Quantitative and Qualitative Airborne Mycobiota Surveillance in High-Risk Hospital Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Air Examination
2.3. Microbiological Proceeding
2.4. Susceptibility to Triazole Derivatives
2.5. Statistical Analysis
3. Results
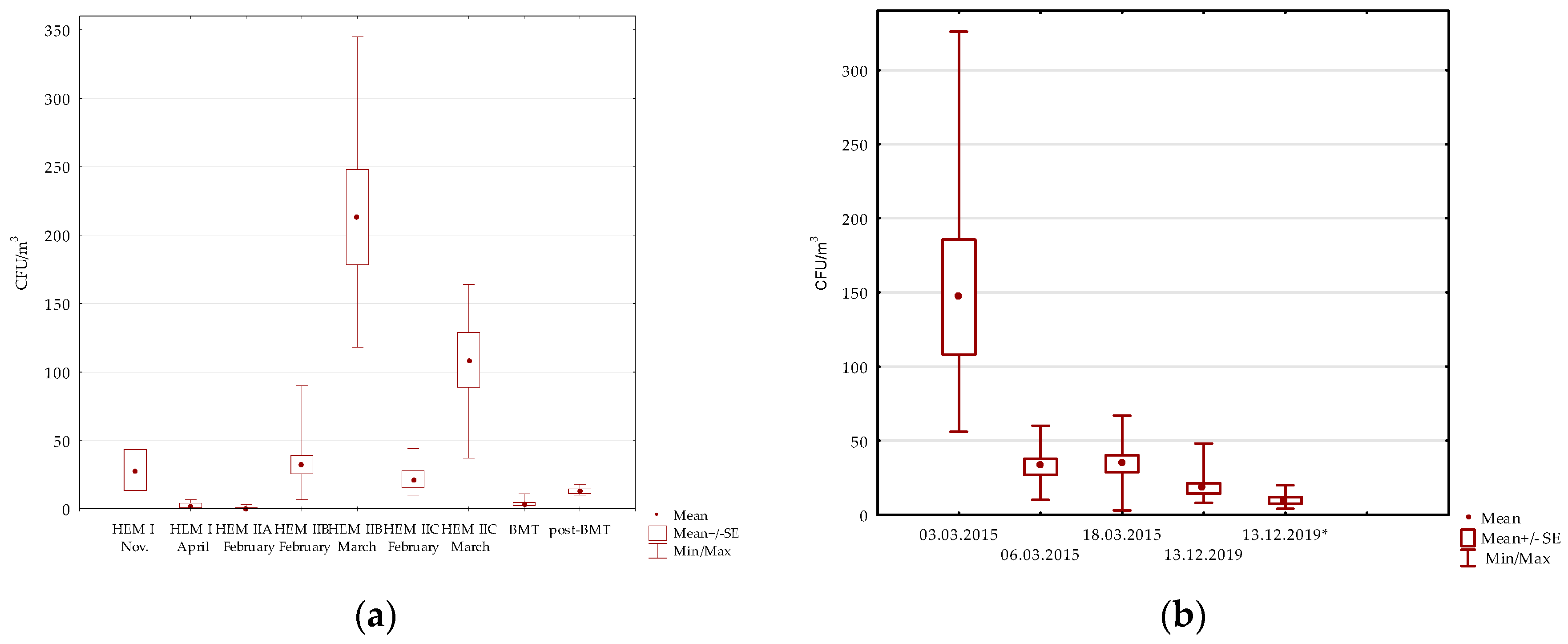
3.1. Air Examination
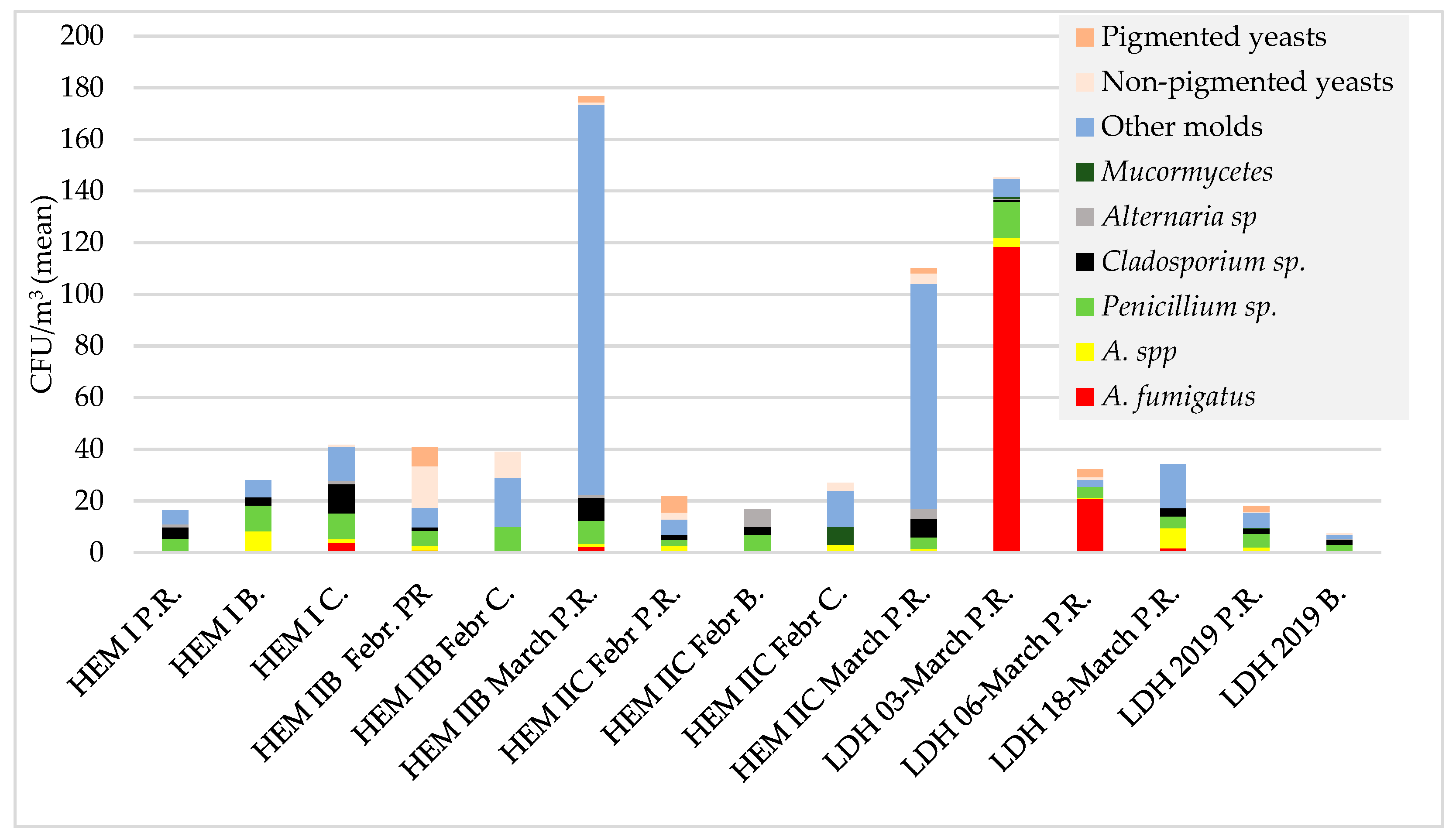
3.1.1. Hematology Wards
Pulmonology Hospital
3.2. Aspergillus Fumigatus Species Complex Susceptibility to Triazole Derivatives
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niculita-Hirzel, H.; Wild, P.; Hirzel, A.H. Season, Vegetation Proximity and Building Age Shape the Indoor Fungal Communities’ Composition at City-Scale. J. Fungi 2022, 8, 1045. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, G.; Spieksma, F.T.M. Aerobiologic and clinical aspects of mould allergy in Europe. Allergy 1995, 50, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Pokala, H.R.; Leonard, D.; Cox, J.; Metcalf, P.; McClay, J.; Siegel, J.; Winick, N. Association of hospital construction with the development of healthcare associated environmental mold infections (HAEMI) in pediatric patients with leukemia. Pediatr. Blood Cancer 2014, 61, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-Y.; Myong, J.-P.; Kim, W.-B.; Park, C.; Lee, S.J.; Lee, S.H.; Lee, D.-G. Profiles of Environmental Mold: Indoor and Outdoor Air Sampling in a Hematology Hospital in Seoul, South Korea. Int. J. Environ. Res. Public Health 2018, 15, 2560. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Ananda-Rajah, M.; Belcastro, A.; McMullan, B.; Reid, A.; Dempsey, K.; Athan, E.; Cheng, A.; Slavin, M.A. Consensus guidelines for implementation of quality processes to prevent invasive fungal disease and enhanced surveillance measures during hospital building works, 2014. Intern. Med. J. 2014, 44, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- De Linares, C.; Navarro, D.; Puigdemunt, R.; Belmonte, J. Airborne Alt a 1 Dynamic and Its Relationship with the Airborne Dynamics of Alternaria Conidia and Pleosporales Spores. J. Fungi 2022, 8, 125. [Google Scholar] [CrossRef]
- Bigot, J.; Guillot, L.; Guitard, J.; Ruffin, M.; Corvol, H.; Balloy, V.; Hennequin, C. Bronchial Epithelial Cells on the Front Line to Fight Lung Infection-Causing Aspergillus fumigatus. Front. Immunol. 2020, 22, 1041. [Google Scholar] [CrossRef]
- Vitte, J.; Michel, M.; Malinovschi, A.; Caminati, M.; Odebode, A.; Annesi-Maesano, I.; Caimmi, D.P.; Cassagne, C.; Demoly, P.; Heffler, E.; et al. Fungal exposome, human health and unmet needs: A 2022 update with special focus on allergy. Allergy 2022, 71, 3199–3216. [Google Scholar] [CrossRef]
- The Fungal Infection Trust. Available online: https://www.fungalinfectiontrust.org/how-common-are-fungal-diseases (accessed on 20 February 2023).
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Earle, K.; Valero, C.; Conn, D.P.; Vere, G.; Cook, P.C.; Bromley, M.J.; Bowyer, P.; Gago, S. Pathogenicity and virulence of Aspergillus fumigatus. Virulence 2023, 14, 2172264. [Google Scholar] [CrossRef]
- Denning, D.W.; Cadranel, J.; Beigelman-Aubry, C.; Ader, F.; Chakrabarti, A.; Blot, S.; Ullmann, A.J.; Dimopoulos, G.; Lange, C.; European Society for Clinical Microbiology and Infectious Diseases and European Respiratory Society. Chronic pulmonary aspergillosis: Rationale and clinical guidelines for diagnosis and management. Eur. Respir. J. 2016, 47, 45–68. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, B.; Mahto, K.K.; Meis, J.F.; Chowdhary, A. High-Frequency Direct Detection of Triazole Resistance in Aspergillus fumigatus from Patients with Chronic Pulmonary Fungal Diseases in India. J. Fungi 2020, 6, 67. [Google Scholar] [CrossRef]
- Puerta-Alcalde, P.; Garcia-Vidal, C. Changing Epidemiology of Invasive Fungal Disease in Allogeneic Hematopoietic Stem Cell Transplantation. J. Fungi 2021, 7, 848. [Google Scholar] [CrossRef]
- Commission for Hospital Hygiene and Infection Prevention (KRINKO). Infection Prevention Requirements for the Medical Care of Immunosuppressed Patients: Recommendations of the Commission for Hospital Hygiene and Infection Prevention (KRINKO) at the Robert Koch Institute. GMS Hyg. Infect. Control. 2022, 17, 1–36. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W.; Howard, S.J.; The Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). EUCAST DEFINITIVE DOCUMENT EDef 9.2 Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Conidia Forming Moulds. July 2014. Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST-AFST_EDEF_9_2_Mould_testing_20140815.pdf (accessed on 24 February 2015).
- European Committee on Antimicrobial Susceptibility Testing Breakpoint Tables for Interpretation of MICs for Antifungal Agents Version 10.0. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Clinical_breakpoints/AFST_BP_v10.0_200204_updatd_links_200924.pdf (accessed on 14 February 2020).
- Symoens, F.; Burnod, J.; Lebeau, B.; Viviani, M.; Piens, M.; Tortorano, A.; Nolard, N.; Chapuis, F.; Grillot, R. Hospital-acquired Aspergillus fumigatus infection: Can molecular typing methods identify an environmental source? J. Hosp. Infect. 2002, 52, 60–67. [Google Scholar] [CrossRef]
- de la Rasilla, T.P.-G.; González-Jiménez, I.; Fernández-Arroyo, A.; Roldán, A.; Carretero-Ares, J.L.; García-Clemente, M.; Telenti-Asensio, M.; García-Prieto, E.; Martínez-Suarez, M.; Vázquez-Valdés, F.; et al. COVID-19 Associated Pulmonary Aspergillosis (CAPA): Hospital or Home Environment as a Source of Life-Threatening Aspergillus fumigatus Infection? J. Fungi 2022, 8, 316. [Google Scholar] [CrossRef]
- Rocchi, S.; Sewell, T.R.; Valot, B.; Godeau, C.; Laboissiere, A.; Millon, L.; Fisher, M.C. Molecular Epidemiology of Azole-Resistant Aspergillus fumigatus in France Shows Patient and Healthcare Links to Environmentally Occurring Genotypes. Front. Cell. Infect. Microbiol. 2021, 11, 729476. [Google Scholar] [CrossRef]
- Sehulster, L.M.; Chinn, R.Y.W.; Arduino, M.J.; Carpenter, J.; Donlan, R.; Ashford, D.; Besser, R.; Fields, B.; McNeil, M.M.; Whitney, C.; et al. Guidelines for Environmental Infection Control in Health-Care Facilities; Recommendations from CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC); American Society for Healthcare Engineering/American Hospital Association: Chicago, IL, USA, 2004. [Google Scholar]
- Nawrot, U.; Usnarska-Zubkiewicz, L.; Pajączkowska, M.; Mokracka-Latajka, G.; Kuliczkowski, K.; Nowicka, J. Fungal Contamination of Air in the Department of Haematology. Pol. J. Environ. Stud. 2010, 19, 967–971. [Google Scholar]
- Picot-Guéraud, R.; Khouri, C.; Brenier-Pinchart, M.-P.; Saviuc, P.; Fares, A.; Sellon, T.; Thiebaut-Bertrand, A.; Mallaret, M.-R. En-suite bathrooms in protected haematology wards: A source of filamentous fungal contamination? J. Hosp. Infect. 2015, 91, 244–249. [Google Scholar] [CrossRef]
- Reboux, G.; Gbaguidi-Haore, H.; Bellanger, A.; Demonmerot, F.; Houdrouge, K.; Deconinck, E.; Bertrand, X.; Millon, L. A 10-year survey of fungal aerocontamination in hospital corridors: A reliable sentinel to predict fungal exposure risk? J. Hosp. Infect. 2014, 87, 34–40. [Google Scholar] [CrossRef]
- Tigli, G.A.; Cekin, Y.; Baysan, B.; Ögϋnc, D.; Ongϋt, G.; Saba, R.; Undar, L.; Mutlu, G.; Vural, T. Evaluation of total fungal air contamination levels and efficiency of the ventilation systems used in adult haematology unit and adult stem cell transplantation unit. Afr. J. Microbiol. Res. 2013, 7, 5606–5609. [Google Scholar] [CrossRef]
- Falvey, D.; Streifel, A. Ten-year air sample analysis of Aspergillus prevalence in a university hospital. J. Hosp. Infect. 2007, 67, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Mareković, I. What’s New in Prevention of Invasive Fungal Diseases during Hospital Construction and Renovation Work: An Overview. J. Fungi 2023, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, U.; Kurzyk, E.; Arendrup, M.C.; Mroczyńska, M.; Włodarczyk, K.; Sulik-Tyszka, B.; Wróblewska, M.; Ussowicz, M.; Zdziarski, P.; Niewińska, K.; et al. Detection of Polish clinical Aspergillus fumigatus isolates resistant to triazoles. Med. Mycol. 2018, 56, 121–124. [Google Scholar] [CrossRef]
- Nawrot, U.; Włodarczyk, K.; Bartoszewicz, M.; Balicka, S.; Pomorska-Wesołowska, M.; Kurzyk, E.; Brillowska-Dąbrowska, A.; Sulik-Tyszka, B.; Basak, G.; Wróblewska, M. Activity of isavuconazole and other triazole derivatives against clinical isolates of Aspergillus fumigatus. Postępy Hig. I Med. Doświadczalnej 2019, 73, 76–80. [Google Scholar] [CrossRef]
| Hospital Ward | Data | Type of Room (Number of Rooms Tested) | Cultured Fungi [CFU/m3] (Number of Positive Rooms/Number of Rooms Tested) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Number of Fungi Range (Mean) | A. fumigatus | Aspergillus spp. | Penicillium sp. | Cladosporium sp. | Alternaria sp | Mucormycetes | Other Molds | Non-Pigmented Yeasts | Pigmented Yeasts | |||
| HEM I | 11.12.2014 | Patient rooms (2) | 13.33–43.33 | - | - | - | 0–26.6 | - | - | 13.3–16.3 | - | - |
| Patient’s bathroom (1) | 36.67 | - | 13.33 | 3.33 | 6.6 | - | - | 13.3 | - | - | ||
| Corridors (3) | 50–86.7 (64.4) | 0–13.3 (2/3) | 0–6.6 (1/3) | 13.4–16.6 (3/3) | 6.6–30 (3/3) | 0–6.6 (1/3) | - | 6.6–36.6 (3/3) | 0–3.3 (1/3) | - | ||
| Nurses’ station (1) | 56 | 0 | 10 | 15 | - | - | - | 30 | - | - | ||
| 14.04.2015 | Patient rooms (4) | 0–6.67 (2.5) (2/4) | - | - | 3.3 (1/4) | - | 0–6.6 (1/4) | - | 0–3.3 (1/4) | - | - | |
| Patient’s bathroom (1) | 20 | - | 3.33 | 16.6 | - | - | - | - | - | - | ||
| Treatment room (1) | 3.33 | - | - | - | - | - | - | 3.3 | - | - | ||
| Nurses’ station (1) | 23.33 | - | 3.33 | 3.33 | 6.6 | - | - | 6.6 | 3.33 | - | ||
| Corridor (2) | 3.33–13.33 | - | - | 0–6.6 | 3.3–3.3 | - | - | 0–3.3 | - | - | ||
| HEM II (part A) | 6.02.2015 | Patient rooms (8) | 0–3.33 (1/8) | - | - | - | - | - | - | 0–3.33 (1/8) | - | - |
| Nurses’ station (1) | 3.33 | - | - | 3.33 | - | - | - | - | - | - | ||
| Corridor (1) | 0 | - | - | - | - | - | - | - | - | - | ||
| Kitchen (1) | 0 | - | - | - | - | - | - | - | - | - | ||
| HEM II (part B) | 10.02.2015 | Patient’s room (11) | 6.6–9 (30) (11/11) | 0–6.67 (2/11) | 0–6.6 (3/11) | 0–16.6 (8/11) | 0–6.6 (3/11) | - | - | 0–13.3 (10/11) | 0–30 (6/11) | 0–26.6 (6/11) |
| Corridor (1) | 40 | - | - | 10 | - | - | - | 19 | 10 | - | ||
| 26.03.2015 | Patient rooms (7) | 118–345 (213) (7/7) | 0–7 (3/7) | 0–7 (1/7) | 0–20 (6/7) | 3–20 (7/7) | 0–7 (1/7) | - | 83–288 (151) (7/7) | 0–7 (1/7) | 0–17 (1/7) | |
| HEM II (part C) | 20.02.2015 | Patient rooms (5) | 10–44 (20) (5/5) | - | 0–7 (2/5) | 0–5 (3/5) | 0–7 (2/5) | - | - | 0–1 (3/5) | 0–14 (1/5) | 0–24 (2/5) |
| Patient’s bathroom (1) | 17 | - | - | 7 | 3 | 7 | - | - | - | - | ||
| Treatment room (1) | 7 | - | - | - | - | - | - | 7 | - | - | ||
| Corridor (1) | 27 | - | 3 | - | - | - | 7 | 14 | 3 | - | ||
| 26.03.2015 | Patient rooms (6) | 37–164 (108) | - | 0–7 (2/6) | 0–17 (4/6) | 0–27 (4/6) | 0–10 (3/6) | - | 24–153 (87) | 0–10 (4/6) | 0–7 (3/6) | |
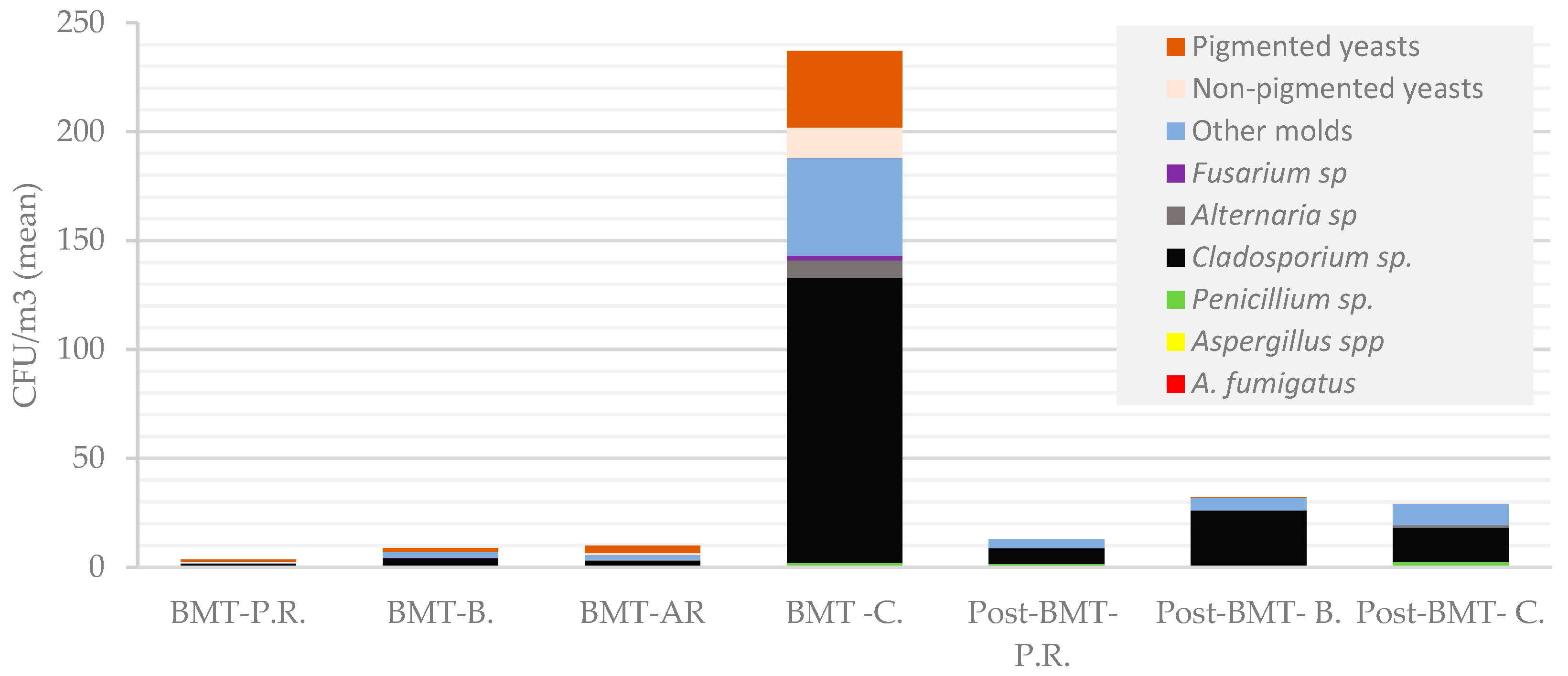
| BMT | 22.10.2019 | Patient isolation rooms (8) | 0–11 (3.5) | - | - | - | 0–4 (5/8) | 0–1 (1/8) | - | 0–1 (2/8) | 0–1 (1/8) | 0–8 (2/8) |
| Sluices (8) | 2–36 (10) | - | - | 0–4 (2/8) | 0–10 (3/8) | 0–2 (1/8) | - | 0–14 (2/8) | 0–6 (1/8) | 0–18 (2/8) | ||
| Corridor (1) | 237 | 1 | - | 1 | 131 | 8 | - | 47 F | 14 | 35 | ||
| Bathrooms (8) | 2–32 (8) | 0–2 (1/8) | - | 0–4 (1/8) | 0–14 (7/8) | - | - | 0–6 F (5/8) | - | 0–12 (2/8) | ||
| Post-BMT | 12.11.2019 | Patient rooms (5) | 10–18 (12.8) | - | - | 0–4 (3/5) | 2–12; (5/5) | - | - | 2–8 (5/5) | - | - |
| Bathrooms (5) | 6–28 (19.6) | - | - | 0–2 (1/5) | 4–22; (5/5) | - | - | 2–12 (5/5) | - | 0–2 (1/5) | ||
| Corridor (1) | 54 | - | - | 4 | 15 | 2 | - | 32 | 1 | - | ||
| Nurses’ counter | 18 | - | - | 4 | 2 | - | - | 12 | - | - | ||
| Communication routes BMT/post-BMT | 12.11.2019 | Elevator I | 36 | 2 | 28 | 2 | ||||||
| Elevator II | 20 | 4 | 16 | |||||||||
| Corridor by the elevators | 10 | 4 | 6 | |||||||||
| Data | Type of Room (Number of Rooms Tested) | Total Number of Fungi [CFU/m3] Range (Mean) | Cultured Fungi [CFU/m3] (Number of Positive Rooms/Number of Rooms Tested) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A. fumigatus | A. spp. | Penicillium sp. | Cladosporium sp., | Alternaria sp. | Mucor mycetes | Other Molds | Non-Pigmented Yeasts | Pigmented yeasts | |||
| 3.03.2015 | Patient rooms (8) | 56–326 (146.8) | 45–300 (118.5 ^) (8/8) | 0–17 (4/8) | 3–26 (14.1^) (8/8) | 0–6 (1/8) | - | 0–3 (2/8) | 0–32 (7/8) | 0–3 (1/8) | - |
| 6.03.2015 | Patient rooms (8) | 10–60 (32.3) | 7–37 (20.8 ^) (8/8) | 0–3 (1/8) | 0–17 (6/8) | - | - | - | 0–7 (5/8) | 0–7 (1/8) | 0–17 (2/8) |
| 18.03.2015 | Patient rooms (8) | 3–67 (34.4) | 0–7 (4/8) | 0–23 (4/8) | 0–13 (5/8) | 0–10 (4/8) | - | - | 0–33 (7/8) | - | - |
| Treatment room | 40 | 0 | 3 | 0 | 10 | 30 | |||||
| 13.12.2019 | Patient rooms (10) | 8–48 (18) | 0–4 (1/10) | 0–4 (6/10) | 0–28 (9/10) | 0–8 (5/10) | - | 0–2 (1/10) | 2–16 (6 ^) (10/10) | 0–2 (2/2) | 0–6 (5/10) |
| Bathrooms (4) | 4–10 (8) | 0–6 (3/4) | 0–4 (3/4) | 0–2 (1/4) | 0–2 (3/4) | 0–2 (1/4) | |||||
| Treatment room | 7 | 2 | 2 | 1 | |||||||
| Nurses’ room | 20 | 6 | 2 | 14 | |||||||
| Hospital Ward | Number of Isolates (n = 31) | MIC (mg/L) | ||
|---|---|---|---|---|
| Itraconazole | Voriconazole | Posaconazole | ||
| Lung Diseases (24) | (17) | 0.25 | 0.25 | 0.06 |
| (6) | 1 | 0.25 | 0.06 | |
| (1) | 0.5 | 0.25 | 0.06 | |
| HEM I (1) | (1) | 0.5 | 0.25 | 0.03 |
| HEM II part B (5) | (4) | 0.25 | 0.25 | 0.06 |
| (1) | 1 | 0.5 | 0.06 | |
| HEM II part C (1) | (1) | 1 | 1 | 0.125 |
| MIC50 | (31) | 0.25 | 0.25 | 0.06 |
| MIC90 | (31) | 1 | 0.25 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górzyńska, A.; Grzech, A.; Mierzwiak, P.; Ussowicz, M.; Biernat, M.; Nawrot, U. Quantitative and Qualitative Airborne Mycobiota Surveillance in High-Risk Hospital Environment. Microorganisms 2023, 11, 1031. https://doi.org/10.3390/microorganisms11041031
Górzyńska A, Grzech A, Mierzwiak P, Ussowicz M, Biernat M, Nawrot U. Quantitative and Qualitative Airborne Mycobiota Surveillance in High-Risk Hospital Environment. Microorganisms. 2023; 11(4):1031. https://doi.org/10.3390/microorganisms11041031
Chicago/Turabian StyleGórzyńska, Aleksandra, Aneta Grzech, Paulina Mierzwiak, Marek Ussowicz, Monika Biernat, and Urszula Nawrot. 2023. "Quantitative and Qualitative Airborne Mycobiota Surveillance in High-Risk Hospital Environment" Microorganisms 11, no. 4: 1031. https://doi.org/10.3390/microorganisms11041031
APA StyleGórzyńska, A., Grzech, A., Mierzwiak, P., Ussowicz, M., Biernat, M., & Nawrot, U. (2023). Quantitative and Qualitative Airborne Mycobiota Surveillance in High-Risk Hospital Environment. Microorganisms, 11(4), 1031. https://doi.org/10.3390/microorganisms11041031







