Toxicity and Impact of Silica Nanoparticles on the Configuration of Gut Microbiota in Immunodeficient Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Manufacturing of NMs
2.3. Animals and Treatments
2.4. Sampling for Silica Toxicity Analysis
2.5. Toxicity Analysis after the Exposure of SiNPs
2.6. Feces Sampling for Gut Microbiome Analysis
2.7. DNA Extraction and 16 Metagenomic Sequencing
2.8. Gut Microbiome Community Analysis
2.9. Statistical Analysis
3. Results
3.1. Analysis of SiNPs on Cellular and Hematological Activities of Immunodeficient Mice
3.2. Effect of SiNPs on Gut Microbiome in Immunodeficient Mice
3.2.1. Intestinal Microbiota Profiling in Immunodeficient Mice Prior to SiNP Treatments
3.2.2. Gut Microbiota Profiling in Immunodeficient Mice after Gavage of SiNPs
3.2.3. Gut Microbial Diversity and Abundance at Phylum Level
3.2.4. Comparison of Abundant Intestinal Bacteria and Taxonomic Relationship
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cormode, D.P.; Skajaa, T.; Fayad, Z.A.; Mulder, W.J. Nanotechnology in medical imaging: Probe design and applications. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Rizvi, T.F. Nanotechnology: Scope and application in plant disease management. Plant Pathol. J. 2014, 13, 214–231. [Google Scholar] [CrossRef]
- Moore, J.A.; Chow, J.C. Recent progress and applications of gold nanotechnology in medical biophysics using artificial intelligence and mathematical modeling. Nano Express 2021, 2, 022001. [Google Scholar] [CrossRef]
- Moshed, A.; Sarkar, M.K.I.; Khaleque, M.A. The Application of nanotechnology in medical sciences: New horizon of treatment. Am. J. Biomed. Sci. 2017, 9, 1–14. [Google Scholar] [CrossRef]
- Roszek, B.; De Jong, W.; Geertsma, R.E. Nanotechnology in Medical Applications: State-Of-The-Art in Materials and Devices. 2005. Available online: https://rivm.openrepository.com/bitstream/handle/10029/7265/265001001.pdf?sequence=1&isAllowed=y (accessed on 23 April 2023).
- Adeel, M.; Farooq, T.; White, J.C.; Hao, Y.; He, Z.; Rui, Y. Carbon-based nanomaterials suppress tobacco mosaic virus (TMV) infection and induce resistance in Nicotiana benthamiana. J. Hazard. Mater. 2021, 404, 124167. [Google Scholar] [CrossRef]
- Farooq, A.; Shukur, A.; Astley, C.; Tosheva, L.; Kelly, P.; Whitehead, D.; Azzawi, M. Titania coating of mesoporous silica nanoparticles for improved biocompatibility and drug release within blood vessels. Acta Biomater. 2018, 76, 208–216. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef]
- McNamara, K.; Tofail, S.A. Nanosystems: The use of nanoalloys, metallic, bimetallic, and magnetic nanoparticles in biomedical applications. Phys. Chem. Chem. Phys. 2015, 17, 27981–27995. [Google Scholar] [CrossRef]
- McNamara, K.; Tofail, S.A. Nanoparticles in biomedical applications. Adv. Phys. X 2017, 2, 54–88. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; He, X.-W.; Li, W.-Y.; Zhang, Y.-K. Multifunctional mesoporous silica nanoplatform based on silicon nanoparticles for targeted two-photon-excited fluorescence imaging-guided chemo/photodynamic synergetic therapy in vitro. Talanta 2020, 209, 120552. [Google Scholar] [CrossRef]
- Daund, V.; Chalke, S.; Sherje, A.P.; Kale, P.P. ROS responsive mesoporous silica nanoparticles for smart drug delivery: A review. J. Drug Deliv. Sci. Technol. 2021, 64, 102599. [Google Scholar] [CrossRef]
- Selvarajan, V.; Obuobi, S.; Ee, P.L.R. Silica nanoparticles—A versatile tool for the treatment of bacterial infections. Front. Chem. 2020, 8, 602. [Google Scholar] [CrossRef]
- Li, Y.; Duan, J.; Chai, X.; Yang, M.; Wang, J.; Chen, R.; Sun, Z. Microarray-assisted size-effect study of amorphous silica nanoparticles on human bronchial epithelial cells. Nanoscale 2019, 11, 22907–22923. [Google Scholar] [CrossRef]
- Niculescu, V.-C. Mesoporous silica nanoparticles for bio-applications. Front. Mater. 2020, 7, 36. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, H.; Zhang, Z.; Wen, B.; Cao, H.; Bai, Y.; Che, Q.; Guo, J.; Su, Z. Applications and Biocompatibility of Mesoporous Silica Nanocarriers in the Field of Medicine. Front. Pharmacol. 2022, 13, 829796. [Google Scholar] [CrossRef]
- Almanaa, T.N.; Aref, M.; Kakakhel, M.A.; Elshopakey, G.E.; Mahboub, H.H.; Abdelazim, A.M.; Kamel, S.; Belali, T.M.; Abomughaid, M.M.; Alhujaily, M. Silica Nanoparticle Acute Toxicity on Male Rattus norvegicus Domestica: Ethological Behavior, Hematological Disorders, Biochemical Analyses, Hepato-Renal Function, and Antioxidant-Immune Response. Front. Bioeng. Biotechnol. 2022, 10, 868111. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Zhang, Y.; Zhang, G.; Kang, Y.; Chen, A.; Feng, X.; Shao, L. The toxicity of silica nanoparticles to the immune system. Nanomedicine 2018, 13, 1939–1962. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Joachim, E.; Choi, H.; Kim, K. Toxicity of silica nanoparticles depends on size, dose, and cell type. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1407–1416. [Google Scholar] [CrossRef]
- Duan, J.; Yu, Y.; Shi, H.; Tian, L.; Guo, C.; Huang, P.; Zhou, X.; Peng, S.; Sun, Z. Toxic effects of silica nanoparticles on zebrafish embryos and larvae. PLoS ONE 2013, 8, e74606. [Google Scholar] [CrossRef]
- Azouz, R.A.; Korany, R.M. Toxic impacts of amorphous silica nanoparticles on liver and kidney of male adult rats: An in vivo study. Biol. Trace Elem. Res. 2021, 199, 2653–2662. [Google Scholar] [CrossRef]
- Hassankhani, R.; Esmaeillou, M.; Tehrani, A.A.; Nasirzadeh, K.; Khadir, F.; Maadi, H. In vivo toxicity of orally administrated silicon dioxide nanoparticles in healthy adult mice. Environ. Sci. Pollut. Res. 2015, 22, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Sun, J.; Zhong, G.; Shi, L.; Zhang, D. Biodistribution and toxicity of intravenously administered silica nanoparticles in mice. Arch. Toxicol. 2010, 84, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Dar, G.I.; Saeed, M.; Wu, A. Toxicity of TiO2 nanoparticles. In TiO2 Nanoparticles: Applications in Nanobiotechnology and Nanomedicine; Wiley-VCH: Hoboken, NJ, USA, 2020; pp. 67–103. [Google Scholar]
- Fleddermann, J.; Susewind, J.; Peuschel, H.; Koch, M.; Tavernaro, I.; Kraegeloh, A. Distribution of SiO2 nanoparticles in 3D liver microtissues. Int. J. Nanomed. 2019, 14, 1411–1431. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yu, Y.; Li, Y.; Yu, Y.; Duan, J.; Zou, Y.; Li, Q.; Sun, Z. Oxidative damage and energy metabolism disorder contribute to the hemolytic effect of amorphous silica nanoparticles. Nanoscale Res. Lett. 2016, 11, 57. [Google Scholar] [CrossRef]
- Murugadoss, S.; Lison, D.; Godderis, L.; Van Den Brule, S.; Mast, J.; Brassinne, F.; Sebaihi, N.; Hoet, P.H. Toxicology of silica nanoparticles: An update. Arch. Toxicol. 2017, 91, 2967–3010. [Google Scholar] [CrossRef]
- Yu, T.; Malugin, A.; Ghandehari, H. Impact of silica nanoparticle design on cellular toxicity and hemolytic activity. ACS Nano 2011, 5, 5717–5728. [Google Scholar] [CrossRef]
- Lee, J.H.; Gulumian, M.; Faustman, E.M.; Workman, T.; Jeon, K.; Yu, I.J. Blood biochemical and hematological study after subacute intravenous injection of gold and silver nanoparticles and coadministered gold and silver nanoparticles of similar sizes. BioMed Res. Int. 2018, 2018, 8460910. [Google Scholar] [CrossRef]
- Hagihara, M.; Kuroki, Y.; Ariyoshi, T.; Higashi, S.; Fukuda, K.; Yamashita, R.; Matsumoto, A.; Mori, T.; Mimura, K.; Yamaguchi, N. Clostridium butyricum modulates the microbiome to protect intestinal barrier function in mice with antibiotic-induced dysbiosis. Iscience 2020, 23, 100772. [Google Scholar] [CrossRef]
- Ahlmann, M.; Hempel, G. The effect of cyclophosphamide on the immune system: Implications for clinical cancer therapy. Cancer Chemother. Pharmacol. 2016, 78, 661–671. [Google Scholar] [CrossRef]
- Jafari, S.; Derakhshankhah, H.; Alaei, L.; Fattahi, A.; Varnamkhasti, B.S.; Saboury, A.A. Mesoporous silica nanoparticles for therapeutic/diagnostic applications. Biomed. Pharmacother. 2019, 109, 1100–1111. [Google Scholar] [CrossRef]
- Mamaeva, V.; Rosenholm, J.M.; Bate-Eya, L.T.; Bergman, L.; Peuhu, E.; Duchanoy, A.; Fortelius, L.E.; Landor, S.; Toivola, D.M.; Lindén, M. Mesoporous silica nanoparticles as drug delivery systems for targeted inhibition of Notch signaling in cancer. Mol. Ther. 2011, 19, 1538–1546. [Google Scholar] [CrossRef]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X. Effects of cyclophosphamide on immune system and gut microbiota in mice. Microbiol. Res. 2015, 171, 97–106. [Google Scholar] [CrossRef]
- Battaglia, M.; Garrett-Sinha, L.A. Bacterial infections in lupus: Roles in promoting immune activation and in pathogenesis of the disease. J. Transl. Autoimmun. 2021, 4, 100078. [Google Scholar] [CrossRef]
- Routy, B.; Gopalakrishnan, V.; Daillère, R.; Zitvogel, L.; Wargo, J.A.; Kroemer, G. The gut microbiota influences anticancer immunosurveillance and general health. Nat. Rev. Clin. Oncol. 2018, 15, 382–396. [Google Scholar] [CrossRef]
- Usuda, H.; Okamoto, T.; Wada, K. Leaky gut: Effect of dietary fiber and fats on microbiome and intestinal barrier. Int. J. Mol. Sci. 2021, 22, 7613. [Google Scholar] [CrossRef]
- Yang, J.; Liu, K.-X.; Qu, J.-M.; Wang, X.-D. The changes induced by cyclophosphamide in intestinal barrier and microflora in mice. Eur. J. Pharmacol. 2013, 714, 120–124. [Google Scholar] [CrossRef]
- Chávez-Galán, L.; Ramon-Luing, L.A.; Torre-Bouscoulet, L.; Pérez-Padilla, R.; Sada-Ovalle, I. Pre-exposure of Mycobacterium tuberculosis-infected macrophages to crystalline silica impairs control of bacterial growth by deregulating the balance between apoptosis and necrosis. PLoS ONE 2013, 8, e80971. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, R.; Wang, B.; Cai, C.; Zheng, L.; Wang, H.; Wang, M.; Ouyang, H.; Zhou, X.; Chai, Z. The effects of orally administered Ag, TiO2 and SiO2 nanoparticles on gut microbiota composition and colitis induction in mice. NanoImpact 2017, 8, 80–88. [Google Scholar] [CrossRef]
- Ogawa, T.; Okumura, R.; Nagano, K.; Minemura, T.; Izumi, M.; Motooka, D.; Nakamura, S.; Iida, T.; Maeda, Y.; Kumanogoh, A. Oral intake of silica nanoparticles exacerbates intestinal inflammation. Biochem. Biophys. Res. Commun. 2021, 534, 540–546. [Google Scholar] [CrossRef]
- Tam, S.Y.J.; Coller, J.K.; Wignall, A.; Gibson, R.J.; Khatri, A.; Barbé, C.; Bowen, J.M. Intestinal accumulation of silica particles in a rat model of dextran sulfate sodium-induced colitis. Ann. Gastroenterol. 2019, 32, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Gmoshinski, I.V.; Shipelin, V.A.; Shumakova, A.A.; Trushina, E.N.; Mustafina, O.K.; Safenkova, I.V.; Khotimchenko, S.A.; Nikityuk, D.B.; Tutelyan, V.A. Toxicity evaluation of nanostructured silica orally administered to rats: Influence on immune system function. Nanomaterials 2020, 10, 2126. [Google Scholar] [CrossRef] [PubMed]
- Kusaka, T.; Nakayama, M.; Nakamura, K.; Ishimiya, M.; Furusawa, E.; Ogasawara, K. Effect of silica particle size on macrophage inflammatory responses. PLoS ONE 2014, 9, e92634. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, N.I.; Gonzalez, Z.; Ferrari, B.; Castro, Y. Synthesis of mesoporous silica nanoparticles by sol–gel as nanocontainer for future drug delivery applications. Boletín Soc. Española Cerámica Vidr. 2017, 56, 139–145. [Google Scholar] [CrossRef]
- Yan, F.; Jiang, J.; Chen, X.; Tian, S.; Li, K. Synthesis and characterization of silica nanoparticles preparing by low-temperature vapor-phase hydrolysis of SiCl4. Ind. Eng. Chem. Res. 2014, 53, 11884–11890. [Google Scholar] [CrossRef]
- Li, D.; Zhang, R.; Cui, L.; Chu, C.; Zhang, H.; Sun, H.; Luo, J.; Zhou, L.; Chen, L.; Cui, J. Multiple organ injury in male C57BL/6J mice exposed to ambient particulate matter in a real-ambient PM exposure system in Shijiazhuang, China. Environ. Pollut. 2019, 248, 874–887. [Google Scholar] [CrossRef]
- Chan, W.-T.; Liu, C.-C.; Chiau, J.-S.C.; Tsai, S.-T.; Liang, C.-K.; Cheng, M.-L.; Lee, H.-C.; Yeung, C.-Y.; Hou, S.-Y. In vivo toxicologic study of larger silica nanoparticles in mice. Int. J. Nanomed. 2017, 12, 3421–3432. [Google Scholar] [CrossRef]
- Noosud, J.; Lailerd, N.; Kayan, A.; Boonkaewwan, C. In vitro and in vivo assessment of inhibitory effect of stevioside on pro-inflammatory cytokines. Avicenna J. Phytomed. 2017, 7, 101–107. [Google Scholar]
- Zou, W.; Feng, R.; Yang, Y. Changes in the serum levels of inflammatory cytokines in antidepressant drug-naïve patients with major depression. PLoS ONE 2018, 13, e0197267. [Google Scholar] [CrossRef]
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.V.; Leung, J.M.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014, 158, 705–721. [Google Scholar] [CrossRef]
- Li, G.-Q.; Zhang, T.; Yang, W.-G.; Zhong, H.-L.; Xiao, P.; Liu, L.-W.; Wang, Y.-W.; Chen, H.; Kong, R.; Wang, G. Correction: Gut microbiota patterns associated with somatostatin in patients undergoing pancreaticoduodenectomy: A prospective study. Cell Death Discov. 2020, 6, 105. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Zhu, J.; Cai, R.; Tan, Y.; Wu, X.; Wen, Q.; Liu, Z.; Ouyang, S.-H.; Yin, Z.; Yang, H. Preventive consumption of green tea modifies the gut microbiota and provides persistent protection from high-fat diet-induced obesity. J. Funct. Foods 2020, 64, 103621. [Google Scholar] [CrossRef]
- Ding, Y.; Yan, Y.; Chen, D.; Ran, L.; Mi, J.; Lu, L.; Jing, B.; Li, X.; Zeng, X.; Cao, Y. Modulating effects of polysaccharides from the fruits of Lycium barbarum on the immune response and gut microbiota in cyclophosphamide-treated mice. Food Funct. 2019, 10, 3671–3683. [Google Scholar] [CrossRef]
- Tecza, K.; Pamula-Pilat, J.; Lanuszewska, J.; Butkiewicz, D.; Grzybowska, E. Pharmacogenetics of toxicity of 5-fluorouracil, doxorubicin and cyclophosphamide chemotherapy in breast cancer patients. Oncotarget 2018, 9, 9114–9136. [Google Scholar] [CrossRef]
- Bhavsar, D.; Patel, V.; Sawant, K. Systematic investigation of in vitro and in vivo safety, toxicity and degradation of mesoporous silica nanoparticles synthesized using commercial sodium silicate. Microporous Mesoporous Mater. 2019, 284, 343–352. [Google Scholar] [CrossRef]
- Morales, I.; Guzmán-Martínez, L.; Cerda-Troncoso, C.; Farías, G.A.; Maccioni, R.B. Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front. Cell. Neurosci. 2014, 8, 112. [Google Scholar] [CrossRef]
- Vysakh, A.; Jayesh, K.; Helen, L.R.; Jyothis, M.; Latha, M. Acute oral toxicity and anti-inflammatory evaluation of methanolic extract of Rotula aquatica roots in Wistar rats. J. Ayurveda Integr. Med. 2020, 11, 45–52. [Google Scholar] [CrossRef]
- Chen, D.; Chen, G.; Ding, Y.; Wan, P.; Peng, Y.; Chen, C.; Ye, H.; Zeng, X.; Ran, L. Polysaccharides from the flowers of tea (Camellia sinensis L.) modulate gut health and ameliorate cyclophosphamide-induced immunosuppression. J. Funct. Foods 2019, 61, 103470. [Google Scholar] [CrossRef]
- Chen, Z.; Han, S.; Shi, M.; Liu, G.; Chen, Z.; Chang, J.; Wu, C.; Xiao, Y. Immunomodulatory effects of mesoporous silica nanoparticles on osteogenesis: From nanoimmunotoxicity to nanoimmunotherapy. Appl. Mater. Today 2018, 10, 184–193. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Desouky, E.M.; Hozayen, W.G.; Bin-Jumah, M.; El-Nahass, E.-S.; Soliman, H.A.; Farghali, A.A. Mesoporous silica nanoparticles trigger liver and kidney injury and fibrosis via altering TLR4/NF-κB, JAK2/STAT3 and Nrf2/HO-1 signaling in rats. Biomolecules 2019, 9, 528. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, H.; Forman, H.J. Silica induces macrophage cytokines through phosphatidylcholine-specific phospholipase C with hydrogen peroxide. Am. J. Respir. Cell Mol. Biol. 2007, 36, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Boudard, D.; Aureli, F.; Laurent, B.; Sturm, N.; Raggi, A.; Antier, E.; Lakhdar, L.; Marche, P.N.; Cottier, M.; Cubadda, F. Chronic oral exposure to synthetic amorphous silica (NM-200) results in renal and liver lesions in mice. Kidney Int. Rep. 2019, 4, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhouhua, W.; Jie, Z.; Xinlu, F.; Jinqiang, L.; Yuwen, Q.; Zhiying, H. Renal interstitial fibrosis induced by high-dose mesoporous silica nanoparticles via the NF-κB signaling pathway. Int. J. Nanomed. 2015, 10, 1–22. [Google Scholar] [CrossRef]
- Deng, Y.-D.; Zhang, X.-D.; Yang, X.-S.; Huang, Z.-L.; Wei, X.; Yang, X.-F.; Liao, W.-Z. Subacute toxicity of mesoporous silica nanoparticles to the intestinal tract and the underlying mechanism. J. Hazard. Mater. 2021, 409, 124502. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, C.-S.; Ignacio, R.M.C.; Kim, D.-H.; Sajo, M.E.J.; Maeng, E.H.; Qi, X.-F.; Park, S.-E.; Kim, Y.-R.; Kim, M.-K. Immunotoxicity of silicon dioxide nanoparticles with different sizes and electrostatic charge. Int. J. Nanomed. 2014, 9, 183–193. [Google Scholar] [CrossRef]
- Bosco, N.; Noti, M. The aging gut microbiome and its impact on host immunity. Genes Immun. 2021, 22, 289–303. [Google Scholar] [CrossRef]
- Donati Zeppa, S.; Agostini, D.; Ferrini, F.; Gervasi, M.; Barbieri, E.; Bartolacci, A.; Piccoli, G.; Saltarelli, R.; Sestili, P.; Stocchi, V. Interventions on Gut Microbiota for Healthy Aging. Cells 2022, 12, 34. [Google Scholar] [CrossRef]
- Brandenberger, C.; Rowley, N.L.; Jackson-Humbles, D.N.; Zhang, Q.; Bramble, L.A.; Lewandowski, R.P.; Wagner, J.G.; Chen, W.; Kaplan, B.L.; Kaminski, N.E. Engineered silica nanoparticles act as adjuvants to enhance allergic airway disease in mice. Part. Fibre Toxicol. 2013, 10, 26. [Google Scholar] [CrossRef]
- Chia, S.L.; Leong, D.T. Reducing ZnO nanoparticles toxicity through silica coating. Heliyon 2016, 2, e00177. [Google Scholar] [CrossRef]
- Ji, Y.; Ma, N.; Zhang, J.; Wang, H.; Tao, T.; Pei, F.; Hu, Q. Dietary intake of mixture coarse cereals prevents obesity by altering the gut microbiota in high-fat diet fed mice. Food Chem. Toxicol. 2021, 147, 111901. [Google Scholar] [CrossRef]
- Alou, M.T.; Lagier, J.-C.; Raoult, D. Diet influence on the gut microbiota and dysbiosis related to nutritional disorders. Hum. Microbiome J. 2016, 1, 3–11. [Google Scholar] [CrossRef]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.-Z.; Abe, F.; Osawa, R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 2016, 16, 90. [Google Scholar] [CrossRef]
- Meng, C.; Feng, S.; Hao, Z.; Dong, C.; Liu, H. Changes in gut microbiota composition with age and correlations with gut inflammation in rats. PLoS ONE 2022, 17, e0265430. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Shanahan, F.; O’Toole, P.W. The gut microbiome as a modulator of healthy ageing. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 565–584. [Google Scholar] [CrossRef]
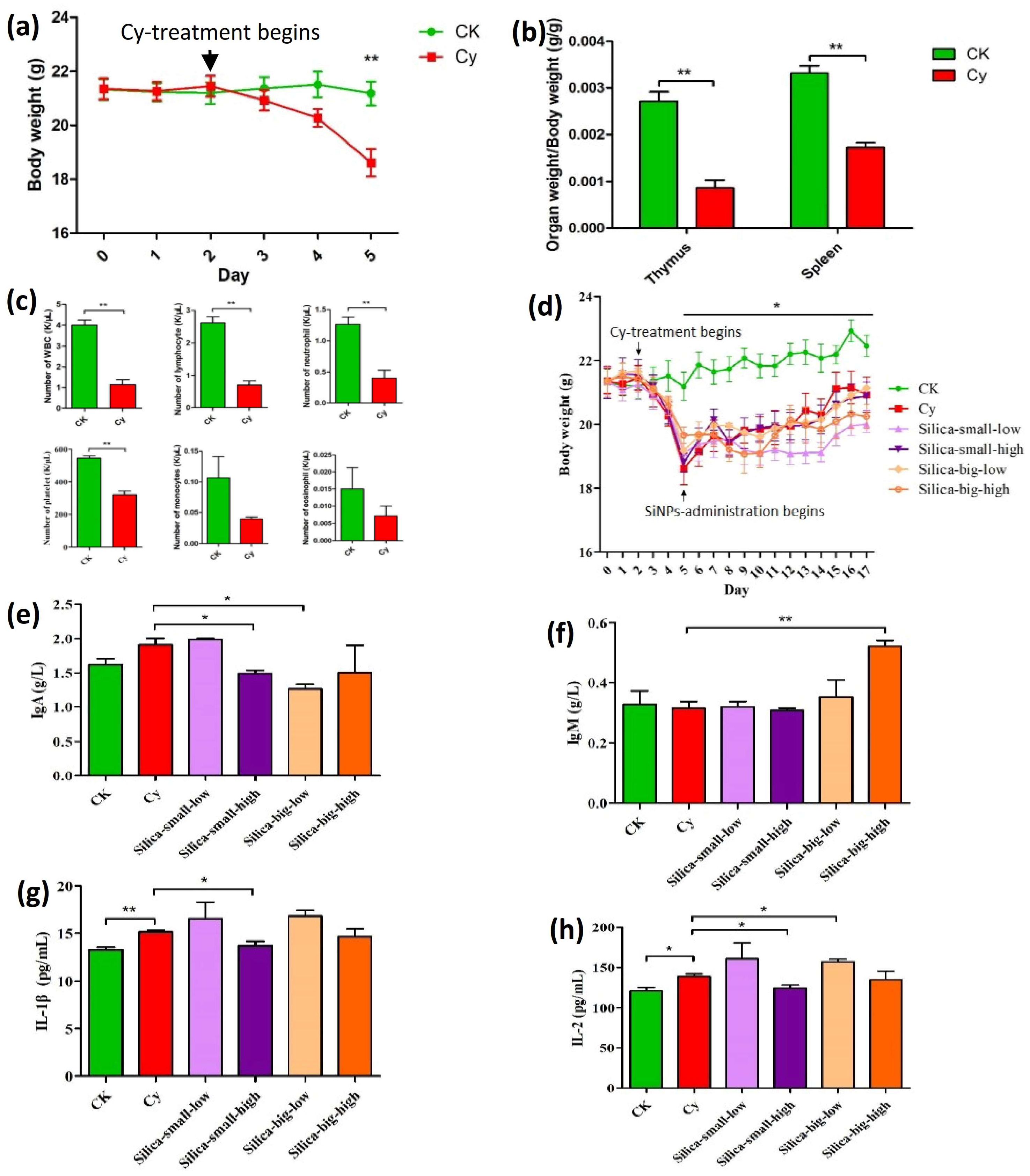
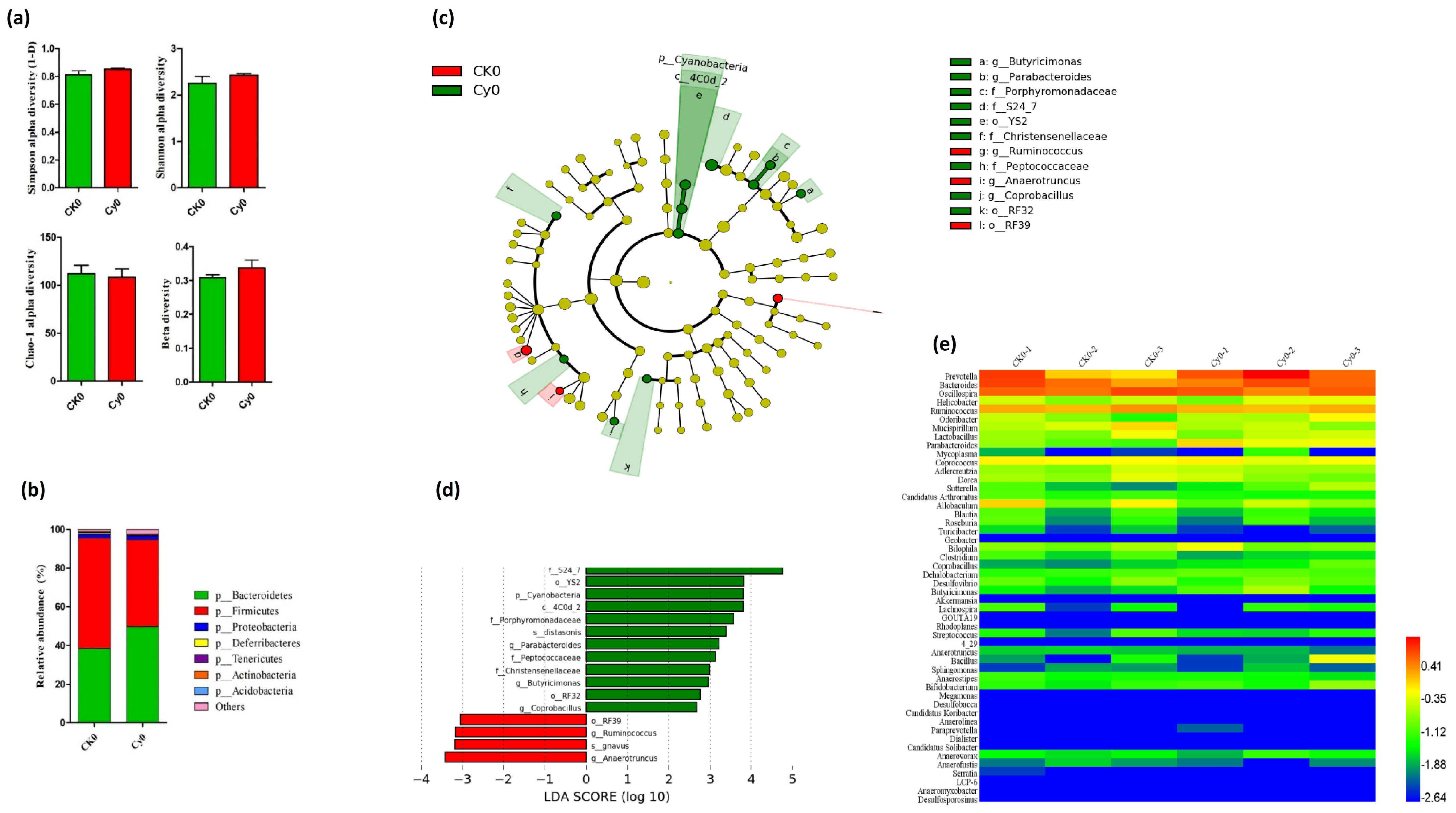
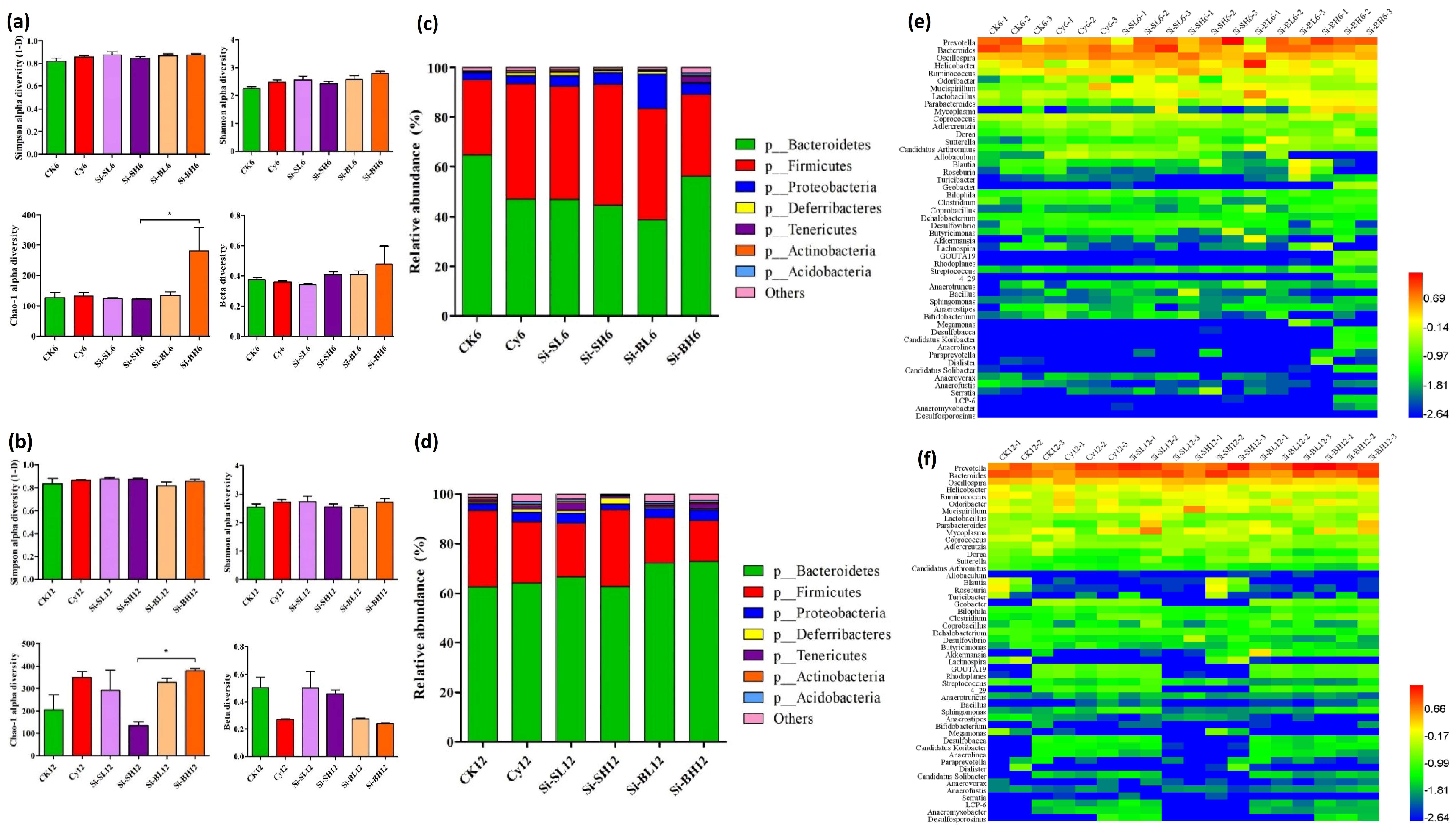

| SiNP Treatment | LDA Analysis of Mice Treated with Cy | LDA Analysis of Immunodeficient Mice Treated with Different Levels of SiNPs | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Comparison of gut microbiota after 6 days of SiNP treatment | CK6 vs. Cy6 | Cy6 vs. Si-SL6 | Cy6 vs. Si-SH6 | Cy6 vs. Si-BL6 | Cy6 vs. Si-BH6 | |||||
| Abundant genera | Abundant genera | Abundant genera | Abundant genera | Abundant genera | ||||||
| CK6 control group | Cy6-immunodeficient group | Cy6-immunodeficient group | Si-SL6-treated group | Cy6-immunodeficient group | Si-SH6-treated group | Cy6-immunodeficient group | Si-BL6-treated group | Cy6-immunodeficient group | Si-BH6-treated group | |
| Prevotella, Anaerofustis | Oscillospira, Anaeroplasma, Allobaculum, Ruminococcus, Odoribacter, Sutterella, Coprococcus | Clostridium, Anaerofustis | Lactobacillus, Sphingomonas, Butyricimonas, Streptococcus | Ochrobactrum, Turicibacter, Dorea, Allobaculum | Coprobacillus, Sphingomonas | Clostridium, Staphylococcus, Desulfovibrio, Anaerovorax, Ochrobactrum, Allobaculum, Ruminococcus | Lactobacillus, Acinetobacter, Sphingomonas | Bacillus, Dehalobacterium, Anaeroplasma, Bilophila, Bifidobacterium, Anaerovorax, Serratia, Allobaculum, Ruminococcus, Oscillospira | Prevotella, Mycoplasma, Parabacteroides, Lactobacillus, Proteus, Mycobacterium, Sutterella, Paraprevotella, Coprobacillus, Turicibacter | |
| Comparison of gut microbiota after 12 days of SiNP treatment | CK12 vs. Cy12 | Cy12 vs. Si-SL12 | Cy12 vs. Si-SH12 | Cy12 vs. Si-BL12 | Cy12 vs. Si-BH12 | |||||
| Abundant genera | Abundant genera | Abundant genera | Abundant genera | Abundant genera | ||||||
| CK12 control group | Cy12-immunodeficient group | Cy12-immunodeficient group | Si-SL12-treated group | Cy12-immunodeficient group | Si-SH12-treated group | Cy12-immunodeficient group | Si-BL12-treated group | Cy12-immunodeficient group | Si-BH12-treated group | |
| Streptococcus, Anaerostipes, Sutterella, Dorea, Bacteroides | Methylibium, Anaeromyxobacter, Desulfococcus, Sphingomonas, Thiobacillus, Aquicella | Dehalobacterium, Anaerofustis, Geothrix, Methylibium | Sutterella | Rhodoplanes, Lactobacillus, Geobacter, Kaistobacter, Syntrophobacter, Desulfococcus, Mycobacterium, Nitrospira, Candidatus Solibacter, Geothrix, Phenylobacterium, Thiobacillus, Sphingomonas, Anaeromyxobacter, Anaerolinea, Candidatus, Koribacter, Desulfobacca | Akkermansia | Streptococcus, Desulfovibrio, Anaeromyxobacter, Dehalobacterium, Nitrospira, Ruminococcus | Prevotella, Akkermansia, Sutterella | Anaerostipes, Dehalobacterium, Anaeromyxobacter, Geothrix, Methylibium, Geobacter, Ruminococcus, Mucispirillum | Prevotella, Sutterella, Akkermansia | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabbir, S.; Hu, Y.; He, X.; Huang, K.; Xu, W. Toxicity and Impact of Silica Nanoparticles on the Configuration of Gut Microbiota in Immunodeficient Mice. Microorganisms 2023, 11, 1183. https://doi.org/10.3390/microorganisms11051183
Shabbir S, Hu Y, He X, Huang K, Xu W. Toxicity and Impact of Silica Nanoparticles on the Configuration of Gut Microbiota in Immunodeficient Mice. Microorganisms. 2023; 11(5):1183. https://doi.org/10.3390/microorganisms11051183
Chicago/Turabian StyleShabbir, Sana, Yanzhou Hu, Xiaoyun He, Kunlun Huang, and Wentao Xu. 2023. "Toxicity and Impact of Silica Nanoparticles on the Configuration of Gut Microbiota in Immunodeficient Mice" Microorganisms 11, no. 5: 1183. https://doi.org/10.3390/microorganisms11051183
APA StyleShabbir, S., Hu, Y., He, X., Huang, K., & Xu, W. (2023). Toxicity and Impact of Silica Nanoparticles on the Configuration of Gut Microbiota in Immunodeficient Mice. Microorganisms, 11(5), 1183. https://doi.org/10.3390/microorganisms11051183









