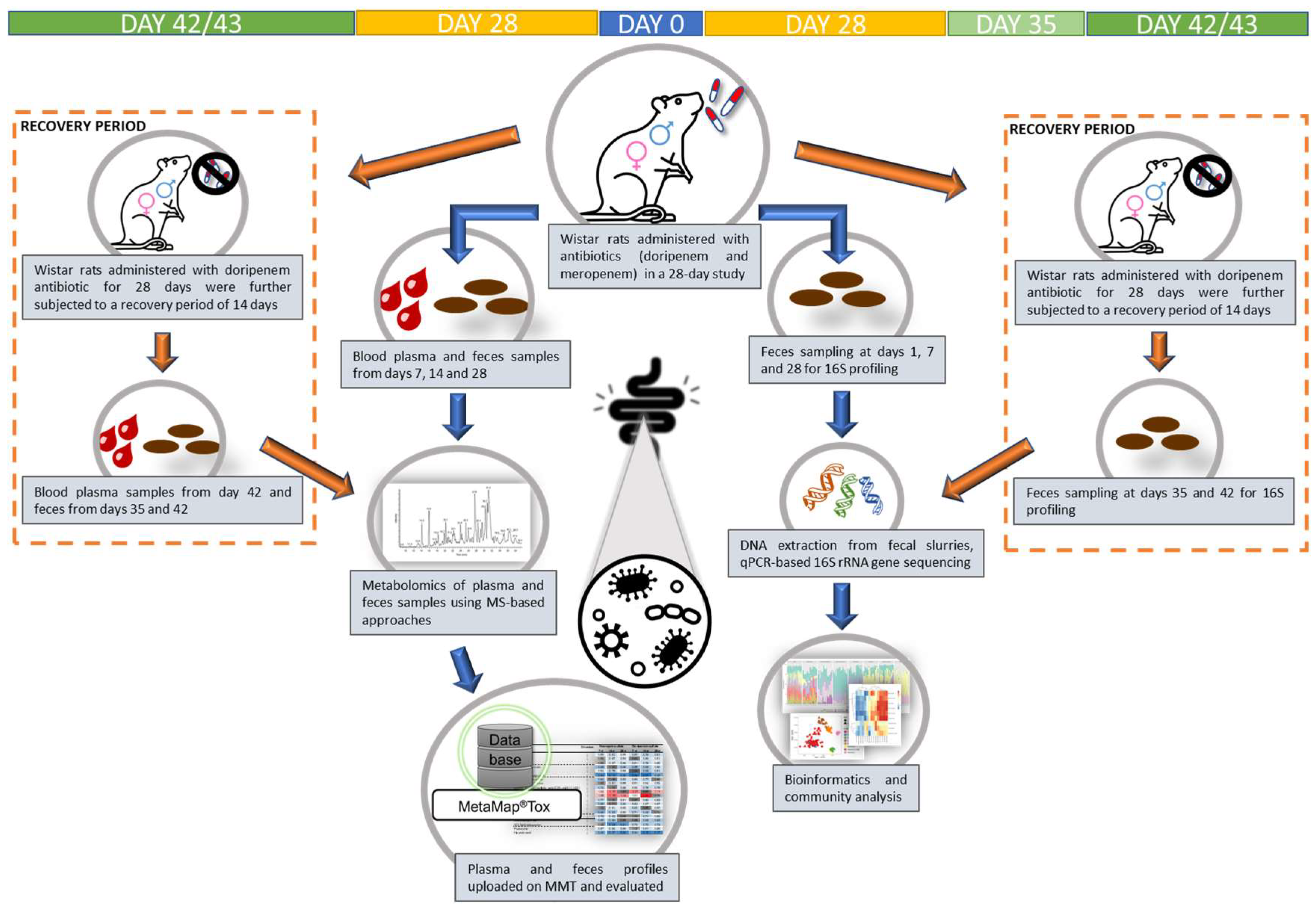
Figure 1.
Schematic representation of the study design for plasma and fecal metabolome analyses and the corresponding gut (fecal) 16S bacterial profiling in a 28-day oral gavage study using meropenem- and doripenem- (administered at high and low doses) treated male and female young adult Wistar rats. Note: For doripenem, an additional one- and two-week recovery period, as indicated by the orange dashed boxes, was included to analyze the reversibility. The top row indicates sampling timeline.
Figure 1.
Schematic representation of the study design for plasma and fecal metabolome analyses and the corresponding gut (fecal) 16S bacterial profiling in a 28-day oral gavage study using meropenem- and doripenem- (administered at high and low doses) treated male and female young adult Wistar rats. Note: For doripenem, an additional one- and two-week recovery period, as indicated by the orange dashed boxes, was included to analyze the reversibility. The top row indicates sampling timeline.
Figure 2.
Line plots representing food consumption (in grams) and body weight (in grams), in male (A,C) and female (B,D) animals, respectively, compared to the controls and different antibiotics treatment groups on days 0, 6, 13, and 27 and additionally on days 35 and 42/43 for the doripenem-recovery groups. The end of meropenem treatment and the start of doripenem-recovery groups have been highlighted with black arrows. Changes in body weight and food consumption were compared between control and treated groups and showed no significant differences (p-value < 0.05).
Figure 2.
Line plots representing food consumption (in grams) and body weight (in grams), in male (A,C) and female (B,D) animals, respectively, compared to the controls and different antibiotics treatment groups on days 0, 6, 13, and 27 and additionally on days 35 and 42/43 for the doripenem-recovery groups. The end of meropenem treatment and the start of doripenem-recovery groups have been highlighted with black arrows. Changes in body weight and food consumption were compared between control and treated groups and showed no significant differences (p-value < 0.05).
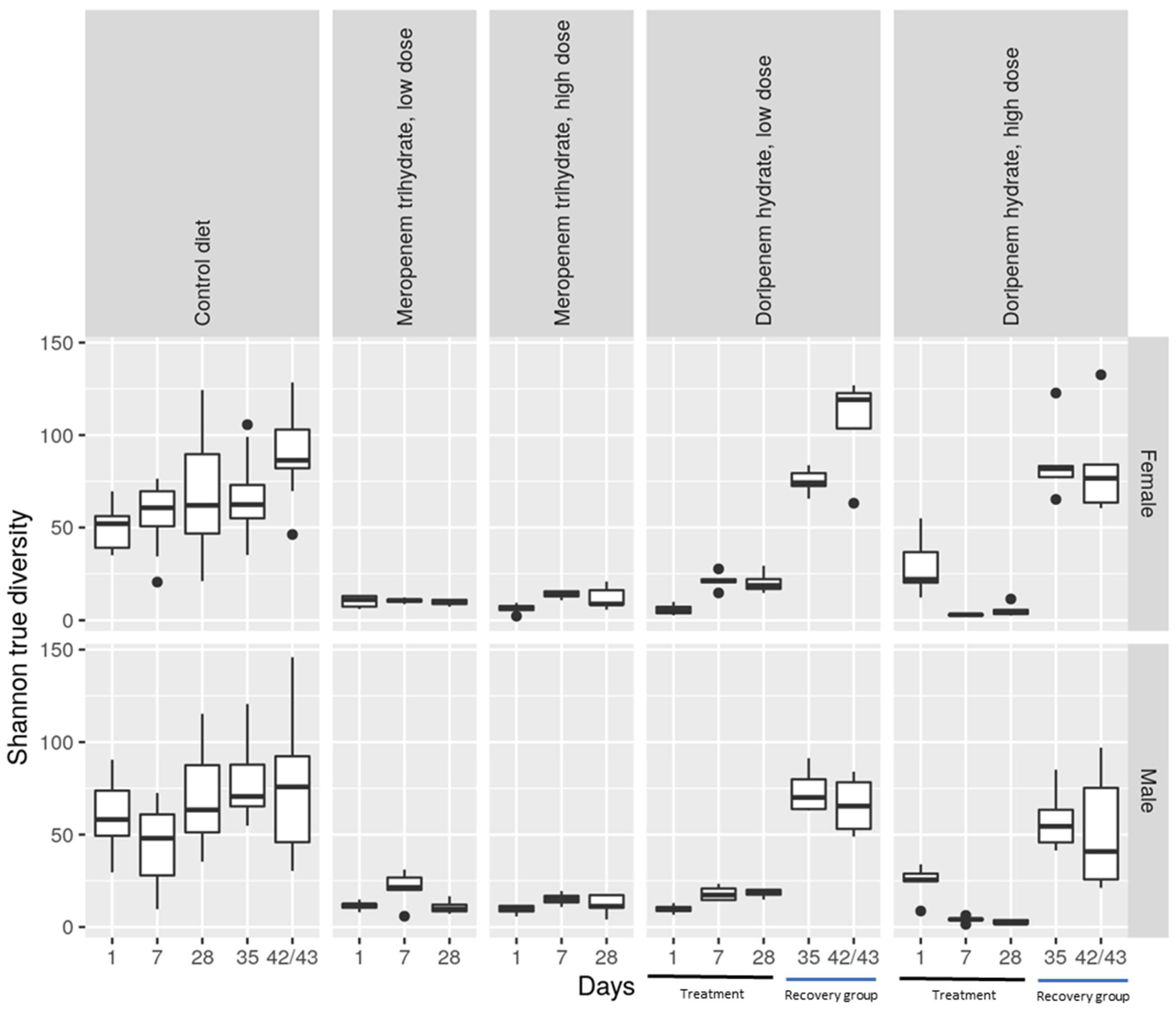
Figure 3.
Shannon true diversity analysis of the antibiotic treatments, meropenem, doripenem, and controls for both male and female Wistar rats. The top row shows data for females (f) and the bottom row shows data for males (m). Doripenem treatment time points (days 1, 7, and 28) and recovery time points (days 35 and 42/43) are indicated with black and blue lines, respectively, on the x-axis. Whiskers denote standard deviations, and solid lines within the boxes indicate the group medians. The dots falling outside the boxes demonstrate the most extreme values.
Figure 3.
Shannon true diversity analysis of the antibiotic treatments, meropenem, doripenem, and controls for both male and female Wistar rats. The top row shows data for females (f) and the bottom row shows data for males (m). Doripenem treatment time points (days 1, 7, and 28) and recovery time points (days 35 and 42/43) are indicated with black and blue lines, respectively, on the x-axis. Whiskers denote standard deviations, and solid lines within the boxes indicate the group medians. The dots falling outside the boxes demonstrate the most extreme values.
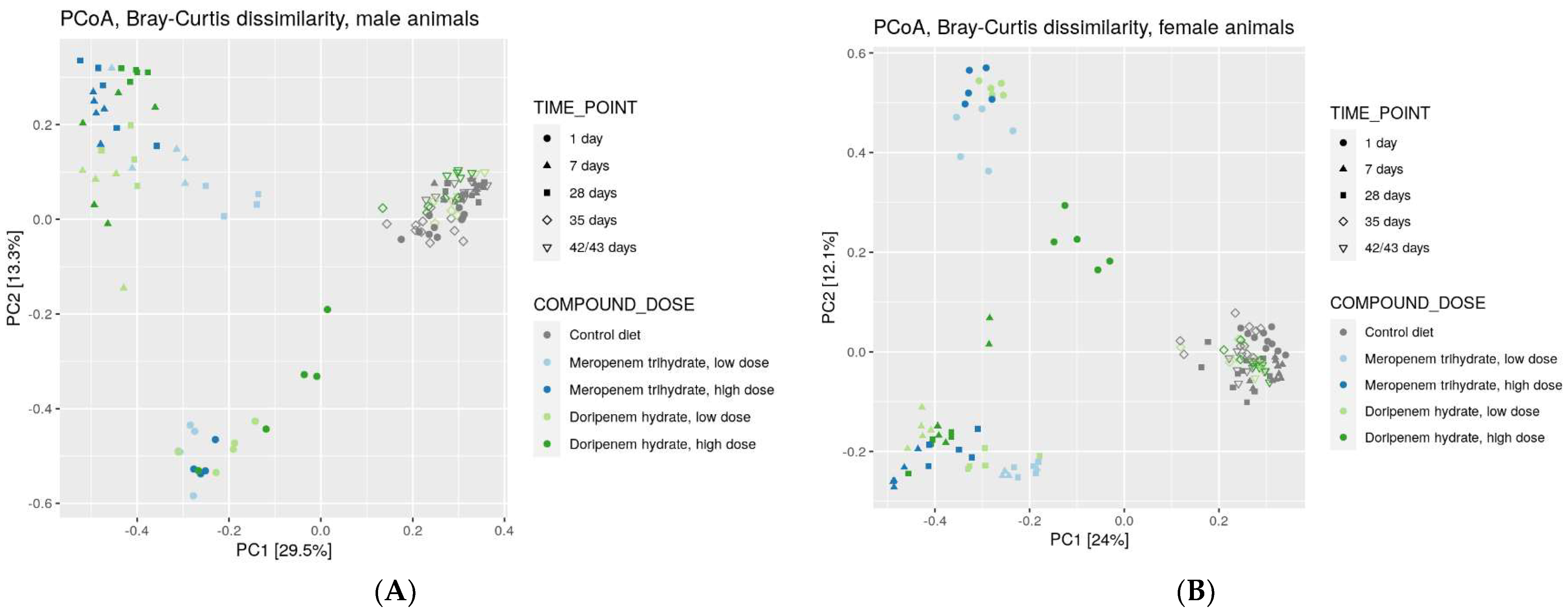
Figure 4.
Principal Coordinate Analysis (PCoA) of bacterial families from controls, meropenem, and doripenem antibiotic-treated rats at different time points. Rank-based clustering with Bray-Curtis distance matrix of samples from (A) males and (B) females are presented.
Figure 4.
Principal Coordinate Analysis (PCoA) of bacterial families from controls, meropenem, and doripenem antibiotic-treated rats at different time points. Rank-based clustering with Bray-Curtis distance matrix of samples from (A) males and (B) females are presented.
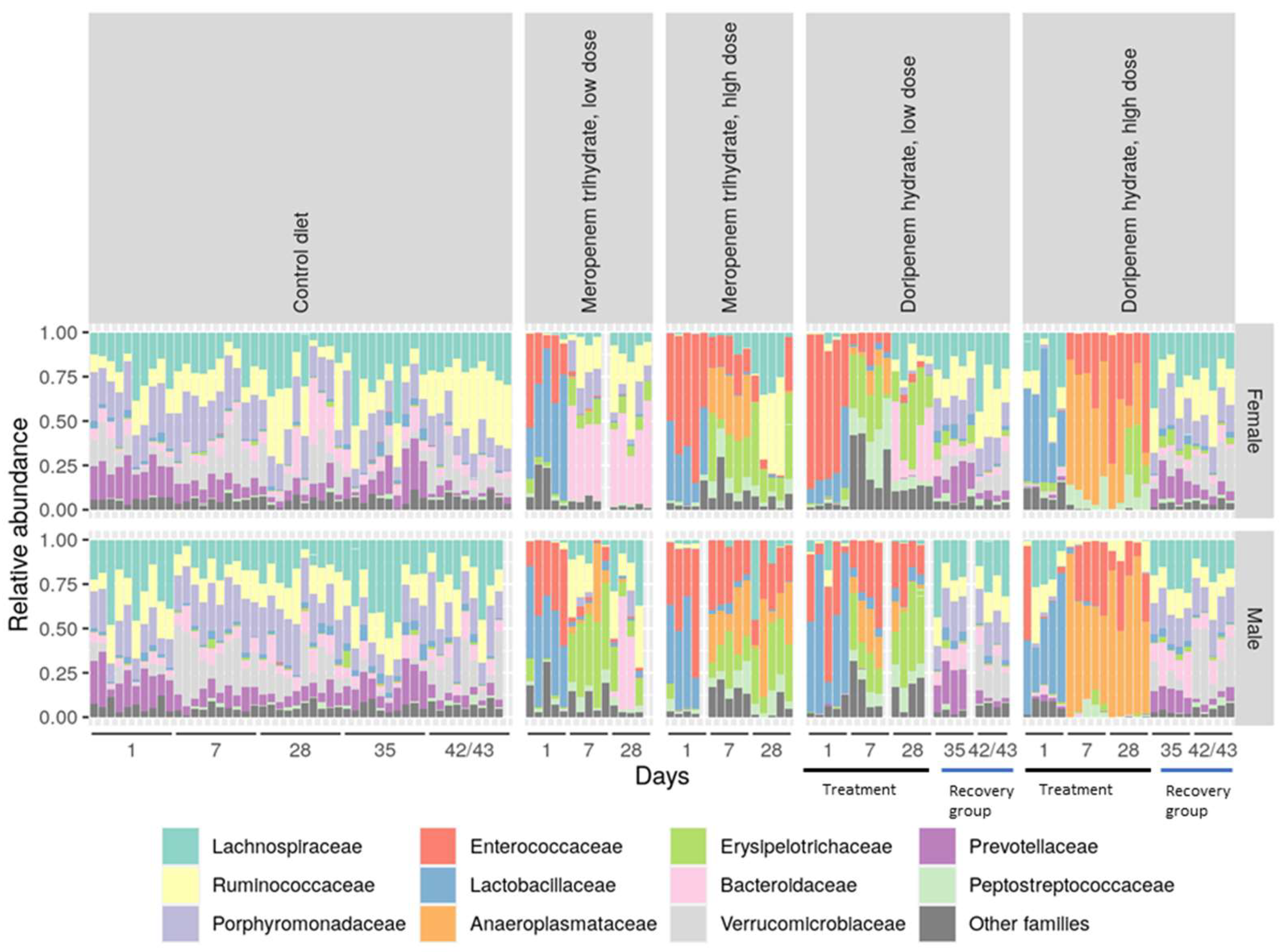
Figure 5.
Stacked bar diagram showing relative abundances of the detected bacterial families in the different experimental groups. Inter-individual variability in the composition can be observed. Dose groups and sexes showed significant differences specifically in samples from days 1, 7, and 28 relative to the controls. Antibiotic-specific effects were observed on the intestinal community composition. For the doripenem group, treatment time points, i.e., days 1, 7, and 28, and recovery time points, i.e., 35 and 42/43, are highlighted with black and blue lines on the x-axis, respectively.
Figure 5.
Stacked bar diagram showing relative abundances of the detected bacterial families in the different experimental groups. Inter-individual variability in the composition can be observed. Dose groups and sexes showed significant differences specifically in samples from days 1, 7, and 28 relative to the controls. Antibiotic-specific effects were observed on the intestinal community composition. For the doripenem group, treatment time points, i.e., days 1, 7, and 28, and recovery time points, i.e., 35 and 42/43, are highlighted with black and blue lines on the x-axis, respectively.
Figure 6.
Stacked bar diagram showing relative abundances of bacterial families that were detected in the controls and doripenem-recovery groups. An indication of the recovery of abundant bacterial families one and two weeks post-doripenem treatment was observed and resulted in abundances comparable to the controls.
Figure 6.
Stacked bar diagram showing relative abundances of bacterial families that were detected in the controls and doripenem-recovery groups. An indication of the recovery of abundant bacterial families one and two weeks post-doripenem treatment was observed and resulted in abundances comparable to the controls.
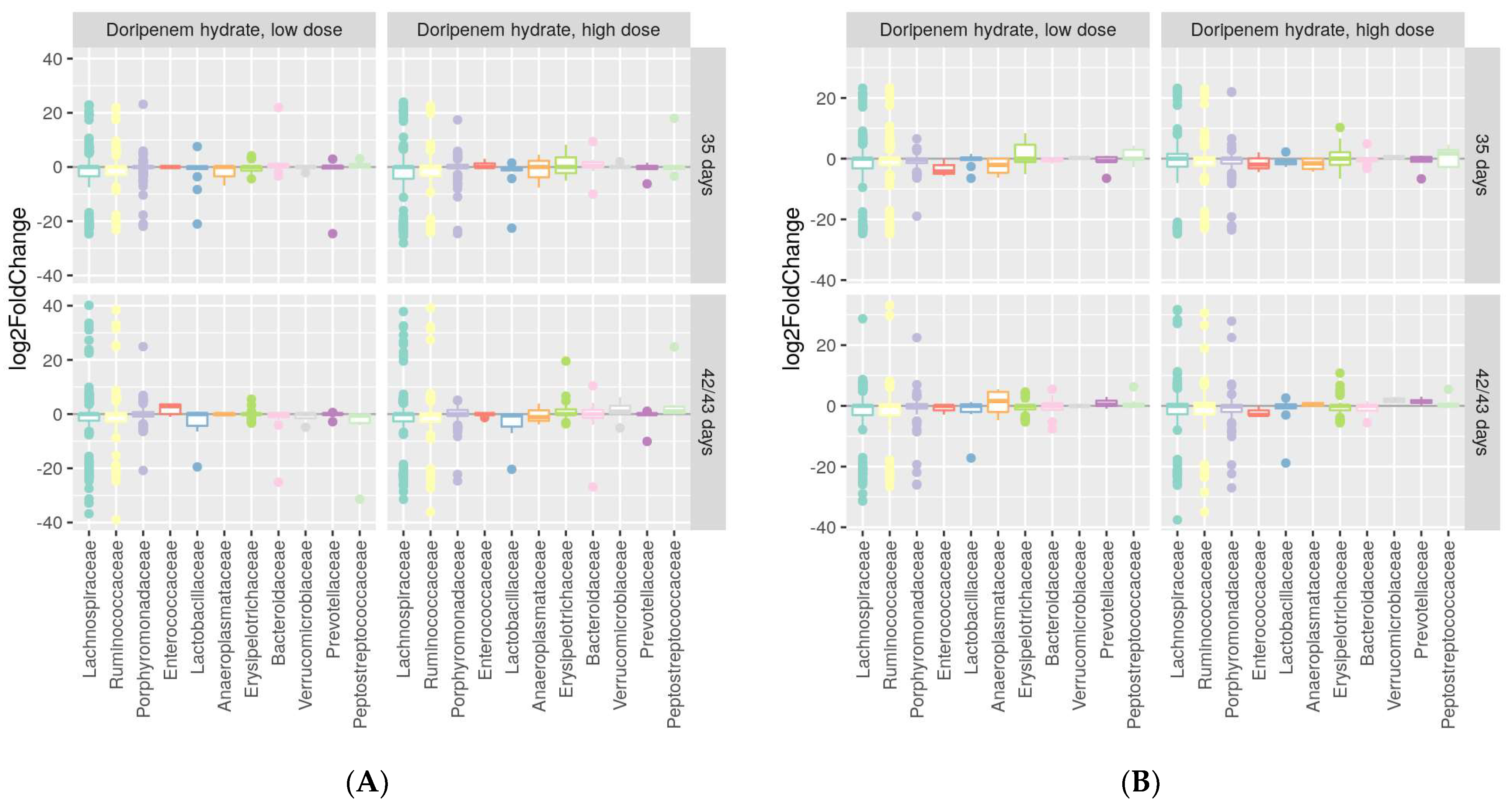
Figure 7.
Differential abundances in dominant bacterial families one and two weeks post-doripenem-treated samples from the low- (LD) and high- (HD) dose groups relative to the controls for males (A) and females (B). Differential abundance analysis was performed using log2FC (log2 fold change) values.
Figure 7.
Differential abundances in dominant bacterial families one and two weeks post-doripenem-treated samples from the low- (LD) and high- (HD) dose groups relative to the controls for males (A) and females (B). Differential abundance analysis was performed using log2FC (log2 fold change) values.
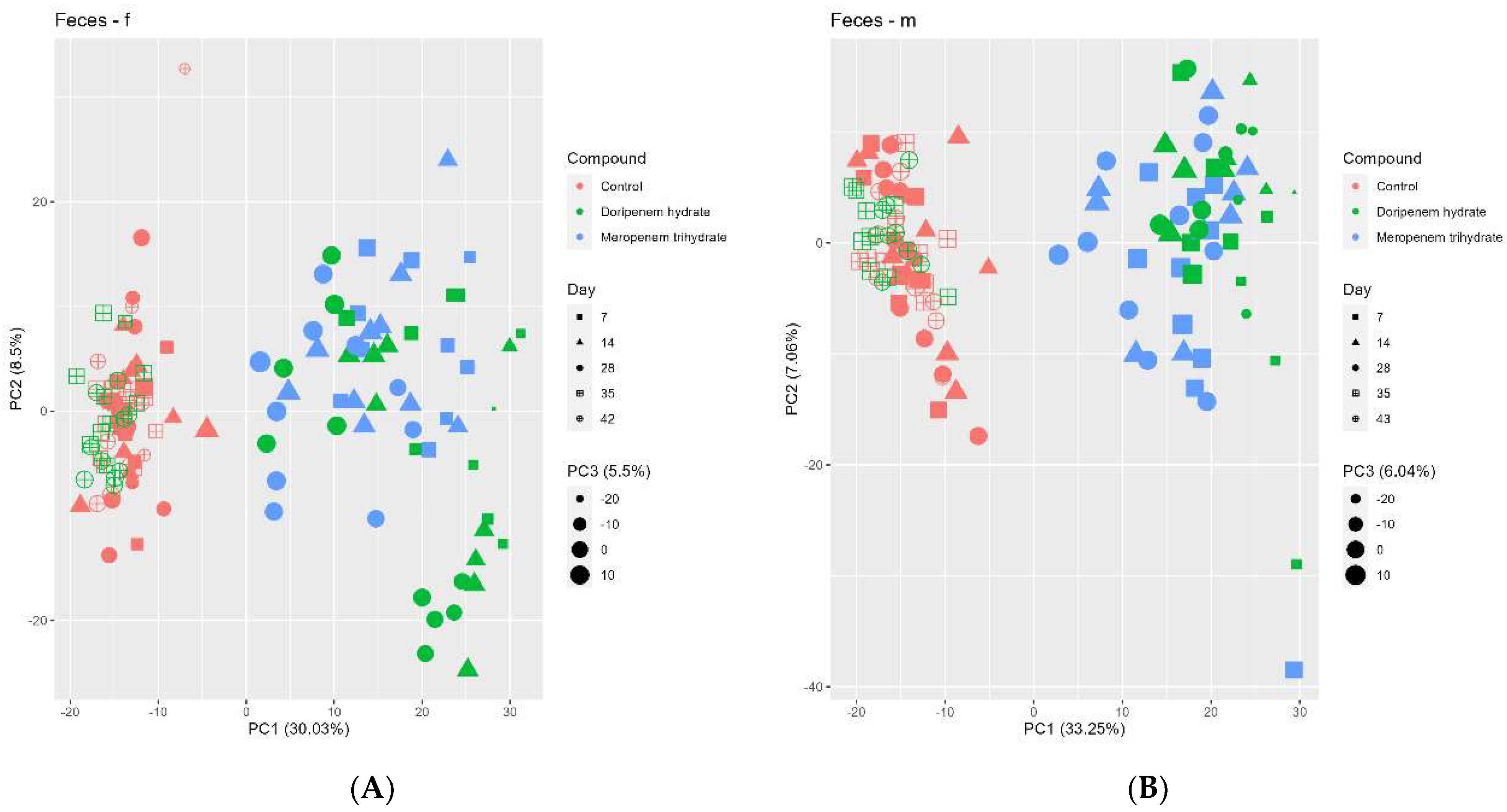
Figure 8.
Principal component analysis (PCA) of the fecal metabolome profiles of controls (red), meropenem- (blue), and doripenem- (green) treated rats. PCA for fecal metabolome profiles of (A) female and (B) male Wistar rats on days 7, 14, and 28 of the study and days 35 and 42/43 for the doripenem-recovery group. The data points are sized by the PC3 value. Different shapes of the data points represent respective sampling time points. Dose group-specific effects were marginal, so the groups were not separated based on the dose levels.
Figure 8.
Principal component analysis (PCA) of the fecal metabolome profiles of controls (red), meropenem- (blue), and doripenem- (green) treated rats. PCA for fecal metabolome profiles of (A) female and (B) male Wistar rats on days 7, 14, and 28 of the study and days 35 and 42/43 for the doripenem-recovery group. The data points are sized by the PC3 value. Different shapes of the data points represent respective sampling time points. Dose group-specific effects were marginal, so the groups were not separated based on the dose levels.
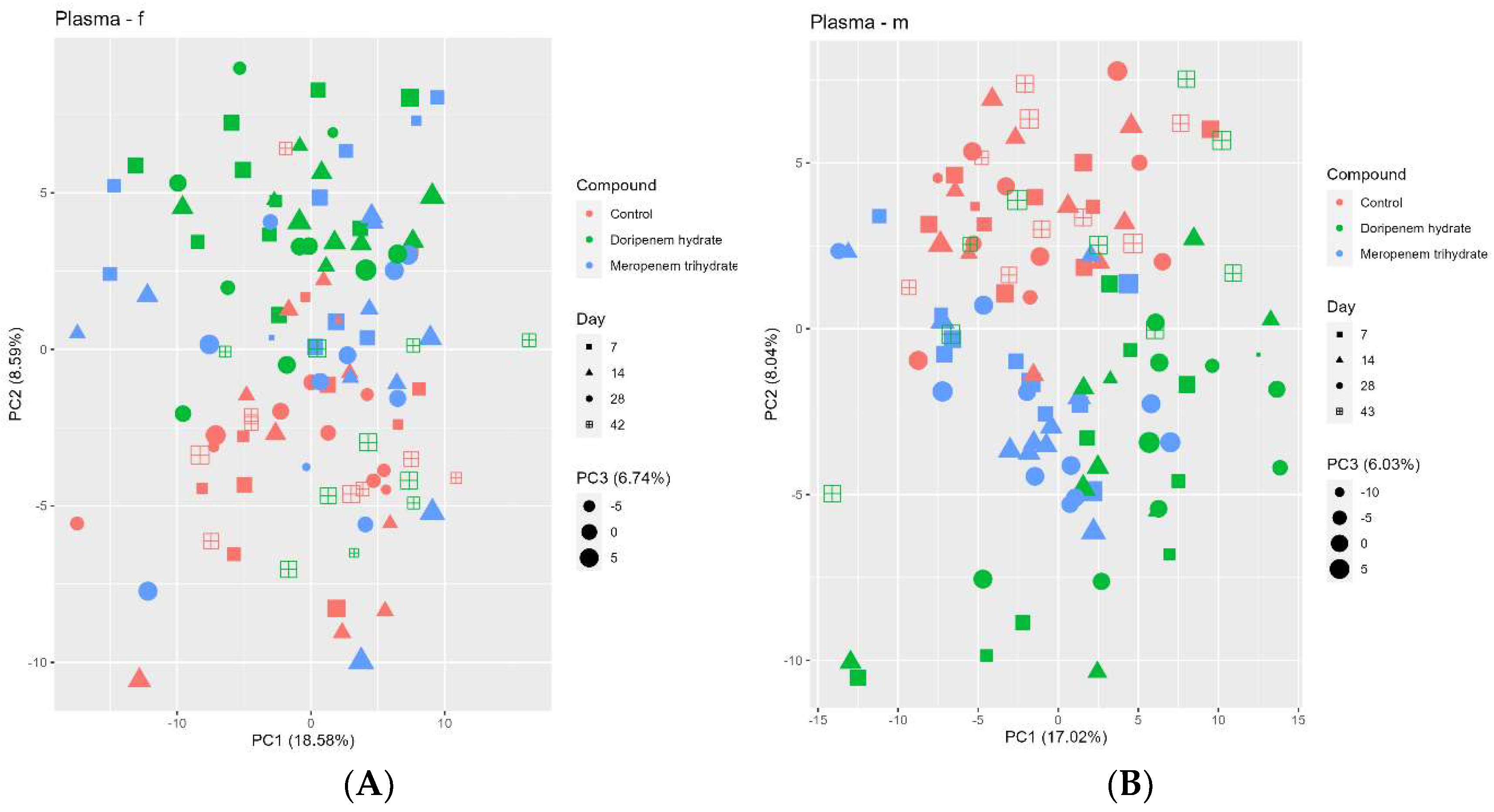
Figure 9.
Principal component analysis (PCA) of the blood plasma metabolome profiles of controls (red), meropenem- (blue), and doripenem- (green) treated rats. PCA for plasma metabolome profiles of (A) female and (B) male Wistar rats on days 7, 14, and 28 of the study and on day 42/43 for the doripenem-recovery group. The data points are sized by the PC3 value. Different shapes of the data points represent respective sampling time points. Dose group-specific effects were marginal, so the groups were not separated based on the dose levels.
Figure 9.
Principal component analysis (PCA) of the blood plasma metabolome profiles of controls (red), meropenem- (blue), and doripenem- (green) treated rats. PCA for plasma metabolome profiles of (A) female and (B) male Wistar rats on days 7, 14, and 28 of the study and on day 42/43 for the doripenem-recovery group. The data points are sized by the PC3 value. Different shapes of the data points represent respective sampling time points. Dose group-specific effects were marginal, so the groups were not separated based on the dose levels.
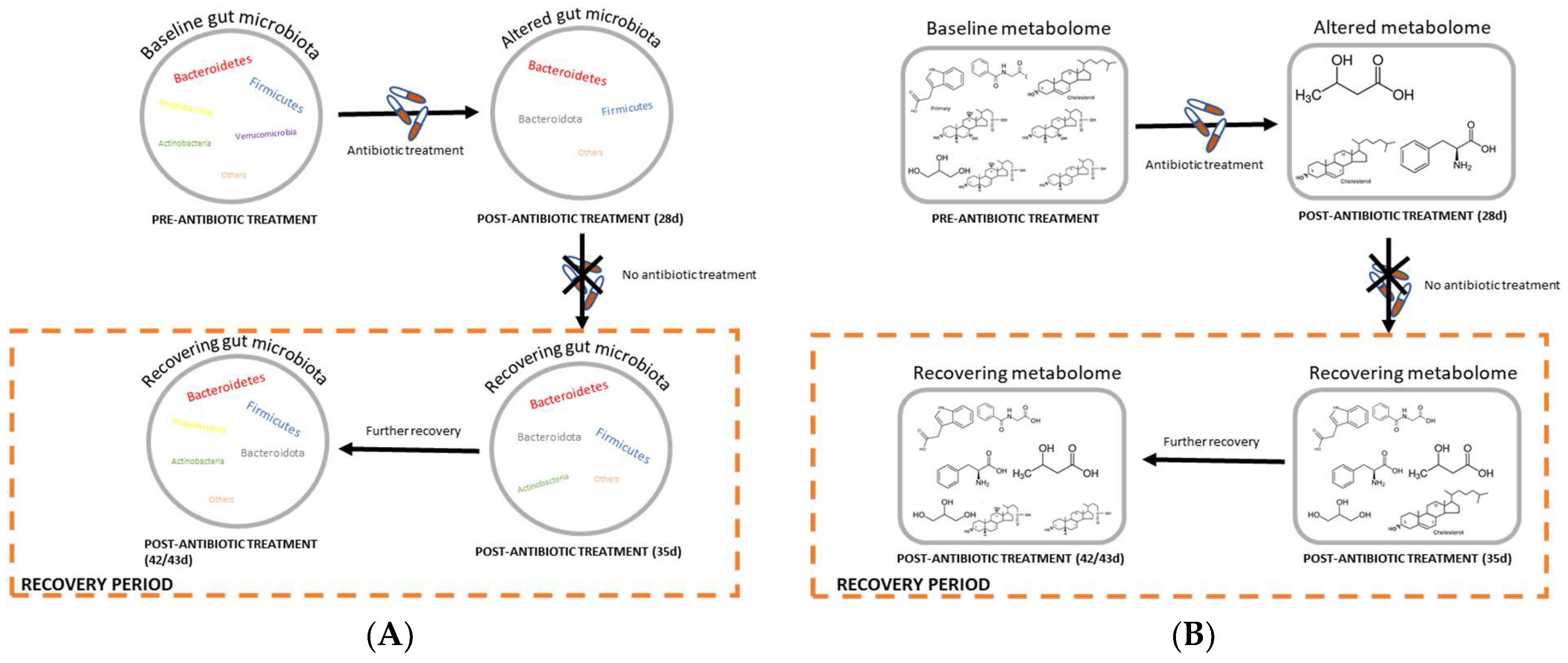
Figure 10.
Schematic diagrams of the recovery processes of (A) gut or fecal microbiota and (B) plasma and feces metabolomes before and after doripenem antibiotic treatments. Baseline state indicates the original or ‘normal’ state of the gut microbiota or metabolomes prior to antibiotic administration, which becomes altered upon 28 days of antibiotic administration. Further, post-antibiotic treatment, a recovery or overcompensation takes place where the gut bacteria as well as the host metabolomes try to return to the ‘normal’ state.
Figure 10.
Schematic diagrams of the recovery processes of (A) gut or fecal microbiota and (B) plasma and feces metabolomes before and after doripenem antibiotic treatments. Baseline state indicates the original or ‘normal’ state of the gut microbiota or metabolomes prior to antibiotic administration, which becomes altered upon 28 days of antibiotic administration. Further, post-antibiotic treatment, a recovery or overcompensation takes place where the gut bacteria as well as the host metabolomes try to return to the ‘normal’ state.
Table 1.
Compounds used, dose levels, and form of preparation. All compounds were administered orally using gavage.
Table 1.
Compounds used, dose levels, and form of preparation. All compounds were administered orally using gavage.
| Treatment | Low Dose
(mg/kg bw/d a) | High Dose
(mg/kg bw/d a) |
|---|
| Meropenem trihydrate | 100 | 300 |
| Doripenem hydrate | 100 | 1000 |
Table 2.
Meropenem-induced BA fold changes in feces of female (f) Wistar rats (N = 5 per group) dosed with 100 (LD) and 300 (HD) mg/kg bw/day observed on days 7, 14, and 28 (f7, f14, and f28). Statistically significant changes (Welch t-test; p-value < 0.05) are shown where red boxes mean a significant increase, yellow boxes mean a significant decrease, and white boxes indicate an insignificant change in the respective fecal BAs.
Table 2.
Meropenem-induced BA fold changes in feces of female (f) Wistar rats (N = 5 per group) dosed with 100 (LD) and 300 (HD) mg/kg bw/day observed on days 7, 14, and 28 (f7, f14, and f28). Statistically significant changes (Welch t-test; p-value < 0.05) are shown where red boxes mean a significant increase, yellow boxes mean a significant decrease, and white boxes indicate an insignificant change in the respective fecal BAs.
| | | Meropenem Trihydrate HD | Meropenem Trihydrate LD |
|---|
| Metabolite | Class | f7 | f14 | f28 | f7 | f14 | f28 |
|---|
| Deoxycholic acid | 2° BA * | 0.01 | 0.01 | 0.06 | 0.09 | 0.16 | 0.17 |
| Cholic acid | 1° BA * | 10.78 | 9.57 | 7.33 | 17.48 | 1.14 | 0.91 |
| Lithocholic acid | 2° BA | 0.02 | 0.03 | 0.02 | 0.08 | 0.15 | 0.15 |
| Taurocholic acid sodium salt | Taurine-conjugated 1° BA | 4.70 | 2.04 | 3.85 | 3.41 | 1.87 | 1.14 |
| Taurolithocholic Acid | Taurine-conjugated 2° BA | 0.04 | 0.12 | 0.15 | 0.41 | 0.61 | 0.78 |
| Taurocholic Acid 3-sulfate | Taurine-conjugated 1° BA | 185.39 | 197.22 | 280.09 | 40.98 | 81.49 | 6.10 |
| Tauro-b-muricholic Acid | Taurine-conjugated 1° BA | 3.51 | 2.01 | 3.42 | 2.14 | 1.30 | 1.10 |
| Taurochenodeoxycholate | Taurine-conjugated 1° BA | 2.08 | 1.43 | 2.60 | 1.71 | 1.56 | 1.17 |
| Hyodeoxycholic acid | 2° BA | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.04 |
| o-Muricholic Acid | 2° BA | 0.19 | 0.04 | 0.03 | 0.60 | 0.12 | 0.13 |
| isoLCA | 2° BA | 0.01 | 0.01 | 0.01 | 0.08 | 0.28 | 0.31 |
Table 3.
Meropenem-induced BA fold changes in feces of male Wistar rats (N = 5 per group) dosed with 100 (LD) and 300 (HD) mg/kg bw/day observed on days 7, 14, and 28 (m7, m14, and m28). Statistically significant changes (Welch t-test; p-value < 0.05) are shown where red boxes mean an increase, yellow boxes mean a significant decrease, and white boxes indicate an insignificant change in the respective fecal BAs.
Table 3.
Meropenem-induced BA fold changes in feces of male Wistar rats (N = 5 per group) dosed with 100 (LD) and 300 (HD) mg/kg bw/day observed on days 7, 14, and 28 (m7, m14, and m28). Statistically significant changes (Welch t-test; p-value < 0.05) are shown where red boxes mean an increase, yellow boxes mean a significant decrease, and white boxes indicate an insignificant change in the respective fecal BAs.
| | | Meropenem Trihydrate HD | Meropenem Trihydrate LD |
|---|
| Metabolite | Class | m7 | m14 | m28 | m7 | m14 | m28 |
|---|
| Deoxycholic acid | 2° BA | 0.01 | 0.01 | 0.00 | 0.20 | 0.21 | 0.24 |
| Cholic acid | 1° BA | 13.78 | 22.75 | 33.78 | 4.61 | 5.80 | 7.61 |
| Lithocholic acid | 2° BA | 0.02 | 0.03 | 0.02 | 0.13 | 0.10 | 0.08 |
| Taurocholic acid sodium salt | Taurine-conjugated 1° BA | 4.49 | 110.41 | 12.63 | 8.93 | 19.40 | 0.33 |
| Taurocholic Acid 3-sulfate | Taurine-conjugated 1° BA | 15.24 | 921.65 | 113.19 | 83.21 | 759.51 | 0.13 |
| Tauro-b-muricholic Acid | Taurine-conjugated 1° BA | 3.50 | 26.50 | 12.70 | 10.01 | 12.34 | 2.03 |
| a-Muricholic Acid | 1° BA | 23.88 | 2.53 | 2.28 | 18.70 | 5.85 | 2.13 |
| b-Muricholic Acid | 1° BA | 8.16 | 2.43 | 2.13 | 5.03 | 1.73 | 3.48 |
| Hyodeoxycholic acid | 2° BA | 0.02 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 |
| o-Muricholic Acid | 2° BA | 0.35 | 0.05 | 0.05 | 0.14 | 0.15 | 0.07 |
| isoLCA | 2° BA | 0.01 | 0.01 | 0.01 | 0.14 | 0.19 | 0.14 |
Table 4.
Doripenem-induced BA fold changes in feces of female Wistar rats (N = 5 per group) dosed with 100 (LD) and 1000 (HD) mg/kg bw/day observed on days 7, 14, and 28 (f7, f14, and f28) and on days 35 and 42 (f35 and f42) for the recovery group. Statistically significant changes (Welch t-test; p-value < 0.05) are shown where red boxes mean an increase, yellow boxes mean a significant decrease, and white boxes indicate an insignificant change in the respective fecal BAs.
Table 4.
Doripenem-induced BA fold changes in feces of female Wistar rats (N = 5 per group) dosed with 100 (LD) and 1000 (HD) mg/kg bw/day observed on days 7, 14, and 28 (f7, f14, and f28) and on days 35 and 42 (f35 and f42) for the recovery group. Statistically significant changes (Welch t-test; p-value < 0.05) are shown where red boxes mean an increase, yellow boxes mean a significant decrease, and white boxes indicate an insignificant change in the respective fecal BAs.
| | | Doripenem HD | Doripenem HD Recovery | Doripenem LD | Doripenem LD Recovery |
|---|
| Metabolite | Class | f7 | f14 | f28 | f35 | f42 | f7 | f14 | f28 | f35 | f43 |
|---|
| Deoxycholic acid | 2° BA | 0 | 0 | 0 | 0.85 | 0.5 | 0.01 | 0 | 0.03 | 1.04 | 0.68 |
| Cholic acid | 1° BA | 3.44 | 2.81 | 5.6 | 1.15 | 1.08 | 15.5 | 15.76 | 15.61 | 1.23 | 0.88 |
| Lithocholic acid | 2° BA | 0.01 | 0.01 | 0.01 | 2.23 | 1.13 | 0.02 | 0.01 | 0.03 | 1.68 | 1.11 |
| Taurocholic acid sodium salt | Taurine-conjugated 1° BA | 8.1 | 6.65 | 5.78 | 0.09 | 0.37 | 3.83 | 2.12 | 2.46 | 0.28 | 0.85 |
| Glycolithocholic Acid | Glycine-conjugated 2° BA | 0.2 | 0.13 | 0.06 | 6.89 | 5.76 | 0.56 | 0.09 | 0.29 | 5.39 | 0.63 |
| Taurolithocholic Acid | Taurine-conjugated 2° BA | 0.03 | 0.02 | 0.01 | 0.9 | 2.66 | 0.2 | 0.03 | 0.4 | 1.25 | 0.89 |
| Taurocholic Acid 3-sulfate | Taurine-conjugated 1° BA | 395.06 | 302.06 | 28.52 | 1.03 | 0.91 | 139.94 | 156.93 | 16.82 | 2.13 | 1.32 |
| Tauro-b-muricholic Acid | Taurine-conjugated 1° BA | 6.8 | 4.47 | 2.27 | 0.39 | 0.82 | 2.02 | 1.59 | 1.73 | 0.82 | 0.85 |
| Taurochenodeoxycholate | Taurine-conjugated 1° BA | 1.65 | 1.94 | 1.95 | 0.92 | 0.86 | 1.63 | 1.18 | 1.87 | 0.98 | 0.77 |
| Taurodeoxycholate | Taurine-conjugated 2° BA | 0.54 | 0.46 | 0.49 | 0.66 | 0.64 | 0.55 | 0.48 | 0.46 | 0.66 | 0.69 |
| Glycodeoxycholate | Glycine-conjugated 2° BA | 0.46 | 0.33 | 0.35 | 1.48 | 1.28 | 0.45 | 0.26 | 0.39 | 1.05 | 0.93 |
| Hyodeoxycholic acid | 2° BA | 0 | 0 | 0 | 6.9 | 3 | 0.03 | 0 | 0.01 | 1.82 | 0.49 |
| o-Muricholic Acid | 2° BA | 0.01 | 0 | 0.01 | 0.74 | 0.08 | 0.28 | 0.08 | 0.08 | 1.3 | 1.25 |
| isoLCA | 2° BA | 0 | 0 | 0.01 | 3.18 | 1.5 | 0.01 | 0 | 0.02 | 1.63 | 1.2 |
Table 5.
Doripenem-induced BA fold changes in feces of male Wistar rats (N = 5 per group) dosed with 100 (LD) and 1000 (HD) mg/kg bw/day observed on days 7, 14, 28 (m7, m14 and m28) and on days 35 and 42 (m35 and m43) for the doripenem-recovery group. Statistically significant changes (Welch t-test; p-value < 0.05) are shown where red boxes mean an increase, yellow boxes mean a significant decrease, and white boxes indicate an insignificant change in the respective fecal BAs.
Table 5.
Doripenem-induced BA fold changes in feces of male Wistar rats (N = 5 per group) dosed with 100 (LD) and 1000 (HD) mg/kg bw/day observed on days 7, 14, 28 (m7, m14 and m28) and on days 35 and 42 (m35 and m43) for the doripenem-recovery group. Statistically significant changes (Welch t-test; p-value < 0.05) are shown where red boxes mean an increase, yellow boxes mean a significant decrease, and white boxes indicate an insignificant change in the respective fecal BAs.
| | | Doripenem HD | Doripenem HD Recovery | Doripenem LD | Doripenem LD Recovery |
|---|
| Metabolite | Class | m7 | m14 | m28 | m35 | m43 | m7 | m14 | m28 | m35 | m43 |
|---|
| Deoxycholic acid | 2° BA | 0.01 | 0 | 0.01 | 0.77 | 1.15 | 0.01 | 0 | 0 | 0.91 | 0.78 |
| Cholic acid | 1° BA | 14.31 | 26.92 | 5.47 | 0.98 | 0.87 | 15.34 | 20.97 | 14.38 | 1 | 0.47 |
| Lithocholic acid | 2° BA | 0.02 | 0.02 | 0.02 | 1.09 | 1.07 | 0.02 | 0.02 | 0.02 | 1.07 | 0.74 |
| Taurocholic acid sodium salt | Taurine-conjugated 1° BA | 16.62 | 73.02 | 11.51 | 0.5 | 0.2 | 6.14 | 15.13 | 4.85 | 0.76 | 0.38 |
| Taurocholic Acid 3-sulfate | Taurine-conjugated 1° BA | 142.02 | 1738.7 | 129.59 | 2.24 | 0.37 | 20.83 | 85.11 | 43.38 | 1.52 | 0.12 |
| Tauro-b-muricholic Acid | Taurine-conjugated 1° BA | 20.19 | 148.81 | 40.8 | 0.7 | 0.67 | 4.03 | 6.14 | 2.65 | 0.64 | 0.45 |
| Hyodeoxycholic acid | 2° BA | 0.01 | 0 | 0.01 | 0.23 | 0.84 | 0.01 | 0 | 0.01 | 0.58 | 0.45 |
| o-Muricholic Acid | 2° BA | 0.08 | 0 | 0 | 2 | 1.32 | 0.14 | 0.03 | 0.02 | 1.05 | 0.6 |
| isoLCA | 2° BA | 0.01 | 0 | 0 | 0.78 | 0.89 | 0 | 0 | 0.01 | 0.96 | 0.55 |
Table 6.
Meropenem-induced plasma metabolite fold changes in female (f) Wistar rats (N = 5 per group) dosed with 100 (LD) and 300 (HD) mg/kg bw/day observed on 7, 14, and 28 days (f7, f14, and f28). Statistically significant changes (Welch t-test; p-value < 0.05) are shown where red boxes mean a significant increase in the respective plasma metabolites and yellow boxes mean a significant reduction compared to control levels.
Table 6.
Meropenem-induced plasma metabolite fold changes in female (f) Wistar rats (N = 5 per group) dosed with 100 (LD) and 300 (HD) mg/kg bw/day observed on 7, 14, and 28 days (f7, f14, and f28). Statistically significant changes (Welch t-test; p-value < 0.05) are shown where red boxes mean a significant increase in the respective plasma metabolites and yellow boxes mean a significant reduction compared to control levels.
| | | Meropenem Trihydrate HD | Meropenem Trihydrate LD |
|---|
| Metabolite | Class | f7 | f14 | f28 | f7 | f14 | f28 |
|---|
| Threonine | Amino acids | 0.87 | 0.80 | 0.84 | 0.93 | 0.99 | 1.05 |
| Glycine | Amino acids | 0.92 | 0.89 | 0.87 | 0.86 | 0.87 | 0.97 |
| Threonine | Amino acids | 0.83 | 0.82 | 0.84 | 0.99 | 0.94 | 1.03 |
| Cysteine | Amino acids | 0.85 | 1.03 | 0.93 | 0.92 | 1.11 | 1.08 |
| 3-Hydroxyindole | Amino acids-related | 0.11 | 0.10 | 0.24 | 0.86 | 0.45 | 0.97 |
| Indole-3-acetic acid | Amino acids-related | 5.60 | 4.82 | 5.66 | 1.68 | 2.44 | 1.94 |
| 3-Indoxylsulfate | Amino acids-related | 0.10 | 0.15 | 0.27 | 0.87 | 0.59 | 1.10 |
| Deoxyribonucleic acids, total | Carbohydrates and related | 0.81 | 0.93 | 1.06 | 0.79 | 0.91 | 0.73 |
| Xylitol | Carbohydrates and related | 1.64 | 1.52 | 1.54 | 0.84 | 0.76 | 1.41 |
| 3-Hydroxybutyrate | Energy metabolism and related | 1.30 | 1.23 | 0.97 | 1.13 | 1.04 | 0.96 |
| 3-Hydroxybutyrate | Energy metabolism and related | 1.41 | 1.32 | 1.15 | 1.29 | 1.12 | 1.02 |
| 3-Methoxytyrosine | Hormones, signal substances and related | 1.43 | 1.44 | 1.44 | 1.18 | 1.20 | 1.24 |
| 17-Hydroxypregnenolone | Hormones, signal substances and related | 0.02 | 0.01 | 0.03 | 0.02 | 0.03 | 0.02 |
| Hippuric acid | Miscellaneous | 0.24 | 0.24 | 0.43 | 0.48 | 0.53 | 0.99 |
| Cytosine | Nucleobases and related | 0.78 | 0.77 | 1.08 | 0.76 | 0.78 | 0.78 |
Table 7.
Meropenem-induced plasma BA fold changes in female (f) Wistar rats (N = 5 per group) dosed with 100 (LD) and 300 (HD) mg/kg bw/day observed on 7, 14, and 28 days (f7, f14, and f28). Statistically significant changes (Welch t-test; p-value < 0.05) are shown where red boxes mean a significant increase in the respective plasma BAs and yellow boxes mean a significant reduction compared to control levels.
Table 7.
Meropenem-induced plasma BA fold changes in female (f) Wistar rats (N = 5 per group) dosed with 100 (LD) and 300 (HD) mg/kg bw/day observed on 7, 14, and 28 days (f7, f14, and f28). Statistically significant changes (Welch t-test; p-value < 0.05) are shown where red boxes mean a significant increase in the respective plasma BAs and yellow boxes mean a significant reduction compared to control levels.
| | | Meropenem Trihydrate HD | Meropenem Trihydrate LD |
|---|
| Metabolite | Class | f7 | f14 | f28 | f7 | f14 | f28 |
|---|
| Deoxycholic acid | 2° BA | 0.04 | 0.04 | 0.24 | 0.49 | 0.82 | 1.03 |
| Lithocholic acid | 2° BA | 0.01 | 0.02 | 0.05 | 1.04 | 0.72 | 1.82 |
| Taurolithocholic Acid | Taurine-conjugated 2° BA | 0.01 | 0.03 | 0.07 | 0.07 | 0.19 | 0.26 |
| Tauroursodeoxycholic Acid | Taurine-conjugated 2° BA | 3.49 | 1.83 | 6.66 | 5.30 | 2.14 | 8.83 |
| Taurodeoxycholate | Taurine-conjugated 2° BA | 0.00 | 0.00 | 0.04 | 0.08 | 0.14 | 0.32 |
| Glycocholic acid | Glycine-conjugated 1° BA | 4.30 | 1.82 | 5.16 | 2.28 | 3.68 | 3.61 |
| Glycochenodeoxycholic acid | Glycine-conjugated 1° BA | 8.51 | 2.62 | 13.20 | 10.69 | 16.07 | 12.32 |
| Glycodeoxycholate | Glycine-conjugated 2° BA | 0.05 | 0.14 | 0.39 | 0.57 | 2.94 | 3.19 |
| Glycoursodeoxycholic acid | Glycine-conjugated 2° BA | 22.25 | 11.61 | 35.10 | 10.72 | 32.78 | 24.98 |
| Hyodeoxycholic acid | 2° BA | 0.03 | 0.03 | 0.03 | 0.14 | 0.05 | 0.18 |
| o-Muricholic Acid | 2° BA | 0.20 | 0.16 | 0.29 | 0.88 | 0.23 | 1.21 |
| isoLCA | 2° BA | 0.06 | 0.48 | 0.34 | 0.07 | 0.35 | 0.72 |
Table 8.
Meropenem-induced plasma metabolite fold changes in male (m) Wistar rats (N = 5 per group) dosed with 100 (LD) and 300 (HD) mg/kg bw/day observed on 7, 14, and 28 days (m7, m14, and m28). Statistically significant changes (Welch t-test; p-value < 0.05) are shown where red boxes mean a significant increase in the respective plasma metabolites and yellow boxes mean a significant reduction compared to control levels.
Table 8.
Meropenem-induced plasma metabolite fold changes in male (m) Wistar rats (N = 5 per group) dosed with 100 (LD) and 300 (HD) mg/kg bw/day observed on 7, 14, and 28 days (m7, m14, and m28). Statistically significant changes (Welch t-test; p-value < 0.05) are shown where red boxes mean a significant increase in the respective plasma metabolites and yellow boxes mean a significant reduction compared to control levels.
| | | Meropenem Trihydrate HD | Meropenem Trihydrate LD |
|---|
| Metabolite | Class | m7 | m14 | m28 | m7 | m14 | m28 |
|---|
| Threonine | Amino acids | 0.86 | 0.82 | 0.75 | 0.90 | 1.04 | 0.97 |
| Threonine | Amino acids | 0.93 | 0.78 | 0.75 | 0.89 | 0.98 | 0.92 |
| Methionine | Amino acids | 0.97 | 0.88 | 0.88 | 1.02 | 0.98 | 1.05 |
| 3-Indoxylsulfate | Amino acids-related | 0.11 | 0.11 | 0.09 | 0.74 | 0.63 | 0.46 |
| Indole-3-acetic acid | Amino acids-related | 3.02 | 6.51 | 9.41 | 5.03 | 4.17 | 6.45 |
| 3-Hydroxyindole | Amino acids-related | 0.12 | 0.11 | 0.14 | 1.07 | 0.64 | 0.48 |
| Lysophosphatidylcholine (C18:2) | Complex lipids, fatty acids and related | 1.07 | 0.96 | 1.14 | 0.97 | 0.96 | 1.17 |
| Citrate | Energy metabolism and related | 1.09 | 1.17 | 1.17 | 1.04 | 1.11 | 1.28 |
| Cortisol | Hormones, signal substances and related | 3.36 | 2.72 | 4.95 | 1.43 | 1.69 | 2.21 |
| Corticosterone | Hormones, signal substances and related | 2.68 | 1.64 | 1.65 | 2.90 | 2.15 | 2.34 |
| 18-Hydroxy-11-deoxycorticosterone | Hormones, signal substances and related | 2.16 | 1.52 | 1.27 | 2.44 | 2.61 | 2.40 |
| Hippuric acid | Miscellaneous | 0.52 | 0.33 | 0.53 | 0.27 | 0.41 | 0.32 |
Table 9.
Meropenem-induced blood plasma BA fold changes in male (m) Wistar rats. Metabolite fold changes in plasma BAs of male Wistar rats (N = 5 per group) dosed with meropenem (100 (LD) and 300 (HD) mg/kg bw/day) observed on 7, 14, and 28 days (m7, m14, and m28). Statistically significant changes (Welch t-test; p-value < 0.05) are shown where red boxes mean a significant increase in the respective plasma BAs and yellow boxes mean a significant reduction compared to control levels.
Table 9.
Meropenem-induced blood plasma BA fold changes in male (m) Wistar rats. Metabolite fold changes in plasma BAs of male Wistar rats (N = 5 per group) dosed with meropenem (100 (LD) and 300 (HD) mg/kg bw/day) observed on 7, 14, and 28 days (m7, m14, and m28). Statistically significant changes (Welch t-test; p-value < 0.05) are shown where red boxes mean a significant increase in the respective plasma BAs and yellow boxes mean a significant reduction compared to control levels.
| | | Meropenem Trihydrate HD | Meropenem Trihydrate LD |
|---|
| Metabolite | Class | m7 | m14 | m28 | m7 | m14 | m28 |
|---|
| Ursodeoxycholic acid | 2° BA | 1.54 | 8.42 | 3.22 | 2.41 | 4.27 | 2.23 |
| Deoxycholic acid | 2° BA | 0.01 | NA | 0.02 | 0.46 | 1.84 | 0.29 |
| Lithocholic acid | 2° BA | 0.02 | 0.14 | 0.02 | 0.14 | 0.59 | 0.07 |
| Taurolithocholic Acid | Taurine-conjugated 2° BA | 0.05 | 0.08 | 0.08 | 0.16 | 0.16 | 0.31 |
| Taurocholic Acid 3-sulfate | Taurine-conjugated 1° BA | 0.22 | 1.10 | 0.31 | 0.65 | 0.30 | 0.34 |
| Tauro-b-muricholic Acid | Taurine-conjugated 1° BA | 1.73 | 4.06 | 1.96 | 0.95 | 1.03 | 1.10 |
| Tauroursodeoxycholic Acid | Taurine-conjugated 2° BA | 1.25 | 7.42 | 5.90 | 1.44 | 1.18 | 0.63 |
| b-Muricholic Acid | 1° BA | 1.45 | 3.56 | 2.23 | 1.51 | 1.65 | 1.88 |
| Taurochenodeoxycholate | Taurine-conjugated 1° BA | 2.42 | 3.53 | 2.05 | 1.17 | 0.68 | 1.22 |
| Taurodeoxycholate | Taurine-conjugated 2° BA | 0.02 | 0.00 | 0.03 | 0.10 | 0.21 | 0.10 |
| Glycodeoxycholate | Glycine-conjugated 2° BA | 0.01 | NA | 0.01 | 0.64 | 3.02 | 0.09 |
| Hyodeoxycholic acid | 2° BA | 0.01 | 0.03 | 0.01 | 0.03 | 0.01 | 0.00 |
| o-Muricholic Acid | 2° BA | 0.11 | 1.23 | 0.07 | 0.12 | 0.14 | 0.09 |
| isoLCA | 2° BA | 0.26 | 2.59 | 0.12 | 0.29 | 0.44 | 0.35 |
Table 10.
Doripenem-induced plasma metabolite fold changes in female (f) Wistar rats (N = 5 per group) dosed with 100 (LD) and 1000 (HD) mg/kg bw/day observed on days 7, 14, and 28 (f7, f14, and f28) and on day 42 (f42) from the doripenem-recovery group. Statistically significant changes (Welch t-test; p-value < 0.05) are shown where red boxes mean a significant increase, yellow boxes mean a significant decrease, and white boxes indicate an insignificant change in the respective plasma metabolites compared to control group.
Table 10.
Doripenem-induced plasma metabolite fold changes in female (f) Wistar rats (N = 5 per group) dosed with 100 (LD) and 1000 (HD) mg/kg bw/day observed on days 7, 14, and 28 (f7, f14, and f28) and on day 42 (f42) from the doripenem-recovery group. Statistically significant changes (Welch t-test; p-value < 0.05) are shown where red boxes mean a significant increase, yellow boxes mean a significant decrease, and white boxes indicate an insignificant change in the respective plasma metabolites compared to control group.
| | | Doripenem HD | Doripenem HD Recovery | Doripenem LD | Doripenem LD Recovery |
|---|
| Metabolite | Class | f7 | f14 | f28 | f42 | f7 | f14 | f28 | f42 |
|---|
| Tyrosine | Amino acids | 1.11 | 0.79 | 0.75 | 0.84 | 0.83 | 0.83 | 0.97 | 1.12 |
| Threonine | Amino acids | 0.83 | 0.77 | 0.80 | 1.06 | 0.92 | 0.90 | 0.90 | 1.02 |
| Tyrosine | Amino acids | 0.88 | 0.82 | 0.80 | 0.84 | 0.86 | 0.86 | 0.95 | 0.98 |
| trans-4-Hydroxyproline | Amino acids-related | 0.90 | 0.81 | 0.89 | 1.07 | 1.11 | 0.93 | 0.93 | 1.14 |
| 3-Hydroxyindole | Amino acids-related | 0.09 | 0.09 | 0.06 | 0.79 | 0.31 | 0.55 | 0.63 | 0.72 |
| Ketoleucine | Amino acids-related | 0.67 | 0.76 | 0.71 | 0.79 | 0.81 | 0.74 | 0.88 | 0.96 |
| Indole-3-propionic acid | Amino acids-related | 0.85 | 0.87 | 0.66 | 0.77 | 1.74 | 0.93 | 0.93 | 0.90 |
| 3-Indoxylsulfate | Amino acids-related | 0.06 | 0.04 | 0.03 | 0.96 | 0.24 | 0.37 | 0.66 | 0.93 |
| Deoxyribonucleic acids, total | Carbohydrates and related | 0.76 | 0.92 | 0.72 | 0.99 | 0.79 | 0.80 | 0.78 | 0.91 |
| Ceramide (d18:1,C24:1) | Complex lipids, fatty acids and related | 0.80 | 0.71 | 0.74 | 1.27 | 1.20 | 1.29 | 1.37 | 1.06 |
| Ceramide (d18:1,C24:0) | Complex lipids, fatty acids and related | 0.73 | 0.68 | 0.62 | 1.25 | 1.02 | 1.25 | 1.19 | 1.09 |
| Citrate | Energy metabolism and related | 1.09 | 1.26 | 1.26 | 1.09 | 1.05 | 1.10 | 1.12 | 0.98 |
| 2-Hydroxybutyrate | Energy metabolism and related | 1.55 | 1.60 | 2.09 | 1.17 | 1.37 | 1.52 | 1.14 | 0.73 |
| 3-Methoxytyrosine | Hormones, signal substances and related | 1.10 | 1.22 | 1.2 | 1.26 | 1.38 | 1.41 | 1.05 | 1.10 |
| 17-Hydroxypregnenolone | Hormones, signal substances and related | 0.01 | 0.01 | 0.02 | 0.83 | 0.01 | 0.04 | 0.53 | 1.25 |
| beta-Sitosterol, total | Miscellaneous | 0.62 | 0.74 | 0.74 | 1.12 | 1.01 | 1.07 | 0.80 | 1.13 |
| Hippuric acid | Miscellaneous | 0.23 | 0.25 | 0.48 | 1.58 | 0.39 | 0.32 | 0.52 | 1.00 |
| Cytosine | Nucleobases and related | 0.86 | 0.77 | 0.71 | 0.88 | 0.89 | 0.64 | 0.83 | 0.75 |
| Allantoin | Nucleobases and related | 0.75 | 0.89 | 0.86 | 1.01 | 0.73 | 0.82 | 1.01 | 0.97 |
| Cholesterol, total | | 0.84 | 0.99 | 0.69 | 1.32 | 1.04 | 1.30 | 0.83 | 1.15 |
Table 11.
Doripenem-induced plasma BA fold changes in female (f) Wistar rats (N = 5 per group) dosed with 100 (LD) and 1000 (HD) mg/kg bw/day observed on days 7, 14, and 28 (f7, f14, and f28) and on day 42 (f42) for the doripenem-recovery group. Statistically significant changes (Welch t-test; p-value < 0.05) are shown where red boxes mean a significant increase, yellow boxes mean a significant decrease, and white boxes indicate an insignificant change in the respective plasma BAs compared to control groups.
Table 11.
Doripenem-induced plasma BA fold changes in female (f) Wistar rats (N = 5 per group) dosed with 100 (LD) and 1000 (HD) mg/kg bw/day observed on days 7, 14, and 28 (f7, f14, and f28) and on day 42 (f42) for the doripenem-recovery group. Statistically significant changes (Welch t-test; p-value < 0.05) are shown where red boxes mean a significant increase, yellow boxes mean a significant decrease, and white boxes indicate an insignificant change in the respective plasma BAs compared to control groups.
| | | Doripenem HD | Doripenem HD Recovery | Doripenem LD | Doripenem LD Recovery |
|---|
| Metabolite | Class | f7 | f14 | f28 | f42 | f7 | f14 | f28 | f42 |
|---|
| Deoxycholic acid | 2° BA | 0.02 | 0.02 | 0.03 | 3.41 | 0.02 | 0.06 | 0.32 | 2.57 |
| Taurocholic acid sodium salt | Taurine-conjugated 1° BA | 0.99 | 3.46 | 1.88 | 0.44 | 0.68 | 0.66 | 0.76 | 0.49 |
| Glycolithocholic Acid | Glycine-conjugated 2° BA | 0.11 | 0.12 | 0.33 | 9.27 | 0.04 | 0.37 | 1.26 | 13.31 |
| Taurolithocholic Acid | Taurine-conjugated 2° BA | 0.01 | 0.01 | 0.04 | 3.43 | 0.01 | 0.03 | 0.18 | 1.32 |
| Taurocholic Acid 3-sulfate | Taurine-conjugated 1° BA | 1.68 | 2.09 | 2.41 | 0.98 | 1.26 | 1.83 | 2.24 | 1.62 |
| Tauro-b-muricholic Acid | Taurine-conjugated 1° BA | 1.24 | 6.05 | 2.74 | 0.78 | 1.48 | 1.52 | 1.22 | 1.21 |
| Tauroursodeoxycholic Acid | Taurine-conjugated 2° BA | 14.68 | 7.45 | 17.31 | 3.88 | 5.43 | 2.66 | 11.53 | 0.97 |
| Taurochenodeoxycholate | Taurine-conjugated 1° BA | 1.29 | 2.94 | 1.91 | 1.30 | 1.38 | 1.33 | 1.28 | 1.12 |
| Taurodeoxycholate | Taurine-conjugated 2° BA | 0.00 | 0.00 | 0.01 | 0.65 | 0.00 | 0.00 | 0.05 | 0.68 |
| Hyodeoxycholic acid | 2° BA | 0.02 | 0.01 | 0.01 | 19.38 | 0.05 | 0.01 | 0.02 | 2.55 |
| o-Muricholic Acid | 2° BA | 0.11 | 0.08 | 0.14 | 0.66 | 0.16 | 0.13 | 0.45 | 1.98 |
| isoLCA | 2° BA | 0.08 | 0.27 | 0.23 | 3.90 | 0.07 | 0.10 | 0.13 | 3.06 |
Table 12.
Doripenem-induced plasma metabolite fold changes in male (m) Wistar rats (N = 5 per group) dosed with 100 (LD) and 1000 (HD) mg/kg bw/day observed on days 7, 14, and 28 (m7, m14, and m28) and on day 42 (m42) for the doripenem-recovery group. Statistically significant changes (Welch t-test; p-value < 0.05) are shown where red boxes mean a significant increase, yellow boxes mean a significant decrease, and white boxes indicate an insignificant change in the respective plasma metabolites compared to control group.
Table 12.
Doripenem-induced plasma metabolite fold changes in male (m) Wistar rats (N = 5 per group) dosed with 100 (LD) and 1000 (HD) mg/kg bw/day observed on days 7, 14, and 28 (m7, m14, and m28) and on day 42 (m42) for the doripenem-recovery group. Statistically significant changes (Welch t-test; p-value < 0.05) are shown where red boxes mean a significant increase, yellow boxes mean a significant decrease, and white boxes indicate an insignificant change in the respective plasma metabolites compared to control group.
| | | Doripenem HD | Doripenem HD Recovery | Doripenem LD | Doripenem LD Recovery |
|---|
| Metabolite | Class | m7 | m14 | m28 | m43 | m7 | m14 | m28 | m43 |
|---|
| Tyrosine | Amino acids | 0.71 | 0.95 | 0.8 | 0.86 | 0.83 | 1.07 | 0.82 | 0.93 |
| Alanine | Amino acids | 0.76 | 0.9 | 1 | 0.90 | 0.9 | 0.86 | 0.96 | 0.96 |
| Glycine | Amino acids | 0.87 | 0.94 | 0.81 | 1.00 | 0.89 | 0.82 | 0.77 | 0.97 |
| Threonine | Amino acids | 0.77 | 0.99 | 0.81 | 1.05 | 0.84 | 0.83 | 0.78 | 0.89 |
| Tyrosine | Amino acids | 0.75 | 0.77 | 0.76 | 0.97 | 0.83 | 1 | 0.92 | 0.94 |
| 3-Hydroxyindole | Amino acids-related | 0.12 | 0.08 | 0.1 | 1.40 | 0.2 | 0.59 | 0.58 | 1.18 |
| 3-Hydroxyisobutyrate | Amino acids-related | 0.99 | 0.83 | 0.72 | 0.95 | 0.86 | 0.92 | 0.76 | 1.12 |
| Ketoleucine | Amino acids-related | 0.67 | 0.68 | 0.86 | 0.80 | 0.84 | 0.82 | 0.9 | 0.74 |
| 3-Indoxylsulfate | Amino acids-related | 0.2 | 0.05 | 0.18 | 1.08 | 0.18 | 0.73 | 0.48 | 0.88 |
| Deoxyribonucleic acids, total | Carbohydrates and related | 0.85 | 0.8 | 0.8 | 1.01 | 1.09 | 0.88 | 0.9 | 1.06 |
| Glucose | Carbohydrates and related | 0.68 | 0.59 | 0.68 | 0.68 | 0.91 | 0.72 | 0.81 | 1.06 |
| Xylitol | Carbohydrates and related | 0.69 | 0.4 | 0.69 | 0.60 | 0.93 | 0.62 | 0.52 | 1.03 |
| Taurocholic acid | Complex lipids, fatty acids and related | 4.45 | 6.15 | 4.22 | 0.51 | 1.67 | 1.41 | 0.95 | 1.41 |
| Docosapentaenoic acid (C22:cis [7,10,13,16,19]5) | Complex lipids, fatty acids and related | 0.86 | 0.65 | 0.58 | 1.13 | 0.8 | 0.67 | 0.64 | 0.49 |
| Lysophosphatidylcholine (C20:4) | Complex lipids, fatty acids and related | 0.94 | 0.77 | 0.84 | 1.13 | 0.95 | 0.9 | 0.85 | 0.84 |
| Pyruvate | Energy metabolism and related | 1.5 | 2.23 | 1.25 | 1.17 | 2.03 | 2.18 | 1.27 | 1.53 |
| Pyruvate | Energy metabolism and related | 1.52 | 2.26 | 1.06 | 1.43 | 2.49 | 2.87 | 1.32 | 1.62 |
| Lactate | Energy metabolism and related | 0.59 | 0.8 | 0.74 | 0.97 | 0.83 | 0.99 | 0.91 | 1.01 |
| 3-Phosphoglycerate (3-PGA) | Energy metabolism and related | 0.43 | 0.27 | 0.72 | 0.83 | 0.56 | 0.35 | 0.92 | 0.94 |
| Pregnenolone | Hormones, signal substances and related | 0.52 | 0.49 | 0.38 | 0.77 | 1.04 | 0.89 | 1.04 | 0.78 |
| Hippuric acid | Miscellaneous | 0.31 | 0.31 | 0.46 | 1.67 | 0.38 | 0.42 | 0.32 | 0.99 |
| Uric acid | Nucleobases and related | 0.8 | 0.81 | 0.91 | 0.83 | 0.8 | 0.75 | 0.94 | 0.94 |
| Cytosine | Nucleobases and related | 0.78 | 0.81 | 0.85 | 0.97 | 1.03 | 0.93 | 0.95 | 1.06 |
| Uric acid | Nucleobases and related | 0.71 | 0.59 | 0.76 | 0.74 | 0.72 | 0.72 | 0.89 | 0.88 |
| Threonic acid | Vitamins, cofactors and related | 0.5 | 0.58 | 0.71 | 0.84 | 0.68 | 0.68 | 0.84 | 1.11 |
| Phosphatidylcholine (C18:0,C20:3) | | 0.85 | 0.72 | 0.64 | 1.28 | 0.78 | 0.64 | 0.7 | 0.79 |
Table 13.
Doripenem-induced plasma BA fold changes in male (m) Wistar rats (N = 5 per group) dosed with 100 (LD) and 1000 (HD) mg/kg bw/day observed on days 7, 14, and 28 (m7, m14, and m28) and on day 42 (m42) for the doripenem-recovery group. Statistically significant changes (Welch t-test; p-value < 0.05) are shown where red boxes mean a significant increase, yellow boxes mean a significant decrease, and white boxes indicate an insignificant change in the respective plasma metabolites compared to the control group in the respective plasma BAs.
Table 13.
Doripenem-induced plasma BA fold changes in male (m) Wistar rats (N = 5 per group) dosed with 100 (LD) and 1000 (HD) mg/kg bw/day observed on days 7, 14, and 28 (m7, m14, and m28) and on day 42 (m42) for the doripenem-recovery group. Statistically significant changes (Welch t-test; p-value < 0.05) are shown where red boxes mean a significant increase, yellow boxes mean a significant decrease, and white boxes indicate an insignificant change in the respective plasma metabolites compared to the control group in the respective plasma BAs.
| | | Doripenem HD | Doripenem HD Recovery | Doripenem LD | Doripenem LD Recovery |
|---|
| Metabolite | Class | m7 | m14 | m28 | m43 | m7 | m14 | m28 | m43 |
|---|
| Ursodeoxycholic acid | 2° BA | 0.09 | 0.38 | 0.18 | 0.55 | 1.11 | 5.52 | 2.15 | 0.13 |
| Deoxycholic acid | 2° BA | 0 | 0 | 0.01 | 1.28 | 0.01 | 0.02 | 0.01 | 0.32 |
| Cholic acid | 1° BA | 0.03 | 0.06 | 0.03 | 1.13 | 0.77 | 1.5 | 0.6 | 0.09 |
| Chenodeoxycholic acid | 1° BA | 0.05 | 0.05 | 0.05 | 0.91 | 0.55 | 1.12 | 0.56 | 3.64 |
| Taurocholic acid sodium salt | Taurine-conjugated 1° BA | 3.6 | 3.71 | 4.27 | 0.68 | 2.27 | 0.86 | 0.83 | 1.41 |
| Glycolithocholic Acid | Glycine-conjugated 2° BA | 0.08 | 0.24 | 0.23 | 0.69 | 0.2 | 0.24 | 0.12 | 0.21 |
| Taurolithocholic Acid | Taurine-conjugated 2° BA | 0.06 | 0.1 | 0.21 | 1.16 | 0.06 | 0.06 | 0.07 | 0.69 |
| Tauro-b-muricholic Acid | Taurine-conjugated 1° BA | 4.21 | 4.48 | 4.86 | 0.90 | 2.46 | 1.4 | 1.19 | 1.39 |
| Tauroursodeoxycholic Acid | Taurine-conjugated 2° BA | 5.01 | 2.21 | 4.79 | 0.85 | 1.71 | 1.38 | 1.66 | 0.99 |
| a-Muricholic Acid | 1° BA | 0.05 | 0.07 | 0.04 | 2.08 | 0.52 | 2.79 | 0.95 | 0.29 |
| Taurochenodeoxycholate | Taurine-conjugated 1° BA | 3.13 | 3.6 | 3.24 | 0.63 | 2.47 | 1.1 | 1.26 | 0.94 |
| Taurodeoxycholate | Taurine-conjugated 2° BA | 0.02 | 0.02 | 0.03 | 1.39 | 0.02 | 0.02 | 0.02 | 1.15 |
| Glycocholic acid | Glycine-conjugated 1° BA | 0.37 | 0.16 | 0.31 | 1.63 | 0.65 | 0.74 | 0.72 | 0.28 |
| Glycochenodeoxycholic acid | Glycine-conjugated 1° BA | 0.32 | 0.12 | 0.2 | 0.78 | 0.62 | 0.76 | 0.55 | 0.41 |
| Glycodeoxycholate | Glycine-conjugated 2° BA | 0 | 0 | NA | 0.45 | 0 | 0 | 0 | 0.30 |
| Hyodeoxycholic acid | 2° BA | 0.04 | 0 | 0 | 0.48 | 0.01 | 0.01 | 0 | 0.72 |
| o-Muricholic Acid | 2° BA | 0.14 | 0.02 | 0.01 | 0.81 | 0.11 | 0.28 | 0.08 | 0.54 |
Table 14.
Percentages of the total number of significantly changed plasma (in light blue) and fecal (in light orange) metabolites at a p-value < 0.05 for the (A) HD and (B) LD groups of doripenem treatment plus the doripenem-recovery group and (C) HD and (D) LD groups of meropenem treatment in both sexes, respectively.
Table 14.
Percentages of the total number of significantly changed plasma (in light blue) and fecal (in light orange) metabolites at a p-value < 0.05 for the (A) HD and (B) LD groups of doripenem treatment plus the doripenem-recovery group and (C) HD and (D) LD groups of meropenem treatment in both sexes, respectively.
| (A) | Doripenem HD, males | | | Doripenem HD, females | | |
| Plasma | Plasma Recovery | Feces | Feces Recovery | Plasma | Plasma Recovery | Feces | Feces Recovery |
| 7d | 14d | 28d | 42d | 7d | 14d | 28d | 35d | 42d | 7d | 14d | 28d | 42d | 7d | 14d | 28d | 35d | 42d |
| % of total sig. changed metabolites | 20.27 | 16.89 | 19.59 | 7.43 | 47.75 | 48.55 | 49.04 | 18 | 14.95 | 19.59 | 12.16 | 18.92 | 18.58 | 58.54 | 54.5 | 54.5 | 24.76 | 22.5 |
| (B) | Doripenem LD, males | | | Doripenem LD, females | | |
| Plasma | Plasma Recovery | Feces | Feces Recovery | Plasma | Plasma Recovery | Feces | Feces Recovery |
| 7d | 14d | 28d | 42d | 7d | 14d | 28d | 35d | 42d | 7d | 14d | 28d | 42d | 7d | 14d | 28d | 35d | 42d |
| % of total sig. changed metabolites | 14.86 | 14.19 | 15.2 | 18.92 | 42.76 | 46.78 | 40.35 | 12.06 | 6.27 | 16.89 | 14.86 | 10.81 | 7.78 | 54.66 | 49.03 | 36.65 | 18 | 12.54 |
| (C) | Meropenem HD, males | Meropenem HD, females |
| Plasma | Feces | Plasma | Feces |
| 7d | 14d | 28d | 7d | 14d | 28d | 7d | 14d | 28d | 7d | 14d | 28d |
| % of total sig. changed metabolites | 10.54 | 11.9 | 14.97 | 44.69 | 50.48 | 47.27 | 15.31 | 10.2 | 13.6 | 56.91 | 43.25 | 42.93 |
| (D) | Meropenem LD, males | Meropenem LD, females |
| Plasma | Feces | Plasma | Feces |
| 7d | 14d | 28d | 7d | 14d | 28d | 7d | 14d | 28d | 7d | 14d | 28d |
| % of total sig. changed metabolites | 6.8 | 7.82 | 12.24 | 46.46 | 34.73 | 32.96 | 9.86 | 12.24 | 8.5 | 49.36 | 45.18 | 31.51 |