Abstract
Using a previously characterized and described abdominal model to define the avian immune response to Salmonella intra-abdominal challenge in chickens, we have adapted this technique for the study of chickens’ immune response to a Campylobacter intra-abdominal challenge. The intra-abdominal Campylobacter infection model facilitates the characterization of peripheral blood leukocyte dynamics and abdominal cell infiltrates. Day-of-hatch Leghorn chickens were injected intra-abdominally (IA) with Campylobacter jejuni [(CJ)1 × 108 colony-forming units (CFUs)]. Changes in peripheral blood leukocyte numbers and abdominal cell infiltrates were monitored at 0, 4, 8, and 24 h post-injection. Peripheral blood leukocyte numbers were also determined for 2 h post-injection. For mortality studies, birds were injected intra-abdominally with 1 × 108 CFUs CJ and mortalities were recorded for 72 h post-injection. In the peripheral blood of CJ-injected chicks, total white blood cell (WBC) numbers began increasing by 2 h post-injection, peaking at 4 h post-injection with the predominant cell type being polymorphonuclear leukocytes (heterophils). Total WBCs declined after 8 h and this decline continued at 24 h, with total WBC numbers approaching control values. The injection of CJ into the abdominal cavity caused a rapid rise in abdominal cell infiltrates with the predominant infiltrating leukocytes being heterophils. Peak abdominal heterophil infiltrates were observed at 8 h post-injection, declining only slightly by 24 h post-injection. Mortality in the CJ challenge groups reached 37%. Mortality in the Salmonella enteritidis positive control groups were greater than 50%. The data suggest that Campylobacter infection does stimulate the innate immune response in chickens when administered IA, however, the immune response and infection is not characterized with the high levels of mortality observed with a Salmonella infection. These data provide a basis for a more definitive characterization of chickens’ immune response to Campylobacter and a model to evaluate intervention strategies to prevent the infection and colonization of poultry.
1. Introduction
Campylobacter infections in humans linked to uncooked or improperly handled poultry products are a major public health concern. The World Health Organization considers Campylobacter one of four key global causes of diarrheal disease [1]. The Centers for Disease Control (CDC) estimates that 2.4 million people have diseases associated with Campylobacter each year and approximately 124 people die from the disease [2]. Campylobacter and its effects on the host have been extensively observed in humans due to the disease that it causes. In its animal hosts (swine, poultry, cattle, etc.), the effects of Campylobacter on immune function have begun to be more thoroughly studied.
Data from previous studies using an intra-abdominal (IA) model of Salmonella enteritidis (SE) infection show that SE causes changes in peripheral blood leukocyte numbers and causes an inflammatory cell influx into the abdominal cavity, normally a cell-free location in the body [3]. Heterophil influx into the abdominal cavity was found to increase significantly at 4 h post-infection [3]. Studies in turkeys also show SE causing changes in peripheral blood leukocyte numbers after oral administration of SE [4]. Meade and colleagues described the effects of Campylobacter on a chicken’s immune system after an oral challenge at four weeks of age and found that Campylobacter had no effect on circulating heterophil numbers but did cause an early rise in peripheral blood monocytes/macrophage [5]. In addition, toll-like receptor (TLR) 21 expression was found to increase and antimicrobial peptide gene expression was found to decrease [5]. In the present study, an intra-abdominal (IA) model of Campylobacter infection was used in day-old Leghorn chicks to test the effects on peripheral blood leukocyte numbers and chick mortality.
2. Materials and Methods
2.1. Animals
Day-of-hatch male Leghorn (Hy-Line W-36®) chicks were obtained from a commercial hatchery (Hy-line International, Bryan, TX, USA). Total number of chicks used for peripheral blood counts: 90 chicks per group over three repetitions; 90 chicks per group over three repetitions for abdominal lavage studies. Total number of chickens used in three repetitions in mortality studies: control 220; CJ 205; SE 200. Chicks were placed into floor pens with wood shavings for bedding and had free access to feed and water. The feed followed the nutritional recommendations of the NRC 7 (National Research Council, 1994). Additional warmth was provided by heat lamps. All protocols involving animals were reviewed and accepted by the Animal Care and Use Committee, USDA, ARS, SPARC.
2.2. Campylobacter Jejuni (CJ)
A poultry field isolate of CJ was obtained from Dr. J. Allen Byrd, USDA, ARS, SPARC. CJ was grown in Mueller Hinton broth (MHB) for 48 h at 42 °C prior to challenge. The CJ was washed once in PBS. CJ was resuspended in the same volume of PBS that it was grown in. Birds were then dosed with this concentration of CJ (0.5 mL/bird). The CJ challenge was diluted and plated (MH plates) to verify the CJ challenge dose per bird.
2.3. Salmonella Enteritidis (SE)
A poultry isolate of SE #97-11771 was obtained from the National Veterinary Services Laboratory (Ames, IA). SE was cultured in tryptic soy broth (TSB) overnight at 41 °C. The bacteria were pelleted (7700 g for 10 min) and washed with ice-cold phosphate-buffered saline (PBS), centrifuged at 7700× g for 10 min, supernatant discarded, and the pellet re-suspended in 1 mL cold PBS and diluted to 1 × 108 colony-forming units (cfu)/mL in PBS using a Spectronic 20D spectrophotometer (Milton Roy Co., Golden, CO, USA) with a 625 nm reference wavelength.
2.4. Peripheral Blood Counts
Five chicks from each of the treatment groups described above were randomly selected at 0, 2, 4, 8, and 24 h post-injection and 0.1 mL of blood from each chick was collected in a capillary tube and placed in 1.9 mL of chicken blood diluent [6]. Total leukocytes were counted using a hemocytometer. At the same time, blood smears were prepared, air-dried, and stained using the Hema 3 system (Curtin Matheson Scientific Co., Houston, TX, USA). Cell counts of lymphocytes, large mononuclear cells (LMNs, monocytes, blast cells), and PMNs (heterophils) were made microscopically using oil immersion. The number of heterophils per mm−3 were calculated for each chick from total and differential leukocyte counts.
2.5. Abdominal Lavage and Cell Infiltrates
Day-old chicks were randomly placed in control and treated groups and were maintained under the conditions described above. Day-of-hatch Leghorn chickens were injected intra-abdominally with Campylobacter jejuni [(CJ)1 × 108 colony-forming units (CFUs)]. From dilution of the 1 × 108 CFU stock, SE groups received 5 × 103 CFU/ bird by IA injection for both the mortality and leukocyte studies. Changes in peripheral blood leukocyte numbers and abdominal cell infiltrates were monitored at 0, 2, 4, 8, and 24 h post-injection. At time 0, a collection was performed on five chicks per repetition to establish a baseline of cell infiltrates into the abdominal cavity. At each collection point, five chicks were randomly selected from the respective experimental groups and euthanized by CO2 asphyxiation before their abdominal cavities were lavaged with a total of 2 mL of Ca2+—Mg2+-free Hank’s balanced salt solution containing 0.1 M disodium ethylene diamine tetra-acetic acid and 0.25% bovine serum albumin (Sigma Chemical Co., St. Louis, MO, USA). The abdominal wash from each chick was maintained separately. The recovered IA cell infiltrates were enumerated as described above. A cytospin smear was prepared for each chick at each time point and stained, and fixed and differential leukocyte counts were performed for each chick IA exudate sample as described earlier. Peripheral blood and IA cell infiltrate studies represent three repetitions (10 birds per group/time point/repetition).
2.6. Mortality Evaluation
Chicks were injected intra-abdominally with 1 × 108 CFUs CJ; 1 × 108 SE and mortalities were recorded for 72 h post-injection. Total number of chickens used in three repetitions in mortality studies: control 220; CJ 205; SE 200. Birds that succumbed in the first 4 h post-infection were not considered CJ- or SE-associated mortalities. Birds whose condition provoked humane euthanasia were considered mortalities for all groups after the first 4 h post-infection.
2.7. Statistical Analysis
Statistical analysis was performed using SigmaStat® statistical software (Jandel Scientific, San Rafael, CA, USA). Differences between the two experimental groups and time points were determined using ANOVA + Tukey (p ≤ 0.05).
3. Results
3.1. Peripheral Blood Leukocytes
As seen in Figure 1a,b, the number of leukocytes in the peripheral blood decreased significantly at 8 h post-injection in the CJ-injected birds. In particular, the number of heterophils in the peripheral blood were observed to decrease at 4 h post-injection, which continued at 8 h post-injection, then increased at 24 h post-injection. Salmonella-injected birds showed a marked increase in peripheral blood heterophils at 4 and 8 h post-injection, then decreasing at 24 h post-injection.
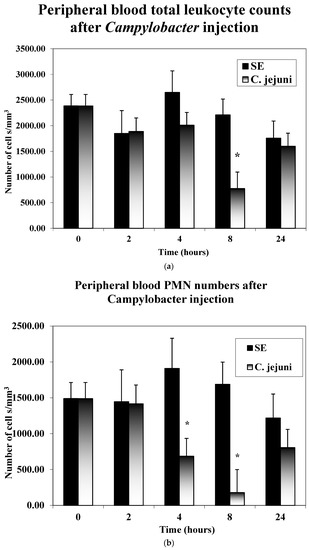
Figure 1.
(a,b) Peripheral blood counts. Five chicks from each of the treatment groups described above were randomly selected at 0, 2, 4, 8, and 24 h post-injection. Blood smears were prepared. Leukocyte cell counts of lymphocytes and large mononuclear cells (LMNs, monocytes, blast cells, heterophils) were made microscopically using oil immersion. The numbers of heterophils per mm−3 were calculated for each chick from the total and differential leukocyte counts. Significant differences are indicated with an asterisk * (p ≤ 0.05).
3.2. Abdominal Cell Infiltrates
All leukocyte types, heterophils, large monocytes, and small monocytes were found to infiltrate the abdominal cavity after injection with CJ and SE (Figure 2). The increase in heterophil cell infiltrates in both the CJ and SE groups began at 4 h post-injection, peaked at 8 h post-injection, and began declining at 24 h post-injection. The increase in heterophil infiltration was greater in the SE group compared to the CJ group.
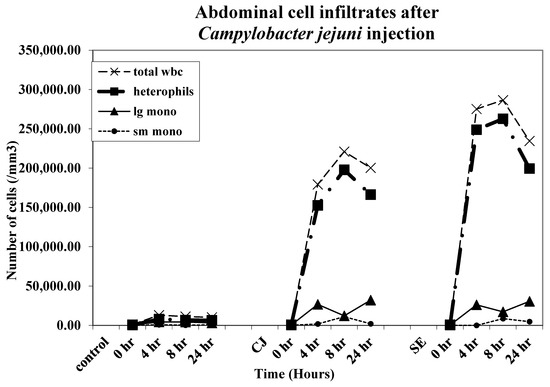
Figure 2.
Abdominal cell infiltrates. Chicks were injected IA with Campylobacter jejuni [(CJ)1 × 108 colony-forming units (CFUs)]. SE groups received 5 × 103 CFU/ bird. Abdominal cell infiltrates were monitored (five chicks/group/timepoint) at 0, 2, 4, 8, and 24 h post-injection. The recovered IA cellular infiltrates were counted on a hemocytometer as described above. A cytospin smear was prepared for each chick at each time point and stained, and fixed and differential leukocyte counts were performed for each chick IA exudate sample as described earlier. Peripheral blood and IA cell infiltrate studies represent three repetitions (15 birds per group/time point/repetition).
3.3. Mortality
Total mortality in all groups was recorded over a 72 h period. Birds that succumbed in the first 4 h after injection were not recorded as associated with CJ or SE injection. Total mortality over 72 h in the CJ group was 37% while mortality in the SE group was 50% (Figure 3).
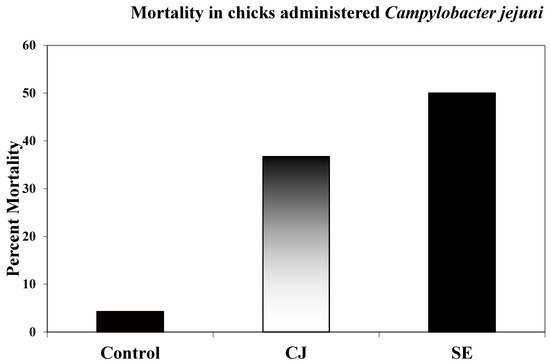
Figure 3.
Chick mortality. Birds were injected intra-abdominally with 1 × 108 CFUs CJ; 1 × 103 SE and mortalities were recorded for 72 h post-injection. Total numbers of chickens used in three repetitions in mortality studies: control 220; CJ 205; SE 200. Birds that succumbed in the first 4 h post-infection were not considered CJ- or SE-associated mortalities. Birds whose condition provoked humane euthanasia were considered mortalities for all groups after the first 4 h post-infection.
4. Discussion
Campylobacter remains one of the most important foodborne pathogens in modern food animal production systems. Poultry constitute a large portion of cases of foodborne illness associated with Campylobacter. The colonization of a chicken’s intestinal tract with Campylobacter does not appear to be detrimental to the chicken and is considered by many as a commensal organism in the GIT tract of poultry and other species [7,8].
The present study found that the IA injection of CJ in chickens resulted in fewer heterophils in the peripheral blood at 4, 8, and 24 h post-injection as compared to SE-injected control birds. Total leukocytes in the peripheral blood were also found to be reduced as compared to the SE-injected control birds at 4 and 8 h post-injection. Abdominal cell infiltrates in the CJ-injected group followed a similar time course pattern as those in the SE-injected group. However, the number of leukocyte infiltrates into the abdomen were reduced compared to the SE-injected control birds. In addition, chicken mortality post-injection was found to be less in the CJ-injected group compared to the SE-injected group (37% vs. 50%, respectively).
In theory, the decrease in heterophils in the CJ-injected group in the peripheral blood would be expected due to an influx of heterophils in the abdominal cavity. However, this trend is not seen in Salmonella-injected birds. Both in the peripheral blood and abdominal cavity, large increases in heterophils are observed in Salmonella-injected birds. The total leukocyte counts in the abdomen and peripheral blood are higher in the Salmonella-injected group. The results would appear to point to a much more robust immune response of the host to Salmonella injection compared to CJ injection. The current literature on Campylobacter and the immune response of chickens appear to support this observation [9,10,11].
The data presented, along with data in the literature, paint a picture of Campylobacter as having host immune interactions that would point to a possible disease process in poultry. However, disease is not observed in naturally infected birds in commercial production systems. Meade and colleagues observed the immune response and leukocyte response in 4-week-old broiler chickens when challenged by the traditional route of oral gavage. By oral challenge, peripheral blood heterophil numbers were found to be unaffected, whereas monocyte numbers were found to increase early on after challenge [5]. The peripheral blood changes observed in the present study associated with Salmonella administration were also observed in 4-week-old broilers upon oral challenge with Salmonella [5]. There may be multiple issues presented by the Campylobacter challenge findings as they are dissimilar to those in the present studies. The use of day-old laying chickens in the study presented here, as well as the route of administration, may account for some differences. Researchers have found that challenging birds prior to three weeks of age with Campylobacter may be inhibited or reduced by maternal antibodies [12]. After three weeks of age, MAB in chicks has diminished to a point where colonization of the chicks’ GI tract with Campylobacter is unfettered and can reach the 100% colonization of birds in commercial settings [12]. Deng et al. have noted that resistance to colonization of the GI tract in chickens is highest in birds from 1 to 2 weeks of age [11]. Also noted was that different breeds and lines of chickens responded differently to Campylobacter challenge, finding that in slower growing breeds, IL-10 mitigates an inflammatory response to Campylobacter as compared to faster growing breeds which do not produce similar levels of IL-10, with subsequent ongoing inflammation and diarrhea in the latter [12,13,14,15]. Meade et al. also found that chickens given Campylobacter orally at 4 weeks of age exhibited a decrease in the gene expression of antimicrobial peptides [5,16]. Taken together, it would appear Campylobacter can cause disease in chickens, perhaps depending on the breed of chicken, but most research points to an altered/decreased immune response to Campylobacter when compared to the immune response elicited by Salmonella in chickens.
The main goal of this study was to test Campylobacter IA injection and compare it to the results of SE IA injection. We have previously used the IA model to investigate the effects of an IA injection of Salmonella on the host, as measured by cell influx into the abdominal cavity and peripheral blood white blood cell dynamics and the use of immune stimulating agents to reduce subsequent mortality and infection in chickens [3,4]. The IA model allows for an observation of the effects of the bacteria on host immunity without the added caveat associated with an oral challenge and unavoidable interactions with the GI flora. Due to the limited nature of the studies presented here, the conclusions made must be narrow in focus. The studies show that Campylobacter administered in the IA model does elicit a cellular response in the abdominal cavity and peripheral blood, and mortality associated with an IA injection is reduced compared to an injection of Salmonella. Further studies designed to achieve a larger, more intricate measurement and characterization of the immune response are planned.
Author Contributions
Conceptualization, M.H.K. and K.J.G.; Validation, C.L.S.; Formal analysis, K.J.G.; Investigation, K.J.G., H.H., C.L.S., J.A.B. and M.H.K.; Data curation, K.J.G., H.H., C.L.S., J.A.B. and M.H.K.; Writing—original draft, K.J.G.; Writing—review & editing, H.H. and M.H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Campylobacter. Available online: www.who.int/news-room/fact-sheets/detail/campylobacter.
- Available online: www.cdc.gov/campylobacter.
- Kogut, M.H.; Moyes, R.; Deloach, J.R. Neutralization of G-CSF inhibits ILK-induced heterophil influx: Salmonella enteritidis-immune lymphokine potentiation of the acute avian inflammatory response. Inflammation 1997, 21, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Genovese, K.J.; Moyes, R.B.; Genovese, L.L.; Lowry, V.K.; Kogut, M.H. Resistance to Salmonella enteritidis organ invasion in day-old turkeys and chickens by transformed T-cell line-produced lymphokines. Avian Dis. 1998, 42, 545. [Google Scholar] [CrossRef] [PubMed]
- Meade, K.G.; Narciandi, F.; Cahalane, S.; Reiman, C.; Allan, B.; O’Farrelly, C. Comparative in vivo infection models yield insights on early host immune response to Campylobacter in chickens. Immunogenetics 2008, 61, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Natt, M.P.; Herrick, C.A. A New Blood Diluent for Counting the Erythrocytes and Leucocytes of the Chicken. Poult. Sci. 1952, 31, 735–738. [Google Scholar] [CrossRef]
- Lee, M.D. Avian Campylobacter Infection. Merk Veterinary Manual. 2021. Available online: www.merckvetmanual.com.
- Hakeem, M.J.; Lu, X. Survival and Control of Campylobacter in Poultry Production Environment. Front. Cell. Infect. Microbiol. 2021, 10, 615049. [Google Scholar] [CrossRef] [PubMed]
- Swaggerty, C.; Pevzner, I.; He, H.; Genovese, K.; Kogut, M. Selection for pro-inflammatory mediators produces chickens more resistant to Campylobacter jejuni. Poult. Sci. 2017, 96, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Swaggerty, C.L.; Kogut, M.H.; Chiang, H.; Wang, Y.; Genovese, K.J.; He, H.; Stern, N.J.; Pevzner, I.Y.; Zhou, H. The Paternal Effect of Campylobacter jejuni Colonization in Ceca in Broilers. Poult. Sci. 2008, 87, 1742–1747. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Dittoe, D.K.; Pavilidis, H.O.; Chaney, W.E.; Yang, Y.; Ricke, S.C. Current Perspectives and Potential of Probiotics to Limit Foodborne Campylobacter in Poultry. Front. Microbiol. 2020, 11, 583429. [Google Scholar] [CrossRef]
- Sahin, O.; Luo, N.; Huang, S.; Zhang, Q. Effect of Campylobacter -Specific Maternal Antibodies on Campylobacter jejuni Colonization in Young Chickens. Appl. Environ. Microbiol. 2003, 69, 5372–5379. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Hess, C.; Hess, M. Re-thinking the chicken–Campylobacter jejuni interaction: A review. Avian Pathol. 2018, 47, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Connerton, P.L.; Richards, P.J.; Lafontaine, G.; O’Kane, P.M.; Ghaffar, N.; Cummings, N.J.; Smith, D.; Fish, N.M.; Connerton, I. The effect of the timing of exposure to Campylobacter jejuni on the gut microbiome and inflammatory responses of broiler chickens. Microbiome 2018, 6, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, S.; Chaloner, G.; Kemmett, K.; Davidson, N.; Williams, N.; Kipar, A.; Humphrey, T.; Wigley, P. Campylobacter jejuni is not merely a commensal in commercial broiler chickens and affects bird welfare. mBio 2014, 5, e01363-14. [Google Scholar] [CrossRef] [PubMed]
- Shaughnessy, R.G.; Meade, K.G.; Cahalane, S.; Allan, B.; Reiman, C.; Callanan, J.J.; O’Farrelly, C. Innate immune gene expression differentiates the early avian intestinal response between Salmonella and Campylobacter. Vet. Immunol. Immunopathol. 2009, 132, 191–198. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).