Metabolic Engineering of Model Microorganisms for the Production of Xanthophyll
Abstract
1. Introduction
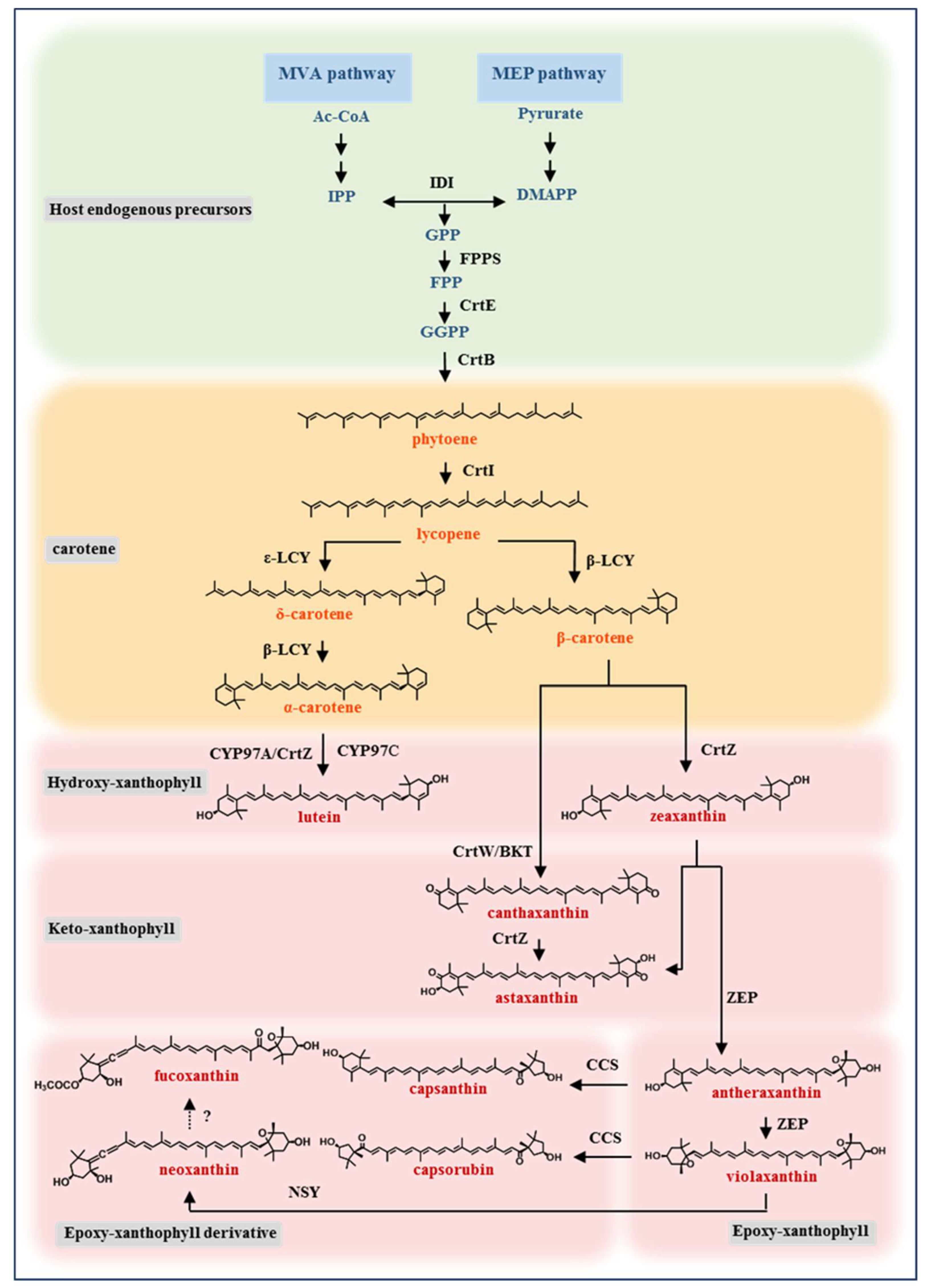
2. Heterologous Synthesis of Xanthophyll in Model Microorganisms
2.1. General Strategy for the Xanthophyll Production by Metabolic Engineering of Model Microorganisms
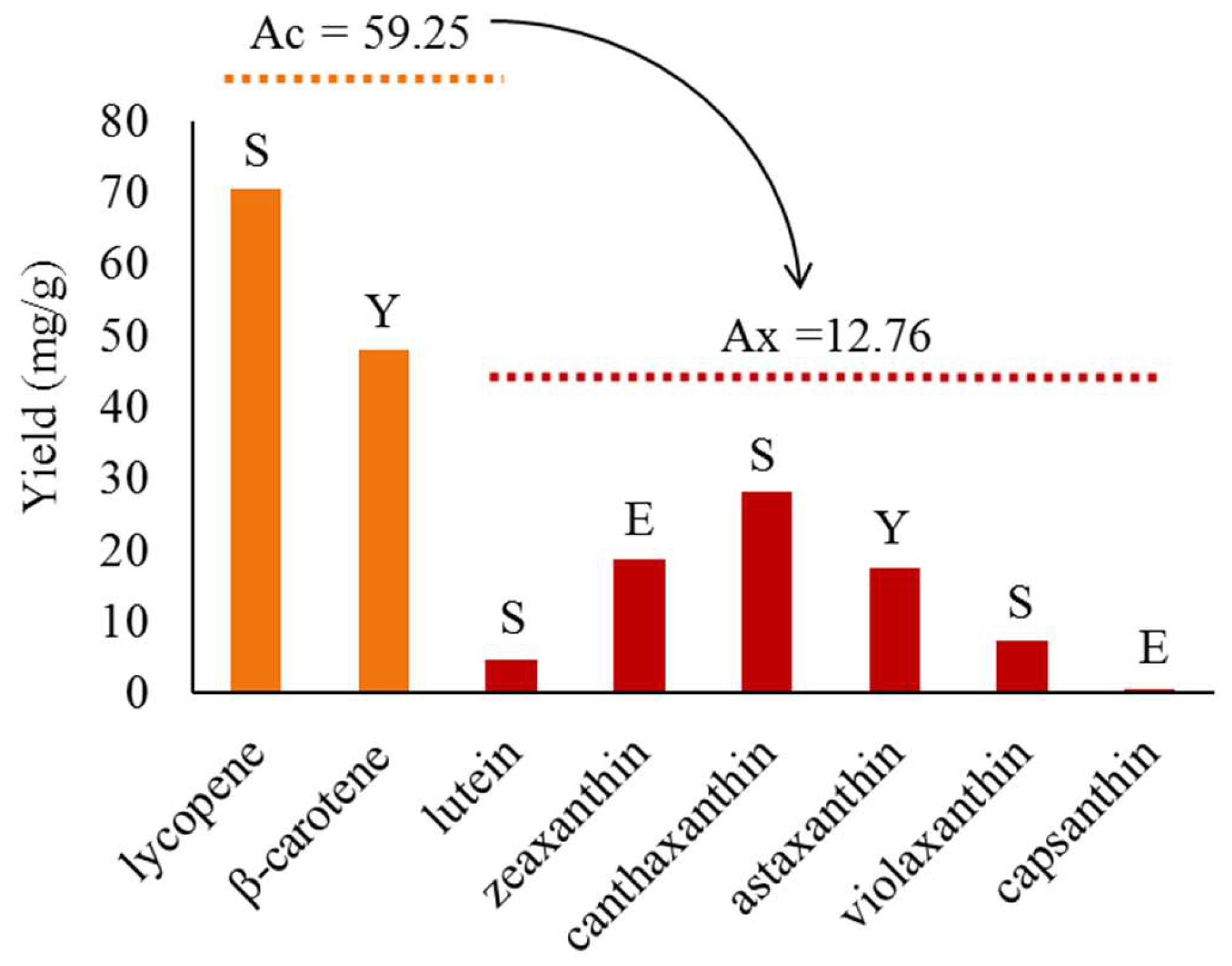
2.2. Progress in Metabolic Engineering Synthesis of Various Xanthophylls
2.2.1. Hydroxy-Xanthophyll
2.2.2. Keto-Xanthophyll
2.2.3. Epoxy-Xanthophyll
2.2.4. Epoxy-Xanthophyll Derivative
| Xanthophyll | Engineering Microbial Hosts | Key Enzymes | Natural Origin Species | Key Expression Cassettes b | Methods or Principles of Host Transformation | Key Strategies | Yield (mg/g DCW) | Titer (mg/L) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| lutein | E. coli | ε-LCY | M. polymorpha | Ptac-IDI-CrtE-CrtB-CrtI-MpLCYb-MpLCYe-CrtZ-TrrnB and PT7-MpCYP97C-TT7- PT7-MpLCYe- TT7 | Electroporation | Selection of ε-LCY and CYP97C from different species, decreasing the activity of β-LCY and increasing the copy number of ε-LCY gene | 2.6 | [56] | |
| β-LCY | M. polymorpha | ||||||||
| CYP97C | M. polymorpha | ||||||||
| CrtZ | P. ananatis | ||||||||
| S. cerevisiae | ε-LCY | A. thaliana | PTER1-tHMG1-TCYC1, PPGK1-CrtE03M-TADH1, PPGK1-CrtYB11M-TADH1, PTEF-CrtI-TCYC1, PACT1-Gal4M9-TADH1, PGAL1-CrtYB-TCYC1, TCYC1-CYP97A3-PGAL1-PGAL10-LUT1-TADH1-TPGK1-FD3-PGAL2-PGAL7-RFNR1-TTPS1, PTEF1-PMSeV-C-At-LCYE-TCYC1, PGAL1-CYP97A3-TCYC1 | Chemical transformation | Selection of ε-LCY from different species, regulation of ratios of CYP97A3 and RFNR1/FD3, and hierarchical dynamic regulation based on the temperature-responsive promoter | 4.53 | 19.92 | [58] | |
| CrtYB | X. dendrorhous | ||||||||
| CYP97A3 | A. thaliana | ||||||||
| Lut1 | A. thaliana | ||||||||
| RFNR1 | A. thaliana | ||||||||
| FD3 | A.s thaliana | ||||||||
| zeaxanthin | E. coli | CrtZ | P. ananatis | PT5-CrtEIBipi-TTR, P37-CrtY- 2CrtZ-TrrnB, pZSPIA44-MevTTIGR-MevBTIGR IS-2 | Electroporation | Introduction and dynamic control of the MVA pathway of S. cerevisiae to increase the precursors supply and prevent the accumulation of toxic metabolites | 18.7 | 58.05 | [46] |
| S. cerevisiae | CrtZ | P. ananatis | PPDC1-CrtE-TPDC1, PTPI1-CrtB-TTPI1, PGPM1-CrtI-TGPM1, PGPD-CrtY-TGPD, PFBA1-CrtZ-TFBA1 | Chemical transformation | Zeaxanthin as a reporter gene for identification of promoter strength | 0.74 | [51] | ||
| Y. Lipolytica | CrtZ | P. ananatis | PTEF1N-CrtE-Txpr2, PTEF1N-CrtB-Txpr2, PTEF1N-CrtI-Txpr2, PTEF1N-CarRP-Txpr2, PTEF1N-CrtZ-Txpr2 | Frozen-EZ Yeast Transformation II Kit | High-copy-number integration of CrtZ gene into ribosomal DNA region | 21.98 in YPD medium (3.2 in YNB medium) | [50] | ||
| astaxanthin | E. coli | CrtZ | P. ananatis | PTM2-CrtEBIA, PT7-RLZ-CrtZ-RLW-CrtW | Screening and regulation of promoters and RBSs | 15.1 | 62 | [75] | |
| CrtW | Brevundimonas sp. SD212 | ||||||||
| S. cerevisiae | CrtZ | A. aurantiacum | A high β-carotene producing strain with PFBA1-CrtZ-TADH1, PTDH3-CrtW-TTDH2 | Homologous recombination | Selection and optimization of combinations of CrtW and CrtZ from different species | 6.05 | [76] | ||
| CrtW | Alcaligenes sp. | ||||||||
| Y. Lipolytica | CrtZ | H. pluvialis | PTEF-carRP-TXPR2, PTEF-thmgR-TXPR2, PTEF-GGS1-TXPR2, PTEF-carB-TXPR2, PTEF-CrtW-linker-RIDD-TXPR2-PTER-CrtZ-linker-RIAD-TXPR2 | Chemical transformation | Selection of CrtW and CrtZ from different species and fine-tuning their transcription | 17.5 | [60] | ||
| BKT | H. pluvialis | ||||||||
| P. pastoris | CrtZ | PAOX1-CrtI-TCYC1, PAOX1-CrtE- CrtZ-TCYC1, PAOX1-CrtYB-CrtW-TCYC1 | CRISPR/Cas9 | Astaxanthin as a reporter gene for marker-less integration of multigene pathways into Pichia pastoris via CRISPR/Cas9 | Approximately 2.5 | [66] | |||
| CrtW | |||||||||
| K. marxianus | CrtZ | H. pluvialis | PKlLac4-CrtZ-TKlLac4, PScGapDH-CrtE-TScGap, PScPGK-CrtZ-TScPGK, PKlGapDH-kanMX-TScGap, PICL-CrtI-T35S, PKlPGK-BKT-TScPGK, PKlADH1-CrtYB-TScGap, PScADH1-tHMG-TScADH1 | Homologous recombination | Increasing the copy number of Hpchyb and BKT genes and modifying the Hpchyb by site-directed mutagenesis | 3.125 in YPL medium, 5.701 in YPG medium | [64] | ||
| BKT | C. reinhardtii | ||||||||
| C. glutamicum | CrtZ | F. pelagi | Ptuf-CrtZ-linker-CrtW | electroporation with xenogeneic plasmid DNA | Fusion expression of CrtZ and CrtW, increasing the expression of upstream enzymes, mediated medium composition | 3.1 | [68] | ||
| CrtW | B. aurantiaca | ||||||||
| canthaxanthin | E. coli | BKT | Anabaena variabilis | PTrc-CrtW | Electroporation | Overexpression of host genes increases the carbon flux into the canthaxanthin biosynthetic pathway | Approximately 10.65 | 24.84 | [87] |
| S. cerevisiae | OBKTM29 (mutant BKT) | H. pluvialis | PGAL1-mBKT-TCYC1-PGAL10-CrtE03-TADH2, TCYC1-PMSeV-C-mBKT-PGAL1-PGAL10-CrtYB-TADH2, TCYC1-PDR3-PGAL1-PGAL10-CrtYB-TADH2 | Homologous recombination and CRISPR/cas9 | Subcellular re-localization of OBKTM29 and its copy number adjustment both in the cytoplasm and on the periplasmic membrane, pleiotropic drug resistance (PDR) regulator overexpression | approximately 20–30 | 168 | [88] | |
| violaxanthin | E. coli | CrtZ | P. ananatis | Plac-CrtE-CrtY-CrtI-CrtB-CrtZ, Plac-ZEP, PT7-gdh | Selection of ZEP from different species and optimization of E. coli strain, expression vector, and ribosome-binding site (RBS) sequence | 0.231 | [95] | ||
| ZEP | C. annuum | ||||||||
| glucose dehydrogenase (gdh) | B. subtilis | ||||||||
| S. cerevisiae | CrtZ | P. ananatis | PTDH3-CrtYB-TCYC1, PTDH3-CrtI-TCYC1, PTDH3-CrtE-TCYC1, PTEF1-CrtZ-linker-trZEP-TADH1, PTEF1-trRFNR1-TADH1, PPGK1-trFD3-TCYC1 | Modified homologous recombination | Selection of CrtZ, ZEP and redox partner from different species and their truncated variants, increasing gene copy number of upstream carotenogenic genes | 7.3 | [96] | ||
| ZEP | H. lacustris | ||||||||
| RFNR1 | A. thaliana | ||||||||
| FD3 | A. thaliana | ||||||||
| capsanthin | E. coli | CrtZ | P. ananatis | Plac-HpIDI-CrtE-CrtY-CrtI-CrtB-CrtZ, Ptac/T7-CCSM40-CaZEP | A particularly high expression of CCS | 0.5 | [97] | ||
| ZEP | C. annuum | ||||||||
| CCS | C. annuum |
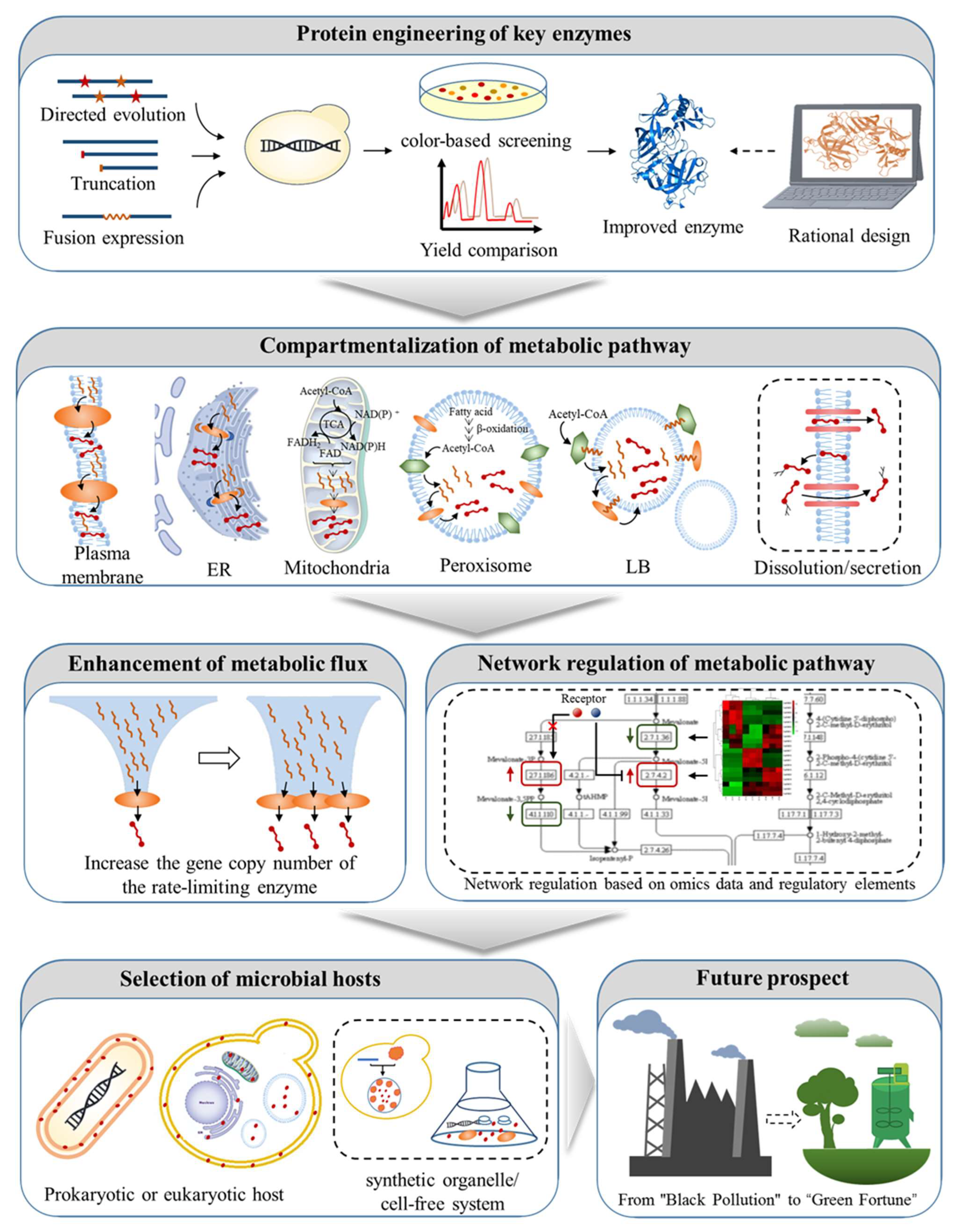
3. Metabolic Engineering Strategies of Model Microorganisms for Xanthophyll Synthesis
3.1. Protein Engineering of Key Enzymes
3.1.1. Directed Evolution of Key Enzymes
3.1.2. Truncation of Transit Peptides
3.1.3. Fusion Expression of Key Enzymes
3.2. Compartmentalization of Metabolic Pathway
3.3. Enhanced Metabolic Flux of Xanthophyll Synthetic Pathway
3.4. Network Regulation of Metabolic Pathway
3.5. Selection of Microbial Hosts
3.5.1. E. coli
3.5.2. S. cerevisiae
3.5.3. P. pastoris
3.5.4. Y. lipolytica
| Key Enzymes | Natural Origin Species | Mutation Sites | Engineering Microbial Hosts | Effect on Activity | Ref. |
|---|---|---|---|---|---|
| ε-LCY | Tagetes erecta | F61N | S. cerevisiae | Enhanced δ-carotene formation by approximately 120% | [57] |
| S401P | Decreased the activity | ||||
| CrtZ | H. pluvialis Flotow N-212 | L288R | S. cerevisiae | Increased the astaxanthin production by ∼33% | [80] |
| B. vesicularis | L53P | Methylomonas sp. 16a | Increased the astaxanthin production | [167] | |
| B. vesicularis | F91S/V140G | Methylomonas sp. 16a | Increased the astaxanthin production | [167] | |
| BKT | H. pluvialis Flotow N-212 | H165R/V264D/F298Y | S. cerevisiae | The canthaxanthin yield was increased approximately 2.4 folds | [81] |
| CrtW | Brevundimonas sp. SD212 | A6T/T105A/L239M | E. colli | Improved astaxanthin production 5.35-fold | [104] |
| S. melonis | F213L/R203W | E. colli | Improved the activity for converting cyclic hydroxylated intermediates into astaxanthin | [168] b | |
| A215T | |||||
| A205V | |||||
| A208V | |||||
| H96L | |||||
| CrtO a | R. erythropolis | A190V | Methylomonas sp. 16a | Increased the astaxanthin production | [167] |
| Key Enzymes | Natural Origin Species | Truncated Length of N-Terminal (Amino Acid) | Engineering Microbial Hosts | Ref. |
|---|---|---|---|---|
| ε-LCY | Marchantia polymorpha | 21 or 47 | S. cerevisiae | [58] |
| CYP97A3 | Arabidopsis | 28 or 49 | E. coli | [53] |
| CYP97C1 | Arabidopsis | 69 | E. coli | [53] |
| BKT | H. pluvialis Flotow N-212 | 7 | S. cerevisiae | [80] |
| ZEP | Haematococcus lacustris | 59 and <100 | S. cerevisiae | [96] |
| ZEP | Arabidopsis thaliana | 57 and <100 | ||
| ZEP | Solanum lycopersicum | 75 and <100 | ||
| CrtZ | Haematococcus lacustris | 69 | ||
| CrtZ | Solanum lycopersicum | 57 |
| Subcellular Organelle | Locating Signal | Engineering Microbial Hosts | Ref. |
|---|---|---|---|
| Endoplasmic reticulum | KDEL | Y. lipolytica | [84,169] |
| Mitochondria | MTS | S. cerevisiae | [121,170] |
| Peroxisome | PTS1 | Y. lipolytica, P. pastoris | [84,169,171] |
| Enhanced PTS1 | S. cerevisiae | [124] | |
| Lipid body | oleosin | Y. lipolytica | [84,169] |
| Outer surface scaffold | S. cerevisiae | [172] | |
| Yeast cell membrane | PMSeV-C | S. cerevisiae | [57] |
| E. coli cell membrane | GlpF | E. coli | [71] |
| The signal peptide of OmpF and TrxA | E. coli | [114] |
4. Current Challenges and Future Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Aziz, E.; Batool, R.; Akhtar, W.; Rehman, S.; Shahzad, T.; Malik, A.; Shariati, M.A.; Laishevtcev, A.; Plygun, S.; Heydari, M.; et al. Xanthophyll: Health benefits and therapeutic insights. Life Sci. 2020, 240, 117104. [Google Scholar] [CrossRef] [PubMed]
- Esteban, R.; Moran, J.F.; Becerril, J.M.; García-Plazaola, J.I. Versatility of carotenoids: An integrated view on diversity, evolution, functional roles and environmental interactions. Environ. Exp. Bot. 2015, 119, 63–75. [Google Scholar] [CrossRef]
- Sun, T.; Yuan, H.; Cao, H.; Yazdani, M.; Tadmor, Y.; Li, L. Carotenoid Metabolism in Plants: The Role of Plastids. Mol. Plant 2018, 11, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Jahns, P.; Holzwarth, A.R. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta 2012, 1817, 182–193. [Google Scholar] [CrossRef]
- Jackson, H.; Braun, C.L.; Ernst, H. The Chemistry of Novel Xanthophyll Carotenoids. Am. J. Cardiol. 2008, 101, 50D–57D. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Wiegertjes, G. Properties of Carotenoids in Fish Fitness: A Review. Mar. Drugs 2020, 18, 568. [Google Scholar] [CrossRef]
- Pereira, A.G.; Otero, P.; Echave, J.; Casais, A.C.; Chamorro, F.; Collazo, N.; Jaboui, A.; Lopes, C.L.; Gandara, J.S.; Prieto, M.A. Xanthophylls from the Sea: Algae as Source of Bioactive Carotenoids. Mar. Drugs 2021, 19, 188. [Google Scholar] [CrossRef]
- Bhosale, P.; Bernstein, P.S. Microbial xanthophylls. Appl. Microbiol. Biotechnol. 2005, 68, 445–455. [Google Scholar] [CrossRef]
- Claudia, S.-D. Engineering novel carotenoids in microorganisms. Curr. Opin. Biotechnol. 2000, 11, 255–261. [Google Scholar]
- Patel, A.K.; Tambat, V.S.; Chen, C.-W.; Chauhan, A.S.; Kumar, P.; Vadrale, A.P.; Huang, C.-Y.; Dong, C.-D.; Singhania, R.R. Recent advancements in astaxanthin production from microalgae: A review. Bioresour. Technol. 2022, 364, 128030. [Google Scholar] [CrossRef]
- Ma, R.; Tao, X.; Chua, E.T.; Ho, S.-H.; Shi, X.; Liu, L.; Xie, Y.; Chen, J. Enhancing astaxanthin production in Haematococcus pluvialis QLD by a pH steady NaHCO3-CO2-C/NH4Cl-N culture system. Algal Res. 2022, 64, 102697. [Google Scholar] [CrossRef]
- Yi, X.; Alper, H.S. Considering Strain Variation and Non-Type Strains for Yeast Metabolic Engineering Applications. Life 2022, 12, 510. [Google Scholar] [CrossRef]
- Ding, Q.; Diao, W.; Gao, C.; Chen, X.; Liu, L. Microbial cell engineering to improve cellular synthetic capacity. Biotechnol. Adv. 2020, 45, 107649. [Google Scholar] [CrossRef]
- Nielsen, J.; Keasling, J.D. Engineering Cellular Metabolism. Cell 2016, 164, 1185–1197. [Google Scholar] [CrossRef]
- Pu, X.; Dong, X.; Li, Q.; Chen, Z.; Liu, L. An update on the function and regulation of methylerythritol phosphate and mevalonate pathways and their evolutionary dynamics. J. Integr. Plant Biol. 2021, 63, 1211–1226. [Google Scholar] [CrossRef]
- Niu, F.; Lu, Q.; Bu, Y.; Liu, J. Metabolic engineering for the microbial production of isoprenoids: Carotenoids and isoprenoid-based biofuels. Synth. Syst. Biotechnol. 2017, 2, 167–175. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, S.; Shao, X.; Park, J.-B.; Jeong, S.-H.; Park, H.-J.; Kwak, W.-J.; Wei, G.; Kim, S.-W. Challenges and tackles in metabolic engineering for microbial production of carotenoids. Microb. Cell Fact. 2019, 18, 55. [Google Scholar] [CrossRef]
- Jing, Y.; Wang, Y.; Zhou, D.; Wang, J.; Li, J.; Sun, J.; Feng, Y.; Xin, F.; Zhang, W. Advances in the synthesis of three typical tetraterpenoids including β-carotene, lycopene and astaxanthin. Biotechnol. Adv. 2022, 61, 108033. [Google Scholar] [CrossRef]
- Jing, Y.; Guo, F.; Zhang, S.; Dong, W.; Zhou, J.; Xin, F.; Zhang, W.; Jiang, M. Recent advances on biological synthesis of lycopene by using industrial yeast. Ind. Eng. Chem. Res. 2021, 60, 3485–3494. [Google Scholar] [CrossRef]
- Zafar, J.; Aqeel, A.; Shah, F.I.; Ehsan, N.; Gohar, U.F.; Moga, M.A.; Festila, D.; Ciurea, C.; Irimie, M.; Chicea, R. Biochemical and Immunological implications of Lutein and Zeaxanthin. Int. J. Mol. Sci. 2021, 22, 10910. [Google Scholar] [CrossRef]
- Gupta, I.; Adin, S.N.; Panda, B.P.; Mujeeb, M. β-Carotene-production methods, biosynthesis from Phaffia rhodozyma, factors affecting its production during fermentation, pharmacological properties: A review. Biotechnol. Appl. Biochem. 2022, 69, 2517–2529. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Sun, J.; Xue, C.; Mao, X. Biotechnological production of zeaxanthin by microorganisms. Trends Food Sci. Technol. 2018, 71, 225–234. [Google Scholar] [CrossRef]
- Basiony, M.; Ouyang, L.; Wang, D.; Yu, J.; Zhou, L.; Zhu, M.; Wang, X.; Feng, J.; Dai, J.; Shen, Y.; et al. Optimization of microbial cell factories for astaxanthin production: Biosynthesis and regulations, engineering strategies and fermentation optimization strategies. Synth. Syst. Biotechnol. 2022, 7, 689–704. [Google Scholar] [CrossRef]
- Wang, D.-N.; Feng, J.; Yu, C.-X.; Zhang, X.-K.; Chen, J.; Wei, L.-J.; Liu, Z.; Ouyang, L.; Zhang, L.; Hua, Q.; et al. Integrated pathway engineering and transcriptome analysis for improved astaxanthin biosynthesis in Yarrowia lipolytica. Synth. Syst. Biotechnol. 2022, 7, 1133–1141. [Google Scholar] [CrossRef]
- Rebelo, B.A.; Farrona, S.; Ventura, M.R.; Abranches, R. Canthaxanthin, a Red-Hot Carotenoid: Applications, Synthe-sis, and Biosynthetic Evolution. Plants 2020, 9, 1039. [Google Scholar] [CrossRef] [PubMed]
- Stachowiak, B.; Szulc, P. Astaxanthin for the food industry. Molecules 2021, 26, 2666. [Google Scholar] [CrossRef]
- Takemura, M.; Sahara, T.; Misawa, N. Violaxanthin: Natural function and occurrence, biosynthesis, and heterologous production. Appl. Microbiol. Biotechnol. 2021, 105, 6133–6142. [Google Scholar] [CrossRef]
- Bouvier, F.; Hugueney, P.; d’Harlingue, A.; Kuntz, M.; Camara, B. Xanthophyll biosynthesis in chromoplasts: Isolation and molecular cloning of an enzyme catalyzing the conversion of 5,6-epoxycarotenoid into ketocarotenoid. Plant J. 1994, 6, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Jeknić, Z.; Morré, J.T.; Jeknić, S.; Jevremović, S.; Subotić, A.; Chen, T.H.H. Cloning and functional characterization of a gene for capsanthin-capsorubin synthase from tiger lily (Lilium lancifolium Thunb. ‘Splendens’). Plant Cell Physiol. 2012, 53, 1899–1912. [Google Scholar] [CrossRef]
- Bouvier, F.; D’Harlingue, A.; Backhaus, R.A.; Kumagai, M.H.; Camara, B. Identification of neoxanthin synthase as a carotenoid cyclase paralog. Eur. J. Biochem. 2000, 267, 6346–6352. [Google Scholar] [CrossRef]
- North, H.M.; Almeida, A.D.; Boutin, J.-P.; Frey, A.; To, A.; Botran, L.; Sotta, B.; Marion-Poll, A. The Arabidopsis ABA-deficient mutant aba4 demonstrates that the major route for stress-induced ABA accumulation is via neoxanthin isomers. Plant J. 2007, 50, 810–824. [Google Scholar] [CrossRef] [PubMed]
- Jian, Y.; Zhang, C.; Wang, Y.; Li, Z.; Chen, J.; Zhou, W.; Huang, W.; Jiang, M.; Zheng, H.; Li, M.; et al. Characterization of the role of the neoxanthin synthase gene BoaNXS in carotenoid biosynthesis in Chinese Kale. Genes 2021, 12, 1122. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Tiwari, A.; Srivastava, S. A Genome-Scale Metabolic Model of Thalassiosira pseudonana CCMP 1335 for a systems-level understanding of its metabolism and biotechnological potential. Microorganisms 2020, 8, 1396. [Google Scholar] [CrossRef] [PubMed]
- Dambek, M.; Eilers, U.; Breitenbach, J.; Steiger, S.; Büchel, C.; Sandmann, G. Biosynthesis of fucoxanthin and diadinoxanthin and function of initial pathway genes in Phaeodactylum tricornutum. J. Exp. Bot. 2012, 63, 5607–5612. [Google Scholar] [CrossRef]
- Chen, D.; Yuan, X.; Zheng, X.; Fang, J.; Lin, G.; Li, R.; Chen, J.; He, W.; Huang, Z.; Fan, W.; et al. Multi-omics analyses provide insight into the biosynthesis pathways of fucoxanthin in Isochrysis galbana. Genom. Proteom. Bioinform. 2022. [Google Scholar] [CrossRef]
- Gómez-García, M.R.; Ochoa-Alejo, N. Biochemistry and molecular biology of carotenoid biosynthesis in chili peppers (Capsicum spp.). Int. J. Mol. Sci. 2013, 14, 19025–19053. [Google Scholar] [CrossRef]
- Palozza, P.; Krinsky, N.I. Astaxanthin and canthaxanthin are potent antioxidants in a membrane model. Arch. Biochem. Biophys. 1992, 297, 291–295. [Google Scholar] [CrossRef]
- Kobayashi, M. In vivo antioxidant role of astaxanthin under oxidative stress in the green alga Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 2000, 54, 550–555. [Google Scholar] [CrossRef]
- Kennedy, L.E.; Abraham, A.; Kulkarni, G.; Shettigar, N.; Dave, T.; Kulkarni, M. Capsanthin, a plant-derived xanthophyll: A review of pharmacology and delivery strategies. AAPS PharmSciTech 2021, 22, 203. [Google Scholar] [CrossRef]
- Larroude, M.; Celinska, E.; Back, A.; Thomas, S.; Nicaud, J.-M.; Ledesma-Amaro, R. A synthetic biology approach to transform Yarrowia lipolytica into a competitive biotechnological producer of β-carotene. Biotechnol. Bioeng. 2018, 115, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Shi, B.; Ye, Z.; Li, X.; Liu, M.; Chen, Y.; Xia, J.; Nielsen, J.; Deng, Z.; Liu, T. Lipid engineering combined with systematic metabolic engineering of Saccharomyces cerevisiae for high-yield production of lycopene. Metab. Eng. 2019, 52, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.J.; Sampson, J.; Candeias, L.P.; Bramley, P.M.; Rice-Evans, C.A. Antioxidant activities of carotenes and xanthophylls. FEBS Lett. 1996, 384, 240–242. [Google Scholar] [CrossRef] [PubMed]
- Doshi, R.; Nguyen, T.; Chang, G. Transporter-mediated biofuel secretion. Proc. Natl. Acad. Sci. USA 2013, 110, 7642–7647. [Google Scholar] [CrossRef]
- Pyne, M.E.; Narcross, L.; Martin, V.J.J. Engineering plant secondary metabolism in microbial systems. Plant Physiol. 2019, 179, 844–861. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.-J.; Cheng, B.-Y.; Zhang, Y.-M.; Tang, L.; Li, Z.; Bu, Y.-F.; Li, X.-R.; Tian, G.-Q.; Liu, J.-Z. Dynamic control of the mevalonate pathway expression for improved zeaxanthin production in Escherichia coli and comparative proteome analysis. Metab. Eng. 2016, 38, 180–190. [Google Scholar] [CrossRef]
- Nishizaki, T.; Tsuge, K.; Itaya, M.; Doi, N.; Yanagawa, H. Metabolic engineering of carotenoid biosynthesis in Escherichia coli by ordered gene assembly in Bacillus subtilis. Appl. Environ. Microbiol. 2007, 73, 1355–1361. [Google Scholar] [CrossRef]
- Li, X.-R.; Tian, G.-Q.; Shen, H.-J.; Liu, J.-Z. Metabolic engineering of Escherichia coli to produce zeaxanthin. J. Ind. Microbiol. Biotechnol. 2015, 42, 627–636. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, D.; Li, S.; Wang, J.; Bi, C.; Zhang, X. Combinatorial modulation of initial codons for improved zeaxanthin synthetic pathway efficiency in Escherichia coli. Microbiologyopen 2019, 8, e930. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, S.; Xiong, X. Metabolic Engineering of non-carotenoid-producing yeast Yarrowia lipolytica for the biosynthesis of zeaxanthin. Front. Microbiol. 2021, 12, 699235. [Google Scholar] [CrossRef]
- Sun, J.; Shao, Z.; Zhao, H.; Nair, N.; Wen, F.; Xu, J.-H.; Zhao, H. Cloning and characterization of a panel of constitutive promoters for applications in pathway engineering in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2012, 109, 2082–2092. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Ning, J.C.; Zhao, H. Coordinated induction of multi-gene pathways in Saccharomyces cerevisiae. Nucleic Acids Res. 2013, 41, e54. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Guo, Q.; Wang, J.; Zhao, S.; He, Y.; Liu, L. Structural basis for plant lutein biosynthesis from α-carotene. Proc. Natl. Acad. Sci. USA 2020, 117, 14150–14157. [Google Scholar] [CrossRef] [PubMed]
- Hannemann, F.; Bichet, A.; Ewen, K.M.; Bernhardt, R. Cytochrome P450 systems--biological variations of electron transport chains. Biochim. Biophys. Acta 2007, 1770, 330–344. [Google Scholar] [CrossRef]
- Iyanagi, T.; Xia, C.; Kim, J.-J.P. NADPH-cytochrome P450 oxidoreductase: Prototypic member of the diflavin reductase family. Arch. Biochem. Biophys. 2012, 528, 72–89. [Google Scholar] [CrossRef]
- Takemura, M.; Kubo, A.; Watanabe, A.; Sakuno, H.; Minobe, Y.; Sahara, T.; Murata, M.; Araki, M.; Harada, H.; Terada, Y.; et al. Pathway engineering for high-yield production of lutein in Escherichia coli. Synth. Biol. 2021, 6, ysab012. [Google Scholar] [CrossRef]
- Bian, Q.; Zhou, P.; Yao, Z.; Li, M.; Yu, H.; Ye, L. Heterologous biosynthesis of lutein in S. cerevisiae enabled by temporospatial pathway control. Metab. Eng. 2021, 67, 19–28. [Google Scholar] [CrossRef]
- Bian, Q.; Jiao, X.; Chen, Y.; Yu, H.; Ye, L. Hierarchical dynamic regulation of Saccharomyces cerevisiae for enhanced lutein biosynthesis. Biotechnol. Bioeng. 2023, 120, 536–552. [Google Scholar] [CrossRef]
- Takemura, M.; Maoka, T.; Misawa, N. Biosynthetic routes of hydroxylated carotenoids (xanthophylls) in Marchantia polymorpha, and production of novel and rare xanthophylls through pathway engineering in Escherichia coli. Planta 2015, 241, 699–710. [Google Scholar] [CrossRef]
- Zhu, H.Z.; Jiang, S.; Wu, J.J.; Zhou, X.R.; Liu, P.Y.; Huang, F.H.; Wan, X. Production of high levels of 3 S,3’ S-astaxanthin in Yarrowia lipolytica via iterative metabolic engineering. J. Agric. Food Chem. 2022, 70, 2673–2683. [Google Scholar] [CrossRef]
- Le-Feuvre, R.; Moraga-Suazo, P.; Gonzalez, J.; Martin, S.S.; Henríquez, V.; Donoso, A.; Muñoz, C.A. Biotechnology applied to Haematococcus pluvialis Fotow: Challenges and prospects for the enhancement of astaxanthin accumulation. J. Appl. Phycol. 2020, 32, 3831–3852. [Google Scholar] [CrossRef]
- Zhu, X.; Meng, C.; Sun, F.; Wei, Z.; Chen, L.; Chen, W.; Tong, S.; Du, H.; Gao, J.; Ren, J.; et al. Sustainable production of astaxanthin in microorganisms: The past, present, and future. Crit. Rev. Food Sci. Nutr. 2022, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-J.; Thia, C.; Lin, H.-Y.; Liu, H.-L.; Ho, F.-J.; Wu, J.-T.; Shih, M.-C.; Li, W.-H.; Huang, C.-C. Integrating an algal β-carotene hydroxylase gene into a designed carotenoid-biosynthesis pathway increases carotenoid production in yeast. Bioresour. Technol. 2015, 184, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-J.; Chang, J.-J.; Lin, H.-Y.; Thia, C.; Kao, Y.-Y.; Huang, C.-C.; Li, W.-H. Metabolic engineering a yeast to produce astaxanthin. Bioresour. Technol. 2017, 245, 899–905. [Google Scholar] [CrossRef]
- Araya-Garay, J.M.; Ageitos, J.M.; Vallejo, A.J.; Veiga-Crespo, P.; Sánchez-Pérez, A.; Villa, T.G. Construction of a novel Pichia pastoris strain for production of xanthophylls. AMB Express 2012, 2, 24. [Google Scholar] [CrossRef]
- Gao, J.; Xu, J.; Zuo, Y.; Ye, C.; Jiang, L.; Feng, L.; Huang, L.; Xu, Z.; Lian, J. Synthetic biology toolkit for marker-less integration of multigene pathways into Pichia pastoris via CRISPR/Cas9. ACS Synth. Biol. 2022, 11, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Henke, N.A.; Heider, A.E.S.; Peters-Wendisch, P.; Wendisch, V.F. Production of the marine carotenoid astaxanthin by metabolically engineered Corynebacterium glutamicum. Mar. Drugs. 2016, 14, 124. [Google Scholar] [CrossRef]
- Henke, N.A.; Wendisch, V.F. Improved astaxanthin production with Corynebacterium glutamicum by application of a membrane fusion protein. Mar. Drugs 2019, 17, 621. [Google Scholar] [CrossRef]
- Zhang, M.; Gong, Z.; Tang, J.; Lu, F.; Li, Q.; Zhang, X. Improving astaxanthin production in Escherichia coli by co-utilizing CrtZ enzymes with different substrate preference. Microb. Cell Fact. 2022, 21, 71. [Google Scholar] [CrossRef]
- Nogueira, M.; Enfissi, M.A.E.; Welsch, R.; Beyer, P.; Zurbriggen, D.M.; Fraser, D.P. Construction of a fusion enzyme for astaxanthin formation and its characterisation in microbial and plant hosts: A new tool for engineering ketocarotenoids. Metab. Eng. 2019, 52, 243–252. [Google Scholar] [CrossRef]
- Gong, Z.; Wang, H.; Tang, J.; Bi, C.; Li, Q.; Zhang, X. Coordinated expression of astaxanthin biosynthesis genes for improved astaxanthin production in Escherichia coli. J. Agric. Food Chem. 2020, 68, 14917–14927. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhu, X.; Wu, T.; Wang, W.; Zhao, D.; Bi, C.; Zhang, X. Optimizing the localization of astaxanthin enzymes for improved productivity. Biotechnol. Biofuels. 2018, 11, 278. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Bu, Y.-F.; Liu, J.-Z. Metabolic Engineering of Escherichia coli for Producing astaxanthin as the predominant carotenoid. Mar. Drugs 2017, 15, 296. [Google Scholar] [CrossRef] [PubMed]
- Scaife, M.A.; Burja, A.M.; Wright, P.C. Characterization of cyanobacterial beta-carotene ketolase and hydroxylase genes in Escherichia coli, and their application for astaxanthin biosynthesis. Biotechnol. Bioeng. 2009, 103, 944–955. [Google Scholar] [CrossRef]
- Zhang, C.; Seow, V.Y.; Chen, X.; Too, H.-P. Multidimensional heuristic process for high-yield production of astaxanthin and fragrance molecules in Escherichia coli. Nat. Commun. 2018, 9, 1858. [Google Scholar] [CrossRef]
- Qi, D.-D.; Jin, J.; Liu, D.; Jia, B.; Yuan, Y.-J. In vitro and in vivo recombination of heterologous modules for improving biosynthesis of astaxanthin in yeast. Microb. Cell Fact. 2020, 19, 103. [Google Scholar] [CrossRef]
- Jiang, G.; Yang, Z.; Wang, Y.; Yao, M.; Chen, Y.; Xiao, W.; Yuan, Y. Enhanced astaxanthin production in yeast via combined mutagenesis and evolution. Biochem. Eng. J. 2020, 156, 107519. [Google Scholar] [CrossRef]
- Jin, J.; Wang, Y.; Yao, M.; Gu, X.; Li, B.; Liu, H.; Ding, M.; Xiao, W.; Yuan, Y. Astaxanthin overproduction in yeast by strain engineering and new gene target uncovering. Biotechnol. Biofuels. 2018, 11, 230. [Google Scholar] [CrossRef]
- Wang, R.; Gu, X.; Yao, M.; Pan, C.; Liu, H.; Xiao, W.; Wang, Y.; Yuan, Y. Engineering of β-carotene hydroxylase and ketolase for astaxanthin overproduction in Saccharomyces cerevisiae. Front. Chem. Sci. Eng. 2017, 11, 89–99. [Google Scholar] [CrossRef]
- Zhou, P.; Li, M.; Shen, B.; Yao, Z.; Bian, Q.; Ye, L.; Yu, H. Directed coevolution of β-carotene ketolase and hydroxylase and its application in temperature-regulated biosynthesis of astaxanthin. J. Agric. Food Chem. 2019, 67, 1072–1080. [Google Scholar] [CrossRef]
- Zhou, P.; Xie, W.; Li, A.; Wang, F.; Yao, Z.; Bian, Q.; Zhu, Y.; Yu, H.; Ye, L. Alleviation of metabolic bottleneck by combinatorial engineering enhanced astaxanthin synthesis in Saccharomyces cerevisiae. Enzym. Microb. Technol. 2017, 100, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Ye, L.; Xie, W.; Lv, X.; Yu, H. Highly efficient biosynthesis of astaxanthin in Saccharomyces cerevisiae by integration and tuning of algal crtZ and bkt. Appl. Microbiol. Biotechnol. 2015, 99, 8419–8428. [Google Scholar] [CrossRef] [PubMed]
- Ukibe, K.; Hashida, K.; Yoshida, N.; Takagi, H. Metabolic engineering of Saccharomyces cerevisiae for astaxanthin production and oxidative stress tolerance. Appl. Environ. Microbiol. 2009, 75, 7205–7211. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, J.; Huang, S.; Stephanopoulos, G. Targeting pathway expression to subcellular organelles improves astaxanthin synthesis in Yarrowia lipolytica. Metab. Eng. 2021, 68, 152–161. [Google Scholar] [CrossRef]
- Tramontin, L.R.R.; Kildegaard, K.R.; Sudarsan, S.; Borodina, I. Enhancement of astaxanthin biosynthesis in oleaginous Yeast Yarrowia lipolytica via microalgal pathway. Microorganisms 2019, 7, 472. [Google Scholar] [CrossRef]
- Kildegaard, K.R.; Pérez, B.A.; Belda, D.D.; Khangura, J.K.; Holkenbrink, C.; Borodina, I. Engineering of Yarrowia lipolytica for production of astaxanthin. Synth. Syst. Biotechnol. 2017, 2, 287–294. [Google Scholar] [CrossRef]
- Scaife, M.A.; Prince, C.A.; Norman, A.; Armenta, R.E. Progress toward an Escherichia coli canthaxanthin bioprocess. Process Biochem. 2012, 47, 2500–2509. [Google Scholar] [CrossRef]
- Chen, M.; Li, M.; Ye, L.; Yu, H. Construction of canthaxanthin-producing yeast by combining spatiotemporal regulation and pleiotropic drug resistance engineering. ACS Synth. Biol. 2022, 11, 325–333. [Google Scholar] [CrossRef]
- Marinn, E.; Nussaume, L.; Quesada, A.; Gonneau, M.; Sotta, B.; Hugueney, P.; Frey, A.; Poll, A.M. Molecular identification of zeaxanthin epoxidase of Nicotiana plumbaginifolia, a gene involved in abscisic acid biosynthesis and corresponding to the ABA locus of Arabidopsis thaliana. EMBO J. 1996, 15, 2331–2342. [Google Scholar] [CrossRef]
- Bouvier, F.; d’Harlingue, A.; Hugueney, P.; Marini, E.; Polli, A.M.; Camara, B. Xanthophyll biosynthesis. Cloning, expression, functional reconstitution, and regulation of beta-cyclohexenyl carotenoid epoxidase from pepper (Capsicum annuum). J. Biol. Chem. 1996, 271, 28861–28867. [Google Scholar] [CrossRef]
- Zhu, C.; Yamamura, S.; Nishihara, M.; Koiwa, H.; Sandmann, G. cDNAs for the synthesis of cyclic carotenoids in petals of Gentiana lutea and their regulation during flower development. Biochim. Biophys. Acta 2003, 1625, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Eilers, U.; Dietzel, L.; Breitenbach, J.; Büchel, C.; Sandmann, G. Identification of genes coding for functional zeaxanthin epoxidases in the diatom Phaeodactylum tricornutum. J. Plant Physiol. 2016, 192, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhou, Q.; Wu, Y.; Chen, X.; Zhong, F. Properties and mechanisms of flavin-dependent monooxygenases and their applications in natural product synthesis. Int. J. Mol. Sci. 2022, 23, 2622. [Google Scholar] [CrossRef] [PubMed]
- Iyanagi, T. Roles of Ferredoxin-NADP+ Oxidoreductase and Flavodoxin in NAD(P)H-dependent electron transfer systems. Antioxidants 2022, 11, 2143. [Google Scholar] [CrossRef]
- Takemura, M.; Kubo, A.; Higuchi, Y.; Maoka, T.; Sahara, T.; Yaoi, K.; Ohdan, K.; Umeno, D.; Misawa, N. Pathway engineering for efficient biosynthesis of violaxanthin in Escherichia coli. Appl. Microbiol. Biotechnol. 2019, 103, 9393–9399. [Google Scholar] [CrossRef]
- Cataldo, V.F.; Arenas, N.; Salgado, V.; Camilo, C.; Ibáñez, F.; Agosin, E. Heterologous production of the epoxycarotenoid violaxanthin in Saccharomyces cerevisiae. Metab. Eng. 2020, 59, 53–63. [Google Scholar] [CrossRef]
- Babili, S.A.; Hugueney, P.; Schledz, M.; Welsch, R.; Frohnmeyer, H.; Laule, O.; Beyer, P. Identification of a novel gene coding for neoxanthin synthase from Solanum tuberosum. FEBS Lett. 2000, 485, 168–172. [Google Scholar] [CrossRef]
- Bakare, O.O.; Fadaka, A.O.; Akanbi, M.O.; Akinyede, K.A.; Klein, A.; Keyster, M. Evaluation of selected carotenoids of Lycopersicon esculentum variants as therapeutic targets for Alzheimer’s disease: An in silico approach. BMC Mol. Cell Biol. 2021, 22, 49. [Google Scholar] [CrossRef]
- Kumagai, M.H.; Keller, Y.; Bouvier, F.; Clary, D.; Camara, B. Functional integration of non-native carotenoids into chloroplasts by viral-derived expression of capsanthin-capsorubin synthase in Nicotiana benthamiana. Plant J. 1998, 14, 305–315. [Google Scholar] [CrossRef]
- Piano, D.; Cocco, E.; Guadalupi, G.; Kalaji, H.M.; Kirkpatrick, J.; Farci, D. Characterization under quasi-native conditions of the capsanthin/capsorubin synthase from Capsicum annuum L. Plant Physiol. Biochem. 2019, 143, 165–175. [Google Scholar] [CrossRef]
- Mialoundama, A.S.; Heintz, D.; Jadid, N.; Nkeng, P.; Rahier, A.; Deli, J.; Camara, B.; Bouvier, F. Characterization of plant carotenoid cyclases as members of the flavoprotein family functioning with no net redox change. Plant Physiol. 2010, 153, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Hugueney, P.; Badillo, A.; Chen, H.C.; Klein, A.; Hirschberg, J.; Camara, B.; Kuntz, M. Metabolism of cyclic carotenoids: A model for the alteration of this biosynthetic pathway in Capsicum annuum chromoplasts. Plant J. 1995, 8, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Furubayashi, M.; Kubo, A.; Takemura, M.; Otani, Y.; Maoka, T.; Terada, Y.; Yaoi, K.; Ohdan, K.; Misawa, N.; Mitani, Y. Capsanthin Production in Escherichia coli by Overexpression of Capsanthin/Capsorubin Synthase from Capsicum annuum. J. Agric. Food Chem. 2021, 69, 5076–5085. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, Y.; Xu, J.-Y.; Li, Q.-Y.; Tang, J.-L.; Jia, S.-R.; Bi, C.-H.; Dai, Z.-B.; Zhu, X.-N.; Zhang, X.-L. Engineering CrtW and CrtZ for improving biosynthesis of astaxanthin in Escherichia coli. Chin. J. Nat. Med. 2020, 18, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Crameri, A.; Raillard, S.A.; Bermudez, E.; Stemmer, W.P. DNA shuffling of a family of genes from diverse species accelerates directed evolution. Nature 1998, 391, 288–291. [Google Scholar] [CrossRef]
- Sen, S.; Dasu, V.V.; Mandal, B. Developments in directed evolution for improving enzyme functions. Appl. Biochem. Biotechnol. 2007, 143, 212–223. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, J. Hydrogen peroxide-induced astaxanthin biosynthesis and catalase activity in Xanthophyllomyces dendrorhous. Appl. Microbiol. Biotechnol. 2006, 73, 663–668. [Google Scholar] [CrossRef]
- Xie, W.; Lv, X.; Ye, L.; Zhou, P.; Yu, H. Construction of lycopene-overproducing Saccharomyces cerevisiae by combining directed evolution and metabolic engineering. Metab. Eng. 2015, 30, 69–78. [Google Scholar] [CrossRef]
- Wise, R.R. Plastids: The Anabolic Factories of Plant Cells. Encycl. Cell Biol. 2016, 2, 324–330. [Google Scholar]
- Li, L.; Yuan, H. Chromoplast biogenesis and carotenoid accumulation. Arch. Biochem. Biophys. 2013, 539, 102–109. [Google Scholar] [CrossRef]
- Liu, P.; Sun, L.; Sun, Y.; Shang, F.; Yan, G. Decreased fluidity of cell membranes causes a metal ion deficiency in recombinant Saccharomyces cerevisiae producing carotenoids. J. Ind. Microbiol. Biotechnol. 2016, 43, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Park, S.-H.; Kim, S.; Kim, S.-W.; Hahn, J.-S. Efficient production of lycopene in Saccharomyces cerevisiae by enzyme engineering and increasing membrane flexibility and NAPDH production. Appl. Microbiol. Biotechnol. 2019, 103, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Liu, J.-Z. Enhanced Astaxanthin Production in Escherichia coli via Morphology and Oxidative Stress Engineering. J. Agric. Food Chem. 2019, 67, 11703–11709. [Google Scholar] [CrossRef]
- Park, S.Y.; Binkley, R.M.; Kim, W.J.; Lee, M.H.; Lee, S.Y. Metabolic engineering of Escherichia coli for high-level astaxanthin production with high productivity. Metab. Eng. 2018, 49, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Hammer, S.K.; Avalos, J.L. Harnessing yeast organelles for metabolic engineering. Nat. Chem. Biol. 2017, 13, 823–832. [Google Scholar] [CrossRef]
- Hegde, R.S.; Keenan, R.J. The mechanisms of integral membrane protein biogenesis. Nat. Rev. Mol. Cell Biol. 2022, 23, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Bhaduri, S.; Scott, N.A.; Neal, S.E. The Role of the Rhomboid Superfamily in ER Protein Quality Control: From Mechanisms and Functions to Diseases. Cold Spring Harb. Perspect. Biol. 2023, 15, a041248. [Google Scholar] [CrossRef]
- Li, H.; Sun, S. Protein Aggregation in the ER: Calm behind the Storm. Cells 2021, 10, 3337. [Google Scholar] [CrossRef]
- Kim, J.-E.; Jang, I.-S.; Son, S.-H.; Ko, Y.-J.; Cho, B.-K.; Kim, S.C.; Lee, J.Y. Tailoring the Saccharomyces cerevisiae endoplasmic reticulum for functional assembly of terpene synthesis pathway. Metab. Eng. 2019, 56, 50–59. [Google Scholar] [CrossRef]
- Arendt, P.; Miettinen, K.; Pollier, J.; Rycke, R.D.; Callewaert, N.; Goossens, A. An endoplasmic reticulum-engineered yeast platform for overproduction of triterpenoids. Metab. Eng. 2017, 40, 165–175. [Google Scholar] [CrossRef]
- Yuan, J.; Ching, C.-B. Mitochondrial acetyl-CoA utilization pathway for terpenoid productions. Metab. Eng. 2016, 38, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Kulagina, N.; Besseau, S.; Papon, N.; Courdavault, V. Peroxisomes: A New Hub for Metabolic Engineering in Yeast. Front. Bioeng. Biotechnol. 2021, 9, 659431. [Google Scholar] [CrossRef] [PubMed]
- Koch, B.; Schmidt, C.; Daum, G. Storage lipids of yeasts: A survey of nonpolar lipid metabolism in Saccharomyces cerevisiae, Pichia pastoris, and Yarrowia lipolytica. FEMS Microbiol. Rev. 2014, 38, 892–915. [Google Scholar] [CrossRef]
- DeLoache, W.C.; Russ, Z.N.; Dueber, J.E. Towards repurposing the yeast peroxisome for compartmentalizing heterologous metabolic pathways. Nat. Commun. 2016, 7, 11152. [Google Scholar] [CrossRef]
- Dusséaux, S.; Wajn, W.T.; Liu, Y.; Ignea, C.; Kampranis, S.C. Transforming yeast peroxisomes into microfactories for the efficient production of high-value isoprenoids. Proc. Natl. Acad. Sci. USA 2020, 117, 31789–31799. [Google Scholar] [CrossRef] [PubMed]
- Vizeacoumar, F.J.; Torres-Guzman, J.C.; Bouard, D.; Aitchison, J.D.; Rachubinski, R.A. Pex30p, Pex31p, and Pex32p form a family of peroxisomal integral membrane proteins regulating peroxisome size and number in Saccharomyces cerevisiae. Mol. Biol. Cell 2004, 15, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.K.; Alper, H.S. Yarrowia lipolytica: More than an oleaginous workhorse. Appl. Microbiol. Biotechnol. 2019, 103, 9251–9262. [Google Scholar] [CrossRef]
- Verwaal, R.; Jiang, Y.; Wang, J.; Daran, J.M.; Sandmann, G.; Berg, J.A.; Ooyen, A.J. Heterologous carotenoid production in Saccharomyces cerevisiae induces the pleiotropic drug resistance stress response. Yeast 2010, 27, 983–998. [Google Scholar] [CrossRef]
- Gao, S.; Tong, Y.; Zhu, L.; Ge, M.; Zhang, Y.; Chen, D.; Jiang, Y.; Yang, S. Iterative integration of multiple-copy pathway genes in Yarrowia lipolytica for heterologous β-carotene production. Metab. Eng. 2017, 41, 192–201. [Google Scholar] [CrossRef]
- Gajdoš, P.; Ledesma-Amaro, R.; Nicaud, J.-M.; Čertík, M.; Rossignol, T. Overexpression of diacylglycerol acyltransferase in Yarrowia lipolytica affects lipid body size, number and distribution. FEMS Yeast Res. 2016, 16, fow062. [Google Scholar] [CrossRef]
- Verwaal, R.; Wang, J.; Meijnen, J.-P.; Visser, H.; Sandmann, G.; Berg, J.A.; Ooyen, A.J.J. High-level production of beta-carotene in Saccharomyces cerevisiae by successive transformation with carotenogenic genes from Xanthophyllomyces dendrorhous. Appl. Environ. Microbiol. 2007, 73, 4342–4350. [Google Scholar] [CrossRef] [PubMed]
- Rossum, H.M.; Kozak, B.U.; Pronk, J.T.; Maris, A.J.A. Engineering cytosolic acetyl-coenzyme A supply in Saccharomyces cerevisiae: Pathway stoichiometry, free-energy conservation and redox-cofactor balancing. Metab. Eng. 2016, 36, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, B.R.; Nøhr, J.; Douthwaite, S.; Hansen, L.V.; Iversen, J.J.L.; Visser, J.; Ruijter, G.J.G. Increased NADPH concentration obtained by metabolic engineering of the pentose phosphate pathway in Aspergillus niger. FEBS J. 2005, 272, 1313–1325. [Google Scholar] [CrossRef] [PubMed]
- Meadows, A.L.; Hawkins, K.M.; Tsegaye, Y.; Antipov, E.; Kim, Y.; Raetz, L.; Dahl, R.H.; Tai, A.; Mahatdejkul-Meadows, T.; Xu, L.; et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nature 2016, 537, 694–697. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, C.; Liu, Q.; Xu, Q.; Qian, Z.; Peng, Q.; Yu, J.; Xu, M.; Zhou, X.; Zhang, Y.; et al. Engineered ethanol-driven biosynthetic system for improving production of acetyl-CoA derived drugs in Crabtree-negative yeast. Metab. Eng. 2019, 54, 275–284. [Google Scholar] [CrossRef]
- Hackett, S.R.; Zanotelli, V.R.T.; Xu, W.; Goya, J.; Park, J.O.; Perlman, D.H.; Gibney, P.A.; Botstein, D.; Storey, J.D.; Rabinowitz, J.D. Systems-level analysis of mechanisms regulating yeast metabolic flux. Science 2016, 354, aaf2786. [Google Scholar] [CrossRef]
- Mukherjee, M.; Blair, R.H.; Wang, Z.Q. Machine-learning guided elucidation of contribution of individual steps in the mevalonate pathway and construction of a yeast platform strain for terpenoid production. Metab. Eng. 2022, 74, 139–149. [Google Scholar] [CrossRef]
- Naseri, G.; Balazadeh, S.; Machens, F.; Kamranfar, I.; Messerschmidt, K.; Mueller-Roeber, B. Plant-Derived Transcription Factors for Orthologous Regulation of Gene Expression in the Yeast Saccharomyces cerevisiae. ACS Synth. Biol. 2017, 6, 1742–1756. [Google Scholar] [CrossRef]
- Brophy, J.A.N.; Voigt, C.A. Principles of genetic circuit design. Nat. Methods 2014, 11, 508–520. [Google Scholar] [CrossRef]
- Lv, Y.; Qian, S.; Du, G.; Chen, J.; Zhou, J.; Xu, P. Coupling feedback genetic circuits with growth phenotype for dynamic population control and intelligent bioproduction. Metab. Eng. 2019, 54, 109–116. [Google Scholar] [CrossRef]
- Lu, Q.; Zhou, X.-L.; Liu, J.-Z. Adaptive laboratory evolution and shuffling of Escherichia coli to enhance its tolerance and production of astaxanthin. Biotechnol. Biofuels Bioprod. 2022, 15, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, Y.; Xu, Y.; Zhang, J.; Ma, L.; Qi, Q.; Wang, Q. Development of bifunctional biosensors for sensing and dynamic control of glycolysis flux in metabolic engineering. Metab. Eng. 2021, 68, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Farmer, W.R.; Liao, J.C. Improving lycopene production in Escherichia coli by engineering metabolic control. Nat. Biotechnol. 2000, 18, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Nielsen, L.K.; Kampranis, S.C.; Vickers, C.E. Engineered protein degradation of farnesyl pyrophosphate synthase is an effective regulatory mechanism to increase monoterpene production in Saccharomyces cerevisiae. Metab. Eng. 2018, 47, 83–93. [Google Scholar] [CrossRef]
- Rinaldi, M.; Ferraz, C.A.; Scrutton, N.S. Alternative metabolic pathways and strategies to high-titre terpenoid production in Escherichia coli. Nat. Prod. Rep. 2022, 39, 90–118. [Google Scholar] [CrossRef]
- Rong, Y.; Jensen, S.I.; Lindorff-Larsen, K.; Nielsen, A.T. Folding of heterologous proteins in bacterial cell factories: Cellular mechanisms and engineering strategies. Biotechnol. Adv. 2023, 63, 108079. [Google Scholar] [CrossRef]
- Moser, S.; Pichler, H. Identifying and engineering the ideal microbial terpenoid production host. Appl. Microbiol. Biotechnol. 2019, 103, 5501–5516. [Google Scholar] [CrossRef]
- Schwarzhans, J.-P.; Luttermann, T.; Geier, M.; Kalinowski, J.; Friehs, K. Towards systems metabolic engineering in Pichia pastoris. Biotechnol. Adv. 2017, 35, 681–710. [Google Scholar] [CrossRef]
- Frigerio, C.; Nisio, E.D.; Galli, M.; Colombo, C.V.; Negri, R.; Clerici, M. The Chromatin Landscape around DNA Double-Strand Breaks in Yeast and Its Influence on DNA Repair Pathway Choice. Int. J. Mol. Sci. 2023, 24, 3248. [Google Scholar] [CrossRef]
- Lian, J.; Mishra, S.; Zhao, H. Recent advances in metabolic engineering of Saccharomyces cerevisiae: New tools and their applications. Metab. Eng. 2018, 50, 85–108. [Google Scholar] [CrossRef]
- Kim, S.-J.; Lee, J.-E.; Lee, D.Y.; Park, H.; Kim, K.H.; Park, Y.-C. Multi-omic characterization of laboratory-evolved Saccharomyces cerevisiae HJ7-14 with high ability of algae-based ethanol production. Appl. Microbiol. Biotechnol. 2018, 102, 8989–9002. [Google Scholar] [CrossRef]
- Yao, Z.; Wang, Q.; Dai, Z. Recent Advances in Directed Yeast Genome Evolution. J. Fungi 2022, 8, 635. [Google Scholar] [CrossRef]
- Pradipta, P.; Das, M.; Kundu, P.; Ghosh, A. Recent advances in systems and synthetic biology approaches for developing novel cell-factories in non-conventional yeasts. Biotechnol. Adv. 2021, 47, 107695. [Google Scholar]
- Bilal, M.; Ji, L.; Xu, Y.; Xu, S.; Lin, Y.; Iqbal, H.M.N.; Cheng, H. Bioprospecting Kluyveromyces marxianus as a Robust Host for Industrial Biotechnology. Front. Bioeng. Biotechnol. 2022, 10, 851768. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Huang, H.; Zhang, Y.; Du, G.; Chen, J. Recent advances of molecular toolbox construction expand Pichia pastoris in synthetic biology applications. World J. Microbiol. Biotechnol. 2017, 33, 19. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gong, J.-S.; Su, C.; Li, H.; Li, H.; Rao, Z.-M.; Xu, Z.-H.; Shi, J.-S. Pathway engineering facilitates efficient protein expression in Pichia pastoris. Appl. Microbiol. Biotechnol. 2022, 106, 5893–5912. [Google Scholar] [CrossRef]
- Guerfal, M.; Ryckaert, S.; Jacobs, P.P.; Ameloot, P.; Craenenbroeck, K.V.; Derycke, R.; Callewaert, N. The HAC1 gene from Pichia pastoris: Characterization and effect of its overexpression on the production of secreted, surface displayed and membrane proteins. Microb. Cell Fact. 2010, 9, 49. [Google Scholar] [CrossRef]
- Raschmanová, H.; Weninger, A.; Knejzlík, Z.; Melzoch, K.; Kovar, K. Engineering of the unfolded protein response pathway in Pichia pastoris: Enhancing production of secreted recombinant proteins. Appl. Microbiol. Biotechnol. 2021, 105, 4397–4414. [Google Scholar] [CrossRef]
- Byrne, B. Pichia pastoris as an expression host for membrane protein structural biology. Curr. Opin. Struct. Biol. 2015, 32, 9–17. [Google Scholar] [CrossRef]
- Peña, D.A.; Gasser, B.; Zanghellini, J.; Steiger, M.G.; Mattanovich, D. Metabolic engineering of Pichia pastoris. Metab. Eng. 2018, 50, 2–15. [Google Scholar] [CrossRef]
- Gasser, B.; Mattanovich, D. A yeast for all seasons—Is Pichia pastoris a suitable chassis organism for future bioproduction? FEMS Microbiol. Lett. 2018, 365, 4805–4814. [Google Scholar] [CrossRef] [PubMed]
- Hagman, A.; Säll, T.; Piškur, J. Analysis of the yeast short-term Crabtree effect and its origin. FEBS J. 2014, 281, 4805–4814. [Google Scholar] [CrossRef] [PubMed]
- Nocon, J.; Steiger, M.; Mairinger, T.; Hohlweg, J.; Rußmayer, H.; Hann, S.; Gasser, B.; Mattanovich, D. Increasing pentose phosphate pathway flux enhances recombinant protein production in Pichia pastoris. Appl. Microbiol. Biotechnol. 2016, 100, 5955–5963. [Google Scholar] [CrossRef] [PubMed]
- Waterham, H.R.; Cregg, J.M. Peroxisome biogenesis. Bioessays 1997, 19, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mawgoud, A.M.; Markham, K.A.; Palmer, C.M.; Liu, N.; Stephanopoulos, G.; Alper, H.S. Metabolic engineering in the host Yarrowia lipolytica. Metab. Eng. 2018, 50, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Bhutada, G.; Kavšček, M.; Hofer, F.; Gogg-Fassolter, G.; Schweiger, M.; Darnhofer, B.; Kordiš, D.; Birner-Gruenberger, R.; Natter, K. Characterization of a lipid droplet protein from Yarrowia lipolytica that is required for its oleaginous phenotype. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1193–1205. [Google Scholar] [CrossRef]
- Tang, X.S.; Cheng, Q.; Shyr, J.Y.; Tao, L. Bioproduction of Astaxanthin Using Mutant Carotenoid Ketolase and Carotenoid Hydroxylase. Genes. Patent WO 2006/072078 A2, 6 July 2006. [Google Scholar]
- Cheng, Q.; Tao, L. Mutant Carotenoid Ketolases. Patent WO 2007/120426 A1, 25 October 2007. [Google Scholar]
- Yang, K.; Qiao, Y.; Li, F.; Xu, Y.; Yan, Y.; Madzak, C.; Yan, J. Subcellular engineering of lipase dependent pathways directed towards lipid related organelles for highly effectively compartmentalized biosynthesis of triacylglycerol derived products in Yarrowia lipolytica. Metab. Eng. 2019, 55, 231–238. [Google Scholar] [CrossRef]
- Yee, D.A.; DeNicola, A.B.; Billingsley, J.M.; Creso, J.G.; Subrahmanyam, V.; Tang, Y. Engineered mitochondrial production of monoterpenes in Saccharomyces cerevisiae. Metab. Eng. 2019, 55, 76–84. [Google Scholar] [CrossRef]
- Lee, P.C.; Yoon, Y.; Dannert, C.S. Investigation of cellular targeting of carotenoid pathway enzymes in Pichia pastoris. J. Biotechnol. 2009, 140, 227–233. [Google Scholar] [CrossRef]
- Lin, J.-L.; Zhu, J.; Wheeldon, I. Synthetic protein scaffolds for biosynthetic pathway colocalization on lipid droplet membranes. ACS Synth. Biol. 2017, 6, 1534–1544. [Google Scholar] [CrossRef]
- Singh, R.K.; Lee, J.-K.; Selvaraj, C.; Singh, R.; Li, J.; Kim, S.-Y.; Kalia, V.C. Protein engineering approaches in the post-genomic era. Curr. Protein Pept. Sci. 2018, 19, 5–15. [Google Scholar] [CrossRef]
- Wilson, M.A. Mapping Enzyme Landscapes by Time-Resolved Crystallography with Synchrotron and X-ray Free Electron Laser Light. Annu. Rev. Biophys. 2022, 51, 79–98. [Google Scholar] [CrossRef]
- Delhommel, F.; Martínez-Lumbreras, S.; Sattler, M. Combining NMR, SAXS and SANS to characterize the structure and dynamics of protein complexes. Methods Enzymol. 2023, 678, 263–297. [Google Scholar] [PubMed]
- Giri, N.; Roy, R.S.; Cheng, J. Deep learning for reconstructing protein structures from cryo-EM density maps: Recent advances and future directions. Curr. Opin. Struct. Biol. 2023, 79, 102536. [Google Scholar] [CrossRef] [PubMed]
- McCoy, A.J.; Sammito, M.D.; Read, R.J. Implications of AlphaFold2 for crystallographic phasing by molecular replacement. Acta Crystallogr. D Struct. Biol. 2022, 78 Pt 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Wei, S.-P.; Qian, Z.-G.; Hu, C.-F.; Pan, F.; Chen, M.-T.; Lee, S.Y.; Xia, X.-X. Formation and functionalization of membraneless compartments in Escherichia coli. Nat. Chem. Biol. 2020, 16, 1143–1148. [Google Scholar] [CrossRef]
- Lau, Y.H.; Giessen, T.W.; Altenburg, W.J.; Silver, P.A. Prokaryotic nanocompartments form synthetic organelles in a eukaryote. Nat. Commun. 2018, 9, 1311. [Google Scholar] [CrossRef]
- Zhao, E.M.; Suek, N.; Wilson, M.Z.; Dine, E.; Pannucci, N.L.; Gitai, Z.; Avalos, J.L.; Toettcher, J.E. Light-based control of metabolic flux through assembly of synthetic organelles. Nat. Chem. Biol. 2019, 15, 589–597. [Google Scholar] [CrossRef]
- Wilding, K.M.; Schinn, S.-M.; Long, E.A.; Bundy, B.C. The emerging impact of cell-free chemical biosynthesis. Curr. Opin. Biotechnol. 2018, 53, 115–121. [Google Scholar] [CrossRef]
- Vilkhovoy, M.; Adhikari, A.; Vadhin, S.; Varner, J.D. The evolution of cell free biomanufacturing. Processes 2020, 8, 675. [Google Scholar] [CrossRef]
- Luo, S.; Yu, L.; Song, J.; Wu, C.; Li, Y.; Zhang, C. Hybridization of glucosyl stevioside and hydroxypropyl methylcellulose to improve the solubility of lutein. Food Chem. 2022, 394, 133490. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lim, X.; Bouin, A.; Lautier, T.; Zhang, C. High-level de novo biosynthesis of glycosylated zeaxanthin and astaxanthin in Escherichia coli. Bioresour. Bioprocess. 2021, 8, 67. [Google Scholar] [CrossRef]
- Bu, X.; Lin, J.-Y.; Cheng, J.; Yang, D.; Duan, C.-Q.; Koffas, M.; Yan, G.L. Engineering endogenous ABC transporter with improving ATP supply and membrane flexibility enhances the secretion of β-carotene in Saccharomyces cerevisiae. Biotechnol. Biofuels. 2020, 13, 168. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gao, C.; Guo, L.; Hu, G.; Luo, Q.; Liu, J.; Nielsen, J.; Chen, J.; Liu, L. DCEO Biotechnology: Tools to design, construct, evaluate, and optimize the metabolic pathway for biosynthesis of chemicals. Chem. Rev. 2018, 118, 4–72. [Google Scholar] [CrossRef]
- Garrett, D.A.; Failla, M.L.; Sarama, R.J. Development of an in vitro digestion method to assess carotenoid bioavailability from meals. J. Agric. Food Chem. 1999, 47, 4301–4309. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Prasad, P.; Lokesh, V.; Shang, X.; Shin, J.; Keum, Y.-S.; Lee, J.-H. Carotenoids: Dietary sources, extraction, encapsulation, bioavailability, and health benefits-a review of recent advancements. Antioxidants 2022, 11, 795. [Google Scholar] [CrossRef]
- Panozzo, A.; Lemmens, L.; Loey, A.V.; Manzocco, L.; Nicoli, M.C.; Hendrickx, M. Microstructure and bioaccessibility of different carotenoid species as affected by high pressure homogenisation: A case study on differently coloured tomatoes. Food Chem. 2013, 141, 4094–4100. [Google Scholar] [CrossRef]
- Lin, J.-H.; Lee, D.-J.; Chang, J.-S. Lutein production from biomass: Marigold flowers versus microalgae. Bioresour. Technol. 2015, 184, 421–428. [Google Scholar] [CrossRef]
- Stahl, W. Macular carotenoids: Lutein and zeaxanthin. Dev. Ophthalmol. 2005, 38, 70–88. [Google Scholar]
- Honda, M.; Murakami, K.; Osawa, Y.; Kawashima, Y.; Hirasawa, K.; Kuroda, I. Z-isomers of astaxanthin exhibit greater bioavailability and tissue accumulation efficiency than the all- E-isomer. J. Agric. Food Chem. 2021, 69, 3489–3495. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hwang, S.; Lee, S.-M. Metabolic engineering for the utilization of carbohydrate portions of lignocellulosic biomass. Metab. Eng. 2022, 71, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Zha, J.; Yuwen, M.; Qian, W.; Wu, X. Yeast-based biosynthesis of natural products from xylose. Front. Bioeng. Biotechnol. 2021, 9, 634919. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, D.; Hong, J. Carotenoid production from nondetoxified xylose mother liquid or corncob hydrolysate with engineered Kluyveromyces marxianus. Bioresour. Technol. 2022, 364, 128080. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Song, D.; Zhu, H. Metabolic engineering of Saccharomyces cerevisiae for enhanced carotenoid production from xylose-glucose mixtures. Front. Bioeng. Biotechnol. 2020, 8, 435. [Google Scholar] [CrossRef]
- Sun, L.; Atkinson, C.A.; Lee, Y.-G.; Jin, Y.-S. High-level β-carotene production from xylose by engineered Saccharomyces cerevisiae without overexpression of a truncated HMG1 (tHMG1). Biotechnol. Bioeng. 2020, 117, 3522–3532. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Peng, H.; Yang, C.; Guo, W.; Wang, M.; Li, G.; Liu, D. Metabolic Engineering of Model Microorganisms for the Production of Xanthophyll. Microorganisms 2023, 11, 1252. https://doi.org/10.3390/microorganisms11051252
Wang N, Peng H, Yang C, Guo W, Wang M, Li G, Liu D. Metabolic Engineering of Model Microorganisms for the Production of Xanthophyll. Microorganisms. 2023; 11(5):1252. https://doi.org/10.3390/microorganisms11051252
Chicago/Turabian StyleWang, Nan, Huakang Peng, Caifeng Yang, Wenfang Guo, Mengqi Wang, Gangqiang Li, and Dehu Liu. 2023. "Metabolic Engineering of Model Microorganisms for the Production of Xanthophyll" Microorganisms 11, no. 5: 1252. https://doi.org/10.3390/microorganisms11051252
APA StyleWang, N., Peng, H., Yang, C., Guo, W., Wang, M., Li, G., & Liu, D. (2023). Metabolic Engineering of Model Microorganisms for the Production of Xanthophyll. Microorganisms, 11(5), 1252. https://doi.org/10.3390/microorganisms11051252








