branches, and some monocotyledons as saprobes in terrestrial habitats [30]. The unicellular ascospores of Amphisphaeria members typically have J+ or J− apical rings, solitary or aggregated ventral membranes under undeveloped perithecia or absent perithecia; and a coelomycetous asexual morphology with a light- to dark-brown color, oval to fusiform shape, and 1-3-noded ventral conidia [21,22,23,24]. The species of Amphisphaeria that have been reported are sexual morphs or coelomycetous asexual morphs, and no studies have reported hyphomycetous asexual morphs in this genus so far. In addition, Amphisphaeriaceous taxa have not been recorded as phytopathogens [30]. Based on phylogenetic analysis and morphological evidence, we introduce the first endophytic fungus of Amphisphaeria in this study. It is distinguished as a hyphomycetous asexual morph.
Amphisphaeria orixae X. J. Wang, Y. Z. Lu & Z. Zhang, sp. nov., Figure 2.
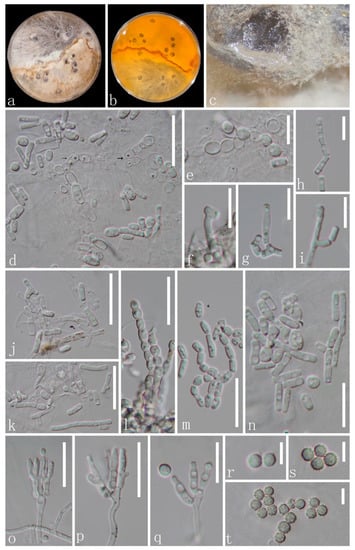
Figure 2.
Amphisphaeria orixae (GZAAS 22-2031, Holotype). (a–c) Colonies on PDA mixed with polypropylene; (d–n) conidiophores, thallic conidiogenesis; (e,l) alternate-arthric; (h–k,m–n) thallic-arthric) and conidia; (o–q) conidiophores with conidiogenous cells (blastic) and conidia; (r–t) conidia. Scale bars: (e–i,q) = 10 μm, (d,j–p) = 20 μm; (r–t) = 5 μm.
Index Fungorum number: IF 900170; Facesoffungi number: FoF 13911.
Etymology—Name referring to the host “Orixa japonica”, using the genitive case meaning “of Orixa”.
Holotype: GZAAS 22-2031.
Description: Endophytic in the roots of O. japonica. Sexual morph: Undetermined. Asexual morph: Two modes of development during conidiogenesis. (1) Thallic (d–n). Conidiophores hypha-like or reduced. Conidiogenous cells holothallic, narrowly cylindrical, frequently undifferentiated, and hyaline, forming conidia by random thallic-arthric disarticulation or alternate-arthric conidiogenesis. Conidia produced by thallic-arthric conidiogenesis, aseptate, micro guttulate, hyaline, polymorphic, bacilliform, cylindrical or cuneiform (2–13 × 1.5–2 µm, n = 23); conidia produced by alternate-arthric conidiogenesis, aseptate, guttulate, globose to subglobose (5.5–7.5 × 4.5–6.5 µm ( = 6 × 5 µm, n = 12)], ellipsoidal 3–6.5 × 3–4.5 ( = 4.5 × 3.5 µm, n = 18). (2) Phialidic (o–t). Conidiophores hyaline, penicillate, monoverticillate to biverticillate, with a stipe and apical series of branches subtending the phialides. Phialides 5.5–10 × 2–3 µm ( = 7.5 × 2.5 µm, n = 15), lageniform to cylindrical, straight or curved, and hyaline. Conidia 2.8–3.5 µm diam, globose, aseptate, hyaline, and smooth when young, and pale yellow, verrucose, or echinulate when mature.
Material examined: CHINA, Guizhou Province, Qingzhen city, from the healthy roots of O. japonica, 14 September 2020, Hong-Bo Wang, Jg1 (Holotype: GZAAS 22-2031, dried culture), ex-type culture: GZCC 22-2031; ibid., Jg2 (GZAAS 22-2032); living culture: GZCC 22-2032.
Culture characteristics: Colonies whole, superficial, flat, slow growing on PDA, reaching 10 mm diam after 10 d at 28 °C, light yellow to brownish yellow with a clear brown irregular boundary in the middle. Vegetative hyphae septate, branched, hyaline, or yellow.
Notes: Amphisphaeria orixae is the first hyphomycetous asexual morph reported in this genus based on molecular DNA data, and it morphologically differs from all existing Amphisphaeria species. Amphisphaeria camelliae (HKAS 107021, MT756615) was the closest species to our newly obtained isolates based on the BLAST results of LSU (99.08% similarity). The phylogenetic analyses showed support values with 98% MLBS/1.00 PP (Figure 1) indicating that the two new isolates of A. orixae form a distinct clade within the genus and are a sister clade to A. camelliae (HKAS 107021 and MFLUCC 20-0122) and A. uniseptata (CBS 114967). Moreover, A. orixae is also the first endophyte in the genus Amphisphaeria.
3.3. Structure Elucidation
Compound 1 was isolated as a white powder, and its molecular formula was determined as C10H10O5 by high-resolution mass spectrometry (HRESIMS) (m/z 209.0465[M-H]+, calcd. for C10H9O5, 209.0455), indicating 6 degrees of unsaturation. UV spectra indicated the presence of a benzophenone moiety in the compound because of the maximum absorption at 267 and 314 nm. The IR spectrum indicated the existence of groups such as hydroxyl (3366 cm−1), carbonyl (1669 cm−1), benzene ring (1621, 1499 cm−1), and methyl (1468 cm−1). The 1H-NMR spectral data (Table 2) showed the presence of a single aromatic proton signal δH 6.35 (1H, s), a hydroxylated methine signal δH 4.89 (1H, dd, J = 2.4, 2.4 Hz), two oxygenated methylene signals (δH 4.59 (1H, dd, J = 12.6, 2.4 Hz) and 4.48 (1H, dd, J = 12.6, 2.4 Hz)), and a methyl signal δH 2.16 (3H, s). The 13C-NMR spectral data (Table 2) give a total of 10 carbon signals, consisting of one ester carbonyl, six aromatic carbons corresponding to a benzene ring, one oxygenated methylene carbon, one hydroxylated sp3 methine, and a methyl. These data were quite similar to those of the known analog (R)-4-acetyl-6,8-dihydroxy-5-methylisochroman-1-one (4), with the main difference being that signals for the sp3 methine C-10 and the attached acetyl substituent in 4 were replaced by signals for one hydroxylated sp3 methine at δc/H 62.1/4.89 in 1, which indicates that a hydroxyl rather than an acetyl substituent as in 4 is located at C-10 of 1. This deduction was further confirmed by COSY correlations of H-10/H2-9 and HMBC correlations (Figure 3A) from H-10 to C-4; H2-9 to C-5 and C-7; H3-11 to C-1, C-5, and C-6; and H-2 to C-1, C-3, and C-4. To ascertain the absolute configuration of 1, The conformations of the isomers of compound 1 were generated using the iMTD-GC method embedded in the Crest program [31]. ECD calculations for 1 (10S) and ent-1 (10R) were performed using Gaussian software, the results of which were compared with experimental values (detailed ECD calculations are in the Supplementary Materials). As shown in Figure 3B, the experiment ECD curve matched well with the calculated curve for 1, thus assigning its absolute configuration as 10S.

Table 2.
1H- and 13C-NMR spectral data of compound 1 (δ in ppm, J in Hz) in CD3OD.
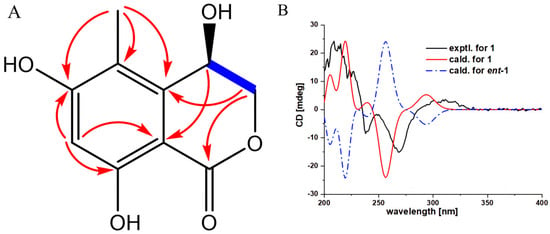
Figure 3.
The key HMBC and H–H COSY correlations and experimental and calculated ECD curves for compound 1. (A) HMBC correlations of compounds 1; (B) Calculated and experimental ECD spectra of compound 1.
Compounds 2–13 were identified as known compounds based on their spectral features and by comparison with the literature (Figure 4): (3R,4R)-4-acetyl-3-ethoxy-6,8-dihydroxy-5-methylisochroman-1-one (2) [32]; (3R,4R)-4-acetyl-6,8-dihydroxy-3-methoxy-5-methylisochroman-1-one (3) [33]; (R)-4-acetyl-6,8-dihydroxy-5-methylisochroman-1-one (4) [34]; kokusaginine (5) [35]; dictamnine (6) [36]; lunidonine (7) [37]; orixinone (8) [38]; N-methylpreskimmianine (9) [39]; isoplatydesmine (10) [40]; S-ribalinine (11) [41]; (-)-(S)-edulinine (12) [42,43]; and orixalone A (13) [44], respectively. In the Supplementary Materials, the NMR data for well-known compounds are described in detail.
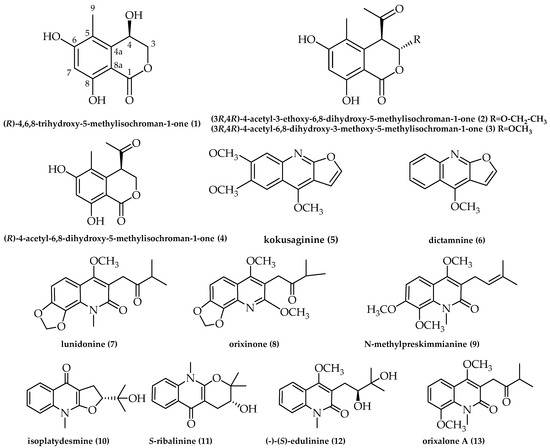
Figure 4.
Chemical structures of compounds 1–13.
Compound 2, C14H16O6, yellow oil, HREIMS m/z 279.0879 [M-H]+ (calcd. for C14H15O6, 279.0862), 1H-NMR (600 MHz, MeOD, δ, ppm) δH 6.34 (1H, s, H-7), 5.83 (1H, d, J = 1.2 Hz, H-3), 4.30 (1H, d, J = 1.2 Hz, H-4), 3.91–3.88 (1H, m, H- 1’), 3.76–3.74 (1H, m, H-1’), 2.18 (3H, s, H-12), 2.04 (3H, s, H-13), 1.17 (3H, t, J = 7.2, 1.2 Hz, H-2’); 13C-NMR (150 MHz, MeOD, δ, ppm) δC 204.6 (C-11), 169.5 (C-1), 165.0 (C-5), 163.7 (C-8), 136.2 (C-10), 117.4 (C-5), 102.3 (-CH), 102.2 (-CH), 101.1 (C-9), 66.5 (-CH2), 55.0 (-CH), 29.1 (-CH3), 15.3 (-CH3), 11.1 (-CH3).
Compound 3, C13H14O6, brown solid, HREIMS m/z 265.0722 [M-H]+ (calcd. for C13H13O6, 265.0722), 1H-NMR (600 MHz, DMSO-d6, δ, ppm), δH 6.36 (1H, s, H-7), 5.84 (1H, d, J = 5.4 Hz, H-3), 4.48 (1H, s, H-4), 3.45 (3H, s, H-11), 2.27 (3H, d, J = 1.8 Hz, H-13), 1.94 (3H, s, H-14); 13C-NMR (150 MHz, DMSO-d6, δ, ppm) δC 202.9 (C-12), 167.2 (C-1), 163.3 (C-6), 161.2 (C-8), 135.0 (C-10), 115.6 (C-5), 101.0 (C-3), 101.0 (C-7), 99.8 (C-9), 56.3 (C-11), 52.8 (C-4), 29.4 (C-13), 10.8 (C-14).
Compound 4, C12H12O5, light-yellow crystal, ESI-MS m/z 259.1 [M + Na]+; 1H-NMR (600 MHz, DMSO-d6, δ, ppm), δH 11.00 (2H, s, H-3), 6.37 (1H, s, H-7), 4.93 (1H, dd, J = 11.4, 5.4 Hz, H-3a), 4.61 (1H, dd, J = 12, 4.2 Hz, H-3b), 4.28 (1H, d, J = 3 Hz, H-4), 2.28 (3H, s, H-12), 1.93 (3H, S, H-13); 13C-NMR (150 MHz, DMSO-d6, δ, ppm) δC 205.1 (C-11), 169.1 (C-1), 162.9 (C-6), 161.4 (C-8), 137.8 (C-10), 114.5 (C-5), 101.2 (C-7), 100.4 (C-9), 68.1 (C-3), 47.7 (C-4), 28.6 (C-12), 10.7 (C-13).
Compound 5, C14H13NO4, white solid, ESI-MS m/z 260.1 [M + H]+; 1H-NMR (400 MHz, CDCl3, δ, ppm), δH 7.58 (1H, d, J = 2.8 Hz, H-2′), 7.48 (1H, s, H-5), 7.34 (1H, s, H-8), 7.05 (1H, d, J = 2.4 Hz, H-3′), 4.44 (3H, s, 4-OCH3), 4.03 (6H, d, J = 2 Hz, 6-OCH3, 7-OCH3); 13C-NMR (100 MHz, CDCl3, δ, ppm), δC 163.2 (C-2), 155.7 (C-4), 152.7 (C-6), 147.9 (C-7), 142.7 (C-8a), 142.6 (C-2′), 113.1 (C-3), 106.8 (C-8), 104.8 (C-3′), 102.3 (C-4a), 100.3 (C-5), 59.0 (4-OCH3), 56.2 (6-OCH3), 56.1 (7-OCH3).
Compound 6, C12H9NO2, yellow crystal, ESI-MS m/z 200.1 [M + H]+; 1H-NMR (400 MHz, CDCl3, δ, ppm), δH 8.25 (1H, dd, J = 8.4, 0.8 Hz, H-5), 8.01 (1H, d, J = 8.4 Hz, H-8), 7.67 (1H, td, J = 6.8, 1.2 Hz, H-7), 7.60 (1H, d, J = 2.8 Hz, H-2), 7.43 (1H, td, J = 6.8, 1.2 Hz, H-6), 7.04 (1H, d, J = 2.8 Hz, H-3, 4.41 (3H, s, -OCH3); 13C-NMR (100 MHz, CDCl3, δ, ppm), δC 163.9 (C-9a), 156.9 (C-4), 145.7 (C-8a), 143.6 (C-2), 129.7 (C-7), 127.8 (C-8), 123.8 (C-6), 122.5 (C-5), 118.8 (C-3a), 104.8 (C-3), 103.4 (C-4a), 59.4 (-OCH3).
Compound 7, C17H19NO5, white crystal, ESI-MS m/z 340.1 [M + Na]+; 1H-NMR (400 MHz, CDCl3, δ, ppm), δH 7.38 (1H, d, J = 8.8 Hz, H-5), 6.82 (1H, d, J = 8.4 Hz, H-6), 6.03 (2H, s, OCH2O), 3.85 (6H, d, J = 5.2 Hz, N-CH3, -OCH3), 3.78 (2H, s, H-11), 2.87 (1H, m, H-13), 1.22 (3H, s, CH3), 1.20 (3H, s, CH3); 13C-NMR (150 MHz, CDCl3, δ, ppm), δC 212.0 (C-12), 163.9 (C-2), 162.2 (C-4), 149.6 (C-7), 133.9 (C-8), 126.1 (C-10), 118.3 (C-5), 115.5 (C-3), 114.3 (C-9), 104.8 (C-6), 101.2 (OCH2O), 62.2 (OCH3), 41.3 (C-13), 37.0 (C-11), 32.8 (N-CH3), 18.5 (C-14, 15).
Compound 8, C17H19NO5, colorless crystal, HREIMS: m/z: 340.1149 [M + Na]+ (calcd. for C14H15O6Na, 340.1149), 1H-NMR (600 MHz, MeOD, δ, ppm), δH 7.53 (1H, d, J = 8.4 Hz, H-4), 7.08 (1H, d, J = 8.4 Hz, H-3), 6.16 (2H, s, H-18), 3.98 (3H, s, H- 17), 3.92 (2H, s, H-11), 3.89 (3H, s, H-16), 2.87 (1H, m, H- 3), 1.19 (6H, d, J = 6.6 Hz, H-14, H-15); 13C-NMR (150 MHz, MeOD, δ, ppm), δC 214.4 (C-12), 164.9 (C-8), 163.9 (C-6), 149.1 (C-2), 141.7 (C-10), 134.0 (C-1), 118.8 (C-7), 117.2 (C-4), 110.9 (C-5), 108.5 (C-3), 103.3 (CH3), 63.1 (CH3), 54.4 (CH3), 41.9 (C-13), 37.0 (C-11), 18.8 (-CH3, -CH3).
Compound 9, C18H23NO4, colorless crystal, ESI-MS m/z 318.3 [M + H]+; 1H-NMR (600 MHz, CDCl3, δ, ppm), δH 7.55 (1H, d, J = 9 Hz, H-5), 6.90 (1H, d, J = 9 Hz, H-6), 5.25 (1H, m, H-12), 3.95 (6H, d, J = 6 Hz, OCH3, OCH3), 3.87 (3H, s, OCH3), 3.77 (3H, s, N-CH3), 3.36 (2H, d, J = 6.6 Hz, H-11), 1.80 (3H, s, CH3), 1.68 (3H, d, J = 1.2 Hz, CH3); 13C-NMR (150 MHz, CDCl3, δ, ppm), δC 165.6 (C-2), 160.2 (C-4), 154.9 (C-7), 137.1 (C-8), 134.3 (C-13), 132.4 (C-10), 121.9 (C-12), 120.0 (C-3), 119.3 (C-5), 114.0 (C-9), 107.4 (C-6), 61.8 (OCH3), 61.8 (OCH3), 56.4 (OCH3), 34.1 (N-CH3), 25.9 (CH3), 24.4 (C-11), 18.1 (CH3).
Compound 10, C15H17NO3, white crystal, ESI-MS m/z 282.1 [M + Na]+; 1H-NMR (600 MHz, MeOD, δ, ppm), δH 8.30 (1H, dd, J = 7.8, 1.2 Hz, H-5), 7.74 (1H, ddd, J = 1.8, 7.2, 8.4 Hz, H-7), 7.69 (1H, d, J = 8.4 Hz, H-8), 7.43 (1H, m, H-6), 4.98 (1H, td, J = 8.4, 2.4 Hz, H-3), 3.81 (3H, s, N-Me), 3.24 (2H, d, J = 8.4 Hz, H-2), 1.38 (3H, s, 2′-Me), 1.26 (3H, s, 3′-Me); 13C-NMR (150 MHz, MeOD, δ, ppm), δC 174.9 (C-4), 164.3 (C-3b), 140.0 (C-8a), 132.7 (C-7), 126.7 (C-4a), 126.4 (C-5), 124.8 (C-6), 116.5 (C-8), 101.7 (C-3a),93.5 (C-2), 72.2 (C-1′), 32.2 (N-Me),28.1 (C-3), 25.8 (C-3), 24.7 (C-2′).
Compound 11, C15H17NO3, white crystal, ESI-MS m/z 282.1 [M + Na]+; 1H-NMR (600 MHz, MeOD, δ, ppm), δH 8.30 (1H, dd, J = 8.4, 1.2 Hz, H-6), 7.74 (1H, dd, J = 9, 1.8 Hz, H-7), 7.71 (1H, d, J = 8.4 Hz, H-9), 7.40 (1H, td, J = 6.6, 1.2 Hz, H-8), 3.88 (1H, t, J = 5.4 Hz, H-3), 3.79 (3H, s, N-Me), 2.95 (1H, dd, J = 16.8, 4.8 Hz, H-4b), 2.73 (1H, dd, J = 16.8, 6 Hz, H-4a), 1.48 (3H, s, 1′-Me), 1.46 (3H, s, 2′-Me); 13C-NMR (150 MHz, MeOD, δ, ppm), δC 178.4 (C-5), 156.9 (C-9b), 140.4 (C-9a), 133.2 (C-8), 126.5 (C-7), 124.5 (C-5b), 124.2 (C-6), 116.6 (C-9), 98.5 (C-5a), 84.3 (C-2), 69.0 (C-3), 31.2 (N-Me), 26.8 (C-4), 25.3 (C-1′), 22.1 (C-2′).
Compound 12, C16H21NO4, white solid, HREIMS m/z 314.1359 [M + Na]+ (calcd. for C16H21NO4Na, 314.1359), 1H-NMR (600 MHz, MeOD, δ, ppm), δH 7.90 (1H, d, J = 7.8 Hz, H-5), 7.64 (1H, t, J = 8.4 Hz, H-7), 7.60 (1H, d, J = 9 Hz, H-8), 7.34 (1H, t, J = 7.2 Hz, H-6), 3.99 (3H, s, 4-OCH3), 3.74 (3H, d, J = 1.2 Hz, N-CH3), 3.70 (1H,dd, J = 10.2, 2.4 Hz, H-12), 3.03 (1H, dd, J =13.2, 1.2 Hz, H-11), 2.81 (1H, t, J = 10.2 Hz, H-10), 1.29 (3H, S, CH3), 1.28 (3H, s, CH3); 13C-NMR (150 MHz, MeOD, δ, ppm), δC 166.8 (C-2), 163.7 (C-4), 140.3 (C-10), 131.9 (C-7), 124.7 (C-5), 123.7 (C-6), 122.0 (C-9), 119.1 (C-3), 116.0 (C-8), 78.7 (C-12), 74.0 (C-13), 63.0 (4-OCH3), 30.5 (N-CH3), 28.6 (C-11), 26.0 (CH3), 25.0 (CH3).
Compound 13, C17H21NO4, white powder, ESI-MS m/z 326.1 [M + Na]+; 1H-NMR (600 MHz, CDCl3, δ, ppm), δH 7.44 (1H, dd, J = 7.8, 1.2 Hz, H-5), 7.17 (1H, t, J = 7.8 Hz, H-6), 7.06 (1H, dd, J = 8.4, 1.2 Hz, H-7), 3.92 (3H, s, 1-NCH3), 3.88 (3H, s, 8-OCH3), 3.85 (3H, s, 4-OCH3), 3.81 (2H, S, H-1′), 2.88 (1H, m, 3′), 1.22 (3H, s, 3′-OCH3), 1.21 (3H, s, 3′-OCH3); 13C-NMR (150 MHz, CDCl3, δ, ppm), δC 211.9 (C-2′), 164.7 (C-2), 161.8 (C-4), 149.0 (C-8), 131.1 (C-8a), 122.7 (C-6), 119.9 (C-4a), 118.0 (C-3), 116.1 (C-5), 114.0 (C-7), 62.1 (4-OCH3), 56.8 (8-OCH3), 41.3 (C-3′), 37.2 (C-1′), 35.7 (1-NCH3), 18.5 (3′-CH3), 18.5 (3′-CH3).
3.4. Antitumor Bioassay
The in vitro cytotoxic activity of the compounds against MCF-7, HepG2, and PANC-1 was analyzed using the CCK-8 assay with Adriamycin as a positive control (Figure 5). The results demonstrated that none of the tested compounds exhibited significant antitumor activity compared to Adriamycin (Table 3). Although compound 3 demonstrated the best antitumor activity against MCF-7, with an IC50 value of 51.9 µM, it displayed weak activity against the other two tumor cells. The compounds tested showed considerable differences in their IC50 values compared to the positive control, indicating that the compounds do not have significant antitumor activity. The IC50 values for Adriamycin were found to be 2.03 µM, 3.85 µM, and 3.44 µM when tested on MCF-7, HepG2, and PANC-1, respectively. Additionally, the inhibitory effect of Adriamycin on tumor cells was observed to be dose-dependent, as the cell viability decreased with the increase in the drug concentration. For the assay, the concentrations of Adriamycin were set at 0.2, 0.5, 1, 2, 5, 10, 20, 50, and 100 µM, whereas for the tested compounds, they were set at 5, 20, 50, 100, and 200 µM for MCF-7 tumor cells and 50, 100, and 200 µM for HepG2 and PANC-1 tumor cells.
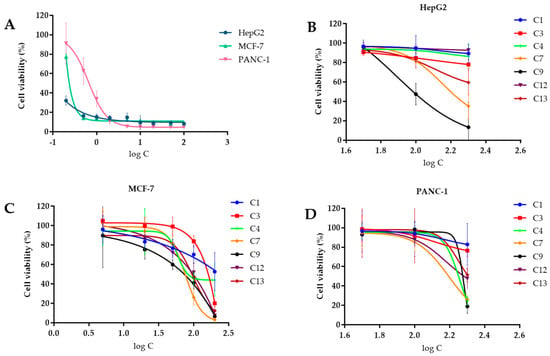
Figure 5.
Effect of compounds on the cell viability of different tumor cells. (A) Effect of Adriamycin on the cell viability of three tumor cells; (B) effect of compounds on the cell viability of HePG2 tumor cells; (C) effect of compounds on the cell viability of MCF–7 tumor cells; (D) effect of compounds on the cell viability of PANC–1 tumor cells.

Table 3.
Cytotoxicity of compounds on three tumor cell lines (half-maximal inhibitory concentration (IC50): µM).
4. Discussion
Endophytic fungi are an important source for discovering naturally active substances [45]. In this study, a new endophytic fungus, viz., Amphisphaeria orixae, was isolated from the medicinal plant O. japonica. The fungi in Amphisphaeria are usually saprobes on woody branches and some monocotyledons, including grasses in terrestrial, mangrove, and freshwater habitats [21,22,23,24,30]. It is noteworthy that A. orixae is the first endophyte and first hyphomycetous asexual morph in Amphisphaeria, which broadens the morphological characteristics and lifestyles of this genus. The asexual sporulation of the new species Amphisphaeria orixae is relatively complex and includes phialidic and thallic conidiogenesis. This discovery is important for future taxonomic studies on this genus, as the generic boundaries of the family Amphisphaeriaceae have traditionally been based on sexual characteristics, and the delimitation of genera is still ambiguous and challenging. Furthermore, thirteen secondary metabolites, including a new isocoumarin, were isolated from the rice fermentation product of this fungus. All 13 compounds obtained in this study were isolated from the genus Amphisphaeria for the first time. Among them, compounds 1–4 are coumarins, and compounds 5–13 are quinoline alkaloids. The experimental strain was isolated from the roots of O. japonica. Interestingly, compounds 5, 7–9, and 12–13 were also obtained from the roots of the O. japonica, as reported by Liu et al. and Huang et al. [10,46], which provided evidence that the endophytic fungus is able to produce the same or similar compounds as the host. Moreover, these compounds are known to have significant antifungal, insecticidal, and food-repelling activities from the reported literature, with kokusaginine (5) having LC50 values of 16.66µg/mL and 5.32µg/mL against Bursaphelenchus xylophilus and Meloidogynein congnita, respectively, and lunidonine (7) not only killed Meloidogynein congnita and Anopheles sinensis but also showed a strong food-repelling effect on Ostrinia furnacalis [10,46]. These findings indicate that Amphisphaeria orixae may be a promising source of bioactive compounds with potential applications in agriculture and medicine. In summary, the discovery of this new endophytic fungus and its secondary metabolites further highlights the importance of exploring the microbial diversity of medicinal plants for the discovery of novel biologically active compounds.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/microorganisms11051268/s1; Figure S1: 1H-NMR spectrum of compound 1; Figure S2: 13C-NMR spectrum of compound 1; Figure S3: HRESIMS spectrum of compound 1; Figure S4: IR spectrum of compound 1; Figure S5: UV spectrum of compound 1; Figure S6: HSQC spectrum of compound 1; Figure S7: HMBC spectrum of compound 1; Figure S8: 1H-1H COSY spectrum of compound 1; Figure S9: 1H-NMR spectrum of compound 2; Figure S10: 13C-NMR spectrum of compound 2; Figure S11:1H-NMR spectrum of compound 3; Figure S12: 13C-NMR spectrum of compound 3; Figure S13: 1H-NMR spectrum of compound 4; Figure S14: 13C-NMR spectrum of compound 4; Figure S15: 1H-NMR spectrum of compound 5; Figure S16: 13C-NMR spectrum of compound 5; Figure S17: 1H-NMR spectrum of compound 6; Figure S18: 13C-NMR spectrum of compound 6; Figure S19: 1H-NMR spectrum of compound 7; Figure S20: 13C-NMR spectrum of compound 7; Figure S21: 1H-NMR spectrum of compound 8; Figure S22: 13C-NMR spectrum of compound 8; Figure S23: 1H-NMR spectrum of compound 9; Figure S24: 13C-NMR spectrum of compound 9; Figure S25: 1H-NMR spectrum of compound 10; Figure S26: 13C-NMR spectrum of compound 10; Figure S27: 1H-NMR spectrum of compound 11; Figure S28: 13C-NMR spectrum of compound 11; Figure S29: 1H-NMR spectrum of compound 12; Figure S30: 13C-NMR spectrum of compound 12; Figure S31: 1H-NMR spectrum of compound 13; Figure S32: 13C-NMR spectrum of compound 13. Figure S33: ECD calculation details of compound 1.
Author Contributions
Conceptualization, Z.Z. and Y.L.; methodology, X.W., P.Z., L.Z., J.M. and J.Z.; software, X.W., P.Z. and L.Z.; investigation, X.W.; writing—original draft preparation, X.W.; writing—review and editing, Z.Z., Y.L. and D.N.W.; project administration, Z.Z. and Y.L.; funding acquisition, Z.Z. and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Youth Science and Technology Talent Development Project from the Guizhou Provincial Department of Education (QJHKYZ [2022]345), the Guizhou Province high-level talent innovation and entrepreneurship merit funding project (No. 202104), and the academic emerging project of Guizhou Institute of Technology (GZLGXM-15).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data analyzed in this study are available within the manuscript and are available from the corresponding authors upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jeewon, R.; Ittoo, J.; Mahadeb, D.; Jaufeerally-Fakim, Y.; Wang, H.K.; Liu, A.R. DNA based identification and phylogenetic characterisation of endophytic and saprobic fungi from Antidesma madagascariense, a medicinal plant in Mauritius. J. Mycol. 2013, 2013, 781914. [Google Scholar] [CrossRef]
- Thi Minh Le, T.; Thi Hong Hoang, A.; Thi Bich Le, T.; Thi Bich Vo, T.; Van Quyen, D.; Hoang Chu, H. Isolation of endophytic fungi and screening of Huperzine A–producing fungus from Huperzia serrata in Vietnam. Sci. Rep. 2019, 9, 16152. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.S.; Jain, R.; Bhardwaj, P.; Thakur, A.; Kumari, M.; Bhushan, S.; Kumar, S. Plant Probiotics–Endophytes pivotal to plant health. Microbiol. Res. 2022, 263, 127148. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, L.; Pan, Y.; Zheng, X.; Liang, X.; Sheng, L.; Wang, Q. Research advances on endophytic fungi and their bioactive metabolites. Bioprocess. Biosyst. Eng. 2022, 46, 165–170. [Google Scholar] [CrossRef]
- Funayama, S.; Tanaka, R.; Kumekawa, Y.; Noshita, T.; Mori, T.; Kashiwagura, T.; Murata, K. Rat small intestine muscle relaxation alkaloids from Orixa japonica leaves. Biol. Pharm. Bull. 2001, 24, 100–102. [Google Scholar] [CrossRef]
- Lu, Q.T.; Zhang, J.Y.; Sun, Y.R.; Tang, X.; Lu, Y.Z.; Zhang, Z. Diaporthe orixae sp. nov., an endophytic species isolated from Orixa japonica in southern China. Phytotaxa 2022, 544, 37–51. [Google Scholar] [CrossRef]
- Nhoek, P.; Ahn, S.; Pel, P.; Kim, Y.M.; Huh, J.; Kim, H.W.; Chin, Y.W. Alkaloids and Coumarins with Adiponectin-Secretion-Promoting Activities from the Leaves of Orixa japonica. J. Nat. Prod. 2023, 86, 138–148. [Google Scholar] [CrossRef]
- Liu, X.C.; Lai, D.; Liu, Q.Z.; Zhou, L.; Liu, Q.; Liu, Z.L. Bioactivities of a new pyrrolidine alkaloid from the root barks of Orixa japonica. Molecules 2016, 21, 1665. [Google Scholar] [CrossRef]
- Choe, S.B.; Kang, S.T. Investigation of antimicrobial activity and stability of Orixa japonica Thunb. leaf extract. Korean J. Food Sci. Technol. 2014, 46, 39–43. [Google Scholar] [CrossRef]
- Huang, L.; Jian, J.Y.; Fan, Y.M.; Liu, Q.; Jin, J.; Yuan, C.M.; Gu, W.; Hao, X.J. Quinoline Alkaloids from the Roots of Orixa japonica with the Anti-Pathogenic Fungi Activities. Chem. Biodivers. 2022, 20, e202201097. [Google Scholar]
- Kim, N.K.; Kweon, D.; Kim, S.K. Effects of natural compounds from various plant eradicate the persister cell of Edwardsiella tarda treated with antibiotics of florfenicol and amoxicillin. J. Life Sci. 2012, 22, 788–793. [Google Scholar] [CrossRef]
- Nakashima, K.I.; Higuchi, Y.; Tomida, J.; Kawamura, Y.; Inoue, M. Two new α-pyrone derivatives from the endophytic Diaporthe sp. ECN371. J. Nat. Med. 2022, 76, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhou, W.Y.; Xu, G.M.; Zhou, X.J. Research progress on natural products of fungi of Diaporthe sp. China J. Chin. Mater. Med. 2021, 46, 1717–1726. [Google Scholar]
- Sugimoto, K.; Oda, S. Efficient production of fungal spores by the combination of reduction of nitrogen source content and embedding of hydrophobic polymer in an agar plate. J. Biosci. Bioeng. 2021, 131, 390–395. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, D.T.; Lee, S.B.; Taylor, J.W.; Sninsky, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacterial. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Lu, Y.Z.; Boonmee, S.; Dai, D.Q.; Liu, J.K.; Hyde, K.D.; Bhat, D.J.; Kang, J.C. Four new species of Tubeufia (Tubeufiaceae, Tubeufiales) from Thailand. Mycol. Prog. 2017, 16, 403–417. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In Nucleic Acids Symposium Series; Oxford Academic: Oxford, UK, 1999; Volume 41, pp. 95–98. [Google Scholar]
- Senanayake, I.C.; Lian, T.T.; Mai, X.M.; Camporesi, E.; Zeng, Y.J.; Tian, S.L.; Xie, N. Taxonomy and phylogeny of Amphisphaeria acericola sp. nov. from Italy. Phytotaxa 2019, 403, 285–292. [Google Scholar] [CrossRef]
- Samarakoon, M.C.; Maharachchikumbura, S.S.; Liu, J.K.; Hyde, K.D.; Promputtha, I.; Stadler, M. Molecular phylogeny and morphology of Amphisphaeria (=Lepteutypa) (Amphisphaeriaceae). J. Fungi 2020, 6, 174. [Google Scholar] [CrossRef] [PubMed]
- Samarakoon, M.C.; Hyde, K.D.; Maharachchikumbura, S.S.; Stadler, M.; Gareth Jones, E.B.; Promputtha, I.; Liu, J.K. Taxonomy, phylogeny, molecular dating and ancestral state reconstruction of Xylariomycetidae (Sordariomycetes). Fungal Divers. 2022, 112, 1–88. [Google Scholar] [CrossRef]
- Dissanayake, L.S.; Samarakoon, M.C.; Mortimer, P.E.; Lu, Y.Z.; Li, Q.R.; Hyde, K.D.; Kang, J.C. Morpho-molecular characterization of two novel amphisphaeriaceous species from Yunnan, China. Phytotaxa 2020, 446, 144–158. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Nuin, P. MrMTgui. v 1.0. MrModelTest/ModelTest Graphical Interface for Windows/Linux. 2007. Available online: http://www.genedrift.org/mtgui.php (accessed on 8 May 2023).
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A. FigTree: Tree Figure Drawing Tool, Version 1.2.2; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, UK, 2008. [Google Scholar]
- Shimodaira, H.; Hasegawa, M. CONSEL: For assessing the confidence of phylogenetic tree selection. Bioinformatics 2001, 17, 1246–1247. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Aptroot, A.; Hyde, K.D. Revision of the genus Amphisphaeria. Hong Kong SAR, China. Fungal Divers. Res. Ser. 2004, 13, 1–168. [Google Scholar]
- Pracht, P.; Bohle, F.; Grimme, S. Automated Exploration of the Low-Energy Chemical Space with Fast Quantum Chemical Methods. Phys. Chem. Chem. Phys. 2022, 22, 7169–7192. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Chen, Y.; Liu, Z.; Tan, H.; Zhang, W. Pyrone and isocoumarin derivatives from the endophytic fungus Cytospora rhizophorae. Org. Biomol. Chem. 2022, 20, 4900–4904. [Google Scholar] [CrossRef]
- Krohn, K.; Flörke, U.; Rao, M.S.; Steingröver, K.; Aust, H.J.; Draeger, S.; Schulz, B. Metabolites from fungi 15. New isocoumarins from an endophytic fungus isolated from the Canadian thistle Cirsium arvense. Nat. Prod. Lett. 2001, 15, 353–361. [Google Scholar] [CrossRef]
- Mulholland, D.A.; Cheplogoi, P.; Crouch, N.R. Secondary metabolites from Kirkia acuminata and Kirkia wilmsii (Kirkiaceae). Biochem. Syst. Ecol. 2003, 7, 793–797. [Google Scholar] [CrossRef]
- Cardoso-Lopes, E.M.; Maier, J.A.; Da Silva, M.R.; Regasini, L.O.; Simote, S.Y.; Lopes, N.P.; Young, M.C.M. Alkaloids from stems of Esenbeckia leiocarpa Engl. (Rutaceae) as potential treatment for Alzheimer disease. Molecules 2010, 15, 9205–9213. [Google Scholar] [CrossRef] [PubMed]
- Sriphana, U.; Thongsri, Y.; Prariyachatigul, C.; Pakawatchai, C.; Yenjai, C. Clauraila E from the roots of Clausena harmandiana and antifungal activity against Pythium insidiosum. Arch. Pharmacal Res. 2013, 36, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Rüegger, A.; Stauffacher, D. Lunidin und Lunidonin, zwei neue Alkaloide aus Lunasia amara BLANCOvar. repanda (LAUTERB. et K. SCHUM.) LAUTERB. Helv. Chim. Acta. 1963, 46, 2329–2336. [Google Scholar] [CrossRef]
- Donnelly, W.J.; Grundon, M.F. Quinoline alkaloids. Part XII. Alkaloids and coumarins of Orixa japonica thunb. Identification of a new quinoline alkaloid, orixinone. J. Chem. Soc. Perkin Trans. 1 1972, 2116–2118. [Google Scholar] [CrossRef]
- Ayafor, J.F.; Sondengam, B.L.; Ngadjui, B. Veprisine and N-methylpreskimmianine: Novel 2-quinolones from Vepris louisii. Tetrahedron Lett. 1980, 21, 3293–3294. [Google Scholar] [CrossRef]
- Rollinger, J.M.; Schuster, D.; Danzl, B.; Schwaiger, S.; Markt, P.; Schmidtke, M.; Stuppner, H. In silico target fishing for rationalized ligand discovery exemplified on constituents of Ruta graveolens. Planta Med. 2009, 75, 195–204. [Google Scholar] [CrossRef]
- Parhoodeh, P.; Rahmani, M.; Mohd, N.; Mohd, H.; Sukari, A.; Lian Ee, G.C. Alkoloid constituents of Haplophyllum laeviusculum (Rutaceae). Sains Malays. 2012, 41, 47–52. [Google Scholar]
- Puricelli, L.; Innocenti, G.; Monache, G.D.; Caniato, R.; Filippini, R.; Cappelletti, E.M. In vivo and in vitro production of alkaloids by Haplophyllum patavinum. Nat. Prod. Lett. 2002, 16, 95–100. [Google Scholar] [CrossRef]
- Yang, Z.D.; Zhang, D.B.; Ren, J.; Yang, M.J. Skimmianine, a furoquinoline alkaloid from Zanthoxylum nitidum as a potential acetylcholinesterase inhibitor. Med. Chem. Res. 2012, 21, 722–725. [Google Scholar] [CrossRef]
- Ito, C.; Itoigawa, M.; Furukawa, A.; Hirano, T.; Murata, T.; Kaneda, N.; Hisada, Y.; Okuda, K.; Furukawa, H. Quinolone alkaloids with nitric oxide Production inhibitory activity from Orixa japonica. J. Nat. Prod. 2004, 67, 1800–1803. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liang, W.; Zhang, Z.; Wang, Y.; Zhang, S.; Liu, J.; Chang, J.; Ji, C.; Zhu, D. Polyketide Derivatives from the Endophytic Fungus Phaeosphaeria sp. LF5 Isolated from Huperzia serrata and Their Acetylcholinesterase Inhibitory Activities. J. Fungi 2022, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-C. Bioactive Consitituents Isolated from Orixa japonica and Toddalia asiatica. Ph.D. Thesis, China Agricultural University, Beijing, China, 2017. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).