Effect of Essential Oils of Apiaceae, Lamiaceae, Lauraceae, Myrtaceae, and Rutaceae Family Plants on Growth, Biofilm Formation, and Quorum Sensing in Chromobacterium violaceum, Pseudomonas aeruginosa, and Enterococcus faecalis
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Essential Oils (EOs) and Their Extraction
2.3. GC-MS Analysis of Essential Oils
2.4. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.5. Biofilm Formation Evaluation
2.6. Violacein Production Evaluation
2.7. Gelatinase Activity Evaluation
2.8. Genomic DNA Extraction
2.9. Quantification of Global 5-Methylcytosine Levels
2.10. Quantification of Global N6-Methyladenosine Levels
2.11. Statistical Analysis
3. Results
3.1. Characterization of the Essential Oils
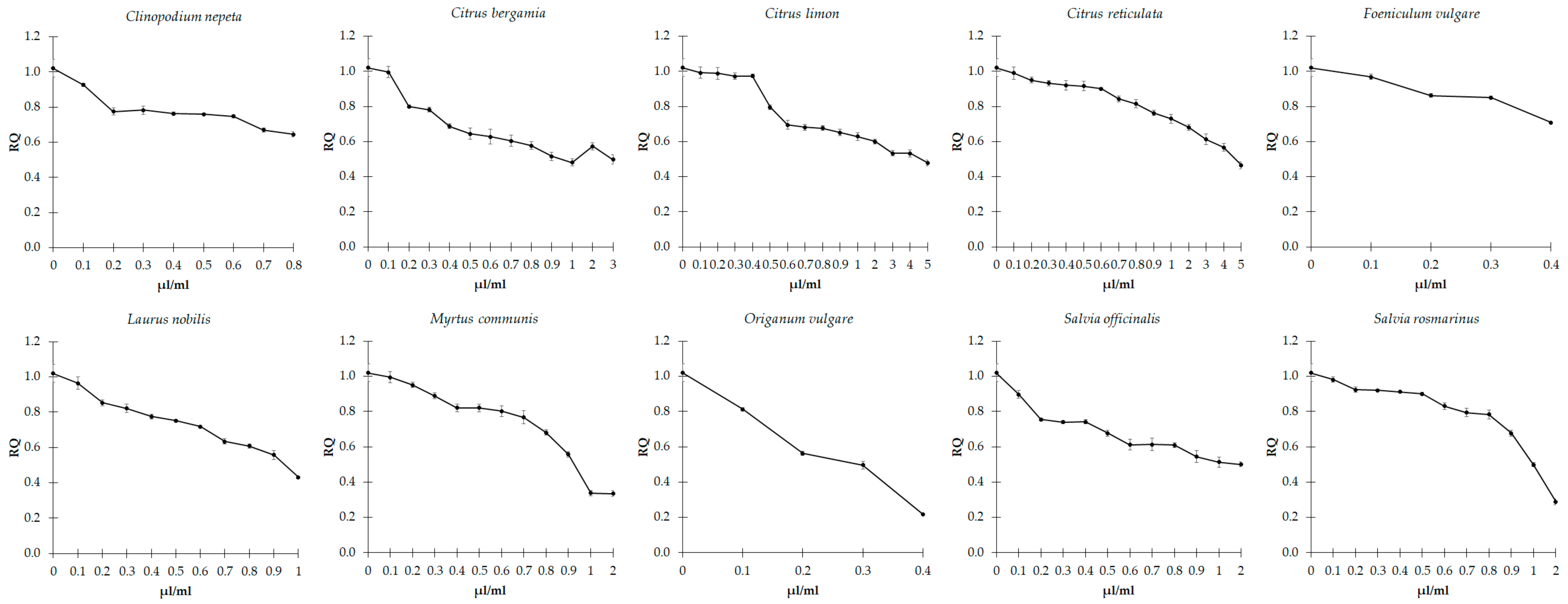
3.2. Effects of EOs on Cell Growth
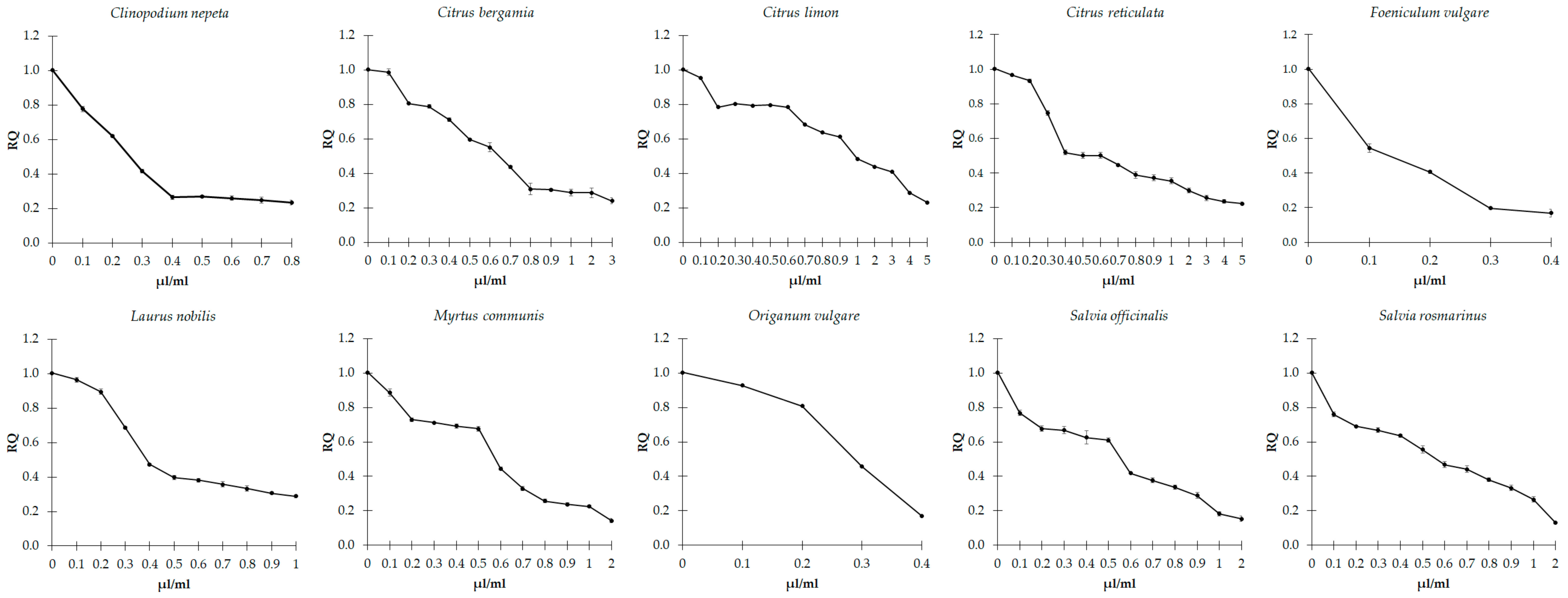
3.3. Effects of EOs on Biofilm Formation
3.4. Effects of EOs on Quorum Sensing
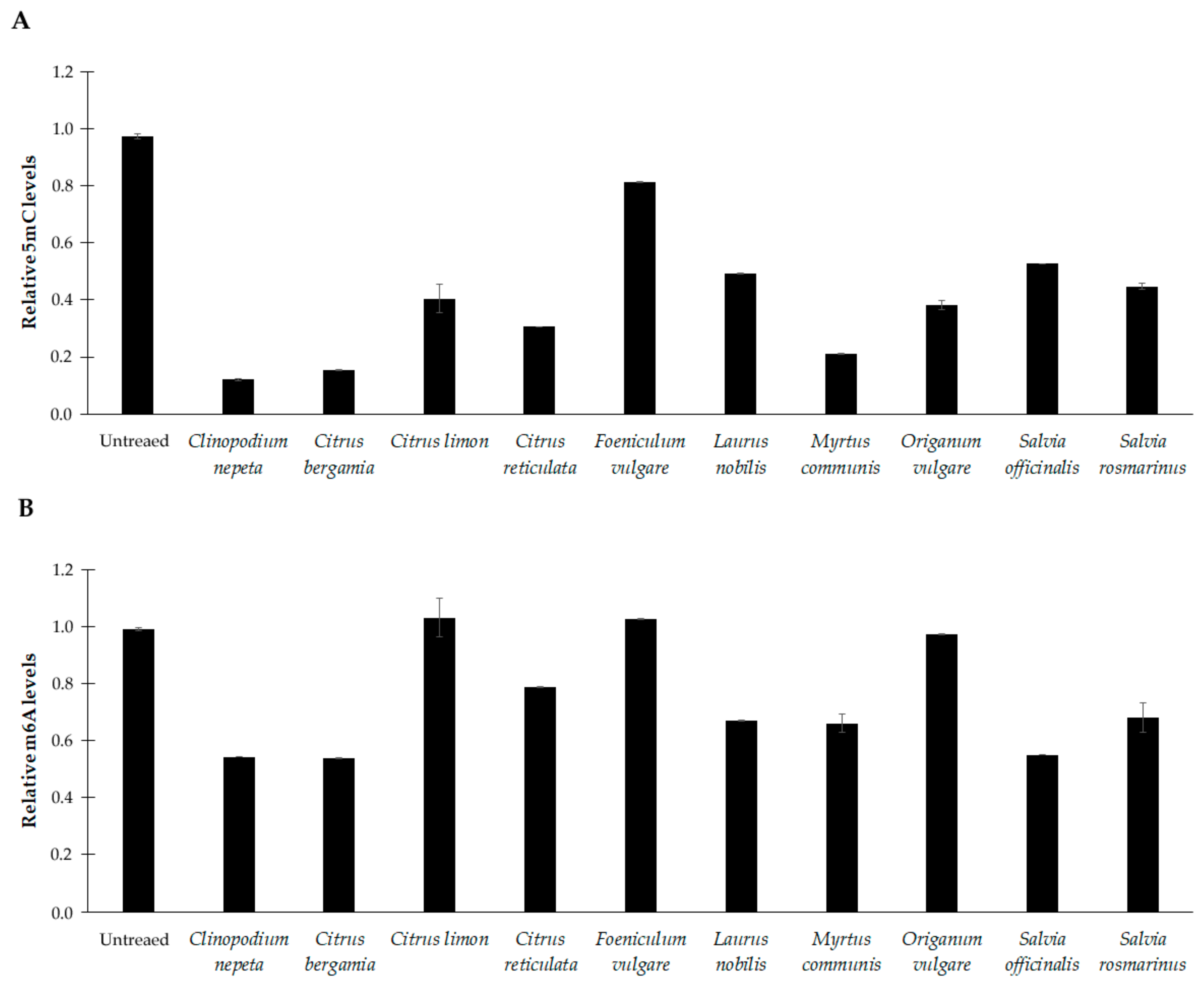
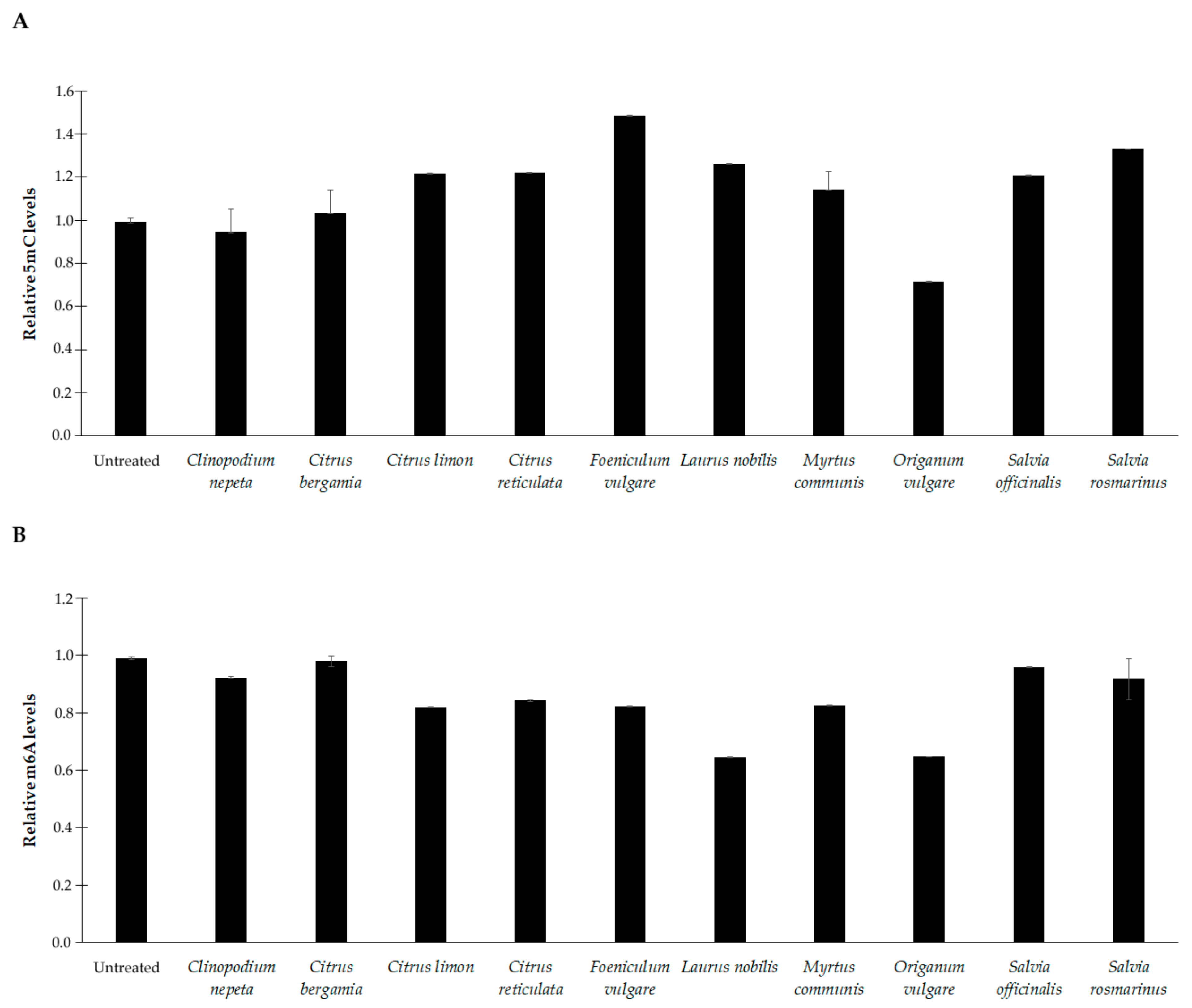
3.5. Effects of EOs on DNA Methylation Profiles
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bunse, M.; Daniels, R.; Gründemann, C.; Heilmann, J.; Kammerer, D.R.; Keusgen, M.; Lindequist, U.; Melzig, M.F.; Morlock, G.E.; Schulz, H.; et al. Essential Oils as Multicomponent Mixtures and Their Potential for Human Health and Well-Being. Front. Pharmacol. 2022, 13, 956541. [Google Scholar]
- Redondo-Blanco, S.; Fernández, J.; López-Ibáñez, S.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Plant Phytochemicals in Food Preservation: Antifungal Bioactivity: A Review. J. Food Prot. 2020, 83, 163–171. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential oils: Extraction, bioactivities, and their uses for food preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils-a review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar]
- Dorman, H.J.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Rezzoug, M.; Bakchiche, B.; Gherib, A.; Roberta, A.; Flamini, G.; Kilinçarslan, Ö.; Mammadov, R.; Bardaweel, S.K. Chemical composition and bioactivity of essential oils and Ethanolic extracts of Ocimum basilicum L. and Thymus algeriensis Boiss. & Reut. from the Algerian Saharan Atlas. BMC Complement. Altern. Med. 2019, 19, 146. [Google Scholar]
- Bardaweel, S.K.; Bakchiche, B.; ALSalamat, H.A.; Rezzoug, M.; Gherib, A.; Flamini, G. Chemical composition, antioxidant, antimicrobial and antiproliferative activities of essential oil of Mentha spicata L. (Lamiaceae) from Algerian Saharan atlas. BMC Complement. Altern. Med. 2018, 18, 201. [Google Scholar]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar]
- Camele, I.; Elshafie, H.S.; Caputo, L.; De Feo, V. Anti-quorum Sensing and Antimicrobial Effect of Mediterranean Plant Essential Oils Against Phytopathogenic Bacteria. Front. Microbiol. 2019, 10, 2619. [Google Scholar]
- Al-Haidari, R.A.; Shaaban, M.I.; Ibrahim, S.R.M.; Mohamed, G.A. Anti-quorum sensing activity of some medicinal plants. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 67–71. [Google Scholar] [CrossRef]
- Koh, C.L.; Sam, C.K.; Yin, W.F.; Tan, L.Y.; Krishnan, T.; Chong, Y.M.; Chan, K.G. Plant-derived natural products as sources of anti-quorum sensing compounds. Sensors 2013, 13, 6217–6228. [Google Scholar]
- D’Aquila, P.; Paparazzo, E.; Crudo, M.; Bonacci, S.; Procopio, A.; Passarino, G.; Bellizzi, D. Antibacterial Activity and Epigenetic Remodeling of Essential Oils from Calabrian Aromatic Plants. Nutrients 2022, 14, 391. [Google Scholar] [CrossRef]
- Fikry, S.; Khalil, N.; Salama, O. Chemical profiling, biostatic and biocidal dynamics of Origanum vulgare L. essential oil. AMB Express 2019, 9, 41. [Google Scholar] [CrossRef]
- Merghni, A.; Haddaji, N.; Bouali, N.; Alabbosh, K.F.; Adnan, M.; Snoussi, M.; Noumi, E. Comparative Study of Antibacterial, Antibiofilm, Antiswarming and Antiquorum Sensing Activities of Origanum vulgare Essential Oil and Terpinene-4-ol against Pathogenic Bacteria. Life 2022, 12, 1616. [Google Scholar] [CrossRef]
- Benzaid, C.; Belmadani, A.; Djeribi, R.; Rouabhia, M. The Effects of Mentha × piperita Essential Oil on C. albicans Growth, Transition, Biofilm Formation, and the Expression of Secreted Aspartyl Proteinases Genes. Antibiotics 2019, 8, 10. [Google Scholar] [CrossRef]
- De Sousa Guedes, J.P.; de Souza, E.L. Investigation of damage to Escherichia coli, Listeria monocytogenes and Salmonella Enteritidis exposed to Mentha arvensis L. and M. piperita L. essential oils in pineapple and mango juice by flow cytometry. Food Microbiol. 2018, 76, 564–571. [Google Scholar] [CrossRef]
- Marwa, C.; Fikri-Benbrahim, K.; Ou-Yahia, D.; Farah, A. African peppermint (Mentha piperita) from Morocco: Chemical composition and antimicrobial properties of essential oil. J. Adv. Pharm. Technol. Res. 2017, 8, 86–90. [Google Scholar]
- Kerekes, E.B.; Deák, É.; Takó, M.; Tserennadmid, R.; Petkovits, T.; Vágvölgyi, C.; Krisch, J. Anti-biofilm forming and anti-quorum sensing activity of selected essential oils and their main components on food-related micro-organisms. J. Appl. Microbiol. 2013, 115, 933–942. [Google Scholar] [CrossRef]
- Sousa, R.M.O.F.; Cunha, A.C.; Fernandes-Ferreira, M. The potential of Apiaceae species as sources of singular phytochemicals and plant-based pesticides. Phytochemistry 2021, 187, 112714. [Google Scholar]
- Uritu, C.M.; Mihai, C.T.; Stanciu, G.D.; Dodi, G.; Alexa-Stratulat, T.; Luca, A.; Leon-Constantin, M.M.; Stefanescu, R.; Bild, V.; Melnic, S.; et al. Medicinal Plants of the Family Lamiaceae in Pain Therapy: A Review. Pain Res. Manag. 2018, 2018, 7801543. [Google Scholar]
- Damasceno, C.S.B.; Fabri Higaki, N.T.; Dias, J.F.G.; Miguel, M.D.; Miguel, O.G. Chemical Composition and Biological Activities of Essential Oils in the Family Lauraceae: A Systematic Review of the Literature. Planta Med. 2019, 85, 1054–1072. [Google Scholar] [CrossRef]
- Saber, F.R.; Munekata, P.E.S.; Rizwan, K.; El-Nashar, H.A.S.; Fahmy, N.M.; Aly, S.H.; El-Shazly, M.; Bouyahya, A.; Lorenzo, J.M. Family Myrtaceae: The treasure hidden in the complex/diverse composition. Crit. Rev. Food Sci. Nutr. 2023, 7, 1–19. [Google Scholar]
- Li, Y.; Liu, S.; Zhao, C.; Zhang, Z.; Nie, D.; Tang, W.; Li, Y. The Chemical Composition and Antibacterial and Antioxidant Activities of Five Citrus Essential Oils. Molecules 2022, 27, 7044. [Google Scholar] [CrossRef]
- The Royal Botanic Gardens, Kew, Harvard University Herbaria & Libraries and Australian National Herbarium. International Plant Names Index. 2023. Available online: http://www.ipni.org (accessed on 25 April 2023).
- Mittal, R.P.; Rana, A.; Jaitak, V. Essential Oils: An Impending Substitute of Synthetic Antimicrobial Agents to Overcome Antimicrobial Resistance. Curr. Drug Targets 2019, 20, 605–624. [Google Scholar] [PubMed]
- Chandra, H.; Bishnoi, P.; Yadav, A.; Patni, B.; Mishra, A.P.; Nautiyal, A.R. Antimicrobial Resistance and the Alternative Resources with Special Emphasis on Plant-Based Antimicrobials—A Review. Plants 2017, 6, 16. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar]
- Xu, J.G.; Liu, T.; Hu, Q.P.; Cao, X.M. Chemical Composition, Antibacterial Properties and Mechanism of Action of Essential Oil from Clove Buds against Staphylococcus aureus. Molecules 2016, 21, 1194. [Google Scholar] [CrossRef]
- Ciprandi, A.; da Silva, W.M.; Santos, A.V.; de Castro Pimenta, A.M.; Carepo, M.S.; Schneider, M.P.; Azevedo, V.; Silva, A. Chromobacterium violaceum: Important insights for virulence and biotechnological potential by exoproteomic studies. Curr. Microbiol. 2013, 67, 100–106. [Google Scholar] [CrossRef]
- Jurado-Martín, I.; Sainz-Mejías, M.; McClean, S. Pseudomonas aeruginosa: An Audacious Pathogen with an Adaptable Arsenal of Virulence Factors. Int. J. Mol. Sci. 2021, 22, 3128. [Google Scholar] [CrossRef] [PubMed]
- Brinkwirth, S.; Ayobami, O.; Eckmanns, T.; Markwart, R. Hospital-acquired infections caused by enterococci: A systematic review and meta-analysis, WHO European Region, 1 January 2010 to 4 February 2020. Eurosurveillance 2021, 26, 2001628. [Google Scholar]
- Anderson, A.C.; Jonas, D.; Huber, I.; Karygianni, L.; Wölber, J.; Hellwig, E.; Arweiler, N.; Vach, K.; Wittmer, A.; Al-Ahmad, A. Enterococcus faecalis from Food, Clinical Specimens, and Oral Sites: Prevalence of Virulence Factors in Association with Biofilm Formation. Front. Microbiol. 2016, 6, 1534. [Google Scholar] [CrossRef]
- Debbabi, H.; Mokni, R.E.; Chaieb, I.; Nardoni, S.; Maggi, F.; Caprioli, G.; Hammami, S. Chemical Composition, Antifungal and Insecticidal Activities of the Essential Oils from Tunisian Clinopodium nepeta subsp. nepeta and Clinopodium nepeta subsp. glandulosum. Molecules 2020, 25, 2137. [Google Scholar] [CrossRef]
- Arantes, S.M.; Piçarra, A.; Guerreiro, M.; Salvador, C.; Candeias, F.; Caldeira, A.T.; Martins, M.R. Toxicological and pharmacological properties of essential oils of Calamintha nepeta, Origanum virens and Thymus mastichina of alentejo (Portugal). Food Chem. Toxicol. 2019, 133, 110747. [Google Scholar] [CrossRef] [PubMed]
- Thielmann, J.; Muranyi, P.; Kazman, P. Screening essential oils for their antimicrobial activities against the foodborne pathogenic bacteria Escherichia coli and Staphylococcus aureus. Heliyon 2019, 5, e01860. [Google Scholar] [CrossRef] [PubMed]
- Puškárová, A.; Bučková, M.; Kraková, L.; Pangallo, D.; Kozics, K. The antibacterial and antifungal activity of six essential oils and their cyto/genotoxicity to human HEL 12469 cells. Sci. Rep. 2017, 7, 8211. [Google Scholar] [CrossRef]
- Dadalioglu, I.; Evrendilek, G.A. Chemical compositions and antibacterial effects of essential oils of Turkish oregano (Origanum minutiflorum), bay laurel (Laurus nobilis), Spanish lavender (Lavandula stoechas L.), and fennel (Foeniculum vulgare) on common foodborne pathogens. J. Agric. Food Chem. 2004, 52, 8255–8260. [Google Scholar] [CrossRef]
- Ruberto, G.; Baratta, M.T.; Deans, S.G.; Dorman, H.J. Antioxidant and antimicrobial activity of Foeniculum vulgare and Crithmum maritimum essential oils. Planta Med. 2000, 66, 687–693. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef]
- Kavanaugh, N.L.; Ribbeck, K. Selected antimicrobial essential oils eradicate Pseudomonas spp. and Staphylococcus aureus biofilms. Appl. Environ. Microbiol. 2012, 78, 4057–4061. [Google Scholar] [CrossRef]
- Xavier, V.; Finimundy, T.C.; Heleno, S.A.; Amaral, J.S.; Calhelha, R.C.; Vaz, J.; Pires, T.C.S.P.; Mediavilla, I.; Esteban, L.S.; Ferreira, I.C.F.R.; et al. Chemical and Bioactive Characterization of the Essential Oils Obtained from Three Mediterranean Plants. Molecules 2021, 26, 7472. [Google Scholar] [CrossRef]
- Nostro, A.; Roccaro, A.S.; Bisignano, G.; Marino, A.; Cannatelli, M.A.; Pizzimenti, F.C.; Cioni, P.L.; Procopio, F.; Blanco, A.R. Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Med. Microbiol. 2007, 56, 519–523. [Google Scholar] [CrossRef]
- Bomfim de Barros, D.; de Oliveira E Lima, L.; Alves da Silva, L.; Cavalcante Fonseca, M.; Ferreira, R.C.; Diniz Neto, H.; da Nóbrega Alves, D.; da Silva Rocha, W.P.; Scotti, L.; de Oliveira Lima, E.; et al. α-Pinene: Docking Study, Cytotoxicity, Mechanism of Action, and Anti-Biofilm Effect against Candida albicans. Antibiotics 2023, 12, 480. [Google Scholar] [CrossRef]
- Di Napoli, M.; Castagliuolo, G.; Badalamenti, N.; Maresca, V.; Basile, A.; Bruno, M.; Varcamonti, M.; Zanfardino, A. Antimicrobial, Antibiofilm, and Antioxidant Properties of Essential Oil of Foeniculum vulgare Mill. Leaves. Plants 2022, 11, 3573. [Google Scholar] [CrossRef] [PubMed]
- Polito, F.; Kouki, H.; Khedhri, S.; Hamrouni, L.; Mabrouk, Y.; Amri, I.; Nazzaro, F.; Fratianni, F.; De Feo, V. Chemical Composition and Phytotoxic and Antibiofilm Activity of the Essential Oils of Eucalyptus bicostata, E. gigantea, E. intertexta, E. obliqua, E. pauciflora and E. tereticornis. Plants 2022, 11, 3017. [Google Scholar] [CrossRef] [PubMed]


| Essential Oil | Family | Extraction Method | Chemical Composition | % | Retention Index | Cultivation Area | Time of Collection | Batch N. |
|---|---|---|---|---|---|---|---|---|
| Clinopodium nepeta | Lamiaceae | Hydro distillation from fresh collected material | piperitone oxide | 34.28 | 16.14 | Briatico, Province of Vibo Valentia, Calabria Region, Southern Italy Michele Crudo Farm | July 2021 | 20210715 |
| piperitenone oxide | 18.23 | 19.72 | ||||||
| (+)-limonene | 15.8 | 8.49 | ||||||
| (+)-pulegone | 13.75 | 15.6 | ||||||
| menthone | 8.32 | 12.77 | ||||||
| isolegylacetate | 3.64 | 17.92 | ||||||
| 1-terpine-4-ol | 1.4 | 13.56 | ||||||
| (+)-neomenthol | 1.37 | 13.25 | ||||||
| β-pinene | 1.22 | 6.92 | ||||||
| Citrus bergamia | Rutaceae | Mechanical extraction through industrial cold expression process from fresh fruit | (+)-limonene | 15.89 | 34.28 | Bovalino, Province of Reggio Calabria, Calabria Region, Southern Italy Luigi Frammartino Farm | December 2021 | 20211221 |
| lynalyl acetate | 10.78 | 11.54 | ||||||
| (+)-linalool | 9.38 | 6.79 | ||||||
| α-terpinene | 8.44 | 38.88 | ||||||
| β-pinene | 6.87 | 5.49 | ||||||
| α-pinene | 5.64 | 1.22 | ||||||
| Citrus limon (L.) | Rutaceae | Hydro distillation from fresh collected material | (+)-limonene | 14.13 | 3.01 | Bovalino, Province of Reggio Calabria, Calabria Region, Southern Italy Luigi Frammartino Farm | November 2021 | 2021118 |
| α-terpinene | 13.59 | 1.26 | ||||||
| β-pinene | 10.28 | 1.67 | ||||||
| α-terpineol | 9.36 | 11.91 | ||||||
| α-terpinolene | 8.42 | 74.41 | ||||||
| 1-Terpine-4-ol | 6.84 | 4.34 | ||||||
| Citrus reticulata | Rutaceae | Hydro distillation from fresh collected material | (+)-sabinene | 12.6 | 1.44 | Briatico, Province of Vibo Valentia, Calabria Region, Southern Italy Michele Crudo Farm | February 2021 | 20210215 |
| (+)-linalool | 10.76 | 18.27 | ||||||
| α-phellandrene | 10.27 | 1.37 | ||||||
| β-cis-ocimene | 9.36 | 1.14 | ||||||
| (+)-limonene | 8.96 | 6.45 | ||||||
| β-myrcene | 8.42 | 5.04 | ||||||
| β-pinene | 7.76 | 6.54 | ||||||
| α-pinene | 7.16 | 2.37 | ||||||
| β-citronellal | 6.86 | 2.35 | ||||||
| α-terpinolene | 6.72 | 50.91 | ||||||
| α-terpinene | 5.63 | 1.93 | ||||||
| Foeniculum vulgare subsp. piperitum | Apiaceae | Hydro distillation from fresh collected material | estragole | 17.21 | 14.54 | Briatico, Province of Vibo Valentia, Calabria Region, Southern Italy Michele Crudo Farm | September 2021 | 20210918 |
| α-pinene | 14.19 | 45.33 | ||||||
| anethal | 10.41 | 11.24 | ||||||
| fenchone | 8.42 | 8.49 | ||||||
| α-limonene | 7.72 | 2.51 | ||||||
| α-phellandrene | 7.18 | 1.05 | ||||||
| β-pinene | 6.86 | 1.65 | ||||||
| β-myrcene | 5.63 | 14.71 | ||||||
| Laurus nobilis L. | Lauraceae | Hydro distillation from fresh collected material | eucalyptol | 21.07 | 1.51 | Briatico, Province of Vibo Valentia, Calabria Region, Southern Italy Michele Crudo Farm | April 2021 | 20210413 |
| (+)-sabinene | 19.18 | 6.48 | ||||||
| (+)-linalool | 13.6 | 1.29 | ||||||
| terpinyl acetate | 10.79 | 7.38 | ||||||
| α-pinene | 8.56 | 56.61 | ||||||
| methyleugenol | 6.73 | 15.74 | ||||||
| 1-terpine-4-ol | 5.64 | 5.65 | ||||||
| Myrtus communis L. | Myrtaceae | Hydro distillation from fresh collected material | eucalyptol | 20.27 | 1.88 | Briatico, Province of Vibo Valentia, Calabria Region, Southern Italy Michele Crudo Farm | May 2021 | 20210521 |
| (−)-myrtenylacetate | 18.43 | 17.04 | ||||||
| α-pinene | 15.92 | 3.88 | ||||||
| (+)-limonene | 14.16 | 2.1 | ||||||
| (+)-linalool | 10.82 | 10.43 | ||||||
| lynalyl acetate | 10.33 | 1.1 | ||||||
| geraniol acetate | 9.44 | 1.09 | ||||||
| α-terpineol | 9.04 | 1.58 | ||||||
| β-ocimene | 8.59 | 33.04 | ||||||
| α-phellandrene | 8.49 | 10.81 | ||||||
| o-cymene | 8.35 | 1.41 | ||||||
| terpinolene | 7.79 | 1.41 | ||||||
| terpinene | 5.69 | 12.33 | ||||||
| Origanum vulgare L. subsp. viridulum | Lamiaceae | Hydro distillation from fresh collected material | p-thymol | 20.48 | 4.88 | Briatico, Province of Vibo Valentia, Calabria Region, Southern Italy Michele Crudo Farm | July 2021 | 20210720 |
| terpinene | 16.77 | 47.31 | ||||||
| p-cymene | 16.42 | 3.52 | ||||||
| β-caryophyllene | 8.63 | 18.52 | ||||||
| β-myrcene | 7.61 | 11.78 | ||||||
| carvacrol | 7.36 | 3.18 | ||||||
| terpinolene | 6.55 | 3.76 | ||||||
| α-thujene (origanene) | 5.15 | 1.23 | ||||||
| α-pinene | 4.96 | 2.73 | ||||||
| Salvia officinalis L. | Lamiaceae | Hydro distillation from fresh collected material | eucalyptol | 22.6 | 5.54 | Briatico, Province of Vibo Valentia, Calabria Region, Southern Italy Michele Crudo Farm | May 2021 | 20210504 |
| (−)-α-thujone | 21.5 | 2.85 | ||||||
| β-pinene | 12.45 | 9.59 | ||||||
| (−)-camphor | 11.44 | 4.35 | ||||||
| α-humulene | 11.07 | 24.14 | ||||||
| (−)-β-thujone | 8.58 | 23.7 | ||||||
| α-pinene | 7.23 | 2.26 | ||||||
| (−)-β-caryophyllene | 6.9 | 15.1 | ||||||
| β-myrcene | 6.75 | 1.13 | ||||||
| C camphene | 6.12 | 1.88 | ||||||
| (+)-sabinene | 5.67 | 3.99 | ||||||
| Salvia rosmarinus | Lamiaceae | Hydro distillation from fresh collected material | eucalyptol | 21.52 | 1.17 | Briatico, Province of Vibo Valentia, Calabria Region, Southern Italy Michele Crudo Farm | April 2021 | 20210421 |
| α-pinene | 13.31 | 2.28 | ||||||
| β-pinene | 12.45 | 3.66 | ||||||
| camphene | 8.56 | 49.29 | ||||||
| (−)-camphor | 7.21 | 1.79 | ||||||
| isoborneol | 6.89 | 9.26 | ||||||
| β -myrcene | 6.1 | 6.7 | ||||||
| (−)-β-caryophyllene | 5.66 | 22.84 |
| Essential Oils | C. violaceum | E. faecalis | ||||||
|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| C. nepeta | 0.80 | 0.07 | 0.80 | 0.07 | 4.00 | 0.00 | 4.00 | 0.00 |
| C. bergamia | 3.00 | 0.00 | 3.00 | 0.00 | 3.00 | 0.00 | 3.00 | 0.00 |
| C. limon | 5.00 | 0.07 | 5.00 | 0.00 | 4.00 | 0.00 | 4.00 | 0.00 |
| C. reticulata | 5.00 | 0.07 | 5.00 | 0.00 | 4.00 | 0.00 | 4.00 | 0.00 |
| F. vulgare | 0.40 | 0.00 | 0.40 | 0.00 | 3.00 | 0.07 | 3.00 | 0.07 |
| L. nobilis | 1.00 | 0.00 | 1.00 | 0.00 | 2.00 | 0.07 | 2.00 | 0.07 |
| M. communis | 2.00 | 0.00 | 2.00 | 0.00 | 3.00 | 0.07 | 3.00 | 0.07 |
| O. vulgare | 0.40 | 0.00 | 0.40 | 0.00 | 0.60 | 0.00 | 0.60 | 0.00 |
| S. officinalis | 2.00 | 0.00 | 2.00 | 0.00 | 3.00 | 0.00 | 3.00 | 0.00 |
| S. rosmarinus | 2.00 | 0.00 | 2.00 | 0.00 | 3.00 | 0.00 | 3.00 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Aquila, P.; Sena, G.; Crudo, M.; Passarino, G.; Bellizzi, D. Effect of Essential Oils of Apiaceae, Lamiaceae, Lauraceae, Myrtaceae, and Rutaceae Family Plants on Growth, Biofilm Formation, and Quorum Sensing in Chromobacterium violaceum, Pseudomonas aeruginosa, and Enterococcus faecalis. Microorganisms 2023, 11, 1150. https://doi.org/10.3390/microorganisms11051150
D’Aquila P, Sena G, Crudo M, Passarino G, Bellizzi D. Effect of Essential Oils of Apiaceae, Lamiaceae, Lauraceae, Myrtaceae, and Rutaceae Family Plants on Growth, Biofilm Formation, and Quorum Sensing in Chromobacterium violaceum, Pseudomonas aeruginosa, and Enterococcus faecalis. Microorganisms. 2023; 11(5):1150. https://doi.org/10.3390/microorganisms11051150
Chicago/Turabian StyleD’Aquila, Patrizia, Giada Sena, Michele Crudo, Giuseppe Passarino, and Dina Bellizzi. 2023. "Effect of Essential Oils of Apiaceae, Lamiaceae, Lauraceae, Myrtaceae, and Rutaceae Family Plants on Growth, Biofilm Formation, and Quorum Sensing in Chromobacterium violaceum, Pseudomonas aeruginosa, and Enterococcus faecalis" Microorganisms 11, no. 5: 1150. https://doi.org/10.3390/microorganisms11051150
APA StyleD’Aquila, P., Sena, G., Crudo, M., Passarino, G., & Bellizzi, D. (2023). Effect of Essential Oils of Apiaceae, Lamiaceae, Lauraceae, Myrtaceae, and Rutaceae Family Plants on Growth, Biofilm Formation, and Quorum Sensing in Chromobacterium violaceum, Pseudomonas aeruginosa, and Enterococcus faecalis. Microorganisms, 11(5), 1150. https://doi.org/10.3390/microorganisms11051150







