Abstract
Microfluidics is a system involving the treatment or manipulation of microscale (10−9 to 10−18 L) fluids using microchannels (10 to 100 μm) contained on a microfluidic chip. Among the different methodologies used to study intestinal microorganisms, new methods based on microfluidic technology have been receiving increasing attention in recent years. The intestinal tracts of animals are populated by a vast array of microorganisms that have been established to play diverse functional roles beneficial to host physiology. This review is the first comprehensive coverage of the application of microfluidics technology in intestinal microbial research. In this review, we present a brief history of microfluidics technology and describe its applications in gut microbiome research, with a specific emphasis on the microfluidic technology-based intestine-on-a-chip, and also discuss the advantages and application prospects of microfluidic drug delivery systems in intestinal microbial research.
1. Introduction
The intestinal tract is not only the largest digestive organ in the human body, but also the habitat of a diverse population of microorganisms. The intestinal microflora include symbiotic microorganisms that colonize the intestinal tract and grow synergistically with epithelial cells [1]. This diverse community, comprising bacteria, fungi, viruses, and bacteriophages, is essential to the maintenance of intestinal homeostasis [2]. Human microbiota gene sequencing studies have revealed that an imbalance in the intestinal microbiota is associated with a range of diseases, including inflammatory bowel disease, metabolic syndrome, non-alcoholic fatty liver disease, and colorectal cancer [3,4,5,6]. However, current knowledge regarding these associations is relatively rudimentary and warrants further extensive investigation [7].
In recent years, the development of microfluidic technology has presented an array of new opportunities for studying the gut microbiome in a more precise and controlled manner. In this context, the intestine-on-a-chip approach provides a convenient platform that can applied to reconstruct the interface between the intestinal lumen and capillary tissues, thereby simulating, in vitro, the in vivo microenvironment (oxygen gradient, oxygen partial pressure, carbon dioxide concentration, and immune components) [8]. In these chips, several channels can be used for co-culturing microvascular endothelial cells, immune cells, and commensal and pathogenic microorganisms obtained from the human body. Thus, by applying microfluidic chips to construct on-chip gut models, microfluidic technology can more accurately simulate the in vivo microenvironment and investigate the interactions between microorganisms and the gut environment. In addition, these chips can be used to fabricate microcapsules to develop microfluidic drug delivery systems [9]. These chips utilize alginate, chitosan, whey protein, and similar biocompatible substances to encapsulate microorganisms within microcapsules, thereby protecting living microorganisms from being directly exposed to the external environment, and also enabling their use in the human body [10,11]. Such microfluidic drug delivery systems can be used to deliver active microorganisms to the intestine, and thereby facilitate investigations assessing the effects of gut microbiota on the host.
Microfluidic technology accordingly has the advantages of simulating the actual physiological environment of the host gut, co-cultivating multiple microorganisms, co-cultivating microorganisms with host cells, and delivering active microorganisms, thereby emphasizing the potential utility of this technology for the study of the gut microbiota and host–microbiota interactions. Microfluidic technology thus provides a powerful tool for in-depth research on the structure and function of the gut microbiota, and its relevance to host health.
2. The Development of Microfluidics
Microfluidic technology was first proposed in 1990 by Manz et al. [12], and was implemented the following year on a flat microchip to achieve capillary electrophoresis. In 1994, Jacobson et al. improved the injection method, thereby enhancing the performance and practicability of microfluidic chip capillary electrophoresis [13]. In 1995, Mathies et al. established high-speed DNA sequencing on chips using microfluidic technology [14]. In 1996, polymerase chain reaction (PCR) and capillary electrophoresis were integrated on microfluidic chips [15], and in the following year, multi-channel capillary electrophoresis DNA sequencing was achieved on microfluidic chips [16]. In 2002, in a report titled “Microfluidic large-scale integrated chip” published in magazine, Thorsen et al. introduced microfluidic chips integrated with thousands of microvalves and hundreds of microreactors [17], marking a fundamental leap from simple electrophoresis technology to elaborate multifunctional integrated laboratories. The history of microfluidic chip development is summarized in Table 1.

Table 1.
A summary of the history of microfluidic technology development.
With ongoing advances in material science, the manufacture of microfluidic chips has become increasingly complex, and their applications are becoming more extensive and interdisciplinary. For example, scientists have performed short-term analyses of cells on microfluidic chips and demonstrated the migration and functions of cells in microchannels [24]. To date, viable cell culture chips have been designed with a controllable microenvironment that can simulate the functions and characteristics of living organs. Microfluidic chips provide a powerful tool to mimic human intestinal physiological and pathological conditions, and can be used to assess the associations of these condition with the gut microbiota, as well as providing a platform for the development and evaluation of drugs [25]. In this latter regard, advances in droplet and electrospray microfluidic technology have enabled the development of microfluidic drug delivery systems that can be used to prepare drug-loaded microcapsules that protect the active components and facilitate the controlled release of drugs and other active substances in vivo [26,27,28]. Figure 1A shows a selection of the different types of microfluidic chips designed to date.
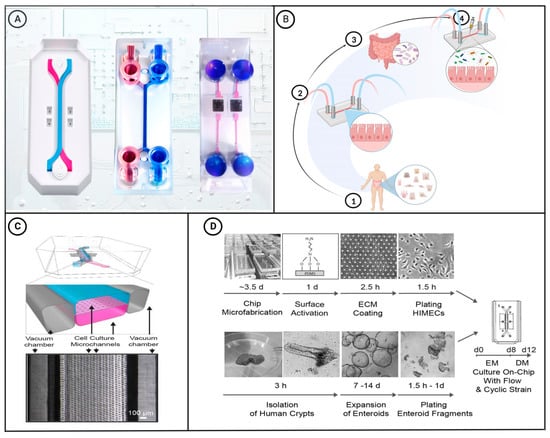
Figure 1.
(A) Schematic diagram of commercially available microfluidic chips. The diagrams on the left and middle show two-channel organ-on-a-chip devices. These chips can be used to create colon-intestine chips, duodenum-intestine chips, liver chips, and other types of organ chips [29]. The two-channel microfluidic chip on the right connects the culture well in its center to the microfluidic channel through a porous membrane. This type of chip is commonly used for research on gas–liquid interfaces and the endothelial–epithelial barrier. (B) Construction of a microfluidic intestinal chip with the capacity for microbial co-culture: ① Intestinal epithelial cells are extracted from the human body via biopsy. ② The obtained epithelial cells are then cultured in a microfluidic intestinal chip. ③ Gut microbiota required for the experiment are extracted. ④ The microbiota are introduced into the microfluidic intestinal chip for co-culture with the epithelial cells. [30]. (C) A schematic cross-sectional view (top) and a phase contrast micrograph of the intestine chip viewed from above (bottom) showing the upper (epithelial; blue) and lower (microvascular; pink) cell culture microchannels separated by a porous, ECM-coated, PDMS membrane sandwiched in between. The membrane is elastic and can be extended and retracted by the application of a cyclic vacuum to the hollow side chambers. This vacuum actuation results in outward deflection of the vertical side walls and lateral extension of the attached horizontal porous elastic membrane, leading to mechanical deformation of the adherent tissue layers cultured in the central channels [31]. (D) This figure shows a step-by-step schematic representation of the procedure for establishing microfluidic co-cultures of primary human intestinal epithelium and intestinal microvascular endothelium in the Intestine Chip [31]. Figure 1C,D have been edited and modified with permission from [31]. Copyright 2018 Authors, licensed under a Creative Commons Attribution 4.0 International License.
3. Application of Microfluidic Intestine-on-a-Chip
Microfluidic intestinal chips create a 3D model of the living intestine established in the chip cavity to accurately reflect the physiological and pathological status of the human body. For example, chips can be designed to simulate the biomechanical characteristics of intestinal peristalsis and fluid flow, thereby enabling intestinal epithelial cells cultured on the chip to differentiate normally and form a miniature intestinal villous structure. Importantly, using these chips, epithelial cells and intestinal microorganisms can be co-cultured for varying lengths of time, from several days to several weeks, thereby facilitating long-term experimentation [30,32]. The process of microfluidic intestinal chip construction is illustrated in Figure 1B. Intestine-on-a-chip systems can simulate the physiological microenvironment of the intestine for culturing gut microorganisms, and in this regard, Kasendra et al. have created a system that combines the epithelial and microvascular endothelial cells obtained from intestinal biopsy samples and simulated the liquid flow and peristaltic movement of the intestinal lumen [31]. The gut-on-a-chip shown in Figure 1C, which is designed to simulate the liquid flow and peristaltic motion in the intestinal lumen, comprises a flexible transparent PDMS support layer with upper and lower microfluidic channels and left and right lateral chambers separated by a porous PDMS membrane coated with an extracellular matrix. The stretchable nature of PDMS enables the chip to adjust to the negative pressure applied to the side chambers in response to cyclic suction applied to the vacuum side via tubing. This cyclic suction causes the central porous PDMS membrane to stretch and the monolayer of Caco-2 cells on the membrane to undergo mechanical deformation, thereby mimicking the physiological peristaltic motion of the intestinal lumen at a frequency of 0.15 Hz. Additionally, the chip can simulate the flow of fluid in the intestinal lumen by controlling the low-rate (30 μL h−1) liquid flow in the central microchannel, which generates a low shear stress of 0.02 dyne cm−2 [33]. Figure 1D presents a schematic depiction of the process of creating microfluidic co-cultures between primary human intestinal epithelium and intestinal microvascular endothelium using the Intestine Chip. Cells cultured on such chips can differentiate into four types of intestinal cells, namely, absorptive, mucus-secreting, intestinal endocrine, and Panetta cells, which spontaneously undergo development to yield intestinal villi and intestinal crypt structures [34]. Using a similar system, Kim et al. co-cultured human intestinal epithelial cells, Caco-2 cells, and intestinal bacteria (Lactobacillus rhamnosus) on an intestine-on-a-chip that simulated the peristaltic movement and fluid flow of the intestinal lumen, with the co-culture of L. rhamnosus and epithelial cells on the chip extending for more than 2 weeks [35]. Compared with cells grown using traditional culture methods, those grown on chips can more closely simulate the barrier function of the intestinal villi, cytochrome P450 activity, and apical mucus secretion.
The intestine-on-a-chip also provides a platform for studying the pathogenic mechanisms of different microorganisms. Using an intestine-on-a-chip, Villenave et al. introduced coxsackievirus B1 (CVB1) to infect villi of the small intestine and reported active replication and apical release of virus particles in epithelial cells, along with cytopathic effects and the production of inflammatory cytokines (IP-10 and IL-8) [36]. The apical release of viral particles on a chip has been similarly reported for Sendai virus [37], human parainfluenza virus type 3 (HPIV3) [38], respiratory syncytial virus [39], and the viruses causing measles [40], mumps [41], and hepatitis A [42]. With respect to bacterial infection, Grassart et al. successfully replicated Shigella flexneri infection on a chip [43] and showed that bacteria successfully accumulated in the intestinal crypts formed on the chip and invaded the intestinal epithelial villi, thereby resulting in a loss of structural integrity. Notably, the cyclic strain and shear stress in the intestinal microenvironment was found to enhance the invasive efficacy and transmission of S. flexneri [43]. Furthermore, Tovaglieri et al. constructed a dual-channel colon chip lined with primary human colon epithelial cells [44], to which they introduced metabolites of mouse or human intestinal microbiota harboring the metabolites enterohemorrhagic Escherichia coli (EHEC). The authors showed that human microbial metabolites enhanced EHEC-induced intestinal epithelial injury, thereby providing evidence that intestine-on-a-chip technology has broader application for translational research studies.
4. Advantages and Disadvantages of Intestine-on-a-Chip
The intestine-on-a-chip system simulates the intestinal environment in a manner that preserves the spatial structure of intestinal epithelial tissue, the interaction between epithelial cells, the interactions between epithelial cells and microorganisms, and peristalsis of the intestinal tract, thereby enabling the accurate replication of a range of different physiological conditions [45]. The physiological microenvironment of a given intestine-on-a-chip is collectively established by the integration of different characteristics, including the 3D intestinal villous structure, multi-lineage cell differentiation, epithelial barrier function, brush border enzyme activity, and mucus production. Moreover, compared with Caco-2 cells cultured using traditional methods, cells cultured on an intestine-on-a-chip are more similar to human duodenal epithelial cells in terms of morphology, structure, multicellular composition, and gene expression patterns [31]. Moreover, these chips can be integrated with in situ visualization instrumentation, such as two-photon or rotating disk microscopes, to visually monitor the co-culture conditions of intestinal epithelium and microorganisms. This coupling is achieved by continuously collecting aliquots of liquid flowing through the chip channels and quantitatively detecting metabolites that reflect the status of a diverse range of processes and conditions, including digestion, secretion, intestinal barrier function, and microbial infection [46].
The intestine-on-a-chip platform provides a controlled micro-platform that facilitates the study of the complex associations between the intestine and microorganisms based on relevant pathophysiological indices. These include the independent or combined effects of microorganisms, lipopolysaccharides (LPSs), immune cells, inflammatory cytokines, and mechanical forces on the maintenance of intestinal homeostasis, which can contribute to elucidating the mechanisms underlying intestinal inflammation, villous damage, and the damaged barrier function of epithelial cells. In addition, the physiological or pathological activities relating to specific areas of the intestinal tract can be simulated by introducing agents such as immune cells or microorganisms to the intestine-on-a-chip to enable a prospective analysis of different intestinal diseases [47].
Despite these multiple advantages, however, these chips have a high technical threshold and a slow, complex process that is not well-suited to rapid analyses. Although some progress has been made in combining bacterial cultures with gut-specific microarrays, the small size of these chips, slow flow rate, and complex technology required to meet the necessary oxygen demand tends to limit the broader application of these chips [48]. In addition, the continuous co-culture of microbiota and cells has yet to be realized using existing intestine-on-a-chip platforms, nor can they integrate the regulatory effects of the endocrine and nervous systems, which renders these systems unsuitable for studying chronic intestinal diseases.
5. Application of Microfluidic Drug Delivery Systems
Drug delivery systems, among which are nanoparticles [49], gel complexes [50], and enteric coating, are routinely applied in research on intestinal microorganisms [51]. As an advanced technology, microfluidics can be used to synthesize functional microcapsules with tailored size, morphology, and structure to deliver active payloads [52,53,54,55]. The drug delivery systems based on this technology can facilitate drug pre-programming and controlled drug delivery, and can be applied to supplement the human body with prebiotics, probiotics, and other active substances [56]. Among microfluidic drug delivery systems, droplet microfluidics uses a fluid to provide a driving force that manipulates liquid droplets, whereas microfluidic electrospray technology utilizes static electricity to manipulate these droplets [57].
Droplet microfluidic technology can be used for delivering active bacteriophages into the intestine, wherein these viruses can influence the intestinal flora by disrupting intestinal bacteria, regulating the immune response, and mediating anti-inflammatory responses. Bacteriophages are an important component of the intestinal microbiota [58], and it has been reported that high-dose phage samples show better antibacterial effects [59,60,61,62,63,64]. Phage delivered via the oral route are typically exposed to gastric acid, bile, and digestive enzymes, which collectively contribute to reducing the phage titer [65], thus reducing the efficacy of phage therapy [66,67]. As shown in Figure 2A, Vinner and Malik used droplet microfluidic chips to microencapsulate bacteriophages, thereby facilitating transport in the active condition [68]. To produce a continuous oil phase, Miglyol 840, a propylene glycol diester of caprylic/capric acid, and castor oil were mixed in a 50:50 ratio, along with 5% (w/v) of the oil-soluble surfactant polyglycerol polyricinoleate (PGPR). The dispersed phase consisted of a mixture of the methyl methacrylate co-methacrylic acid copolymer Eudragit® S100, alginate, and bacteriophages. Using a pressure pump, the two immiscible fluids were brought together at the perpendicular intersection of a T-junction channel, and under the influence of pressure and shear forces, the flowing phase fragmented the dispersed phase, forming droplets, as shown in Figure 2B [69]. Figure 2C shows a real-time micrograph of droplet generation. The resulting emulsion was collected in an acidified oil phase containing 0.05 M p-toluenesulfonic acid in Miglyol and 5% (w/v) PGPR, after which the emulsion was left to crosslink for a minimum of 2 h.
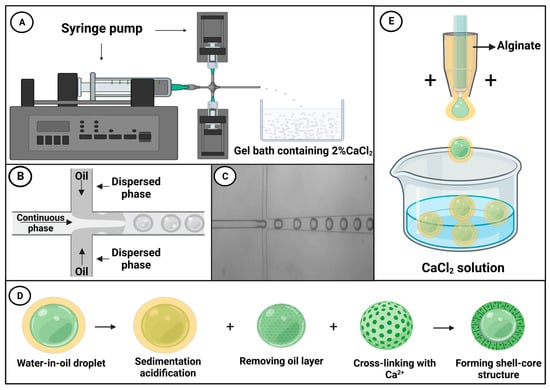
Figure 2.
(A) A simple diagram showing a droplet microfluidic system, in which a droplet microfluidic chip is used to generate droplets that are subsequently cured in a 2% CaCl2 gel to yield microcapsules [68]. (B) A schematic diagram of droplet microfluidic technology [69]. (C) An optical micrograph of the microparticle encapsulation process. (D) Following water-in-oil particle settlement and acidification using an acidified oil, the oil layer and the acidified oil are removed from the droplets, after which CaCl2 is added to crosslink and solidify the alginate ions, thereby generating microspheres with a core–shell structure [70]. (E) A schematic diagram of a device used to produce microcapsules via microfluidic electrospray [71].
Having settled and been acidified by the application of acidified oil, the oil layer and the acidified oil are removed from the liquid drops, and calcium chloride (CaCl2) is added to induce ionic crosslinking and generate microcapsules, as shown in Figure 2D Exposure of these microcapsules to simulated gastric fluid (pH = 1) for 2 h revealed that there was no reduction in phage titer, and the subsequent release of these bacteriophages in simulated intestinal fluid (pH = 7) verified that this pH-induced microcapsule release is suitable for targeted delivery of active microorganisms to the gastrointestinal tract. Moreover, the rate of bacteriophage release (or that of other payloads) can be adjusted by controlling capsule size. Bacteriophages can be wrapped in microcapsules of differing size (50–100 μm), with more rapid release being achieved using smaller capsules. In contrast, whereas larger capsules are also characterized by rapid release during the early stage of delivery, release is slower and more sustained during the latter stages.
When combined with probiotics, microfluidic electrospray technology can be applied for the treatment of metabolic syndrome (MetS). Probiotics are active microorganisms that are beneficial to host health, playing important roles in maintaining gut health by correcting intestinal flora imbalance and protecting against pathogen invasion, which is considered an important strategy for MetS treatment [72,73]. In this context, Zhao et al. used microfluidic electrospray chips to produce binuclear probiotic microcapsules, in which alginate was used as an external phase and the microspheres containing two species of probiotic bacteria (Lactobacillus and Bacillus subtilis) were electrosprayed as an internal phase (Figure 2E) [71]. Ionic cross-linking of the sodium alginate encapsulated the probiotic microspheres within a core-shell structure, which was further covered in a dietary fiber shell that protects the contents from gastric juice attack. The alginate shell was pH-responsive, and, consequently, whereas the contents of these microcapsules are protected from the influence of gastric acid in the stomach, they are efficiently released on entering the intestinal lumen (Figure 3). A mouse model of MetS was generated based on feeding mice a high-fat diet, and these animals exhibited the characteristics of abnormal liver fat metabolism, loss of intestinal barrier proteins, and deformed intestinal villous morphology [74]. Mice to which the probiotic microcapsules had been administered, via gavage, were found to have reduced liver fat deposits and enhanced levels of intestinal barrier proteins. These findings thus indicate that such microcapsules can be used to protect the intestinal barrier, reduce liver fat deposition, and improve MetS. Moreover, such dual-core microcapsules enabled the use of different combinations of probiotic organisms, thereby enabling the exploitation of synergistically beneficial effects.
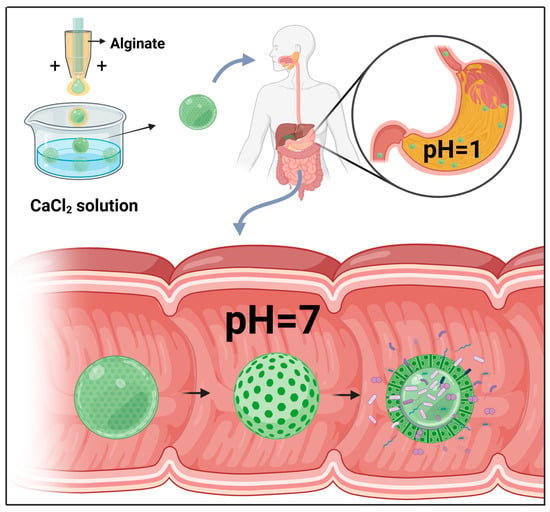
Figure 3.
Oral delivery of microcapsules produced using a liquid droplet microfluidic chip. Given the specific pH-induced release characteristic of these microcapsules, they are protected from attack by gastric fluid, whereas the contents of active microorganisms are effectively released within the intestinal fluid.
Microfluidic electrospray techniques can also be used to generate microcapsules containing detoxified LPS, an outer membrane component of gram-negative bacteria and a causative factor of multiple diseases and disorders, including sepsis, septic shock, and MetS [75,76,77,78]. Within the human body, LPS is mainly derived from intestinal microorganisms [79]. The intestinal barrier plays an important role in combating the harmful effects of different microbial toxins, including LPS [80,81,82], which is inactivated by the action of alkaline phosphatase, thereby playing a vital role in the maintenance of intestinal homeostasis and disease prevention [83,84,85]. The intestinal degradation of microcapsules leads to the release of their contents into the intestine, which thereby contributes to strengthening the bionic barrier, as confirmed by internal computerized tomography imaging and in vivo imaging system analysis [86]. Furthermore, microcapsule contents can be released along the intestinal tract and form a continuous barrier, which in turn blocks LPS permeation and its detrimental effects on intestinal epithelial cells. This effectively suppresses the influence of harmful intestinal microorganisms on the intestinal mucosa, thereby contributing to the maintenance of enteric health [87]. Table 2 summarizes the applications of different microfluidic platforms in gut microbiota research.

Table 2.
A summary of the applications of different microfluidic platforms in gut microbiota research.
6. Advantages and Disadvantages of Microfluidic Drug Delivery Systems
The emergence of microfluidic technology has radically transformed the manufacture and development of microcapsules [89]. Among the available techniques, droplet microfluidic technology is most commonly applied for synthesizing drug carriers and uniform microcapsules [90]. Through a combination of structural design, physical and chemical processes, and functional components, these microcapsules can conveniently carry living intestinal microorganisms. Furthermore, in microfluidic electrospray systems, precise control enables the microbial loading of microcapsules and subsequent release, thereby providing ideal delivery systems [91,92]. Moreover, microfluidic electrospray technology can be used to establish multilayer packaging for protecting the bioactive contents [93,94]. Microfluidic electrospray has thus become one of the commonly used techniques for the manufacture of functional microspheres or microcapsules [95], which can contribute to solving problems such as low biological activity, poor stability, and the uncontrollable release of bioactive contents, thereby enhancing their bioavailability and efficacy.
However, despite the aforementioned advantages, microfluidic drug delivery systems require several components, such as microfluidic chips, high-precision pressure pumps, rotary valves, microvalves, flow meters and manometers, microscopes, microzone spectrometers, and impedance meters, that increase their technical thresholds. In addition, the efficiency of drug release is affected by multiple factors, including the preset parameters, equipment used, wrapping materials, and individual differences, which tend to hinder advances in the research on intestinal microorganisms. Consequently, to enhance the efficacy and cost-effectiveness of microfluidic drug delivery systems, it will be necessary to further study the parameters and materials that affect drug release, develop more efficient microfluidic systems, and examine the potential utility of microfluidic technology in intestinal microbe research. By facilitating screening and optimizing the delivery of intestinal microorganisms and packaging of multiple microorganisms, microfluidic electrospray can provide a basis for research on the effects of a diverse range of microorganisms in the human intestine, as summarized in Table 3.

Table 3.
A comparison of the application of two microfluidic platforms with traditional microbiological platforms (culture of bacteria and 16S rRNA sequencing) in microbiological studies.
7. Discussion
Microfluidic technology can be applied to accurately control the characteristics of different fluids, and as such, it has emerged as a prominent interdisciplinary research field [96]. The development of microfluidic technology will facilitate the construction of complex intestinal disease models, the delivery of active microorganisms, and drug development [97]. In the future, it is anticipated that intestine-on-a-chip systems will replace costly and inefficient sterile tissue culture or traditional animal models for the study of intestinal–microbiota interactions. As intestine-on-a-chip technology advances, the cost of chip fabrication can be further reduced, chip manufacturing technology can be improved, and more advanced chip designs can be implemented to achieve a more effective simulation of the intestinal environment, thereby facilitating continuous co-culture of the gut microbiota and cells. Moreover, these advances will potentially contribute to the development of systems combining intestine-on-a-chip technology with other technologies, such as metabolomics, proteomics, and gene expression profiling, which is predicted to enable us to gain a better understanding of the intestinal microenvironment and the complex interactions among gut microbiota. In addition, studies on the integration of the gut with other systems, such as the nervous and endocrine systems, based on the intestine-on-a-chip, and the development of new technologies for the continuous monitoring of intestinal epithelial and microbial co-culture conditions, will contribute to a more comprehensive understanding of the pathogenesis of intestinal diseases, the development of personalized prevention and treatment strategies for different intestinal diseases, and greater insights into the mechanisms underlying chronic intestinal diseases. Intestine-on-a-chip-based analyses will thus become an indispensable approach for studying the complex interactions between the gut microbiota and the human body.
In tandem with the development of material science, microfluidic drug delivery systems will give rise to the production of multifunctional microcapsules that can encapsulate a larger number of microorganisms and exploit a diverse range of trigger-release strategies, including temperature, pH, and enzymic, which will contribute to more effectively solving the current problems of low bioactivity, poor stability, and uncontrollable drug release. Moreover, these systems can provide a basis for establishing polymicrobial studies via screening and optimization of the delivery and transport of intestinal microorganisms, in addition to systems that facilitate the delivery of multiple microbes. Furthermore, microfluidic drug delivery systems will also enhance the efficacy of oral drug delivery, shorten treatment times, and improve the quality of treatment by enhancing the precision and efficiency of structural designs, physical and chemical processes, and functional components. In addition, new materials or technologies can be applied to increase the capability and quality of microfluidic drug delivery systems, such as biodegradable materials, multilayer encapsulation, and reactive oxygen species inhibitors, and to continue optimization of the technology and enable cost reductions. This will ultimately enhance the performance and efficiency of microfluidic drug delivery systems and lower the technological threshold for examining the potential of microfluidic technology in the study of intestinal microorganisms.
The application of microfluidic technology in the study of intestinal microorganisms will also contribute to accelerating research on the mechanisms underlying intestinal diseases and the development of related drugs. Finally, complex intestinal physiological and pathological phenomena, such as those associated with intestinal barrier functions [98], inflammatory bowel disease [99], and intestinal infection, will be identified, thereby presenting opportunities for the better prevention and control of diseases.
Author Contributions
Writing—original draft preparation, P.Q., J.L. and L.Z.; writing—review and editing, P.Q., J.L., X.Y., L.B. and L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (31960236), the Lanzhou Talent Innovation and Entrepreneurship Project (2019-RC-34), the Natural Science Foundation of Gansu Province (21JR7RA369), the Lanzhou Chengguan District Science and Technology Planning Project (2020SHFZ0029), and the Fund of the first Hospital of Lanzhou University (ldyyyn2019-75).
Data Availability Statement
This is a review article and does not contain any research data.
Acknowledgments
Conflicts of Interest
The authors declare no conflict of interest.
References
- Quigley, E.M.M. Gut Bacteria in Health and Disease. Gastroenterol. Hepatol. 2013, 9, 560–569. [Google Scholar]
- Karlsson, F.H.; Nookaew, I.; Petranovic, D.; Nielsen, J. Prospects for Systems Biology and Modeling of the Gut Microbiome. Trends Biotechnol. 2011, 29, 251–258. [Google Scholar] [CrossRef]
- Puschhof, J.; Pleguezuelos-Manzano, C.; Clevers, H. Organoids and Organs-on-Chips: Insights into Human Gut-Microbe Interactions. Cell Host Microbe 2021, 29, 867–878. [Google Scholar] [CrossRef]
- Arslan, N. Obesity, Fatty Liver Disease and Intestinal Microbiota. World J. Gastroenterol. 2014, 20, 16452–16463. [Google Scholar] [CrossRef]
- Riesenfeld, C.S.; Schloss, P.D.; Handelsman, J. Metagenomics: Genomic Analysis of Microbial Communities. Annu. Rev. Genet. 2004, 38, 525–552. [Google Scholar] [CrossRef] [PubMed]
- Xu, J. Microbial Ecology in the Age of Genomics and Metagenomics: Concepts, Tools, and Recent Advances: Microbial Ecological Genomics. Mol. Ecol. 2006, 15, 1713–1731. [Google Scholar] [CrossRef] [PubMed]
- Round June, L.; Palm Noah, W. Causal Effects of the Microbiota on Immune-Mediated Diseases. Sci. Immunol. 2018, 3, eaao1603. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.N.; Ingber, D.E. Microfluidic Organs-on-Chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Schianti, J.N.; Cerize, N.N.; de Oliveira, A.M.; Derenzo, S.; Góngora-Rubio, M.R. Scaling up of Rifampicin Nanoprecipitation Process in Microfluidic Devices. Prog. Nanotechnol. Nanomater. 2013, 2, 101–107. [Google Scholar] [CrossRef]
- Caillard, R.; Lapointe, N. In Vitro Gastric Survival of Commercially Available Probiotic Strains and Oral Dosage Forms. Int. J. Pharm. 2017, 519, 125–127. [Google Scholar] [CrossRef]
- Yeung, T.W.; Üçok, E.F.; Tiani, K.A.; McClements, D.J.; Sela, D.A. Microencapsulation in Alginate and Chitosan Microgels to Enhance Viability of Bifidobacterium Longum for Oral Delivery. Front. Microbiol. 2016, 7, 494. [Google Scholar] [CrossRef] [PubMed]
- Manz, A.; Graber, N.; Widmer, H.M. Miniaturized Total Chemical Analysis Systems: A Novel Concept for Chemical Sensing. Sens. Actuators B Chem. 1990, 1, 244–248. [Google Scholar] [CrossRef]
- Jacobson, S.C.; Hergenroder, R.; Koutny, L.B.; Warmack, R.J.; Ramsey, J.M. Effects of Injection Schemes and Column Geometry on the Performance of Microchip Electrophoresis Devices. Anal. Chem. 1994, 66, 1107–1113. [Google Scholar] [CrossRef]
- Woolley, A.T.; Mathies, R.A. Ultra-High-Speed DNA Sequencing Using Capillary Electrophoresis Chips. Anal. Chem. 1995, 67, 3676–3680. [Google Scholar] [CrossRef]
- Woolley, A.T.; Hadley, D.; Landre, P.; deMello, A.J.; Mathies, R.A.; Northrup, M.A. Functional Integration of PCR Amplification and Capillary Electrophoresis in a Microfabricated DNA Analysis Device. Anal. Chem. 1996, 68, 4081–4086. [Google Scholar] [CrossRef] [PubMed]
- Woolley, A.T.; Sensabaugh, G.F.; Mathies, R.A. High-Speed DNA Genotyping Using Microfabricated Capillary Array Electrophoresis Chips. Anal. Chem. 1997, 69, 2181–2186. [Google Scholar] [CrossRef]
- Thorsen, T.; Maerkl, S.J.; Quake, S.R. Microfluidic Large-Scale Integration. Science 2002, 298, 580–584. [Google Scholar] [CrossRef]
- Bringer, M.R.; Gerdts, C.J.; Song, H.; Tice, J.D.; Ismagilov, R.F. Microfluidic Systems for Chemical Kinetics That Rely on Chaotic Mixing in Droplets. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2004, 362, 1087–1104. [Google Scholar] [CrossRef]
- Lee, K.G.; Park, T.J.; Soo, S.Y.; Wang, K.W.; Kim, B.I.I.; Park, J.H.; Lee, C.-S.; Kim, D.H.; Lee, S.J. Synthesis and Utilization of E. Coli-Encapsulated PEG-Based Microdroplet Using a Microfluidic Chip for Biological Application. Biotechnol. Bioeng. 2010, 107, 747–751. [Google Scholar] [CrossRef]
- Park, J.; Kerner, A.; Burns, M.A.; Lin, X.N. Microdroplet-Enabled Highly Parallel Co-Cultivation of Microbial Communities. PLoS ONE 2011, 6, e17019. [Google Scholar] [CrossRef]
- Puchberger-Enengl, D.; van den Driesche, S.; Krutzler, C.; Keplinger, F.; Vellekoop, M.J. Hydrogel-Based Microfluidic Incubator for Microorganism Cultivation and Analyses. Biomicrofluidics 2015, 9, 014127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yao, Z.-C.; Ding, Q.; Choi, J.J.; Ahmad, Z.; Chang, M.-W.; Li, J.-S. Tri-Needle Coaxial Electrospray Engineering of Magnetic Polymer Yolk–Shell Particles Possessing Dual-Imaging Modality, Multiagent Compartments, and Trigger Release Potential. ACS Appl. Mater. Interfaces 2017, 9, 21485–21495. [Google Scholar] [CrossRef] [PubMed]
- Quintana, G.; Gerbino, E.; Alves, P.; Simões, P.N.; Rúa, M.L.; Fuciños, C.; Gomez-Zavaglia, A. Microencapsulation of Lactobacillus Plantarum in W/O Emulsions of Okara Oil and Block-Copolymers of Poly(Acrylic Acid) and Pluronic Using Microfluidic Devices. Food Res. Int. 2021, 140, 110053. [Google Scholar] [CrossRef] [PubMed]
- Young, E.W.K. Cells, Tissues, and Organs on Chips: Challenges and Opportunities for the Cancer Tumor Microenvironment. Integr. Biol. 2013, 5, 1096–1109. [Google Scholar] [CrossRef]
- Park, G.-S.; Park, M.H.; Shin, W.; Zhao, C.; Sheikh, S.; Oh, S.J.; Kim, H.J. Emulating Host-Microbiome Ecosystem of Human Gastrointestinal Tract In Vitro. Stem Cell Rev. Rep. 2017, 13, 321–334. [Google Scholar] [CrossRef]
- Shah, R.K.; Kim, J.-W.; Weitz, D.A. Janus Supraparticles by Induced Phase Separation of Nanoparticles in Droplets. Adv. Mater. 2009, 21, 1949–1953. [Google Scholar] [CrossRef]
- Nii, T.; Ishii, F. Encapsulation Efficiency of Water-Soluble and Insoluble Drugs in Liposomes Prepared by the Microencapsulation Vesicle Method. Int. J. Pharm. 2005, 298, 198–205. [Google Scholar] [CrossRef]
- Gañán-Calvo, A.M.; Montanero, J.M.; Martín-Banderas, L.; Flores-Mosquera, M. Building Functional Materials for Health Care and Pharmacy from Microfluidic Principles and Flow Focusing. Adv. Drug Deliv. Rev. 2013, 65, 1447–1469. [Google Scholar] [CrossRef]
- Ewart, L.; Apostolou, A.; Briggs, S.A.; Carman, C.V.; Chaff, J.T.; Heng, A.R.; Jadalannagari, S.; Janardhanan, J.; Jang, K.-J.; Joshipura, S.R.; et al. Performance Assessment and Economic Analysis of a Human Liver-Chip for Predictive Toxicology. Commun. Med. 2022, 2, 154. [Google Scholar] [CrossRef]
- Shin, W.; Kim, H.J. Pathomimetic Modeling of Human Intestinal Diseases and Underlying Host-Gut Microbiome Interactions in a Gut-on-a-Chip. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 146, pp. 135–148. ISBN 978-0-12-814280-6. [Google Scholar]
- Kasendra, M.; Tovaglieri, A.; Sontheimer-Phelps, A.; Jalili-Firoozinezhad, S.; Bein, A.; Chalkiadaki, A.; Scholl, W.; Zhang, C.; Rickner, H.; Richmond, C.A.; et al. Development of a Primary Human Small Intestine-on-a-Chip Using Biopsy-Derived Organoids. Sci. Rep. 2018, 8, 2871. [Google Scholar] [CrossRef]
- Günther, C.; Winner, B.; Neurath, M.F.; Stappenbeck, T.S. Organoids in Gastrointestinal Diseases: From Experimental Models to Clinical Translation. Gut 2022, 71, 1892–1908. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Huh, D.; Hamilton, G.; Ingber, D.E. Human Gut-on-a-Chip Inhabited by Microbial Flora That Experiences Intestinal Peristalsis-like Motions and Flow. Lab Chip 2012, 12, 2165. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Ingber, D.E. Gut-on-a-Chip Microenvironment Induces Human Intestinal Cells to Undergo Villus Differentiation. Integr. Biol. 2013, 5, 1130. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Li, H.; Collins, J.J.; Ingber, D.E. Contributions of Microbiome and Mechanical Deformation to Intestinal Bacterial Overgrowth and Inflammation in a Human Gut-on-a-Chip. Proc. Natl. Acad. Sci. USA 2016, 113, E7–E15. [Google Scholar] [CrossRef]
- Villenave, R.; Wales, S.Q.; Hamkins-Indik, T.; Papafragkou, E.; Weaver, J.C.; Ferrante, T.C.; Bahinski, A.; Elkins, C.A.; Kulka, M.; Ingber, D.E. Human Gut-On-A-Chip Supports Polarized Infection of Coxsackie B1 Virus In Vitro. PLoS ONE 2017, 12, e0169412. [Google Scholar] [CrossRef]
- Villenave, R.; Touzelet, O.; Thavagnanam, S.; Sarlang, S.; Parker, J.; Skibinski, G.; Heaney, L.G.; McKaigue, J.P.; Coyle, P.V.; Shields, M.D.; et al. Cytopathogenesis of Sendai Virus in Well-Differentiated Primary Pediatric Bronchial Epithelial Cells. J. Virol. 2010, 84, 11718–11728. [Google Scholar] [CrossRef]
- Zhang, L.; Bukreyev, A.; Thompson, C.I.; Watson, B.; Peeples, M.E.; Collins, P.L.; Pickles, R.J. Infection of Ciliated Cells by Human Parainfluenza Virus Type 3 in an In Vitro Model of Human Airway Epithelium. J. Virol. 2005, 79, 1113–1124. [Google Scholar] [CrossRef]
- Zhang, L.; Peeples, M.E.; Boucher, R.C.; Collins, P.L.; Pickles, R.J. Respiratory Syncytial Virus Infection of Human Airway Epithelial Cells Is Polarized, Specific to Ciliated Cells, and without Obvious Cytopathology. J. Virol. 2002, 76, 5654–5666. [Google Scholar] [CrossRef]
- Naim, H.Y.; Ehler, E.; Billeter, M.A. Measles Virus Matrix Protein Specifies Apical Virus Release and Glycoprotein Sorting in Epithelial Cells. EMBO J. 2000, 19, 3576–3585. [Google Scholar] [CrossRef]
- Katoh, H.; Nakatsu, Y.; Kubota, T.; Sakata, M.; Takeda, M.; Kidokoro, M. Mumps Virus Is Released from the Apical Surface of Polarized Epithelial Cells, and the Release Is Facilitated by a Rab11-Mediated Transport System. J. Virol. 2015, 89, 12026–12034. [Google Scholar] [CrossRef]
- Blank, C.A.; Anderson, D.A.; Beard, M.; Lemon, S.M. Infection of Polarized Cultures of Human Intestinal Epithelial Cells with Hepatitis A Virus: Vectorial Release of Progeny Virions through Apical Cellular Membranes. J. Virol. 2000, 74, 6476–6484. [Google Scholar] [CrossRef] [PubMed]
- Grassart, A.; Malardé, V.; Gobaa, S.; Sartori-Rupp, A.; Kerns, J.; Karalis, K.; Marteyn, B.; Sansonetti, P.; Sauvonnet, N. Bioengineered Human Organ-on-Chip Reveals Intestinal Microenvironment and Mechanical Forces Impacting Shigella Infection. Cell Host Microbe 2019, 26, 435–444.e4. [Google Scholar] [CrossRef] [PubMed]
- Tovaglieri, A.; Sontheimer-Phelps, A.; Geirnaert, A.; Prantil-Baun, R.; Camacho, D.M.; Chou, D.B.; Jalili-Firoozinezhad, S.; de Wouters, T.; Kasendra, M.; Super, M.; et al. Species-Specific Enhancement of Enterohemorrhagic, E. Coli Pathogenesis Mediated by Microbiome Metabolites. Microbiome 2019, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Mead, B.E.; Safaee, H.; Langer, R.; Karp, J.M.; Levy, O. Engineering Stem Cell Organoids. Cell Stem Cell 2016, 18, 25–38. [Google Scholar] [CrossRef]
- Fatehullah, A.; Tan, S.H.; Barker, N. Organoids as an In Vitro Model of Human Development and Disease. Nat. Cell Biol. 2016, 18, 246–254. [Google Scholar] [CrossRef]
- Tang, H.; Abouleila, Y.; Si, L.; Ortega-Prieto, A.M.; Mummery, C.L.; Ingber, D.E.; Mashaghi, A. Human Organs-on-Chips for Virology. Trends Microbiol. 2020, 28, 934–946. [Google Scholar] [CrossRef]
- Zheng, L.; Kelly, C.J.; Colgan, S.P. Physiologic Hypoxia and Oxygen Homeostasis in the Healthy Intestine. A Review in the Theme: Cellular Responses to Hypoxia. Am. J. Physiol.-Cell Physiol. 2015, 309, C350–C360. [Google Scholar] [CrossRef]
- des Rieux, A.; Fievez, V.; Garinot, M.; Schneider, Y.-J.; Préat, V. Nanoparticles as Potential Oral Delivery Systems of Proteins and Vaccines: A Mechanistic Approach. J. Control. Release 2006, 116, 1–27. [Google Scholar] [CrossRef]
- Zhang, S.; Bellinger, A.M.; Glettig, D.L.; Barman, R.; Lee, Y.-A.L.; Zhu, J.; Cleveland, C.; Montgomery, V.A.; Gu, L.; Nash, L.D.; et al. PH-Responsive Supramolecular Polymer Gel as an Enteric Elastomer for Use in Gastric Devices. Nat. Mater. 2015, 14, 1065–1071. [Google Scholar] [CrossRef]
- Wang, M.; Yang, J.; Li, M.; Wang, Y.; Wu, H.; Xiong, L.; Sun, Q. Enhanced Viability of Layer-by-Layer Encapsulated Lactobacillus Pentosus Using Chitosan and Sodium Phytate. Food Chem. 2019, 285, 260–265. [Google Scholar] [CrossRef]
- Klein, A.M.; Mazutis, L.; Akartuna, I.; Tallapragada, N.; Veres, A.; Li, V.; Peshkin, L.; Weitz, D.A.; Kirschner, M.W. Droplet Barcoding for Single-Cell Transcriptomics Applied to Embryonic Stem Cells. Cell 2015, 161, 1187–1201. [Google Scholar] [CrossRef] [PubMed]
- Macosko, E.Z.; Basu, A.; Satija, R.; Nemesh, J.; Shekhar, K.; Goldman, M.; Tirosh, I.; Bialas, A.R.; Kamitaki, N.; Martersteck, E.M. Highly Parallel Genome-Wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell 2015, 161, 1202–1214. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhang, W.; Abbaspourrad, A.; Ahn, J.; Bader, A.; Bose, S.; Vegas, A.; Lin, J.; Tao, J.; Hang, T. Microfluidic Fabrication of Colloidal Nanomaterials-Encapsulated Microcapsules for Biomolecular Sensing. Nano Lett. 2017, 17, 2015–2020. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, Y.; Liu, D.; Li, J.; Sun, J.; Wei, Q.; Zhao, Y.; Pandol, S.J.; Li, L. Porous Microcapsules Encapsulating β Cells Generated by Microfluidic Electrospray Technology for Diabetes Treatment. NPG Asia Mater. 2022, 14, 39. [Google Scholar] [CrossRef]
- Duncanson, W.J.; Lin, T.; Abate, A.R.; Seiffert, S.; Shah, R.K.; Weitz, D.A. Microfluidic Synthesis of Advanced Microparticles for Encapsulation and Controlled Release. Lab Chip 2012, 12, 2135–2145. [Google Scholar] [CrossRef] [PubMed]
- Weidenbacher, L.; Abrishamkar, A.; Rottmar, M.; Guex, A.G.; Maniura-Weber, K.; deMello, A.J.; Ferguson, S.J.; Rossi, R.M.; Fortunato, G. Electrospraying of Microfluidic Encapsulated Cells for the Fabrication of Cell-Laden Electrospun Hybrid Tissue Constructs. Acta Biomater. 2017, 64, 137–147. [Google Scholar] [CrossRef]
- Rotman, S.G.; Sumrall, E.; Ziadlou, R.; Grijpma, D.W.; Richards, R.G.; Eglin, D.; Moriarty, T.F. Local Bacteriophage Delivery for Treatment and Prevention of Bacterial Infections. Front. Microbiol. 2020, 11, 538060. [Google Scholar] [CrossRef]
- Smith, H.W.; Huggins, M.B.; Shaw, K.M. The Control of Experimental Escherichia Coli Diarrhoea in Calves by Means of Bacteriophages. Microbiology 1987, 133, 1111–1126. [Google Scholar] [CrossRef]
- Biswas, B.; Adhya, S.; Washart, P.; Paul, B.; Trostel, A.N.; Powell, B.; Carlton, R.; Merril, C.R. Bacteriophage Therapy Rescues Mice Bacteremic from a Clinical Isolate of Vancomycin-Resistant Enterococcus Faecium. Infect. Immun. 2002, 70, 204–210. [Google Scholar] [CrossRef]
- Cerveny, K.E.; DePaola, A.; Duckworth, D.H.; Gulig, P.A. Phage Therapy of Local and Systemic Disease Caused by Vibrio Vulnificus in Iron-Dextran-Treated Mice. Infect. Immun. 2002, 70, 6251–6262. [Google Scholar] [CrossRef]
- Wills, Q.F.; Kerrigan, C.; Soothill, J.S. Experimental Bacteriophage Protection against Staphylococcus Aureus Abscesses in a Rabbit Model. Antimicrob. Agents Chemother. 2005, 49, 1220–1221. [Google Scholar] [CrossRef] [PubMed]
- Vonasek, E.; Le, P.; Nitin, N. Encapsulation of Bacteriophages in Whey Protein Films for Extended Storage and Release. Food Hydrocoll. 2014, 37, 7–13. [Google Scholar] [CrossRef]
- Malik, D.J.; Sokolov, I.J.; Vinner, G.K.; Mancuso, F.; Cinquerrui, S.; Vladisavljevic, G.T.; Clokie, M.R.J.; Garton, N.J.; Stapley, A.G.F.; Kirpichnikova, A. Formulation, Stabilisation and Encapsulation of Bacteriophage for Phage Therapy. Adv. Colloid Interface Sci. 2017, 249, 100–133. [Google Scholar] [CrossRef]
- Denou, E.; Bruttin, A.; Barretto, C.; Ngom-Bru, C.; Brüssow, H.; Zuber, S. T4 Phages against Escherichia Coli Diarrhea: Potential and Problems. Virology 2009, 388, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Young, R.; Gill, J.J. Phage Therapy Redux—What Is to Be Done? Science 2015, 350, 1163–1164. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.A.; Sultana, S.; Reuteler, G.; Moine, D.; Descombes, P.; Charton, F.; Bourdin, G.; McCallin, S.; Ngom-Bru, C.; Neville, T. Oral Phage Therapy of Acute Bacterial Diarrhea with Two Coliphage Preparations: A Randomized Trial in Children from Bangladesh. EBioMedicine 2016, 4, 124–137. [Google Scholar] [CrossRef]
- Vinner, G.K.; Malik, D.J. High Precision Microfluidic Microencapsulation of Bacteriophages for Enteric Delivery. Res. Microbiol. 2018, 169, 522–530. [Google Scholar] [CrossRef]
- Rajawat, A.; Tripathi, S. Disease Diagnostics Using Hydrodynamic Flow Focusing in Microfluidic Devices: Beyond Flow Cytometry. Biomed. Eng. Lett. 2020, 10, 241–257. [Google Scholar] [CrossRef]
- Alkayyali, T.; Cameron, T.; Haltli, B.; Kerr, R.G.; Ahmadi, A. Microfluidic and Cross-Linking Methods for Encapsulation of Living Cells and Bacteria—A Review. Anal. Chim. Acta 2019, 1053, 1–21. [Google Scholar] [CrossRef]
- Zhao, C.; Zhu, Y.; Kong, B.; Huang, Y.; Yan, D.; Tan, H.; Shang, L. Dual-Core Prebiotic Microcapsule Encapsulating Probiotics for Metabolic Syndrome. ACS Appl. Mater. Interfaces 2020, 12, 42586–42594. [Google Scholar] [CrossRef]
- Sarao, L.K.; Arora, M. Probiotics, Prebiotics, and Microencapsulation: A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 344–371. [Google Scholar] [CrossRef] [PubMed]
- Dabke, K.; Hendrick, G.; Devkota, S. The Gut Microbiome and Metabolic Syndrome. J. Clin. Investing. 2019, 129, 4050–4057. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, H.; Zhang, C.; Zhao, Y.; Derrien, M.; Rocher, E.; van-Hylckama Vlieg, J.E.; Strissel, K.; Zhao, L.; Obin, M.; et al. Modulation of Gut Microbiota during Probiotic-Mediated Attenuation of Metabolic Syndrome in High Fat Diet-Fed Mice. ISME J. 2015, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hersoug, L.-G.; Møller, P.; Loft, S. Gut Microbiota-Derived Lipopolysaccharide Uptake and Trafficking to Adipose Tissue: Implications for Inflammation and Obesity. Obes. Rev. 2016, 17, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Hersoug, L.-G.; Møller, P.; Loft, S. Role of Microbiota-Derived Lipopolysaccharide in Adipose Tissue Inflammation, Adipocyte Size and Pyroptosis during Obesity. Nutr. Res. Rev. 2018, 31, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Yin, Y.; Li, Z.; Zhang, W. Gut Microbiota-Derived Components and Metabolites in the Progression of Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2019, 11, 1712. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Y. Gut Microbiota Derived Metabolites in Cardiovascular Health and Disease. Protein Cell 2018, 9, 416–431. [Google Scholar] [CrossRef]
- Erridge, C.; Attina, T.; Spickett, C.M.; Webb, D.J. A High-Fat Meal Induces Low-Grade Endotoxemia: Evidence of a Novel Mechanism of Postprandial Inflammation. Am. J. Clin. Nutr. 2007, 86, 1286–1292. [Google Scholar] [CrossRef]
- Neves, A.L.; Coelho, J.; Couto, L.; Leite-Moreira, A.; Roncon-Albuquerque, R. Metabolic Endotoxemia: A Molecular Link between Obesity and Cardiovascular Risk. J. Mol. Endocrinol. 2013, 51, R51–R64. [Google Scholar] [CrossRef]
- Guerville, M.; Boudry, G. Gastrointestinal and Hepatic Mechanisms Limiting Entry and Dissemination of Lipopolysaccharide into the Systemic Circulation. Am. J. Physiol.-Gastrointest. Liver Physiol. 2016, 311, G1–G15. [Google Scholar] [CrossRef]
- Forgie, A.J.; Fouhse, J.M.; Willing, B.P. Diet-Microbe-Host Interactions That Affect Gut Mucosal Integrity and Infection Resistance. Front. Immunol. 2019, 10, 1802. [Google Scholar] [CrossRef] [PubMed]
- Antonini, M.; Lo Conte, M.; Sorini, C.; Falcone, M. How the Interplay Between the Commensal Microbiota, Gut Barrier Integrity, and Mucosal Immunity Regulates Brain Autoimmunity. Front. Immunol. 2019, 10, 1937. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Q.; Ren, J. Microbiota-Immune Interaction in the Pathogenesis of Gut-Derived Infection. Front. Immunol. 2019, 10, 1873. [Google Scholar] [CrossRef] [PubMed]
- Geddes, K.; Philpott, D.J. A New Role for Intestinal Alkaline Phosphatase in Gut Barrier Maintenance. Gastroenterology 2008, 135, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yu, Y.; Zhang, X.; Wu, X.; Ren, J.; Zhao, Y. Biomimetic Intestinal Barrier Based on Microfluidic Encapsulated Sucralfate Microcapsules. Sci. Bull. 2019, 64, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Kurashima, Y.; Kiyono, H. Mucosal Ecological Network of Epithelium and Immune Cells for Gut Homeostasis and Tissue Healing. Annu. Rev. Immunol. 2017, 35, 119–147. [Google Scholar] [CrossRef]
- Valiei, A.; Aminian-Dehkordi, J.; Mofrad, M.R.K. Gut-on-a-Chip Models for Dissecting the Gut Microbiology and Physiology. APL Bioeng. 2023, 7, 011502. [Google Scholar] [CrossRef]
- Ejeta, F. Recent Advances of Microfluidic Platforms for Controlled Drug Delivery in Nanomedicine. Drug Des. Dev. Ther. 2021, 15, 3881–3891. [Google Scholar] [CrossRef]
- Zhao, C.-X. Multiphase Flow Microfluidics for the Production of Single or Multiple Emulsions for Drug Delivery. Adv. Drug Deliv. Rev. 2013, 65, 1420–1446. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Z.; Liu, Y.; Shao, C.; Bian, F.; Zhao, Y. Biomimetic Enzyme Cascade Reaction System in Microfluidic Electrospray Microcapsules. Sci. Adv. 2018, 4, eaat2816. [Google Scholar] [CrossRef]
- Lei, L.; Lv, Q.; Jin, Y.; An, H.; Shi, Z.; Hu, G.; Yang, Y.; Wang, X.; Yang, L. Angiogenic Microspheres for the Treatment of a Thin Endometrium. ACS Biomater. Sci. Eng. 2021, 7, 4914–4920. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; McHugh, K.J.; Webster, J.; Langer, R.; Jaklenec, A. Layer-by-layer Encapsulation of Probiotics for Delivery to the Microbiome. Adv. Mater. 2016, 28, 9486–9490. [Google Scholar] [CrossRef] [PubMed]
- Arya, N.; Chakraborty, S.; Dube, N.; Katti, D.S. Electrospraying: A Facile Technique for Synthesis of Chitosan-based Micro/Nanospheres for Drug Delivery Applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 88, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yu, Y.; Wu, X.; Wang, G.; Gu, G.; Wang, F.; Ren, J.; Zhang, H.; Zhao, Y. Microfluidic Electrospray Niacin Metal-Organic Frameworks Encapsulated Microcapsules for Wound Healing. Research 2019, 2019, 6175398. [Google Scholar] [CrossRef]
- Utada, A.S.; Lorenceau, E.; Link, D.R.; Kaplan, P.D.; Stone, H.A.; Weitz, D.A. Monodisperse Double Emulsions Generated from a Microcapillary Device. Science 2005, 308, 537–541. [Google Scholar] [CrossRef]
- Bein, A.; Shin, W.; Jalili-Firoozinezhad, S.; Park, M.H.; Sontheimer-Phelps, A.; Tovaglieri, A.; Chalkiadaki, A.; Kim, H.J.; Ingber, D.E. Microfluidic Organ-on-a-Chip Models of Human Intestine. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 659–668. [Google Scholar] [CrossRef]
- Marrero, D. Gut-on-a-Chip: Mimicking and Monitoring the Human Intestine. Biosens. Bioelectron. 2021, 15, 113156. [Google Scholar] [CrossRef]
- Rahman, S.; Ghiboub, M.; Donkers, J.M.; van de Steeg, E.; van Tol, E.A.F.; Hakvoort, T.B.M.; de Jonge, W.J. The Progress of Intestinal Epithelial Models from Cell Lines to Gut-On-Chip. Int. J. Mol. Sci. 2021, 22, 13472. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).