Plant and Native Microorganisms Amplify the Positive Effects of Microbial Inoculant
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparing of Experimental Materials
2.2. Experimental Design
2.3. Soil Nutrient and Enzyme Activity Assay
2.4. Statistical Analysis
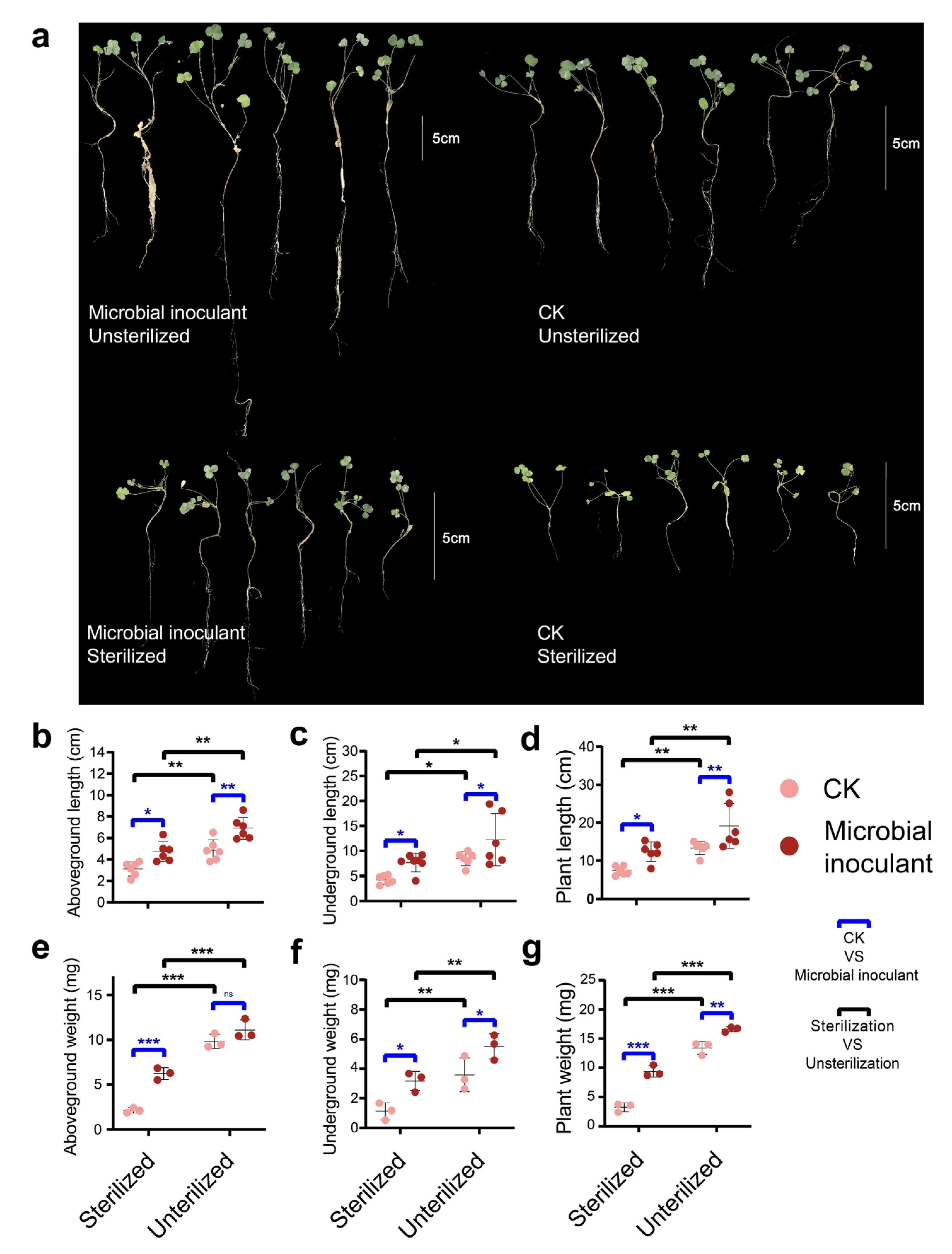
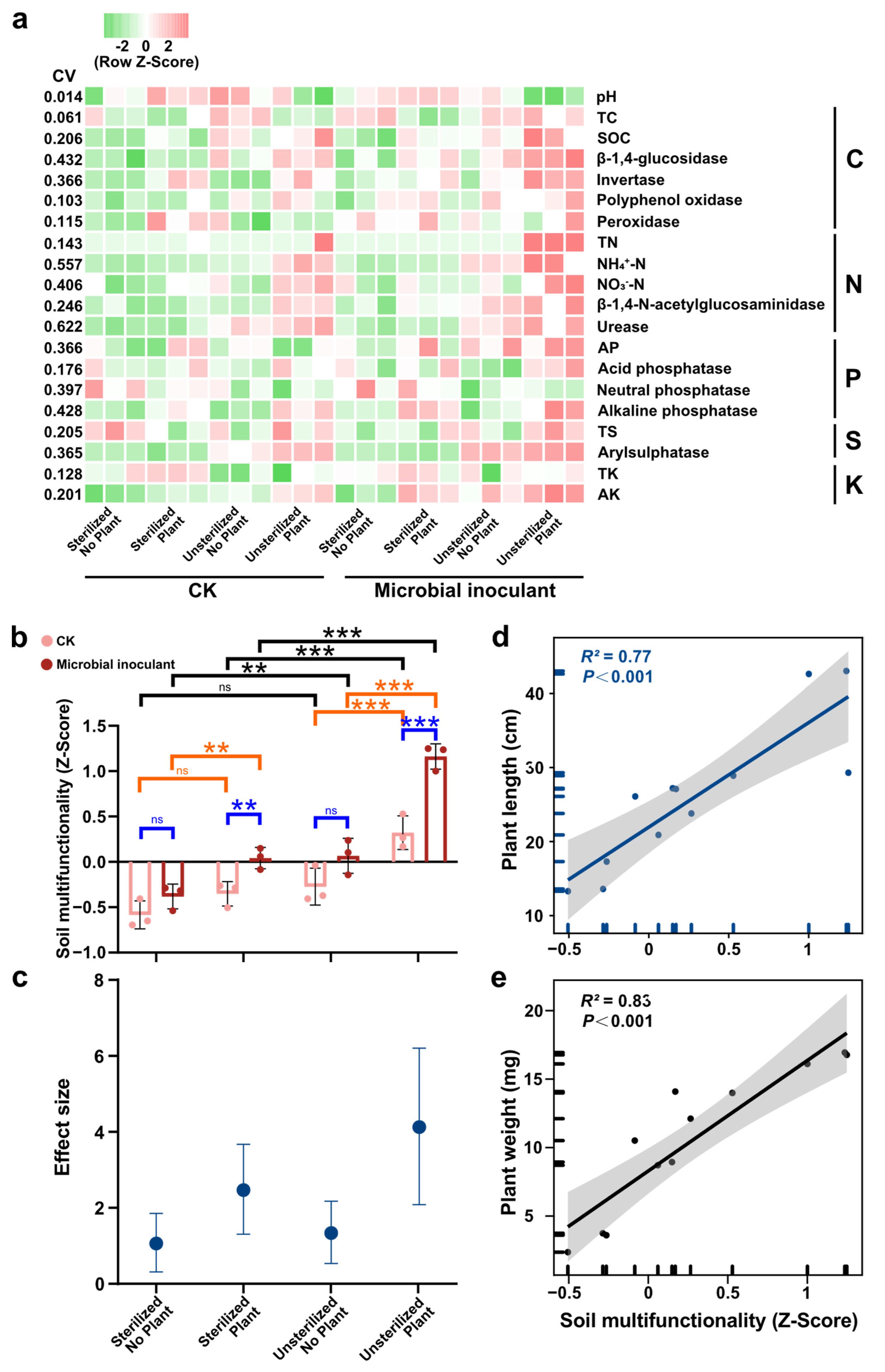
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sonter, L.J.; Dade, M.C.; Watson, J.E.M.; Valenta, R.K. Renewable energy production will exacerbate mining threats to biodiversity. Nat. Commun. 2020, 11, 4174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xi, F. Study on ecological restoration of abandoned mines in China. Acta Ecol. Sin. 2020, 40, 7921–7930. [Google Scholar]
- Wang, F. Occurrence of arbuscular mycorrhizal fungi in mining-impacted sites and their contribution to ecological restoration: Mechanisms and applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1901–1957. [Google Scholar] [CrossRef]
- Rothman, S.E.; Cole, C.A.; Bruns, M.A.; Hall, M. The influence of soil amendments on a native wildflower seed mix in surface mine restoration. Restor. Ecol. 2021, 29, e13440. [Google Scholar] [CrossRef]
- Russell, J.E. Soil Conditions Plants Growth; Daya Books: New Delhi, India, 2002. [Google Scholar]
- Wu, Y.; Zhang, J.; Wang, L.; Wang, Y. A rock-weathering bacterium isolated from rock surface and its role in ecological restoration on exposed carbonate rocks. Ecol. Eng. 2017, 101, 162–169. [Google Scholar] [CrossRef]
- Jia, Z.; Meng, M.; Li, C.; Zhang, B.; Zhai, L.; Liu, X.; Ma, S.; Cheng, X.; Zhang, J. Rock-Solubilizing Microbial Inoculums Have Enormous Potential as Ecological Remediation Agents to Promote Plant Growth. Forests 2021, 12, 357. [Google Scholar] [CrossRef]
- Li, C.; Jia, Z.; Peng, X.; Zhai, L.; Zhang, B.; Liu, X.; Zhang, J. Functions of mineral-solubilizing microbes and a water retaining agent for the remediation of abandoned mine sites. Sci. Total Environ. 2021, 761, 143215. [Google Scholar] [CrossRef]
- Li, C.; Jia, Z.; Yuan, Y.; Cheng, X.; Shi, J.; Tang, X.; Wang, Y.; Peng, X.; Dong, Y.; Ma, S.; et al. Effects of mineral-solubilizing microbial strains on the mechanical responses of roots and root-reinforced soil in external-soil spray seeding substrate. Sci. Total Environ. 2020, 723, 138079. [Google Scholar] [CrossRef]
- Li, C.; Jia, Z.; Zhai, L.; Zhang, B.; Peng, X.; Liu, X.; Zhang, J. Effects of Mineral-Solubilizing Microorganisms on Root Growth, Soil Nutrient Content, and Enzyme Activities in the Rhizosphere Soil of Robinia pseudoacacia. Forests 2021, 12, 60. [Google Scholar] [CrossRef]
- Wu, Y.; Kameshwar, A.K.S.; Zhang, B.; Chen, F.; Qin, W.; Meng, M.; Zhang, J. Genome and transcriptome analysis of rock-dissolving Pseudomonas sp. NLX-4 strain. Bioresour. Bioprocess. 2022, 9, 63. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Guo, X. An Indigenous Soil Bacterium Facilitates the Mitigation of Rocky Desertification in Carbonate Mining Areas. Land Degrad. Dev. 2017, 28, 2222–2233. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Guo, X.; Wang, Y.; Wang, Q. Isolation and characterisation of a rock solubilising fungus for application in mine-spoil reclamation. Eur. J. Soil Biol. 2017, 81, 76–82. [Google Scholar] [CrossRef]
- Li, K.; DiLegge, M.J.; Minas, I.S.; Hamm, A.; Manter, D.; Vivanco, J.M. Soil sterilization leads to re-colonization of a healthier rhizosphere microbiome. Rhizosphere 2019, 2, 100176. [Google Scholar] [CrossRef]
- Qin, S.; Zhou, W.; Lyu, D.; Liu, L. Effects of soil sterilization and biological agent inoculation on the root respiratory metabolism and plant growth of Cerasus sachalinensis Kom. Sci. Hortic. 2014, 170, 189–195. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Oliveira, R.S.; Zhang, C.; Freitas, H. Potential of plant beneficial bacteria and arbuscular mycorrhizal fungi in phytoremediation of metal-contaminated saline soils. J. Hazard Mater. 2019, 379, 120813. [Google Scholar] [CrossRef]
- Ardestani, M.M.; Mudrák, O.; Vicena, J.; Sun, D.; Veselá, H.; Frouz, J. Microbial community from species rich meadow supports plant specialists during meadow restoration. Funct. Ecol. 2022, 36, 1573–1584. [Google Scholar] [CrossRef]
- Van, D.H.; Marcel, G.A.; Bardgett, R.D.; Van, S.; Nico, M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar]
- Hang, X.; Meng, L.; Ou, Y.; Shao, C.; Xiong, W.; Zhang, N.; Liu, H.; Li, R.; Shen, Q.; Kowalchuk, G.A. Trichoderma-amended biofertilizer stimulates soil resident Aspergillus population for joint plant growth promotion. NPJ Biofilms Microbiomes 2022, 8, 57. [Google Scholar] [CrossRef]
- Trivedi, P.; Batista, B.D.; Bazany, K.E.; Singh, B.K. Plant-microbiome interactions under a changing world: Responses, consequences and perspectives. New Phytol. 2022, 234, 1951–1959. [Google Scholar] [CrossRef]
- Liu, H.; Brettell, L.E.; Qiu, Z.; Singh, B.K. Microbiome-mediated stress resistance in plants. Trends Plant Sci. 2020, 25, 733–743. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Delgado-Baquerizo, M.; Luo, X.; Liu, Y.; Van Nostrand, J.D.; Chen, W.; Zhou, J.; Huang, Q. Soil aggregate size-dependent relationships between microbial functional diversity and multifunctionality. Soil Biol. Biochem. 2021, 154, 108143. [Google Scholar] [CrossRef]
- Staff, S.S. Keys to Soil Taxonomy, 12th ed.; Natural Resources Conservation Service, United States Department of Agriculture: Washington, DC, USA, 2014. [Google Scholar]
- Rosenberg, M.S.; Adams, D.C.; Gurevitch, J. MetaWin: Statistical Software for Meta-Analysis with Resampling Tests; Sinauer Associates: Sunderland, MA, USA, 1997. [Google Scholar]
- Zhou, Y.; Staver, A.C. Enhanced activity of soil nutrient-releasing enzymes after plant invasion: A meta-analysis. Ecology 2009, 100, e02830. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, G.S.; Parke, J.L.; Clayton, M.K.; Handelsman, J. Effects of an introduced bacterium on bacterial communities on roots. Ecology 1993, 74, 840–854. [Google Scholar] [CrossRef]
- Silo-Suh, L.A.; Lethbridge, B.J.; Raffel, S.J.; He, H.; Clardy, J.; Handelsman, J. Biological activities of two fungistatic antibiotics produced by Bacillus cereus UW85. Appl. Environ. Microbiol. 1994, 60, 2023–2030. [Google Scholar] [CrossRef]
- Niu, D.; Liu, H.; Jiang, C.; Wang, Y.; Wang, Q.; Jin, H.; Guo, J. The plant growth–promoting rhizobacterium Bacillus cereus AR156 induces systemic resistance in Arabidopsis thaliana by simultaneously activating salicylate-and jasmonate/ethylene-dependent signaling pathways. Mol. Plant Microbe Interact. 2011, 24, 533–542. [Google Scholar] [CrossRef]
- Swain, M.R.; Naskar, S.K.; Ray, R.C. Indole-3-acetic acid production and effect on sprouting of yam (Dioscorea rotundata L.) minisetts by Bacillus subtilis isolated from culturable cowdung microflora. Pol. J. Microbiol. 2007, 56, 103. [Google Scholar]
- Pindi, P.K.; Sultana, T.; Vootla, P.K. Plant growth regulation of Bt-cotton through Bacillus species. 3 Biotech 2014, 4, 305–315. [Google Scholar] [CrossRef]
- Santoyo, G. How plants recruit their microbiome? New insights into beneficial interactions. J. Adv. Res. 2021, 40, 45–58. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef]
- Korenblum, E.; Aharoni, A. Phytobiome metabolism: Beneficial soil microbes steer crop plants’ secondary metabolism. Pest Manag. Sci. 2019, 75, 2378–2384. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, K.; Yamamoto, S.; Sato, H.; Tanabe, A.S.; Hidaka, A.; Toju, H. Mycorrhizal fungi mediate the direction and strength of plant–soil feedbacks differently between arbuscular mycorrhizal and ectomycorrhizal communities. Commun. Biol. 2018, 1, 196. [Google Scholar] [CrossRef] [PubMed]
- Bever, J.D.; Platt, T.G.; Morton, E.R. Microbial population and community dynamics on plant roots and their feedbacks on plant communities. Annu. Rev. Microbiol. 2012, 66, 265–283. [Google Scholar] [CrossRef]
- Han, Q.; Ma, Q.; Chen, Y.; Tian, B.; Xu, L.; Bai, Y.; Chen, W.; Li, X. Variation in rhizosphere microbial communities and its association with the symbiotic efficiency of rhizobia in soybean. ISME J. 2020, 14, 1915–1928. [Google Scholar] [CrossRef]
- Bennett, J.A.; Klironomos, J. Mechanisms of plant–soil feedback: Interactions among biotic and abiotic drivers. New Phytol. 2019, 222, 91–96. [Google Scholar] [CrossRef]
- Liu, X.; Li, C.; Meng, M.; Zhai, L.; Zhang, B.; Jia, Z.; Gu, Z.; Liu, Q.; Zhang, Y.; Zhang, J. Comparative effects of the recovery from sulfuric and nitric acid rain on the soil enzyme activities and metabolic functions of soil microbial communities. Sci. Total Environ. 2020, 714, 136788. [Google Scholar] [CrossRef]
- Fujii, K.; Shibata, M.; Kitajima, K.; Ichie, T.; Kitayama, K.; Turner, B.L. Plant–soil interactions maintain biodiversity and functions of tropical forest ecosystems. Ecol. Res. 2018, 33, 149–160. [Google Scholar] [CrossRef]
- Bennett, J.A.; Maherali, H.; Reinhart, K.O.; Lekberg, Y.; Hart, M.M.; Klironomos, J. Plant-soil feedbacks and mycorrhizal type influence temperate forest population dynamics. Science 2017, 355, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Ardestani, M.M.; Frouz, J. The arbuscular mycorrhizal fungus Rhizophagus intraradices and other microbial groups affect plant species in a copper-contaminated post-mining soil. J. Trace Elem. Med. Biol. 2020, 62, 126594. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Chen, Q.-L.; Ding, J.; Zhu, D.; Hu, H.-W.; Delgado-Baquerizo, M.; Ma, Y.-B.; He, J.-Z.; Zhu, Y.-G. Rare microbial taxa as the major drivers of ecosystem multifunctionality in long-term fertilized soils. Soil Biol. Biochem. 2020, 141, 107686. [Google Scholar] [CrossRef]
- Li, J.; Cui, L.; Delgado-Baquerizo, M.; Wang, J.; Zhu, Y.; Wang, R.; Li, W.; Lei, Y.; Zhai, X.; Zhao, X. Fungi drive soil multifunctionality in the coastal salt marsh ecosystem. Sci. Total Environ. 2022, 818, 151673. [Google Scholar] [CrossRef] [PubMed]
- Mallon, C.A.; Poly, F.; Le Roux, X.; Marring, I.; van Elsas, J.D.; Salles, J.F. Resource pulses can alleviate the biodiversity–invasion relationship in soil microbial communities. Ecology 2015, 96, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Meng, D.; Yang, S.; Xiao, N.; Li, Z.; Liu, Z.; Li, L.; Zeng, X.; Zeng, S.; Yin, H. Invader-resident community similarity contribute to the invasion process and regulate biofertilizer effectiveness. J. Clean. Prod. 2019, 241, 118278. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Jia, Z.; Ma, S.; Liu, X.; Zhang, J.; Müller, C. Plant and Native Microorganisms Amplify the Positive Effects of Microbial Inoculant. Microorganisms 2023, 11, 570. https://doi.org/10.3390/microorganisms11030570
Li C, Jia Z, Ma S, Liu X, Zhang J, Müller C. Plant and Native Microorganisms Amplify the Positive Effects of Microbial Inoculant. Microorganisms. 2023; 11(3):570. https://doi.org/10.3390/microorganisms11030570
Chicago/Turabian StyleLi, Chong, Zhaohui Jia, Shilin Ma, Xin Liu, Jinchi Zhang, and Christoph Müller. 2023. "Plant and Native Microorganisms Amplify the Positive Effects of Microbial Inoculant" Microorganisms 11, no. 3: 570. https://doi.org/10.3390/microorganisms11030570
APA StyleLi, C., Jia, Z., Ma, S., Liu, X., Zhang, J., & Müller, C. (2023). Plant and Native Microorganisms Amplify the Positive Effects of Microbial Inoculant. Microorganisms, 11(3), 570. https://doi.org/10.3390/microorganisms11030570






