Histopathological, Immunohistochemical, Biochemical, and In Silico Molecular Docking Study of Fungal-Mediated Selenium Oxide Nanoparticles on Biomphalaria alexandrina (Ehrenberg, 1831) Snails
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strain and Selenium Nanoparticles Preparation and Characterization
2.2. Snails
2.3. Daphnia Magna
2.4. Bioassays
2.4.1. Toxicological Impact on D. magna
2.4.2. Toxicological Impact on B. alexandrina Snails
Snails’ Egg-Laying Capacity Analysis
Tissue Preparation and Biochemical Analysis
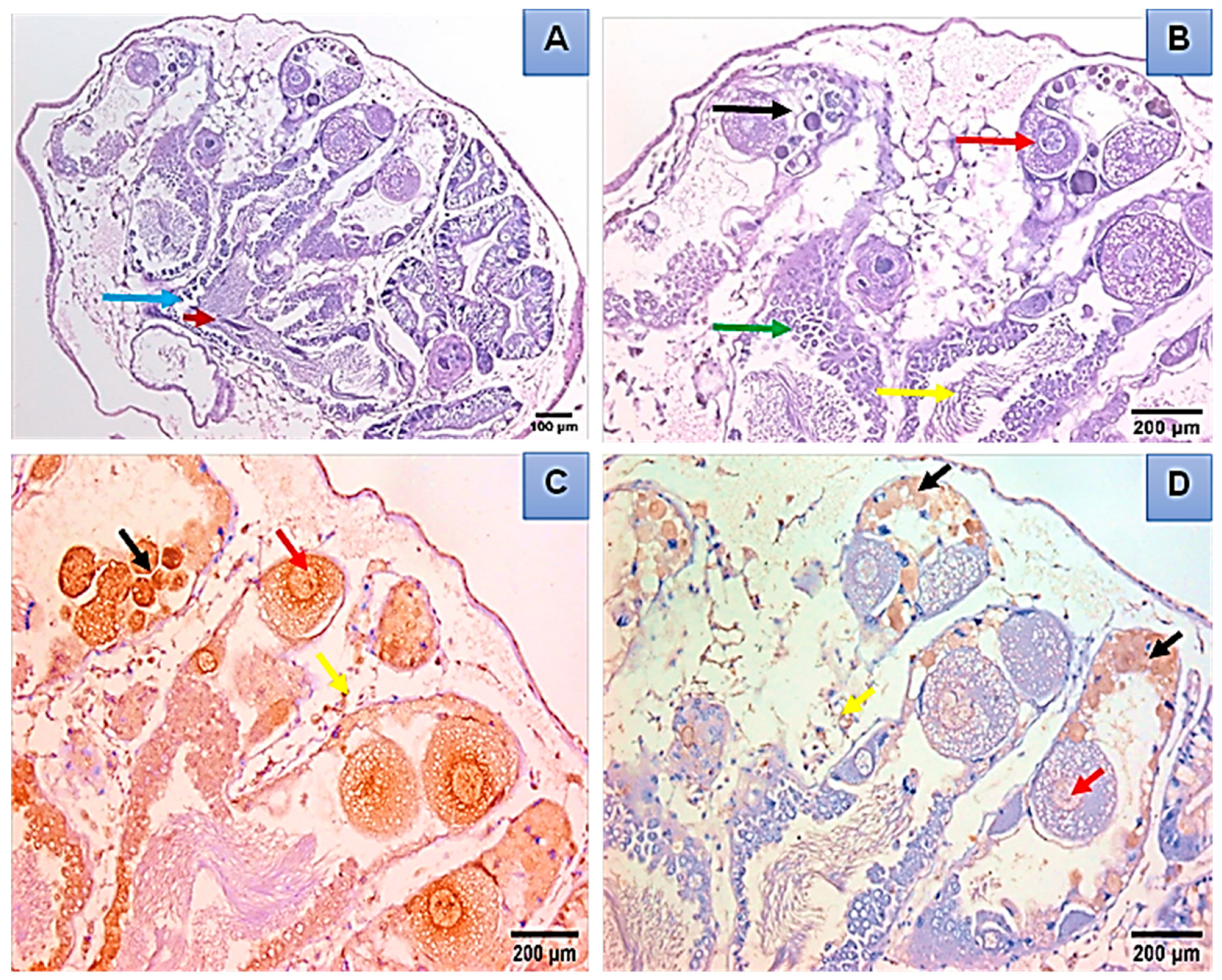
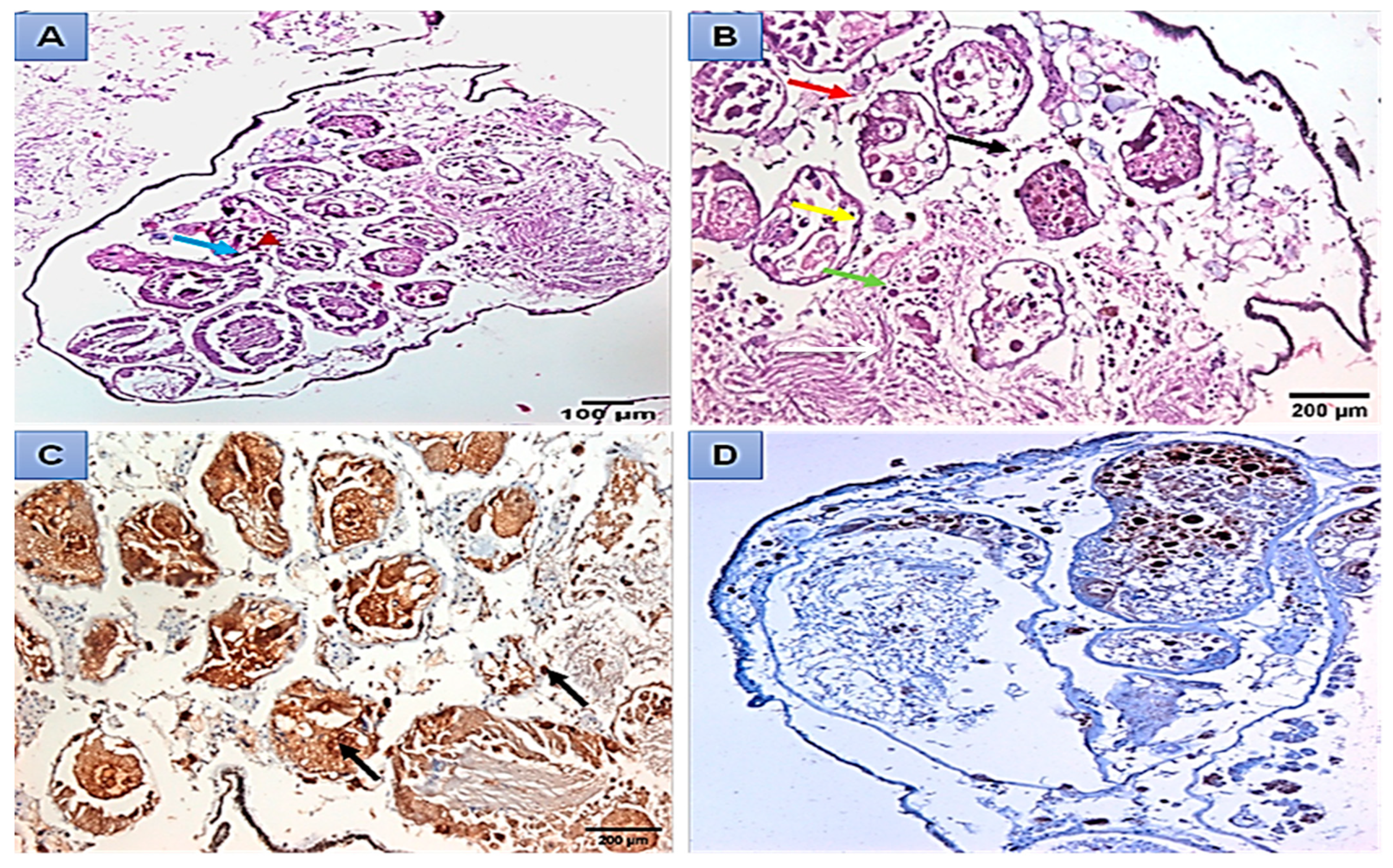
Histopathological and Immunohistochemical Analysis
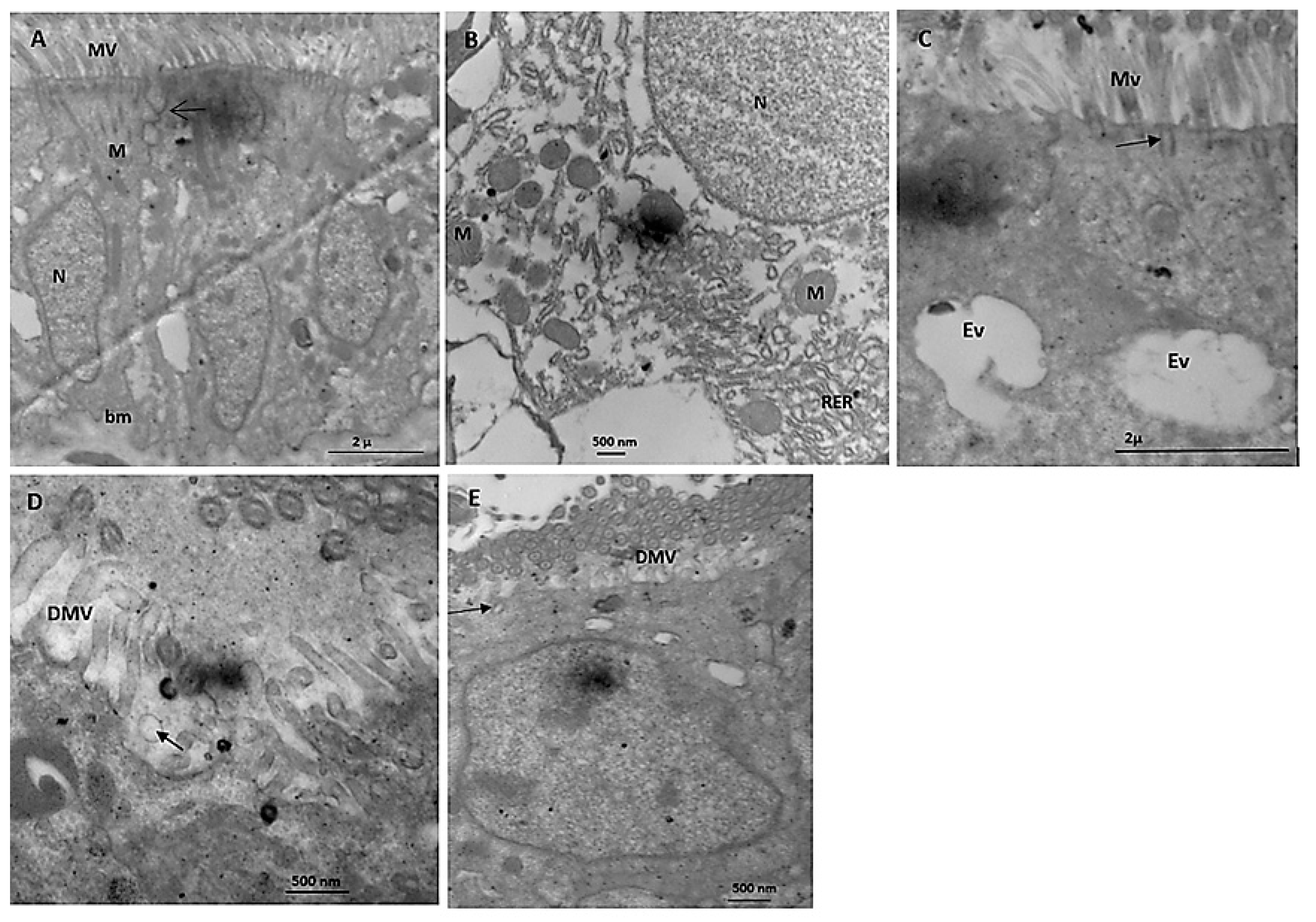
Electron Microscope Study
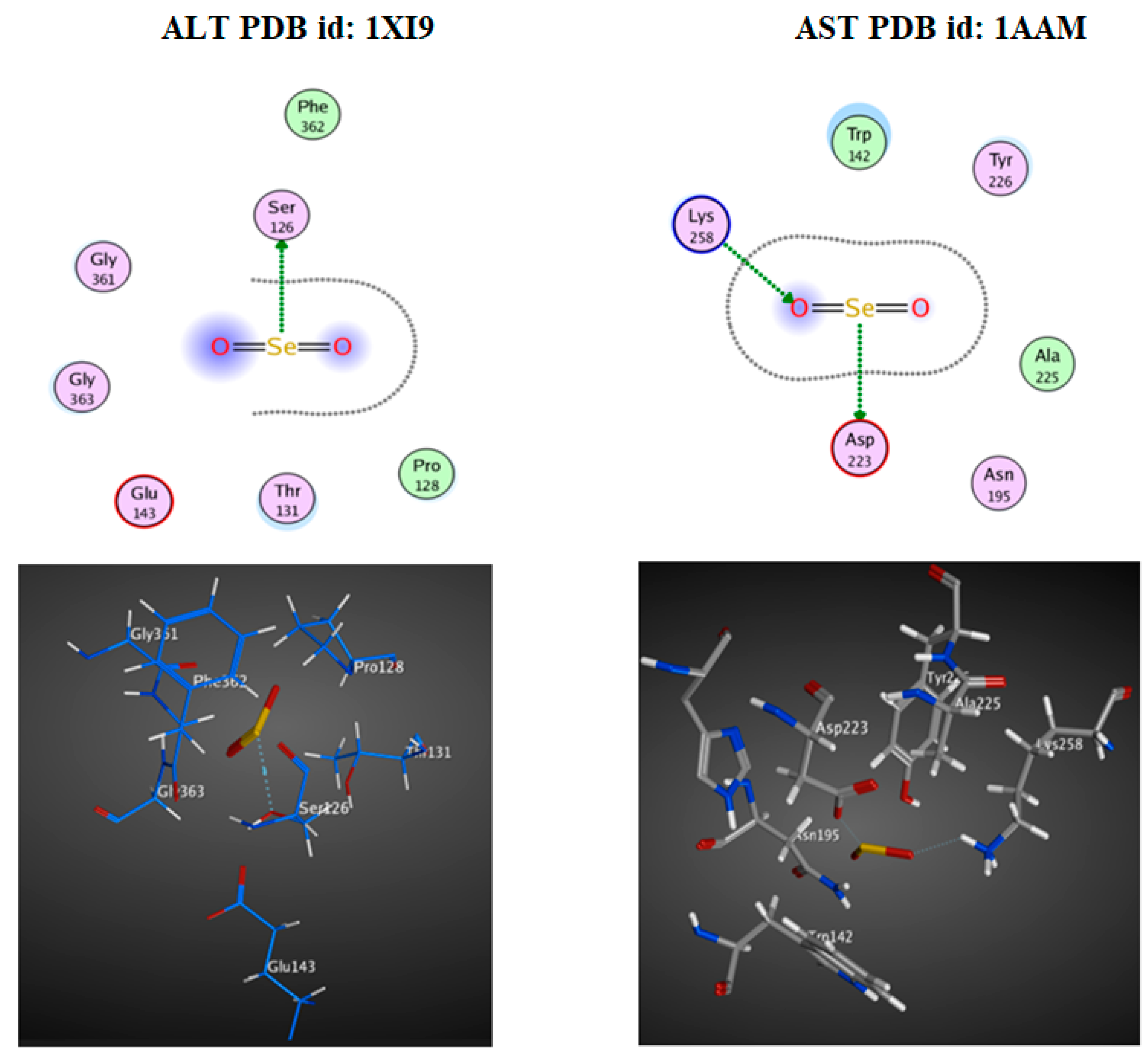
The Molecular Docking Interaction Study
2.5. Statistical Analysis
3. Results
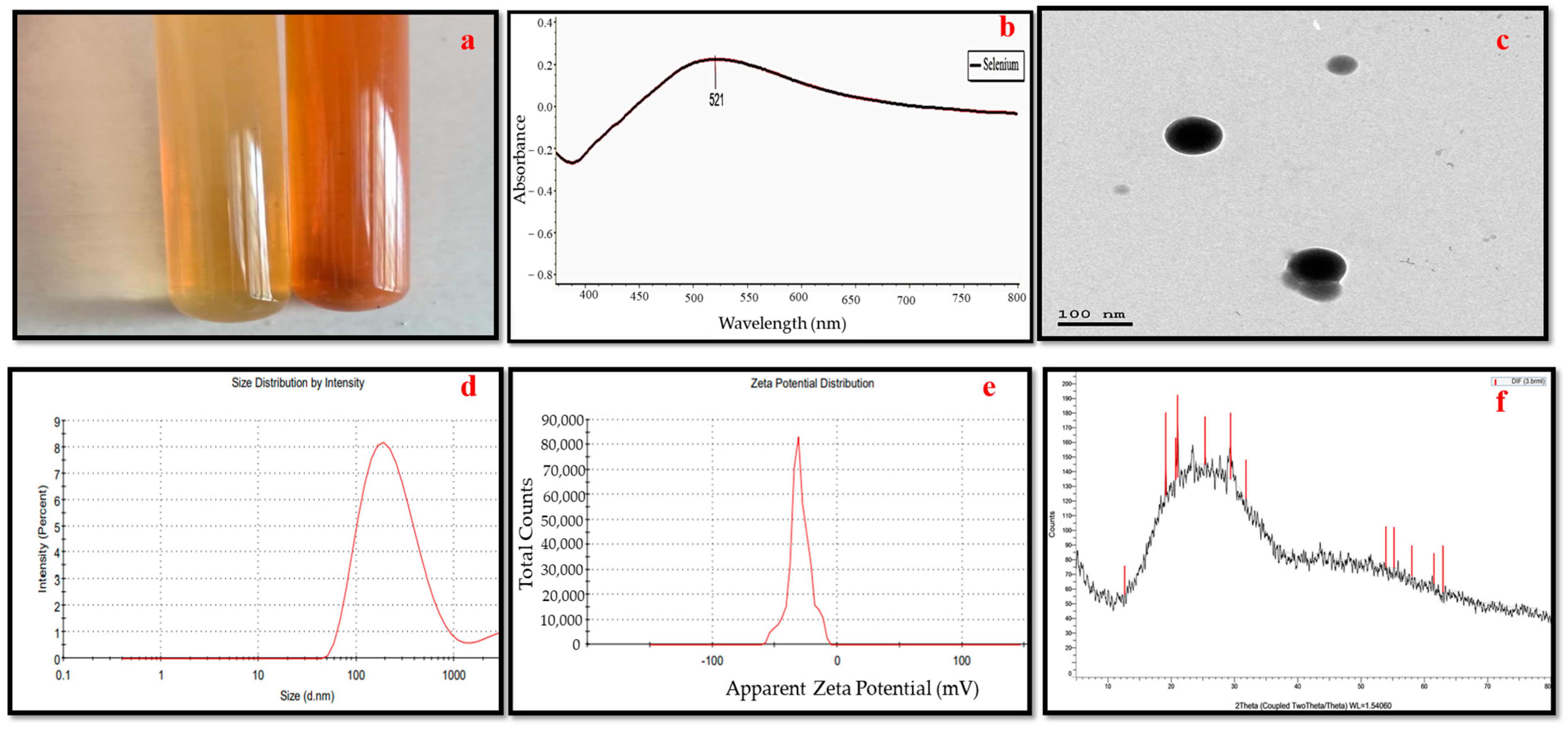
3.1. Selenium Nanoparticles Green Synthesis
3.2. Selenium Nanoparticles’ Characteristics
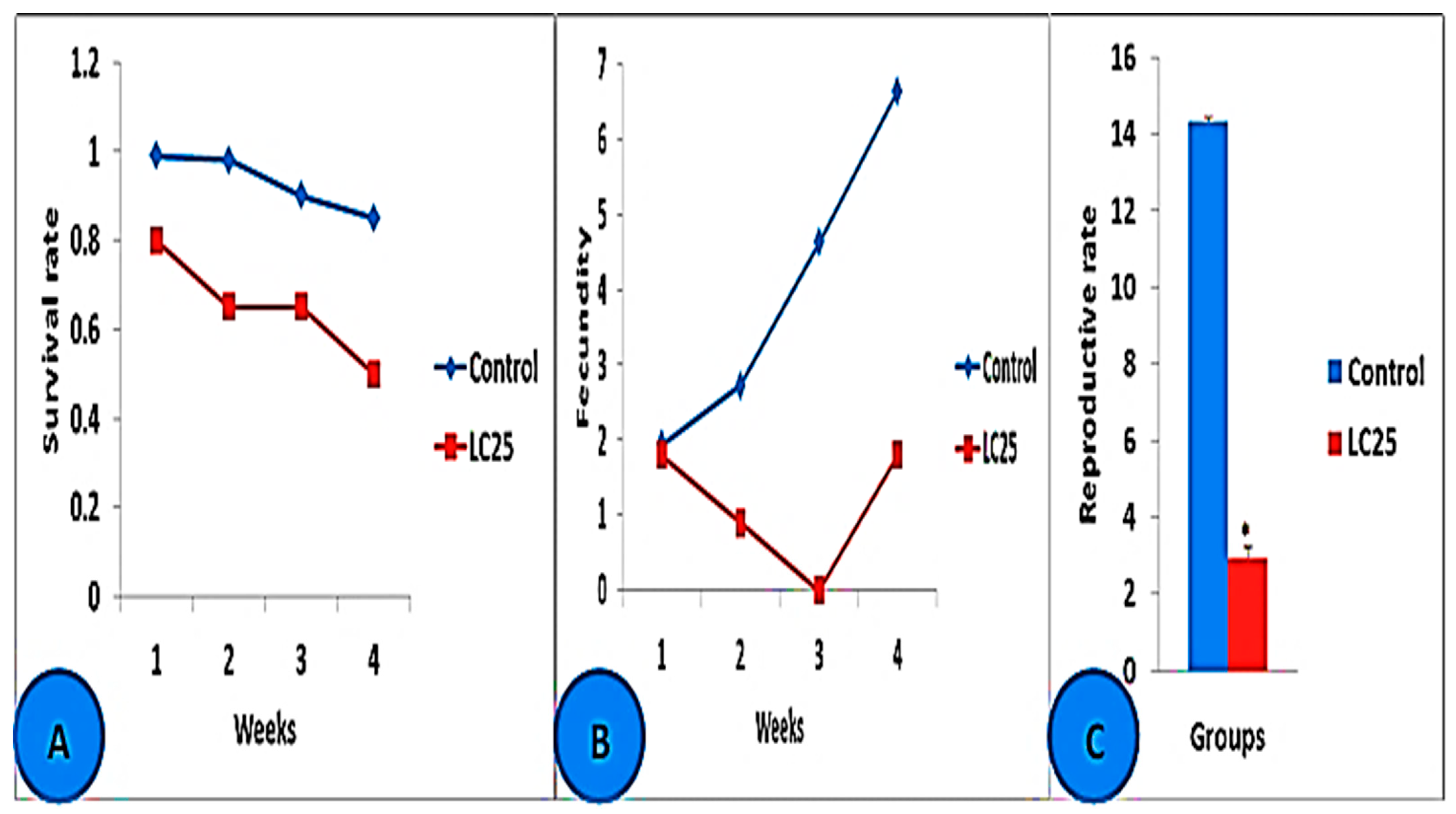
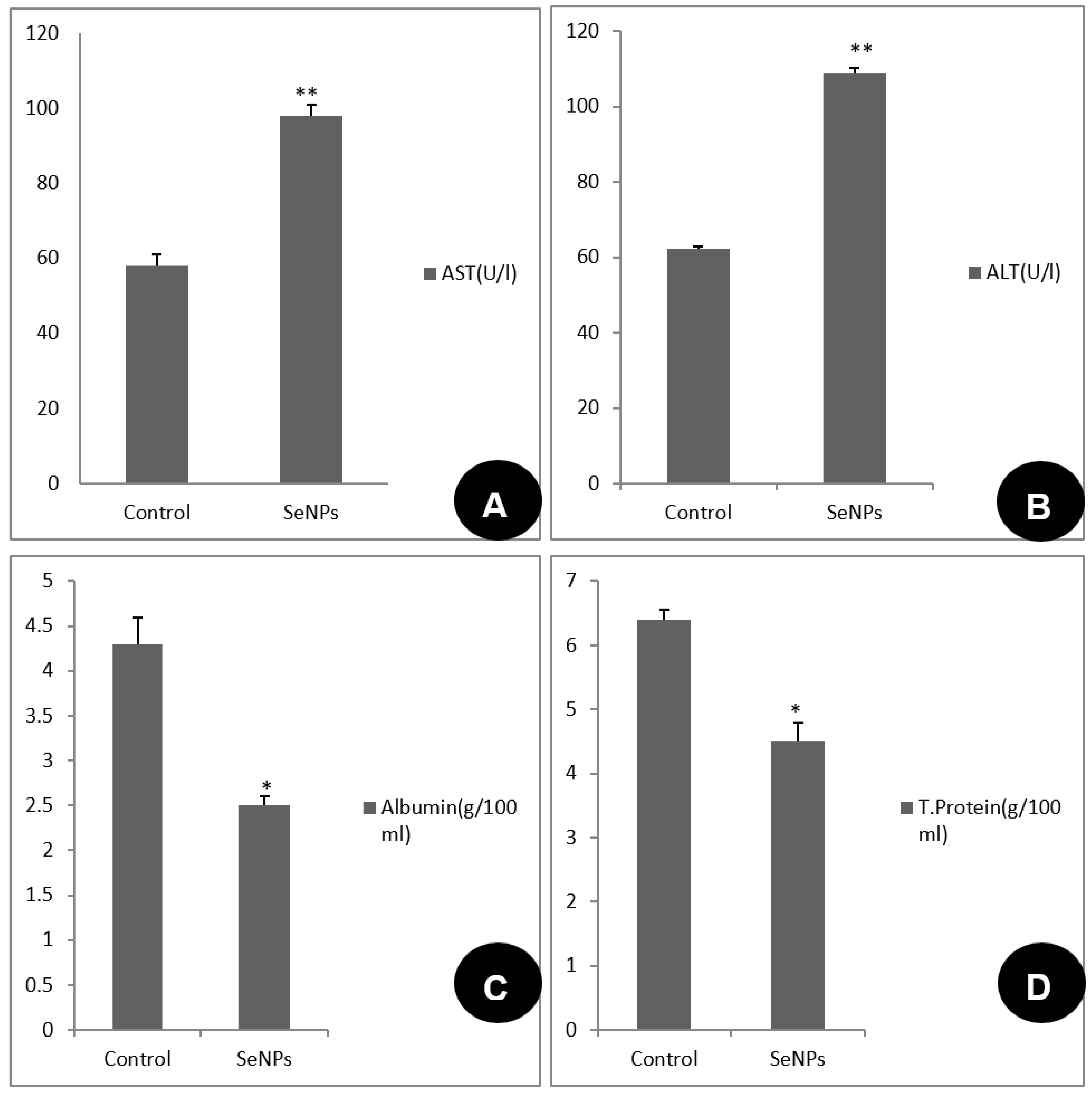
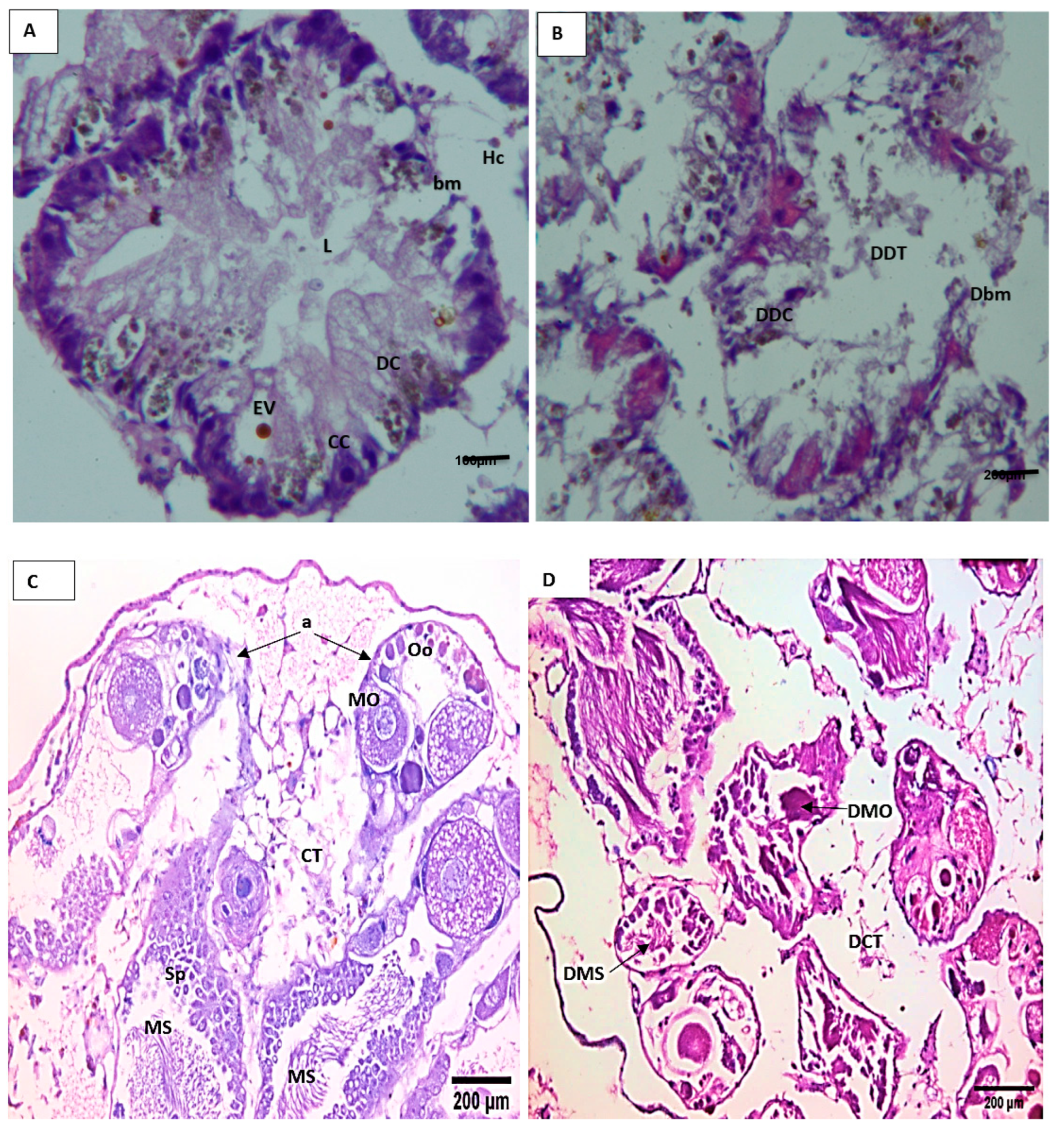
3.3. Toxicological Impact Assessment
3.4. Docking Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murthy, M.K.; Mohanty, C.S.; Swain, P.; Pattanayak, R. Assessment of Toxicity in the Freshwater Tadpole Polypedates Maculatus Exposed to Silver and Zinc Oxide Nanoparticles: A Multi-Biomarker Approach. Chemosphere 2022, 293, 133511. [Google Scholar] [CrossRef] [PubMed]
- Pyrzyńska, K. Determination of Selenium Species in Environmental Samples. Microchim. Acta 2002, 140, 55–62. [Google Scholar] [CrossRef]
- Lemly, A.D. Aquatic Selenium Pollution Is a Global Environmental Safety Issue. Ecotoxicol. Environ. Saf. 2004, 59, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Ungureanu, G.; Boaventura, R.; Botelho, C. Selenium Contaminated Waters: An Overview of Analytical Methods, Treatment Options and Recent Advances in Sorption Methods. Sci. Total Environ. 2015, 521–522, 246–260. [Google Scholar] [CrossRef]
- Filipović, N.; Ušjak, D.; Milenković, M.T.; Zheng, K.; Liverani, L.; Boccaccini, A.R.; Stevanović, M.M. Comparative Study of the Antimicrobial Activity of Selenium Nanoparticles with Different Surface Chemistry and Structure. Front. Bioeng. Biotechnol. 2021, 8, 624621. [Google Scholar] [CrossRef]
- Nayak, V.; Singh, K.R.B.; Singh, A.K.; Singh, R.P. Potentialities of Selenium Nanoparticles in Biomedical Science. New J. Chem. 2021, 45, 2849–2878. [Google Scholar] [CrossRef]
- Hashem, A.H.; Khalil, A.M.A.; Reyad, A.M.; Salem, S.S. Biomedical Applications of Mycosynthesized Selenium Nanoparticles Using Penicillium expansum ATTC 36200. Biol. Trace Elem. Res. 2021, 199, 3998–4008. [Google Scholar] [CrossRef]
- Tan, L.C.; Nancharaiah, Y.V.; van Hullebusch, E.D.; Lens, P.N.L. Selenium: Environmental Significance, Pollution, and Biological Treatment Technologies. Biotechnol. Adv. 2016, 34, 886–907. [Google Scholar] [CrossRef]
- Xia, T.; Li, N.; Nel, A.E. Potential Health Impact of Nanoparticles. Annu. Rev. Public Health 2009, 30, 137–150. [Google Scholar] [CrossRef]
- Caixeta, M.B.; Araújo, P.S.; Gonçalves, B.B.; Silva, L.D.; Grano-Maldonado, M.I.; Rocha, T.L. Toxicity of Engineered Nanomaterials to Aquatic and Land Snails: A Scientometric and Systematic Review. Chemosphere 2020, 260, 127654. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Y.; Leng, X.; Stoll, S. Embryonic Exposure to Selenium Nanoparticles Delays Growth and Hatching in the Freshwater Snail Lymnaea stagnalis. Chemosphere 2022, 307, 136147. [Google Scholar] [CrossRef] [PubMed]
- OECD. Detailed Review Paper (DRP) on Molluscs Life-Cycle Toxicity Testing; OECD: Paris, France, 2010; 182p. [Google Scholar]
- Loker, E.S.; Bayne, C.J. Molluscan Immunobiology: Challenges in the Anthropocene Epoch. Adv. Comp. Immunol. 2018, 343–407. [Google Scholar] [CrossRef]
- Mossalem, H.S.; Ibrahim, A.M. The Ameliorative Potential of the Ethanol Extract of the Plant Ocimum Basilicum on Biomphalaria Alexandrina Snails Exposed to the Insecticide Bestacid. Egypt. J. Aquat. Biol. Fish. 2019, 23, 161–172. [Google Scholar] [CrossRef]
- Abu El Einin, H.M.; Ali, R.E.; Gad El-Karim, R.M.; Youssef, A.A.; Abdel-Hamid, H.; Habib, M.R. Biomphalaria Alexandrina: A Model Organism for Assessing the Endocrine Disrupting Effect of 17β-Estradiol. Environ. Sci. Pollut. Res. 2019, 26, 23328–23336. [Google Scholar] [CrossRef]
- Abdel-Tawab, H.; Ibrahim, A.M.; Hussein, T.; Mohamed, F. Mechanism of Action and Toxicological Evaluation of Engineered Layered Double Hydroxide Nanomaterials in Biomphalaria Alexandrina Snails. Environ. Sci. Pollut. Res. 2022, 29, 11765–11779. [Google Scholar] [CrossRef]
- Sharaf El-Din, A.T.; Mohamed, A.M.; Mohamed, A.H.; El-Hommossany, K.M.; Habib, M.R. Relationship between Some Heavy Metals and Schistosoma Mansoni Infection Rates in Biomphalaria Alexandrina Snails Collected from Different Egyptian Localities. World Appl. Sci. J. 2010, 11, 38–43. [Google Scholar]
- Ghazy, M.M. Aquatic Fauna as a Water Quality Monitoring Device. Ph.D. Thesis, Faculty of Science, Cairo University, Cairo, Egypt, 1997; 169p. [Google Scholar]
- Montassir, L.; Berrebaan, I.; Mellouki, F.; Zkhiri, F.; Boughribil, S.; Bessi, H. Acute Toxicity and Reprotoxicity of Aqueous Extract of a Moroccan Plant (Tetraclinis articulata) on Freshwater Cladoceran Daphnia Magna. J. Mater. Environ. Sci. 2017, 8, 770–776. [Google Scholar]
- Petraretti, M.; Siciliano, A.; Carraturo, F.; Cimmino, A.; De Natale, A.; Guida, M.; Pollio, A.; Evidente, A.; Masi, M. An Ecotoxicological Evaluation of Four Fungal Metabolites with Potential Application as Biocides for the Conservation of Cultural Heritage. Toxins 2022, 14, 407. [Google Scholar] [CrossRef]
- Seremet, O.; Olaru, O.; Gutu, C.; Nitulescu, G.; Ilie, M.; Negres, S.; Zbarcea, C.; Purdel, C.; Spandidos, D.; Tsatsakis, A.; et al. Toxicity of Plant Extracts Containing Pyrrolizidine Alkaloids Using Alternative Invertebrate Models. Mol. Med. Rep. 2018, 17, 7757–7763. [Google Scholar] [CrossRef]
- Huang, X.-J.; Choi, Y.-K.; Im, H.-S.; Yarimaga, O.; Yoon, E.; Kim, H.-S. Aspartate Aminotransferase (AST/GOT) and Alanine Aminotransferase (ALT/GPT) Detection Techniques. Sensors 2006, 6, 756–782. [Google Scholar] [CrossRef]
- Rasool, A.; Zulfajri, M.; Gulzar, A.; Hanafiah, M.M.; Unnisa, S.A.; Mahboob, M. In Vitro Effects of Cobalt Nanoparticles on Aspartate Aminotransferase and Alanine Aminotransferase Activities of Wistar Rats. Biotechnol. Rep. 2020, 26, e00453. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Ghaffar, F.; Ahmed, A.K.; Bakry, F.; Rabei, I.; Ibrahim, A. The Impact of Three Herbicides on Biological and Histological Aspects of Biomphalaria Alexandrina, Intermediate Host of Schistosoma Mansoni. Malacologia 2016, 59, 197–210. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Abdalla, A.M. Impact of Moringa Oleifera Seed Aqueous Extract on Some Biological, Biochemical, and Histological Aspects of Biomphalaria Alexandrina Snails. Environ. Sci. Pollut. Res. 2017, 24, 28072–28078. [Google Scholar] [CrossRef] [PubMed]
- Morad, M.Y.; El-Sayed, H.; El-Khadragy, M.F.; Abdelsalam, A.; Ahmed, E.Z.; Ibrahim, A.M. Metabolomic Profiling, Antibacterial, and Molluscicidal Properties of the Medicinal Plants Calotropis Procera and Atriplex Halimus: In Silico Molecular Docking Study. Plants 2023, 12, 477. [Google Scholar] [CrossRef] [PubMed]
- Morad, M.Y.; El-Sayed, H.; Elhenawy, A.A.; Korany, S.M.; Aloufi, A.S.; Ibrahim, A.M. Myco-Synthesized Molluscicidal and Larvicidal Selenium Nanoparticles: A New Strategy to Control Biomphalaria Alexandrina Snails and Larvae of Schistosoma Mansoni with an In Silico Study on Induced Oxidative Stress. J. Fungi 2022, 8, 262. [Google Scholar] [CrossRef]
- Eveland, L.K.; Haseeb, M.A. Laboratory Rearing of Biomphalaria Glabrata Snails and Maintenance of Larval Schistosomes In Vivo and In Vitro. In Biomphalaria Snails and Larval Trematodes; Springer: New York, NY, USA, 2011; pp. 33–55. [Google Scholar]
- Pellegrino, J.; Goncalves, M. A Simple Method for Collecting Egg Clutches of Biomphalaria Glabrata (Australorbis glabratus) and for Rearing Newly Hatched Snails. J. Parasitol. 1965, 51, 1014. [Google Scholar] [CrossRef]
- Behie, L.A.; Zajic, J.E.; Berk, D.; Brouzes, R.J.P.; Naish, V.A. Standard Primary Toxicity Bioassay Using Daphnia Magna as a Test Species. Water Pollut. Res. Can. 1976, 12, 27–49. [Google Scholar] [CrossRef]
- El-Gindy, M.S.; Radhawy, I.A. Effect of Low Concentrations of Sodium Pentachlorophenate on the Fecundity and Egg Viability of Bulinus Truncatus from Central Iraq. Bull. Endem. Dis. 1965, 7, 44–54. [Google Scholar]
- Reitman, S.; Frankel, S. A Colorimetric Method for the Determination of Serum Glutamic Oxalacetic and Glutamic Pyruvic Transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Doumas, B.T. Standards for Total Serum Protein Assays: A Collaborative Study. Clin. Chem. 1975, 21, 1159–1166. [Google Scholar] [CrossRef]
- Gustafsson, J.E. Improved Specificity of Serum Albumin Determination and Estimation of “Acute Phase Reactants” by Use of the Bromcresol Green Reaction. Clin. Chem. 1976, 22, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Carleton, M.; Drury, R.A.B.; Willington, E.A.; Cammeron, R. Carleton’s Histological Technique; Ulster Med J. Oxford University Press: Oxford, UK, 1967; Volume 36, p. 172. [Google Scholar]
- Ibrahim, A.M.; Ahmed, A.K.; Hammam, O.A.; Abdel-Ghaffar, F. Immunotoxical, Neurotoxical, Histopathological and Immunohistopathological Alterations of Nerium Oleander and Tecoma Stans Methanolic Extract on Biomphalaria Alexandrina Snails. Acta Trop. 2022, 230, 106405. [Google Scholar] [CrossRef] [PubMed]
- Finney, D.J. Probit Analysis; Cambridge University Press: London, UK, 1971; Volume 60, ISBN 1520-6017. [Google Scholar]
- Murray, R. Speigel: Statistical Estimation Theory. In Schaum’s Outline of Theory and Problems of Statistics in S.I. Units, 1st ed.; McGraw Hill B. Company: Singapore, 1981; 156p. [Google Scholar]
- Liao, W.; Yu, Z.; Lin, Z.; Lei, Z.; Ning, Z.; Regenstein, J.M.; Yang, J.; Ren, J. Biofunctionalization of Selenium Nanoparticle with Dictyophora Indusiata Polysaccharide and Its Antiproliferative Activity through Death-Receptor and Mitochondria-Mediated Apoptotic Pathways. Sci. Rep. 2015, 5, 18629. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, D.A. The Contribution of Nano-Selenium in Alleviation of Salinity Adverse Effects on Coriander Plants. J. Soil Sci. Agric. Eng. 2018, 9, 753–760. [Google Scholar] [CrossRef]
- Wang, T.; Liu, W. Emerging Investigator Series: Metal Nanoparticles in Freshwater: Transformation, Bioavailability and Effects on Invertebrates. Environ. Sci. Nano 2022, 9, 2237–2263. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Saleh, H.A.; Zayed, K.M. Colchicum Ritchii Flower: A New Molluscicidal Plant for Biomphalaria Alexandrina Snails and the Infective Stages of Schistosoma Mansoni Alexandrina Snails and the Infective Stages of Schistosoma Mansoni. Molluscan Res. 2021, 41, 289–297. [Google Scholar] [CrossRef]
- Hylton, C.A.; Tsui, M.T.K. Alteration of Acute Toxicity of Inorganic and Methyl Mercury to Daphnia Magna by Dietary Addition. Sci. Rep. 2021, 11, 22865. [Google Scholar] [CrossRef]
- Dunbar, A.M.; Lazorchak, J.M.; Waller, W.T. Acute and Chronic Toxicity of Sodium Selenate to Daphnia Magna Straus. Environ. Toxicol. Chem. 1983, 2, 239–244. [Google Scholar] [CrossRef]
- Okamoto, A.; Yamamuro, M.; Tatarazako, N. Acute Toxicity of 50 Metals to Daphnia Magna. J. Appl. Toxicol. 2015, 35, 824–830. [Google Scholar] [CrossRef]
- Boyum, K.W. The Toxic Effect of Selenium on the Zooplankton, Daphnia Magna and Daphnia Pulicaria, in Water and the Food Source (Chlamydomonas Reinhardtii). Ph.D. Thesis, The University of Wisconsin-Milwaukee, Milwaukee, WI, USA, 1984. [Google Scholar]
- Ingersoll, C.G.; Dwyer, F.J.; May, T.W. Toxicity of Inorganic and Organic Selenium to Daphnia Magna (Cladocera) and Chironomus Riparius (Diptera). Environ. Toxicol. Chem. 1990, 9, 1171–1181. [Google Scholar] [CrossRef]
- Kumar, C.M.V.; Karthick, V.; Inbakandan, D.; Kumar, V.G.; Rene, E.R.; Dhas, T.S.; Ravi, M.; Sowmiya, P.; Das, C.G.A. Effect of Selenium Nanoparticles Induced Toxicity on the Marine Diatom Chaetoceros Gracilis. Process Saf. Environ. Prot. 2022, 163, 200–209. [Google Scholar] [CrossRef]
- Moges, F.D.; Hamdi, H.; Al-Barty, A.; Zaid, A.A.; Sundaray, M.; Parashar, S.K.S.; Gubale, A.G.; Das, B. Effects of Selenium Nanoparticle on the Growth Performance and Nutritional Quality in Nile Tilapia, Oreochromis Niloticus. PLoS ONE 2022, 17, e0268348. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Mohamed, F.; Al-Quraishy, S.; Abdel-Baki, A.A.S.; Abdel-Tawab, H. Green Synthesis of Cerium Oxide / Moringa Oleifera Seed Extract Nano-Composite and Its Molluscicidsal Activities against Biomophalaria Alexanderina. J. King Saud Univ.—Sci. 2021, 33, 101368. [Google Scholar] [CrossRef]
- Verma, R.; Vijayalakshmy, K.; Chaudhiry, V. Detrimental Impacts of Heavy Metals on Animal Reproduction: A Review. J. Entomol. Zool. Stud. 2018, 6, 27–30. [Google Scholar]
- Fahmy, S.R.; Abdel-Ghaffar, F.; Bakry, F.A.; Sayed, D.A. Ecotoxicological Effect of Sublethal Exposure to Zinc Oxide Nanoparticles on Freshwater Snail Biomphalaria Alexandrina. Arch. Environ. Contam. Toxicol. 2014, 67, 192–202. [Google Scholar] [CrossRef]
- Khorrami, M.B.; Sadeghnia, H.R.; Pasdar, A.; Ghayour-Mobarhan, M.; Riahi-Zanjani, B.; Hashemzadeh, A.; Zare, M.; Darroudi, M. Antioxidant and Toxicity Studies of Biosynthesized Cerium Oxide Nanoparticles in Rats. Int. J. Nanomed. 2019, 14, 2915–2926. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.M.; Sayed, D.A. Toxicological Impact of Oxyfluorfen 24% Herbicide on the Reproductive System, Antioxidant Enzymes, and Endocrine Disruption of Biomphalaria Alexandrina (Ehrenberg, 1831) Snails. Environ. Sci. Pollut. Res. 2019, 26, 7960–7968. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Filho, E.C.; de Freitas Muniz, D.H.; de Carvalho, E.L.; Cáceres-Velez, P.R.; Fascineli, M.L.; Azevedo, R.B.; Grisolia, C.K. Effects of AgNPs on the Snail Biomphalaria Glabrata: Survival, Reproduction and Silver Accumulation. Toxics 2019, 7, 12. [Google Scholar] [CrossRef]
- Hasheesh, W.S.; Marie, M.A.S.; El-Deeb, F.A.A.; Sayed, S.S.M. Impact of Asparagus Densiflours and Oreopanax Guatemalensis Plants and Difenoconazole Fungicide on Biochemical Parameters of Biomaphalaria Alexandrina Snails. Austral. J. Basic Appl. Sci. 2011, 5, 366–378. [Google Scholar]
- Ibrahim, A.M.; Bakry, F.A. Assessment of the Molluscicidal Impact of Extracted Chlorophyllin on Some Biochemical Parameters in the Nervous Tissue and Histological Changes in Biomphalaria Alexandrina and Lymnaea Natalensis Snails. Invertebr. Neurosci. 2019, 19, 7. [Google Scholar] [CrossRef]
- Zarai, Z.; Boulais, N.; Karray, A.; Misery, L.; Bezzine, S.; Rebai, T.; Gargouri, Y.; Mejdoub, H. Immunohistochemical Localization of Hepatopancreatic Phospholipase A2 in Hexaplex Trunculus Digestive Cells. Lipids Health Dis. 2011, 10, 91. [Google Scholar] [CrossRef]
- Elwasify, A.Y.H.; Ghanem, M.H.; El-Bamby, M.M.M.; Ali, F.A.F. Effect of Bioaccumulation and Biosedimentation of Some Heavy Metals on Histological Features in the Cichlid Fish, Tilapia Zillii Inhabiting Lake Qarun, Egypt. Egypt. J. Aquat. Biol. Fish. 2021, 25, 695–711. [Google Scholar] [CrossRef]
- Harris, L.; O’Byrne-Ring, N.; Lambkin, H. Characterisation Of Cell Types In Abalone (Haliotis Spp.) Tissues Using Immunohistochemical Techniques. Aquaculture 2006, 261, 1413–1421. [Google Scholar] [CrossRef]
- Nakopoulou, L.; Janinis, J.; Panagos, G.; Comin, G.; Davaris, P. The Immunohistochemical Expression of Proliferating Cell Nuclear Antigen (PCNA/Cyclin) in Malignant and Benign Epithelial Ovarian Neoplasms and Correlation with Prognosis. Eur. J. Cancer 1993, 29, 1599–1601. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Ichimura, K. Proliferation Markers, Proliferating Cell Nuclear Antigen, Ki67, 5-Bromo-2′-Deoxyuridine, and Cyclin D1 in Mouse Olfactory Epithelium. Ann. Otol. Rhinol. Laryngol. 2000, 109, 1046–1048. [Google Scholar] [CrossRef] [PubMed]
- Alao, J.P. The Regulation of Cyclin D1 Degradation: Roles in Cancer Development and the Potential for Therapeutic Invention. Mol. Cancer 2007, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Bartkova, J.; Lukas, J.; Strauss, M.; Bartek, J. Cell Cycle-Related Variation and Tissue-Restricted Expression of Human Cyclin D1 Protein. J. Pathol. 1994, 172, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Tousson, E.; Ali, E.M.M.; Moustafa, A.H.A.; Moselhey, S.S.; El-said, K.S. Proliferating Cell Nuclear Antigen as A Biomarker for Thioacetamide Induced Hepatotoxicity of Rat Liver. Am. J. Zool. Res. 2014, 2, 51–54. [Google Scholar]
- Torlakovic, E.; Nielsen, S.; Vyberg, M. Antibody Selection in Immunohistochemical Detection of Cyclin D1 in Mantle Cell Lymphoma. Am. J. Clin. Pathol. 2005, 124, 782–789. [Google Scholar] [CrossRef]
- Yousef, A.A.A.-H.; EI-Kassas, N.B. Ultrastructure and Histopathological Effects of Some Plant Extracts on Digestive Gland of Biomphalaria Alexandrina and Bulinus Truncatus. J. Basic Appl. Zool. 2013, 66, 27–33. [Google Scholar] [CrossRef]
- Hamed, S.S.; Abdelmeguied, N.E.; Essaway, A.E.; Radwan, M.A.; Hegazy, A.E. Histological and Ultrastructural Changes Induced by Two Carbamate Molluscicides on the Digestive Gland of Eobania Vermiculata. J. Biol. Sci. 2007, 7, 1017–1037. [Google Scholar] [CrossRef]
- Zhou, W.; Tempel, W.; Shah, A.; Chen, L.; Liu, Z.-J.; Lee, D.; Lin, D.; Chang, S.-H.; Brereton, P.S.; Izumi, M.; et al. 1XI9: Alanine Aminotransferase from Pyrococcus Furiosus Pfu-1397077-001; Southeast Collaboratory for Structural Genomics (SECSG): Athens, GA, USA, 2004. [Google Scholar]
- Almo, S.C.; Smith, D.L.; Danishefsky, A.T.; Ringe, D. The Structural Basis for the Altered Substrate Specificity of the R292D Active Site Mutant of Aspartate Aminotransferase from E. coli. Protein Eng. Des. Sel. 1994, 7, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Chu, C.H.; Tsai, S.J.; Yang, R.J. Aspartate Aminotransferase and Alanine Aminotransferase Detection on Paper-Based Analytical Devices with Inkjet Printer-Sprayed Reagents. Micromachines 2016, 7, 9. [Google Scholar] [CrossRef] [PubMed]
| Tested Animals | LC10 (mg/L) | LC25 (mg/L) | LC50 (mg/L) | LC90 (mg/L) |
|---|---|---|---|---|
| D. magna/24 h | 0.70 | 1.04 | 1.62 | 3.77 |
| D. magna/48 h | 0.42 | 0.53 | 0.67 | 1.08 |
| PDB ID | Docking Score (Kcal/mol) | Interaction Type | Amino Acid Residue Involved in Docking |
|---|---|---|---|
| ALT (1XI9) | −1.0 | H-donor | SER 126 |
| AST (1AAM) | −3.8 | H-donor | ASP 223 |
| −2.3 | H-acceptor | LYS 258 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, A.M.; Ghazy, M.; El-Sayed, H.; Abd El-Hameed, R.M.; Khalil, R.G.; Korany, S.M.; Aloufi, A.S.; Hammam, O.A.; Morad, M.Y. Histopathological, Immunohistochemical, Biochemical, and In Silico Molecular Docking Study of Fungal-Mediated Selenium Oxide Nanoparticles on Biomphalaria alexandrina (Ehrenberg, 1831) Snails. Microorganisms 2023, 11, 811. https://doi.org/10.3390/microorganisms11030811
Ibrahim AM, Ghazy M, El-Sayed H, Abd El-Hameed RM, Khalil RG, Korany SM, Aloufi AS, Hammam OA, Morad MY. Histopathological, Immunohistochemical, Biochemical, and In Silico Molecular Docking Study of Fungal-Mediated Selenium Oxide Nanoparticles on Biomphalaria alexandrina (Ehrenberg, 1831) Snails. Microorganisms. 2023; 11(3):811. https://doi.org/10.3390/microorganisms11030811
Chicago/Turabian StyleIbrahim, Amina M., Mahassen Ghazy, Heba El-Sayed, Rehab M. Abd El-Hameed, Rehab G. Khalil, Shereen M. Korany, Abeer S. Aloufi, Olfat A. Hammam, and Mostafa Y. Morad. 2023. "Histopathological, Immunohistochemical, Biochemical, and In Silico Molecular Docking Study of Fungal-Mediated Selenium Oxide Nanoparticles on Biomphalaria alexandrina (Ehrenberg, 1831) Snails" Microorganisms 11, no. 3: 811. https://doi.org/10.3390/microorganisms11030811
APA StyleIbrahim, A. M., Ghazy, M., El-Sayed, H., Abd El-Hameed, R. M., Khalil, R. G., Korany, S. M., Aloufi, A. S., Hammam, O. A., & Morad, M. Y. (2023). Histopathological, Immunohistochemical, Biochemical, and In Silico Molecular Docking Study of Fungal-Mediated Selenium Oxide Nanoparticles on Biomphalaria alexandrina (Ehrenberg, 1831) Snails. Microorganisms, 11(3), 811. https://doi.org/10.3390/microorganisms11030811







