Antibacterial and Photocatalytic Activities of LDH-Based Sorbents of Different Compositions
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents for Synthesis, Photocatalytic and Antimicrobial Tests
2.2. LDH Synthesis
- -
- ZnAl-SO4 LDH is the pristine LDH phase, with no after-synthesis process.
- -
- ZnAl-MMO is the phase obtained by the ZnAl-SO4 LDH annealing at 450 °C for 5 h. This process transforms the pristine LDH structure in ZnAl mixed oxide, whose structure maintains a certain disorder degree.
- -
- ZnAl-CrO4 LDH is the phase resulting from Cr(VI) adsorption, up to saturation, on a pristine ZnAl-SO4 LDH sorbent, according to a protocol described in a previous study [37].
- -
- ZnAl-MMO-CrO4 is the mixed oxide from the annealing process saturated with Cr(VI).
2.3. Characterization Techniques
2.4. Antimicrobial Activity Tests
2.5. Photocatalytic Experiments Setup
3. Results and Discussion
4. Conclusions
- The antimicrobial activity increases after heat treatment is performed, which moreover influences the effect of dose dependence. This result may open interesting new scenarios in drug delivery field applications.
- The photocatalytic activity of the investigated compounds is well described by pseudo-second-order kinetics, despite the calculated values of qe, for both pseudo-first- and pseudo-second-order kinetics are not markedly different.
- The overall performances are affected by adsorption phenomena, and this result is more pronounced for the two compounds without the chromate anion. The results seem to suggest that the presence of chromate ions within the structure may influence the photocatalytic activities of these materials, thus creating new opportunities in recycling exhaust LDH, having adsorbed contaminants like Cr(VI), by turning it into a photocatalyst of promising performances. On the other hand, an increase of catalytic activity following the adsorption of Cr(VI) would hamper the use of this substrate for medical or drug delivery purposes, owing to the well-known toxicity of the adsorbed anion.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Premathilaka, R.W.; Liyanagedera, N.D. Fluoride in Drinking Water and Nanotechnological Approaches for Eliminating Excess Fluoride. J. Nanotechnol. 2019, 2019, 2192383. [Google Scholar] [CrossRef]
- Theiss, F.L.; Couperthwaite, S.J.; Ayoko, G.A.; Frost, R.L. A review of the removal of anions and oxyanions of the halogen elements from aqueous solution by layered double hydroxides. J. Colloid Interface Sci. 2014, 417, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N.; Nguyen, D.T.; Le, G.T.; Tomul, F.; Lima, E.C.; Woo, S.H.; Sarmah, A.K.; Nguyen, H.Q.; Nguyen, P.T.; Nguyen, D.D.; et al. Adsorption mechanism of hexavalent chromium onto layered double hydroxides-based adsorbents: A sytematic in-depth review. J. Hazard. Mater. 2019, 373, 258–270. [Google Scholar] [CrossRef]
- Dias, A.C.; Fontes, M.P.F. Arsenic (V) removal from water using hydrotalcites as adsorbents: A critical review. Appl. Clay Sci. 2020, 191, 105615. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, T.; Li, M.; Yang, Y.; Lu, P.; Ning, P.; Wang, Q. Arsenic removal from water/wastewater using layered double hydroxide derived adsorbents, a critical review. RSC Adv. 2018, 8, 22694–22709. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.-H.; Lim, T.-T.; Dong, Z. Application of layered double hydroxides for removal of oxyanions: A review. Water Res. 2008, 42, 1343–1368. [Google Scholar] [CrossRef]
- Cardinale, A.M.; Carbone, C.; Fortunato, M.; Fabiano, B.; Reverberi, A.P. ZnAl-SO4 Layered Double Hydroxide and Allophane for Cr(VI), Cu(II) and Fe(III) Adsorption in Wastewater: Structure Comparison and Synergistic Effects. Materials 2022, 15, 6887. [Google Scholar] [CrossRef] [PubMed]
- Kobylinska, N.G.; Puzyrnaya, L.M.; Pshinko, G.M. Layered Double Hydroxides as Promising Adsorbents for Purification of Radioactive Polluted Water: A Review. Theor. Exp. Chem. 2022, 58, 221–239. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Mohamed, A.A. A systematic review of layered double hydroxide-based materials for environmental remediation of heavy metals and dye pollutants. Inorg. Chem. Commun. 2023, 148, 110325. [Google Scholar] [CrossRef]
- Yang, F.; Sun, S.; Chen, X.; Chang, Y.; Zha, F.; Lei, Z. Mg–Al layered double hydroxides modified clay adsorbents for efficient removal of Pb 2+, Cu 2+ and Ni 2+ from water. Appl. Clay Sci. 2016, 123, 134–140. [Google Scholar] [CrossRef]
- Zhao, D.; Sheng, G.; Hu, J.; Chen, C.; Wang, X. The adsorption of Pb(II) on Mg2Al layered double hydroxide. Chem. Eng. J. 2011, 171, 167–174. [Google Scholar] [CrossRef]
- Beolchini, F.; Pagnanelli, F.; Reverberi, A.P.; Vegliò, F. Copper Biosorption onto Rhizopus oligosporus: pH-Edge Tests and Related Kinetic and Equilibrium Modeling. Ind. Eng. Chem. Res. 2003, 42, 4881–4887. [Google Scholar] [CrossRef]
- Mourid, E.H.; Lakraimi, M.; Legrouri, A. Removal and Release of the 2,4,5-Trichlorophenoxyacetic Acid Herbicide from Wastewater by Layered Double Hydroxides. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2116–2128. [Google Scholar] [CrossRef]
- Santamaría, L.; Vicente, M.; Korili, S.; Gil, A. Progress in the removal of pharmaceutical compounds from aqueous solution using layered double hydroxides as adsorbents: A review. J. Environ. Chem. Eng. 2020, 8, 104577. [Google Scholar] [CrossRef]
- Yang, Y.; Owino, A.A.; Gao, Y.; Yan, X.; Xu, C.; Wang, J. Occurrence, composition and risk assessment of antibiotics in soils from Kenya, Africa. Ecotoxicology 2016, 25, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.-L.; Lester, E.; Williams, O.; Gomes, R.L. Understanding Layered Double Hydroxide properties as sorbent materials for removing organic pollutants from environmental waters. J. Environ. Chem. Eng. 2021, 9, 105197. [Google Scholar] [CrossRef]
- Li, E.; Liao, L.; Lv, G.; Li, Z.; Yang, C.; Lu, Y. The Interactions between Three Typical PPCPs and LDH. Front. Chem. 2018, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Antoniak-Jurak, K.; Kowalik, P.; Bicki, R.; Michalska, K.; Próchniak, W.; Wiercioch, P. Cu substituted ZnAl2O4 ex-LDH catalysts for medium-temperature WGS—effect of Cu/Zn ratio and thermal treatment on catalyst efficiency. Int. J. Hydrogen Energy 2019, 44, 27390–27400. [Google Scholar] [CrossRef]
- Li, C.; Wei, M.; Evans, D.G.; Duan, X. Layered Double Hydroxide-based Nanomaterials as Highly Efficient Catalysts and Adsorbents. Small 2014, 10, 4469–4486. [Google Scholar] [CrossRef] [PubMed]
- Dewangan, N.; Hui, W.M.; Jayaprakash, S.; Bawah, A.-R.; Poerjoto, A.J.; Jie, T.; Jangam, A.; Hidajat, K.; Kawi, S. Recent progress on layered double hydroxide (LDH) derived metal-based catalysts for CO2 conversion to valuable chemicals. Catal. Today 2020, 356, 490–513. [Google Scholar] [CrossRef]
- Fang, X.; Chen, C.; Jia, H.; Li, Y.; Liu, J.; Wang, Y.; Song, Y.; Du, T.; Liu, L. Progress in Adsorption-Enhanced Hydrogenation of CO2 on Layered Double Hydroxide (LDH) Derived Catalysts. J. Ind. Eng. Chem. 2021, 95, 16–27. [Google Scholar] [CrossRef]
- Bassani, A.; Vianello, C.; Mocellin, P.; Dell’angelo, A.; Spigno, G.; Fabiano, B.; Maschio, G.; Manenti, F. Aprioristic Integration of Process Operations and Risk Analysis: Definition of the Weighted F&EI-Based Concept and Application to AG2S Technology. Ind. Eng. Chem. Res. 2023, 62, 500–510. [Google Scholar] [CrossRef]
- Mohanty, U.A.; Sahoo, D.P.; Paramanik, L.; Parida, K. A critical review on layered double hydroxide (LDH)-derived functional nanomaterials as potential and sustainable photocatalysts. Sustain. Energy Fuels 2023, 7, 1145–1186. [Google Scholar] [CrossRef]
- Wang, C.; Xu, J.; Zhou, Z. A Mini-Review on CO2 Photoreduction by MgAl-LDH Based Materials. Energies 2022, 15, 8117. [Google Scholar] [CrossRef]
- Bi, Z.-X.; Guo, R.-T.; Hu, X.; Wang, J.; Chen, X.; Pan, W.-G. Research progress on photocatalytic reduction of CO2 based on LDH materials. Nanoscale 2022, 14, 3367–3386. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Z.; Wan, S.; Liu, Q.; Ding, J.; Zhong, Q. Facile layer regulation strategy of layered double hydroxide nanosheets for artificial photosynthesis and mechanism insight. Chem. Eng. J. 2022, 434, 134434. [Google Scholar] [CrossRef]
- Li, H.; Zhu, H.; Shi, Y.; Shang, H.; Zhang, L.; Wang, J. Vacancy-Rich and Porous NiFe-Layered Double Hydroxide Ultrathin Nanosheets for Efficient Photocatalytic NO Oxidation and Storage. Environ. Sci. Technol. 2022, 56, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, Y.; Waterhouse, G.I.N.; Zheng, L.; Cao, X.; Teng, F.; Wu, L.-Z.; Tung, C.-H.; O’Hare, D.; Zhang, T. Layered-Double-Hydroxide Nanosheets as Efficient Visible-Light-Driven Photocatalysts for Dinitrogen Fixation. Adv. Mater. 2017, 29, 1703828. [Google Scholar] [CrossRef]
- Zhang, M.; Lai, C.; Li, B.; Xu, F.; Huang, D.; Liu, S.; Qin, L.; Liu, X.; Yi, H.; Fu, Y.; et al. Insightful understanding of charge carrier transfer in 2D/2D heterojunction photocatalyst: Ni-Co layered double hydroxides deposited on ornamental g-C3N4 ultrathin nanosheet with boosted molecular oxygen activation. Chem. Eng. J. 2021, 422, 130120. [Google Scholar] [CrossRef]
- Vennapoosa, C.S.; Varangane, S.; Abraham, B.M.; Perupogu, V.; Bojja, S.; Pal, U. Controlled photoinduced electron transfer from g-C3N4 to CuCdCe-LDH for efficient visible light hydrogen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 40227–40241. [Google Scholar] [CrossRef]
- Grover, A.; Mohiuddin, I.; Lee, J.; Brown, R.J.; Malik, A.K.; Aulakh, J.S.; Kim, K.-H. Progress in pre-treatment and extraction of organic and inorganic pollutants by layered double hydroxide for trace-level analysis. Environ. Res. 2022, 214, 114166. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Yao, Y.; Chen, T.; Kong, D.; Shen, W.; Lee, H.K. Recent advances in the application of layered double hydroxides in analytical chemistry: A review. Anal. Chim. Acta 2020, 1103, 32–48. [Google Scholar] [CrossRef]
- Sohrabi, H.; Khataee, A.; Ghasemzadeh, S.; Majidi, M.R.; Orooji, Y. Layer double hydroxides (LDHs)- based electrochemical and optical sensing assessments for quantification and identification of heavy metals in water and environment samples: A review of status and prospects. Trends Environ. Anal. Chem. 2021, 31, e00139. [Google Scholar] [CrossRef]
- Rad, T.S.; Khataee, A.; Arefi-Oskoui, S.; Rad, S.S.; Orooji, Y.; Gengec, E.; Kobya, M. Graphene-based ZnCr layered double hydroxide nanocomposites as bactericidal agents with high sonophotocatalytic performances for degradation of rifampicin. Chemosphere 2022, 286, 131740. [Google Scholar] [CrossRef]
- Arjomandi-Behzad, L.; Alinejad, Z.; Zandragh, M.R.; Golmohamadi, A.; Vojoudi, H. Facile synthesis of hollow spherical g-C3N4@LDH/NCQDs ternary nanostructure for multifunctional antibacterial and photodegradation activities. iScience 2023, 26, 106213. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.; Dash, B.; Pandey, S. Effect of molecular dimension on gallery height, release kinetics and antibacterial activity of Zn Al layered double hydroxide (LDH) encapsulated with benzoate and its derivatives. Appl. Clay Sci. 2019, 181, 105230. [Google Scholar] [CrossRef]
- Kraus, W.; Nolze, G. Powder Cell for Windows. Powder Diffr. 1998, 13, 256–259. [Google Scholar]
- King, G.; Schwarzenbach Latcon, D. Xtal3.7 System. In The Gnu Xtal System User’s Manual; Hall, S.R., du Boilay, D.J., Olthof-Hazekamp, R., Eds.; University Of Western: Perth, Australia, 2002. [Google Scholar]
- Blanco, I.; Latteri, A.; Cicala, G.; D’angelo, A.; Viola, V.; Arconati, V.; Catauro, M. Antibacterial and Chemical Characterization of Silica-Quercetin-PEG Hybrid Materials Synthesized by Sol–Gel Route. Molecules 2022, 27, 979. [Google Scholar] [CrossRef]
- Catauro, M.; D’angelo, A.; Fiorentino, M.; Gullifa, G.; Risoluti, R.; Ciprioti, S.V. Thermal behavior, morphology and antibacterial properties study of silica/quercetin nanocomposite materials prepared by sol–gel route. J. Therm. Anal. Calorim. 2022, 147, 5337–5350. [Google Scholar] [CrossRef]
- Alberti, S.; Dodero, A.; Sartori, E.; Vicini, S.; Ferretti, M.; Castellano, M. Composite Water-Borne Polyurethane Nanofibrous Electrospun Membranes with Photocatalytic Properties. ACS Appl. Polym. Mater. 2021, 3, 6157–6166. [Google Scholar] [CrossRef]
- Martinelli, A.; Alberti, S.; Caratto, V.; Lova, P.; Locardi, F.; Pampararo, G.; Villa, S.; Ferretti, M. Structural studies on copper and nitrogen doped nanosized anatase. Z. Für Krist. Cryst. Mater. 2018, 233, 867–876. [Google Scholar] [CrossRef]
- Alberti, S.; Basciu, I.; Vocciante, M.; Ferretti, M. Experimental and Physico-Chemical Comparison of ZnO Nanoparticles’ Activity for Photocatalytic Applications in Wastewater Treatment. Catalysts 2021, 11, 678. [Google Scholar] [CrossRef]
- Alberti, S.; Sotiropoulou, M.; Fernández, E.; Solomou, N.; Ferretti, M.; Psillakis, E. UV-254 degradation of nicotine in natural waters and leachates produced from cigarette butts and heat-not-burn tobacco products. Environ. Res. 2021, 194, 110695. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.T.; Alprol, A.E.; Abualnaja, K.M.; El-Beltagi, H.S.; Ramadan, K.M.A.; Ashour, M. The Using of Nanoparticles of Microalgae in Remediation of Toxic Dye from Industrial Wastewater: Kinetic and Isotherm Studies. Materials 2022, 15, 3922. [Google Scholar] [CrossRef]
- Gowda, S.A.; Goveas, L.C.; Dakshayini, K. Adsorption of methylene blue by silver nanoparticles synthesized from Urena lobata leaf extract: Kinetics and equilibrium analysis. Mater. Chem. Phys. 2022, 288, 126431. [Google Scholar] [CrossRef]
- Mondal, U.S.; Das, S.; Somu, P.; Paul, S. Silica sand–supported nano zinc oxide–graphene oxide composite induced rapid photocatalytic decolorization of azo dyes under sunlight and improved antimicrobial activity. Environ. Sci. Pollut. Res. 2023, 30, 17226–17244. [Google Scholar] [CrossRef]
- Ngoc, P.K.; Mac, T.K.; Nguyen, H.T.; Viet, D.T.; Thanh, T.D.; Van Vinh, P.; Phan, B.T.; Duong, A.T.; Das, R. Superior organic dye removal by CoCr2O4 nanoparticles: Adsorption kinetics and isotherm. J. Sci. Adv. Mater. Devices 2022, 7, 100438. [Google Scholar] [CrossRef]
- Pinthong, P.; Praserthdam, P.; Jongsomjit, B. Effect of Calcination Temperature on Mg-Al Layered Double Hydroxides (LDH) as Promising Catalysts in Oxidative Dehydrogenation of Ethanol to Acetaldehyde. J. Oleo Sci. 2019, 68, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Hadnadjev-Kostic, M.; Vulic, T.; Zoric, D.; Marinkovic-Neducin, R. The influence of the UV irradiation intensity on photocatalytic activity of ZnAl layered double hydroxides and derived mixed oxides. Chem. Ind. Chem. Eng. Q. 2012, 18, 295–303. [Google Scholar] [CrossRef]
- Thite, V.D.; Giripunje, S.M. Adsorption and photocatalytic performance of ZnAl layered double hydroxide nanoparticles in removal of methyl orange dye. Nanotechnol. Environ. Eng. 2022, 7, 57–66. [Google Scholar] [CrossRef]
- Lok, C.-N.; Ho, C.-M.; Chen, R.; He, Q.-Y.; Yu, W.-Y.; Sun, H.; Tam, P.K.-H.; Chiu, J.-F.; Che, C.-M. Silver nanoparticles: Partial oxidation and antibacterial activities. JBIC J. Biol. Inorg. Chem. 2007, 12, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Szunerits, S.; Boukherroub, R. Antibacterial activity of graphene-based materials. J. Mater. Chem. B 2016, 4, 6892–6912. [Google Scholar] [CrossRef] [PubMed]
- Hanane, Z.; Kaid, M.; Djamila, I.; Ammam, A.; Villemin, D. Preparation, characterization and antibacterial applications of ZnAl- LDH with the diaminododecylphosphonic acid intercalation. South Asian J. Exp. Biol. 2021, 11, 600–604. [Google Scholar] [CrossRef]
- Pasquet, J.; Chevalier, Y.; Pelletier, J.; Couval, E.; Bouvier, D.; Bolzinger, M.-A. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 263–274. [Google Scholar] [CrossRef]
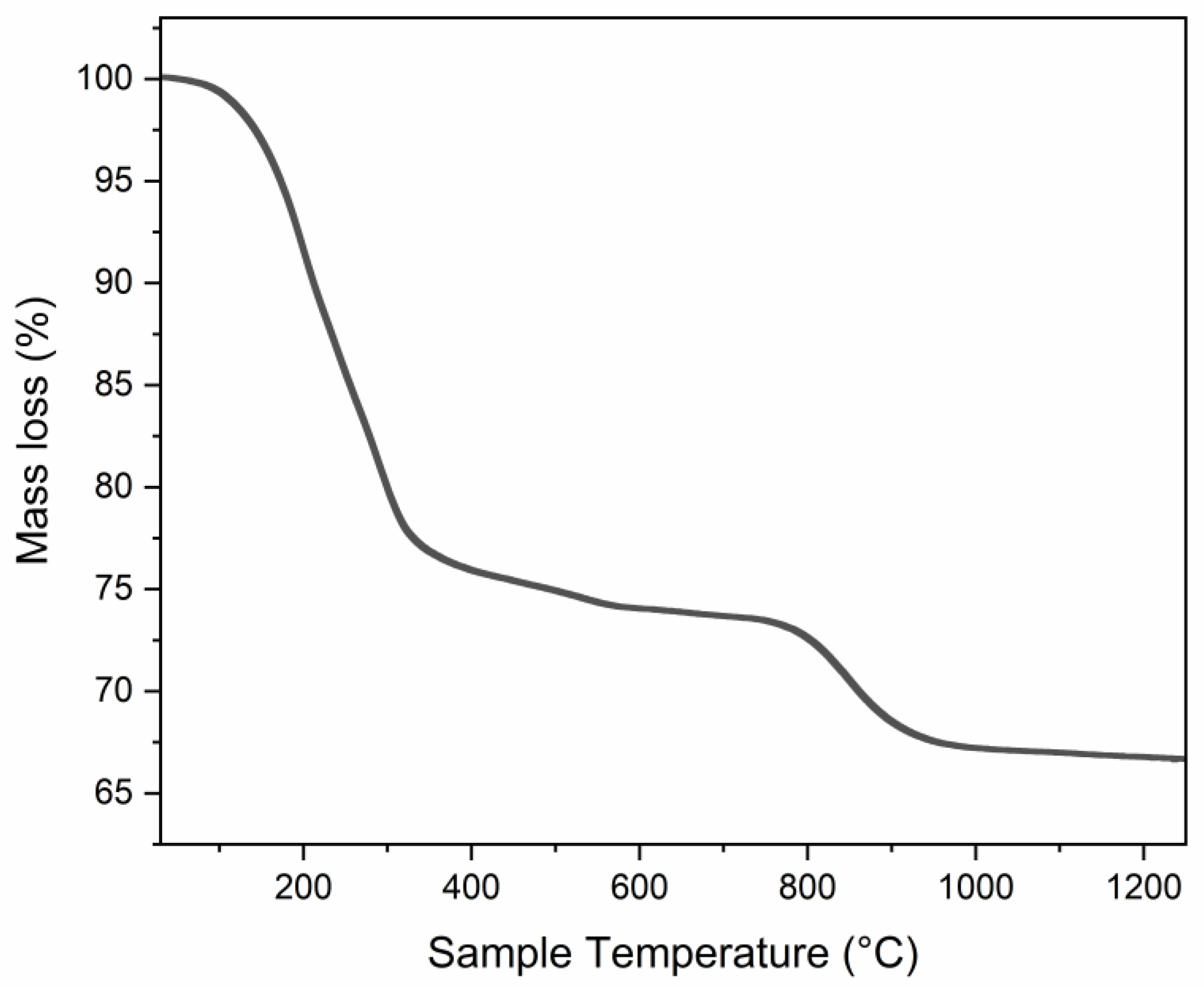
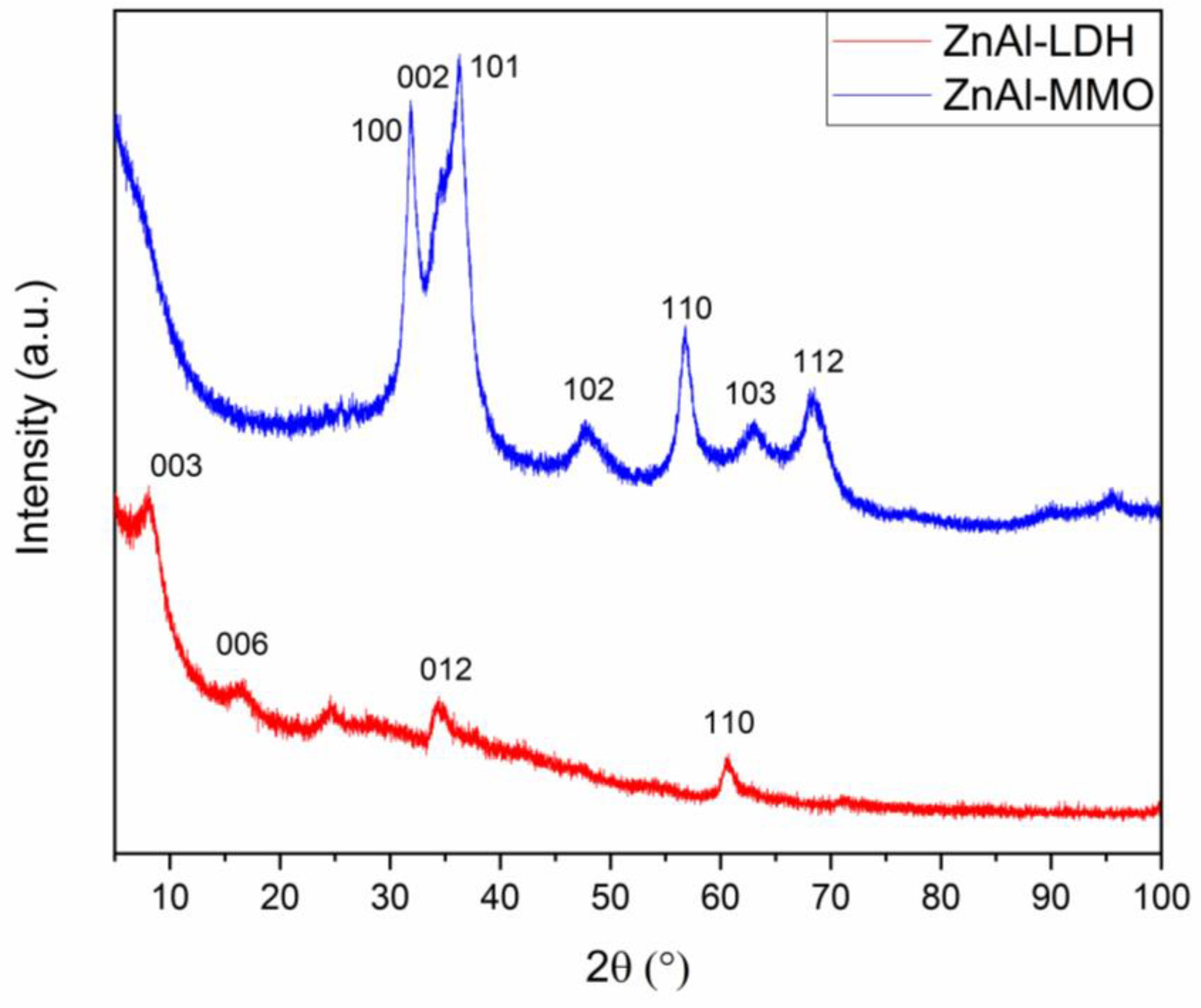
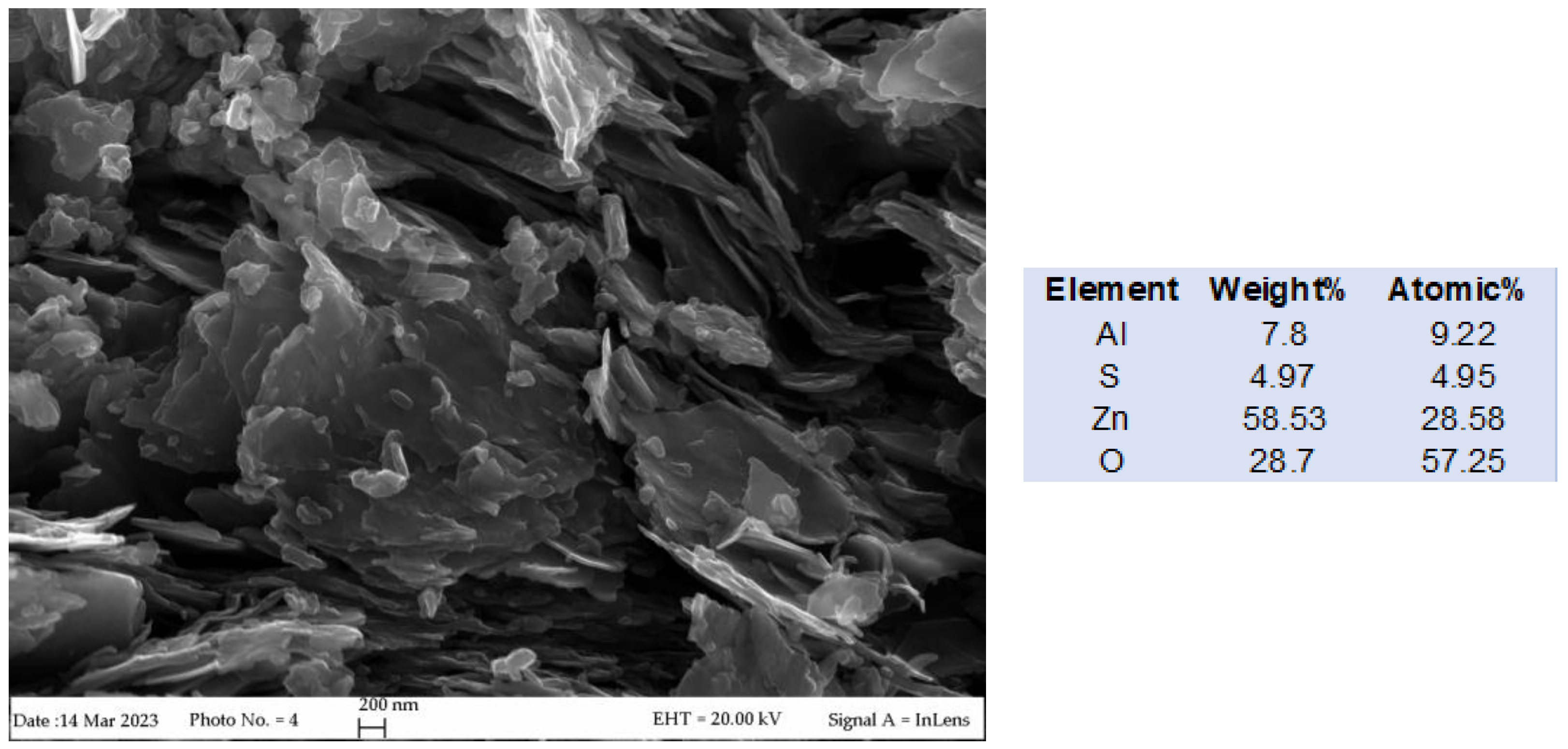
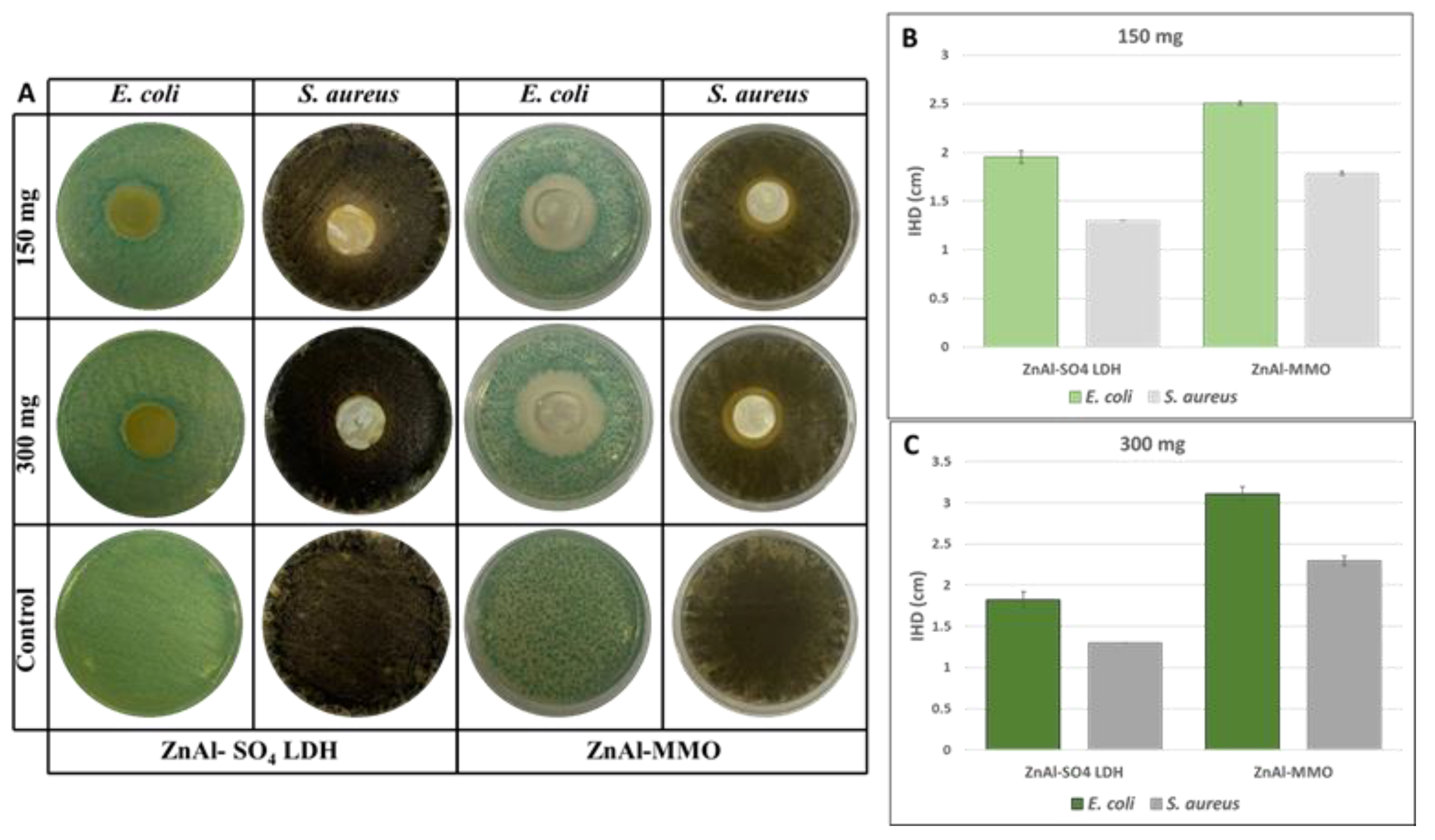

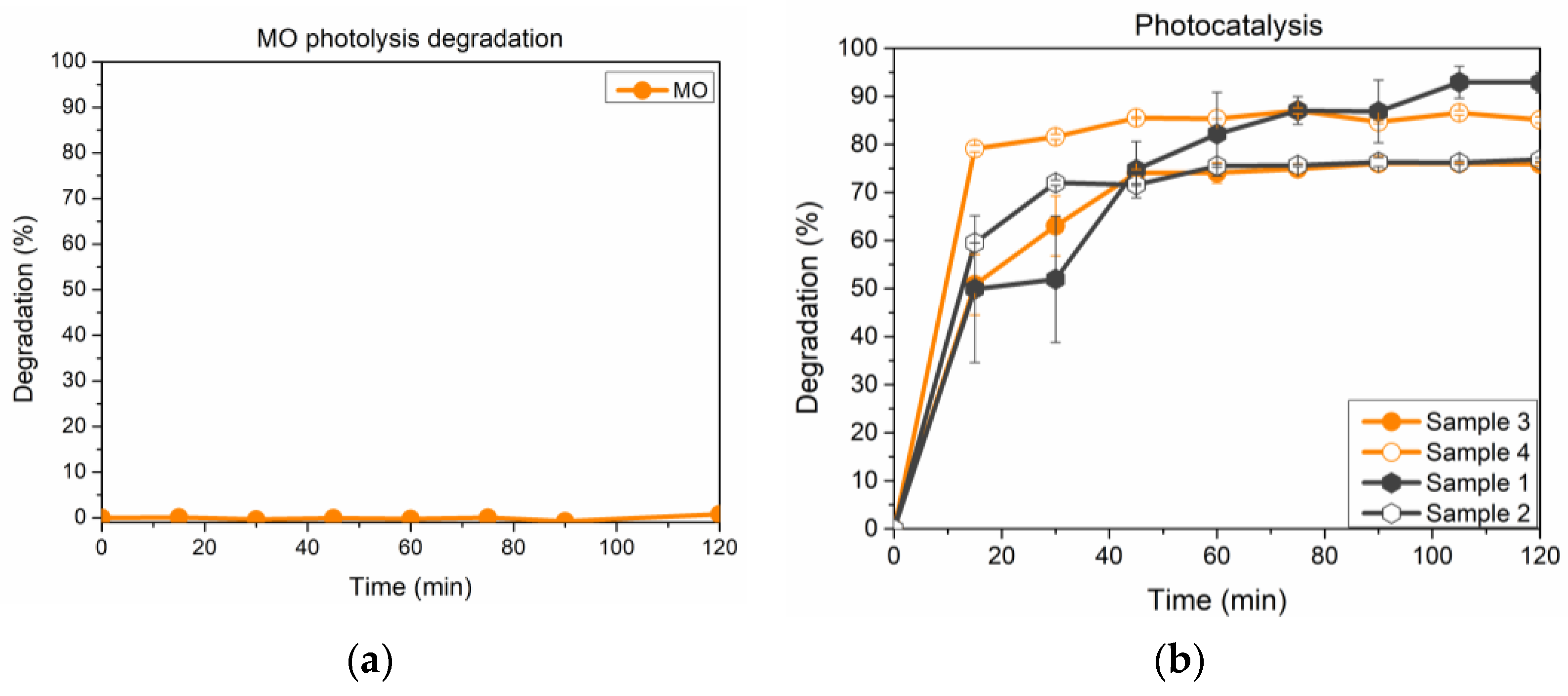
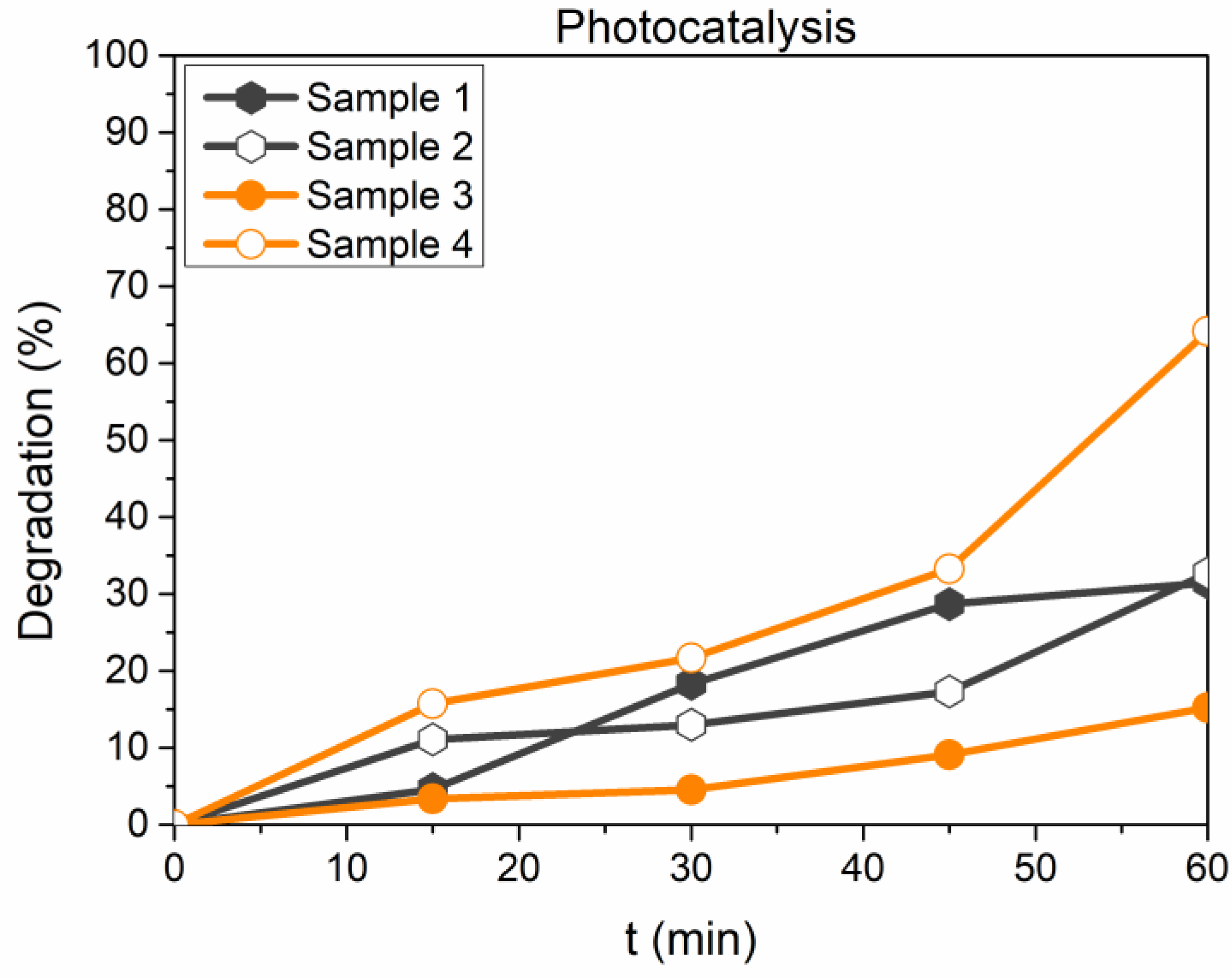
| Sample n. | Composition |
|---|---|
| 1 | ZnAl-SO4 LDH |
| 2 | ZnAl-MMO |
| 3 | ZnAl-CrO4 LDH |
| 4 | ZnAl-MMO-CrO4 |
| Sample n. | Pseudo-First-Order eq. | qe (exp.) (mg g−1) | qe (calc.) (mg g−1) | k1 (min−1) | R2 |
| 1 | y = −0.0038x – 1.9017 | 0.0378 | 0.0125 | 0.0087 | 0.7974 |
| 2 | y = −0.0014x – 2.1001 | 0.0373 | 0.0079 | 0.0032 | 0.575 |
| 3 | y = −0.0043x – 1.7303 | 0.0356 | 0.0186 | 0.0099 | 0.8928 |
| 4 | y = −0.0067x – 1.7674 | 0.0350 | 0.0170 | 0.0154 | 0.6993 |
| Sample n. | Pseudo-Second-Order eq. | qe (exp.) (mg g−1) | qe (calc.) (mg g−1) | k2 (g mg−1 min−1) | R2 |
| 1 | y = 30.877x + 58.531 | 0.0378 | 0.0355 | 16.2886 | 0.9992 |
| 2 | y = 31.732x + 20.434 | 0.0373 | 0.0288 | 49.2766 | 0.9985 |
| 3 | y = 34.666x + 169.79 | 0.0356 | 0.0315 | 7.0777 | 0.9955 |
| 4 | y = 28.122x + 104.29 | 0.0350 | 0.0323 | 7.5831 | 0.9944 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardinale, A.M.; Alberti, S.; Reverberi, A.P.; Catauro, M.; Ghibaudo, N.; Fortunato, M. Antibacterial and Photocatalytic Activities of LDH-Based Sorbents of Different Compositions. Microorganisms 2023, 11, 1045. https://doi.org/10.3390/microorganisms11041045
Cardinale AM, Alberti S, Reverberi AP, Catauro M, Ghibaudo N, Fortunato M. Antibacterial and Photocatalytic Activities of LDH-Based Sorbents of Different Compositions. Microorganisms. 2023; 11(4):1045. https://doi.org/10.3390/microorganisms11041045
Chicago/Turabian StyleCardinale, Anna Maria, Stefano Alberti, Andrea Pietro Reverberi, Michelina Catauro, Nicolò Ghibaudo, and Marco Fortunato. 2023. "Antibacterial and Photocatalytic Activities of LDH-Based Sorbents of Different Compositions" Microorganisms 11, no. 4: 1045. https://doi.org/10.3390/microorganisms11041045
APA StyleCardinale, A. M., Alberti, S., Reverberi, A. P., Catauro, M., Ghibaudo, N., & Fortunato, M. (2023). Antibacterial and Photocatalytic Activities of LDH-Based Sorbents of Different Compositions. Microorganisms, 11(4), 1045. https://doi.org/10.3390/microorganisms11041045









