Microbiome of High-Rank Coal Reservoirs in the High-Production Areas of the Southern Qinshui Basin
Abstract
1. Introduction
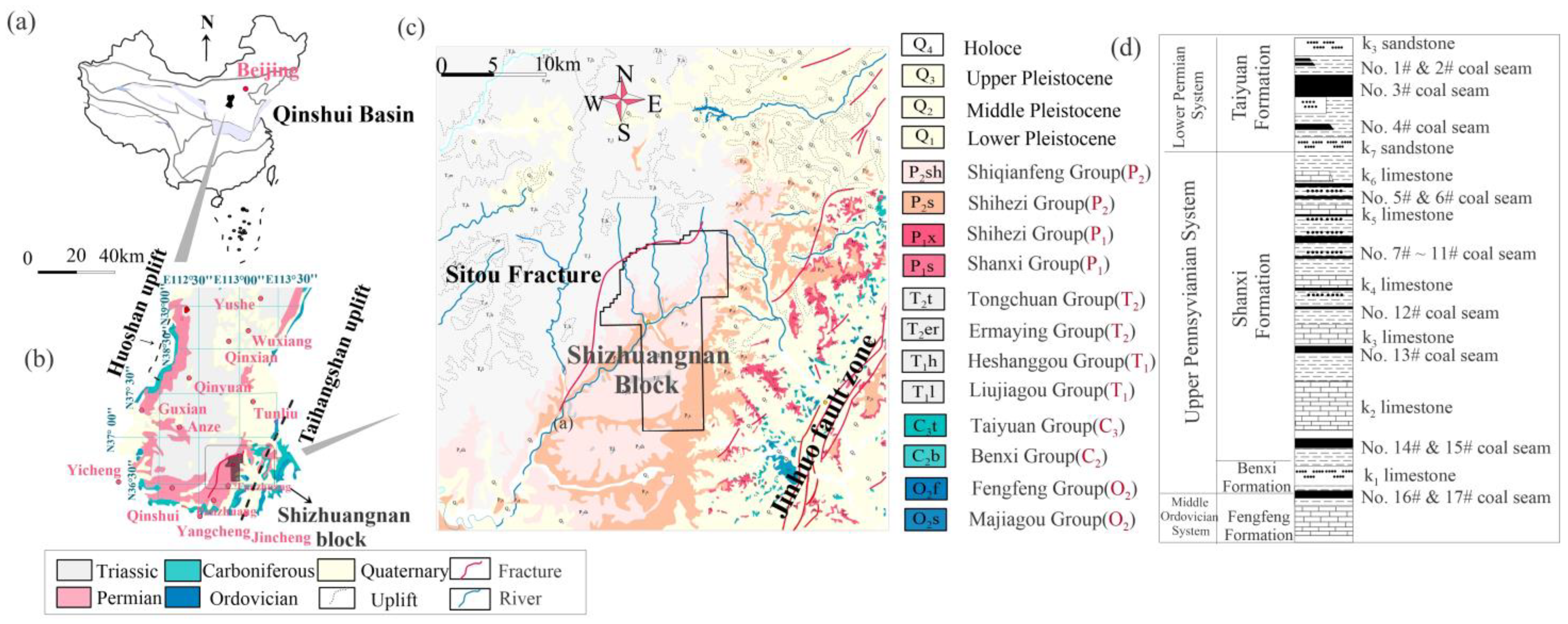
2. Geological and Hydrogeological Conditions
2.1. Main Strata
2.2. Physical Properties of Coal Reservoirs
2.3. Gas Characteristics of the Coal Seam
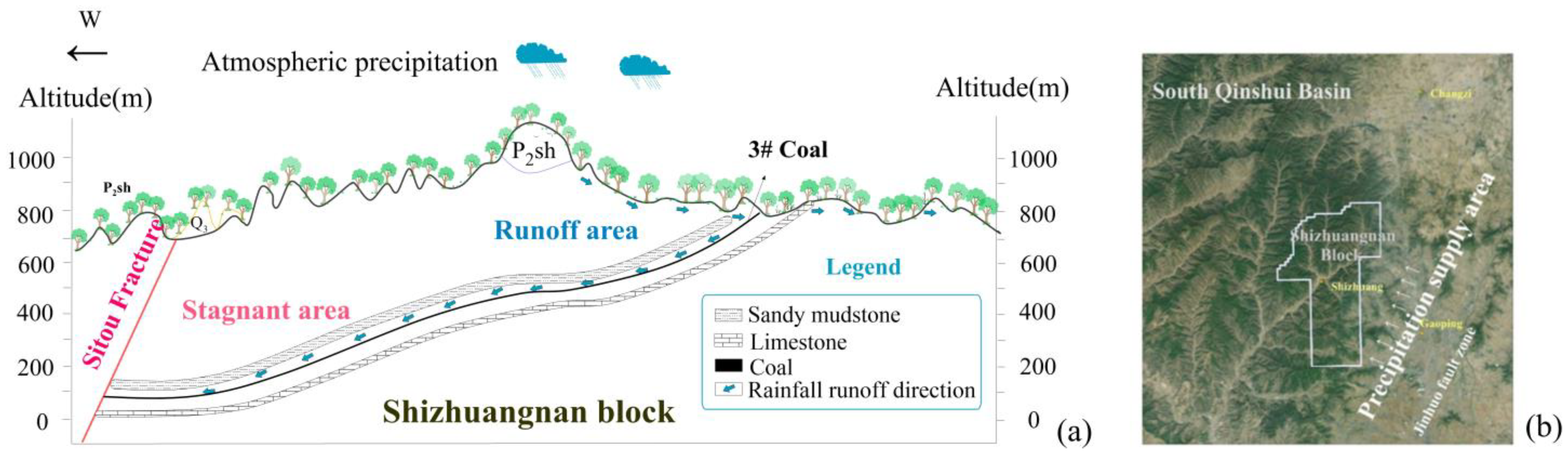
2.4. Hydrogeological Characteristics
3. Materials and Methods
3.1. Sample Collection
3.2. Gene Sequencing Analysis
3.3. Metagenome Sequencing Analysis
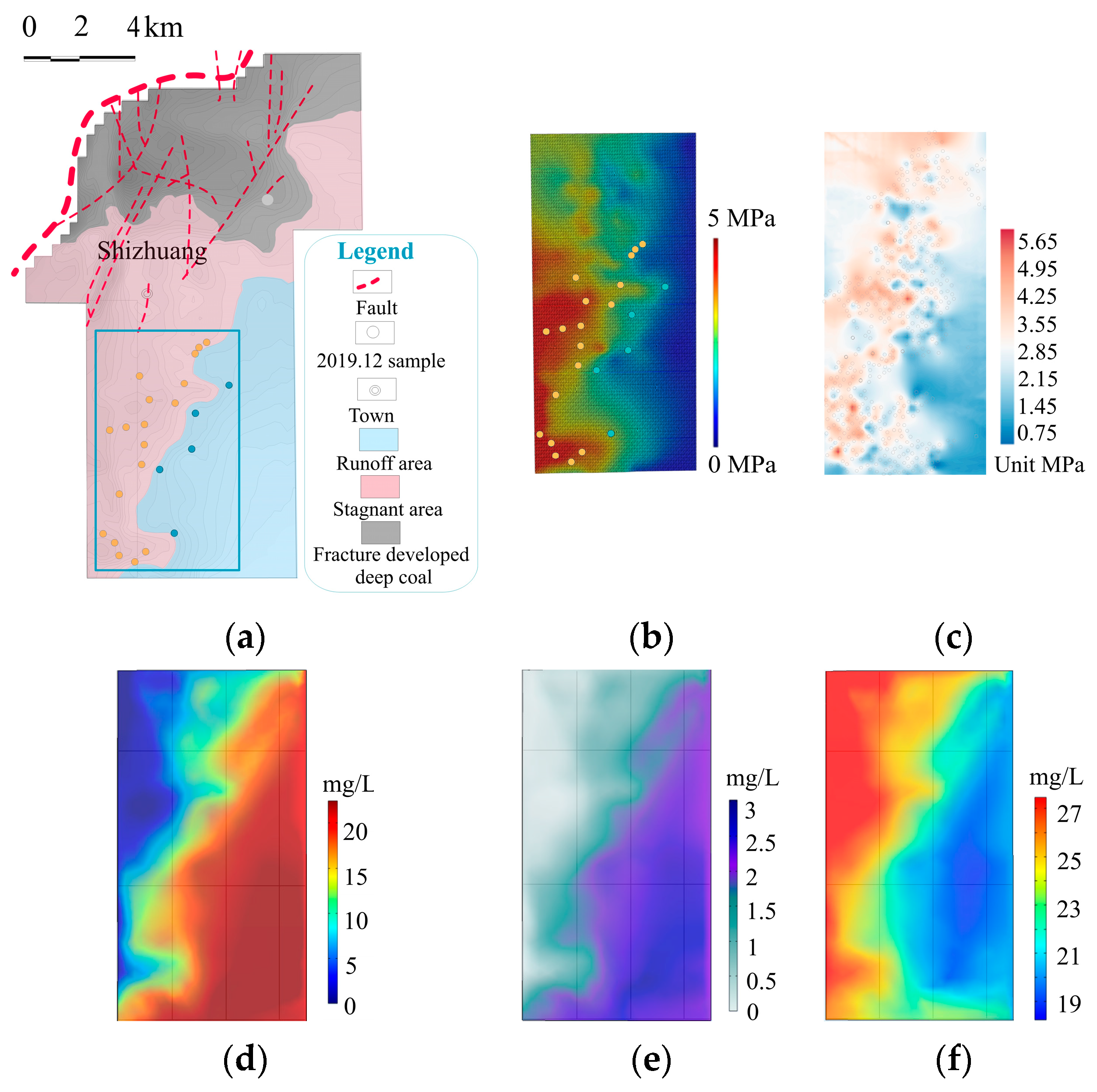
3.4. Hydrodynamic Field Modeling
4. Results
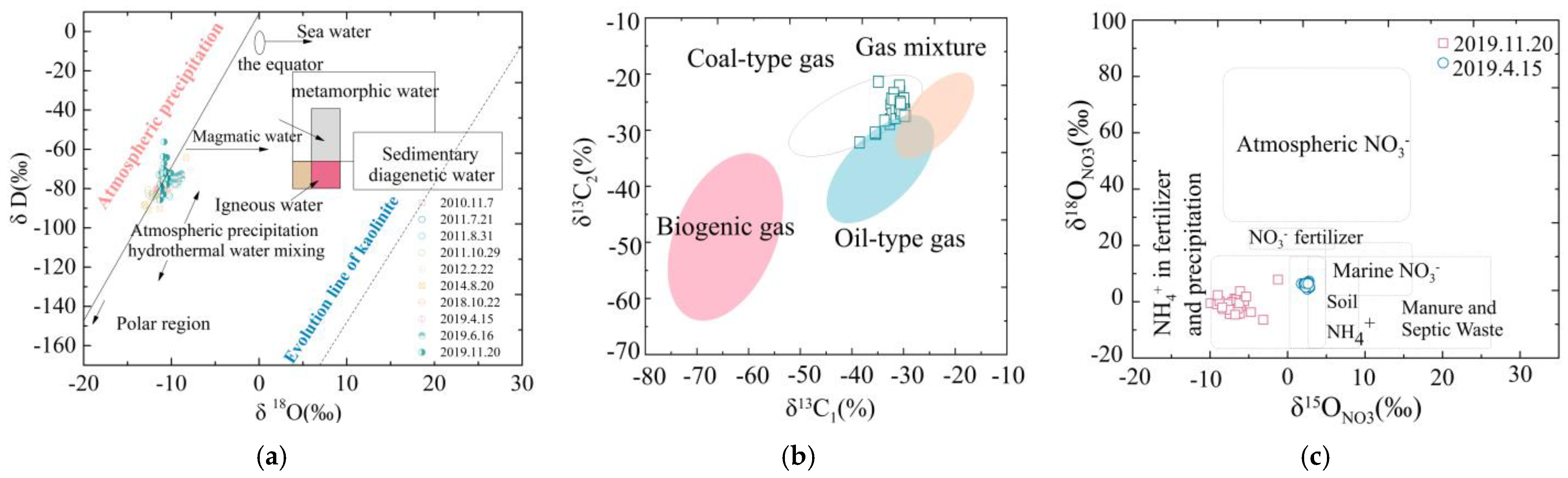
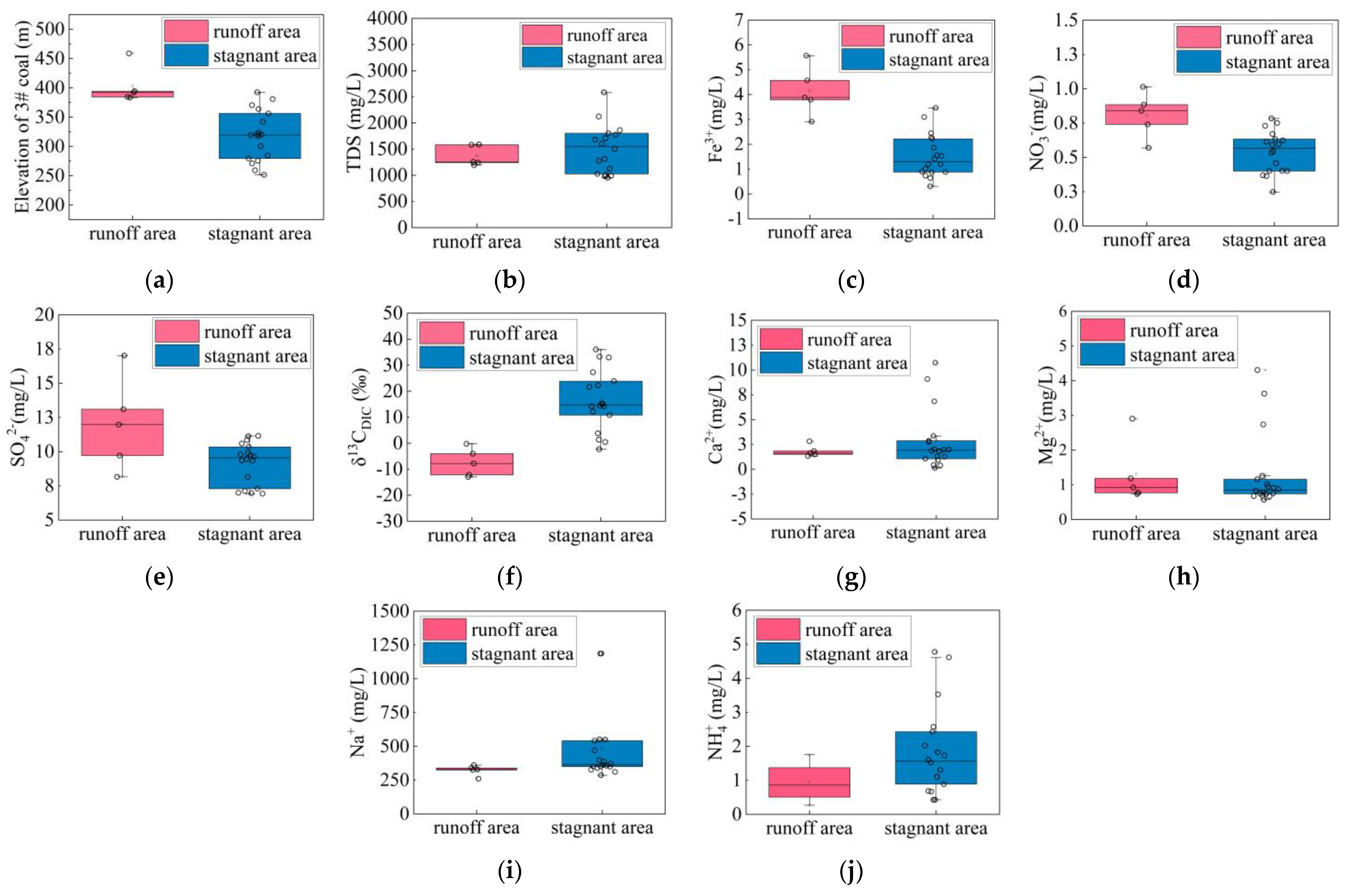
4.1. Geochemical Results
4.2. Runoff and Stagnant Area Division
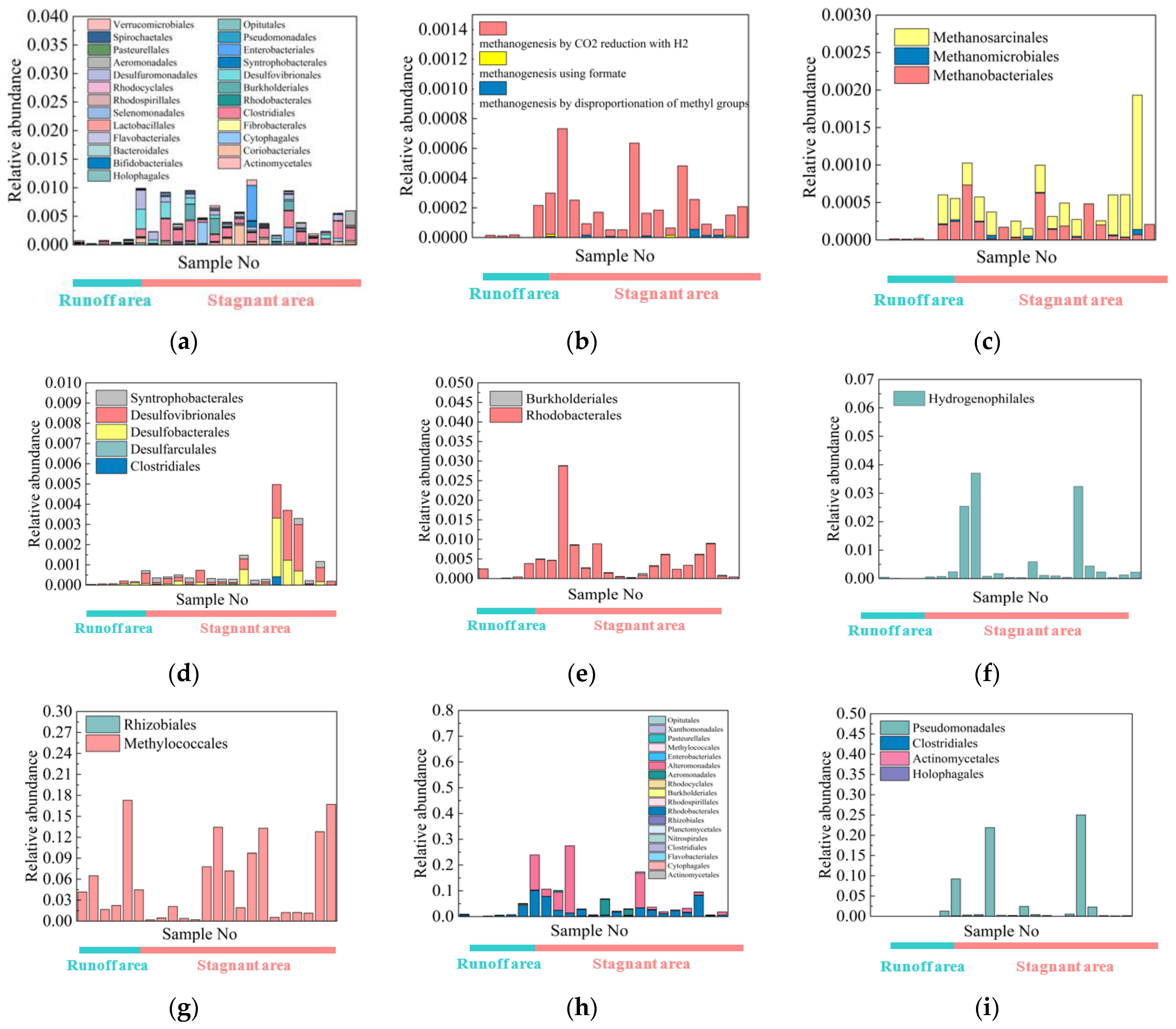
4.3. Microbial Community Structure
4.4. C-N-S-Cycling Species
5. Discussion
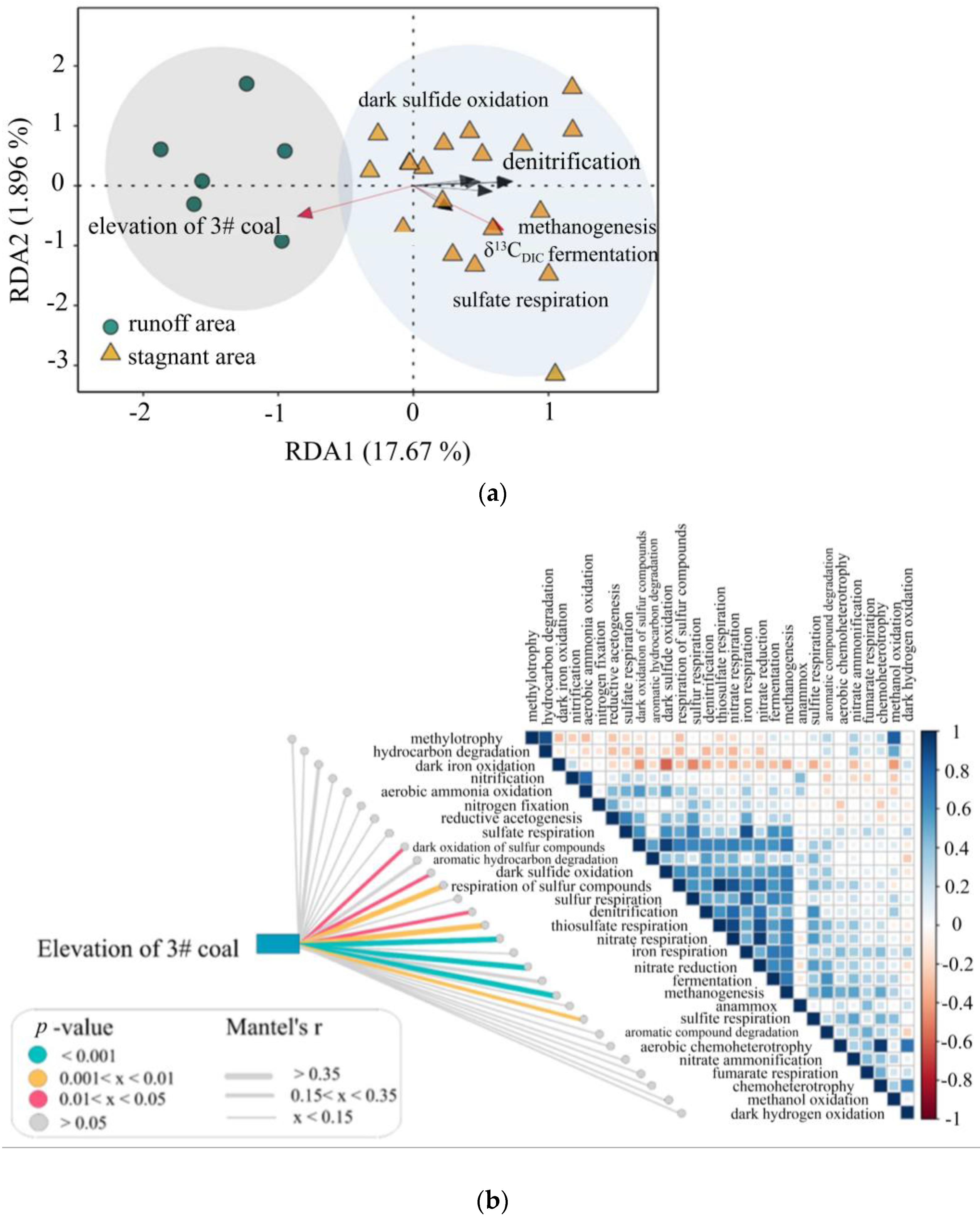
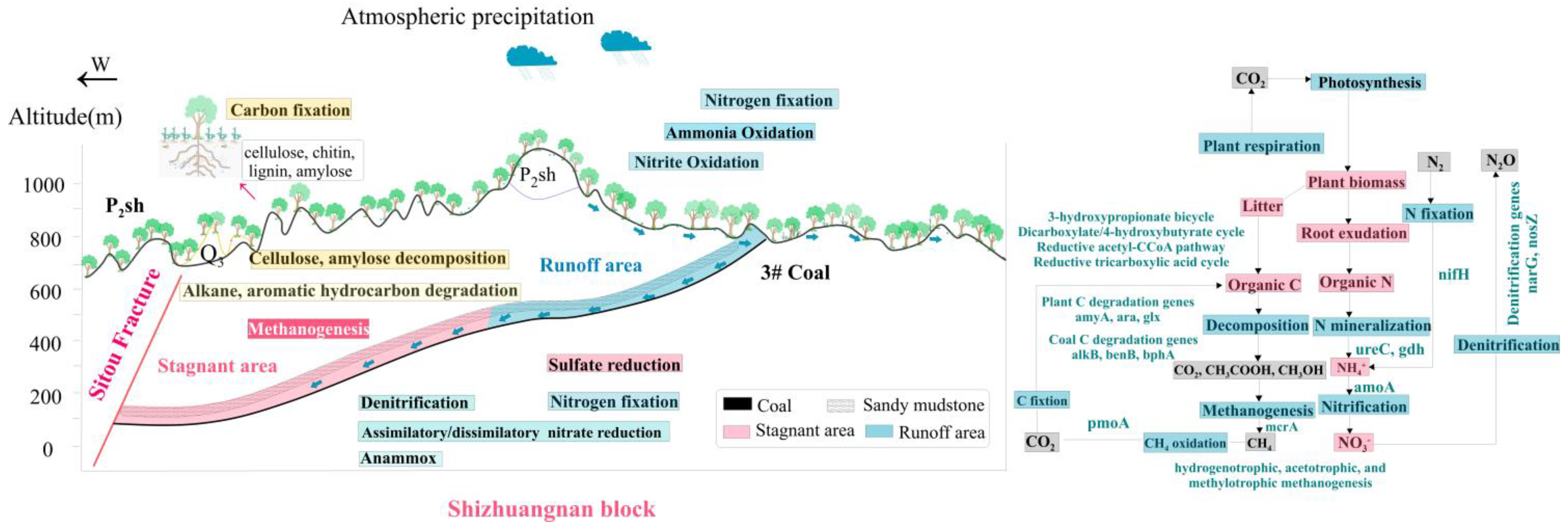
5.1. Hydrochemical Mechanism in the Study Area
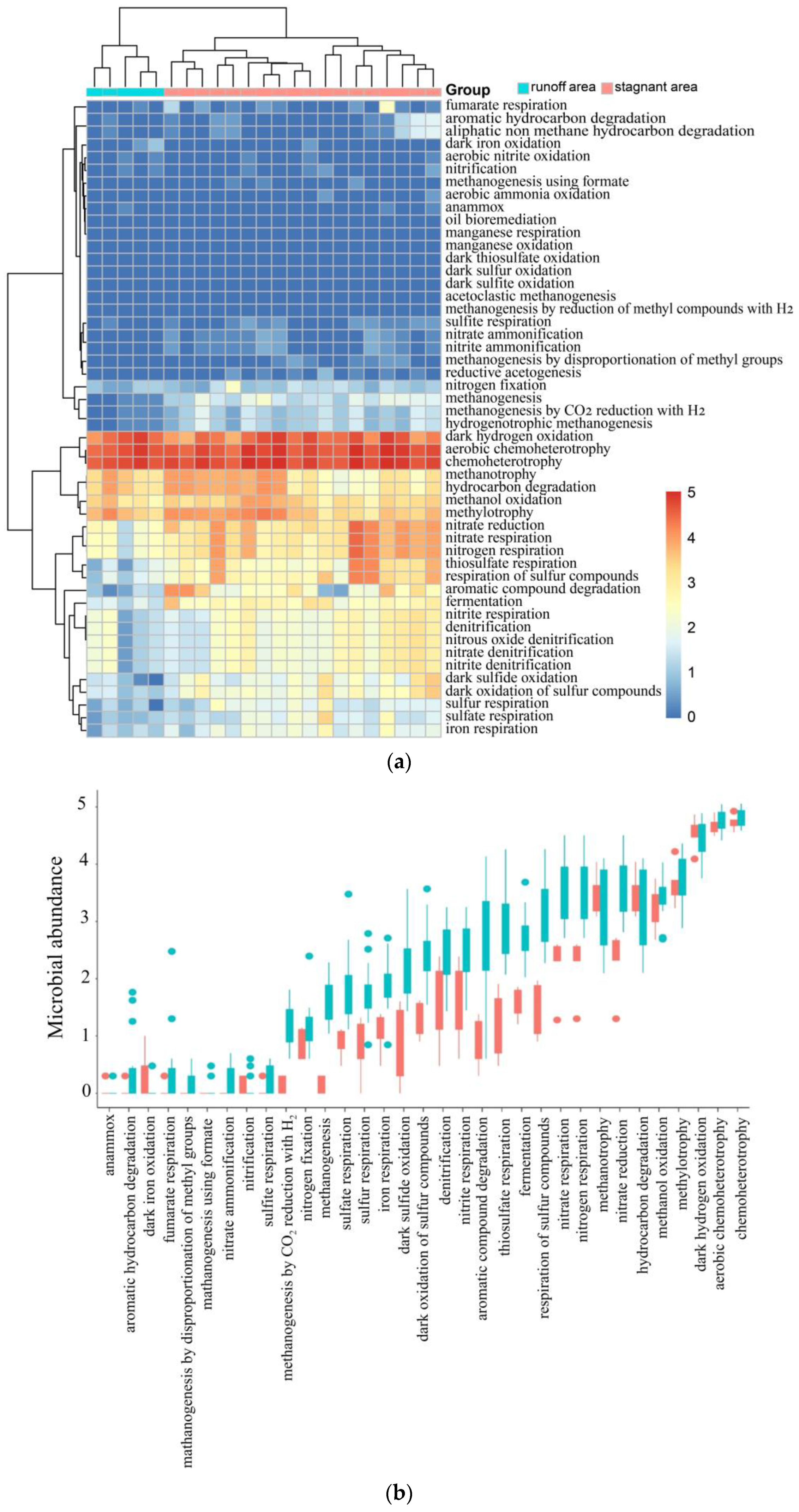
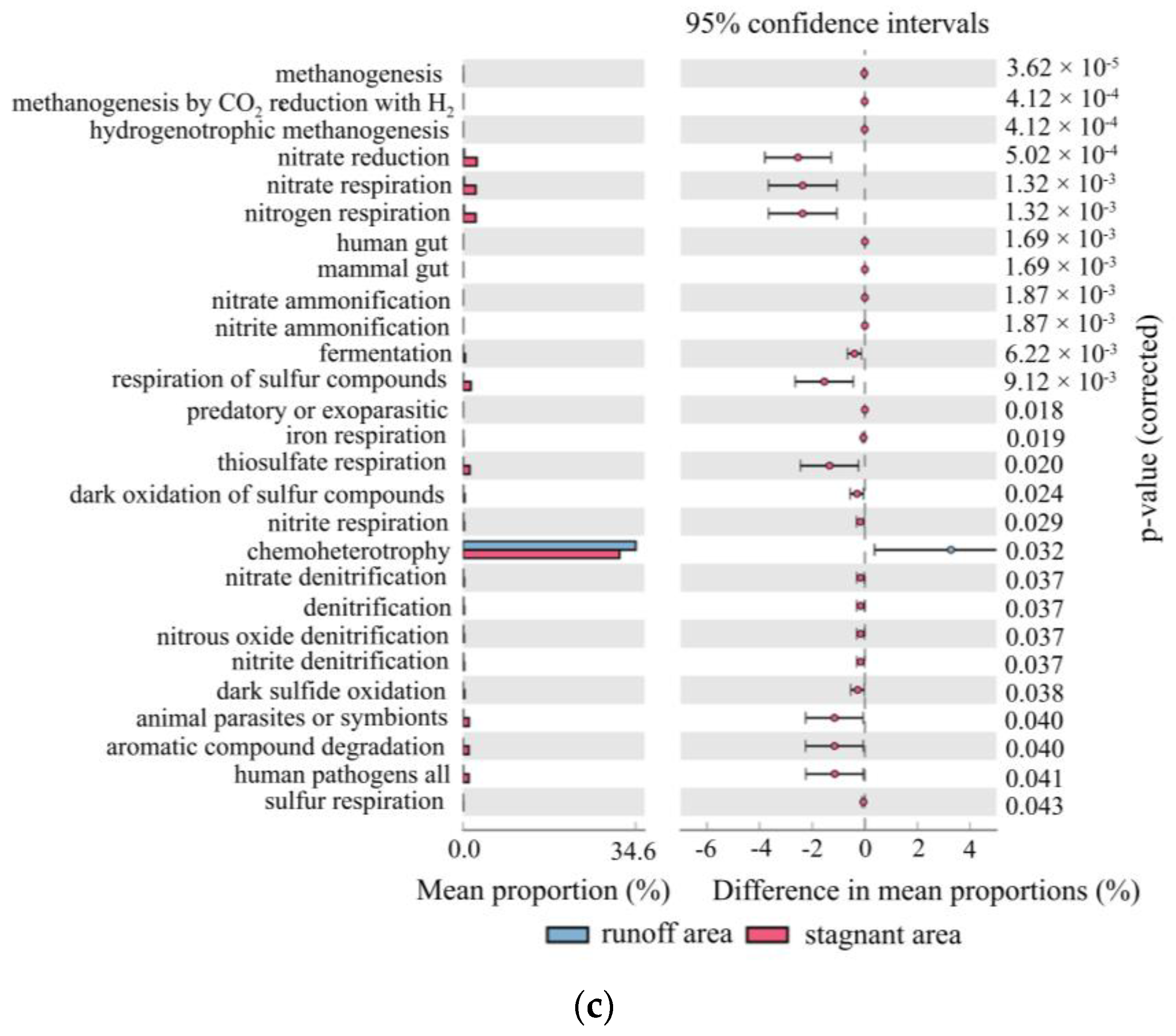
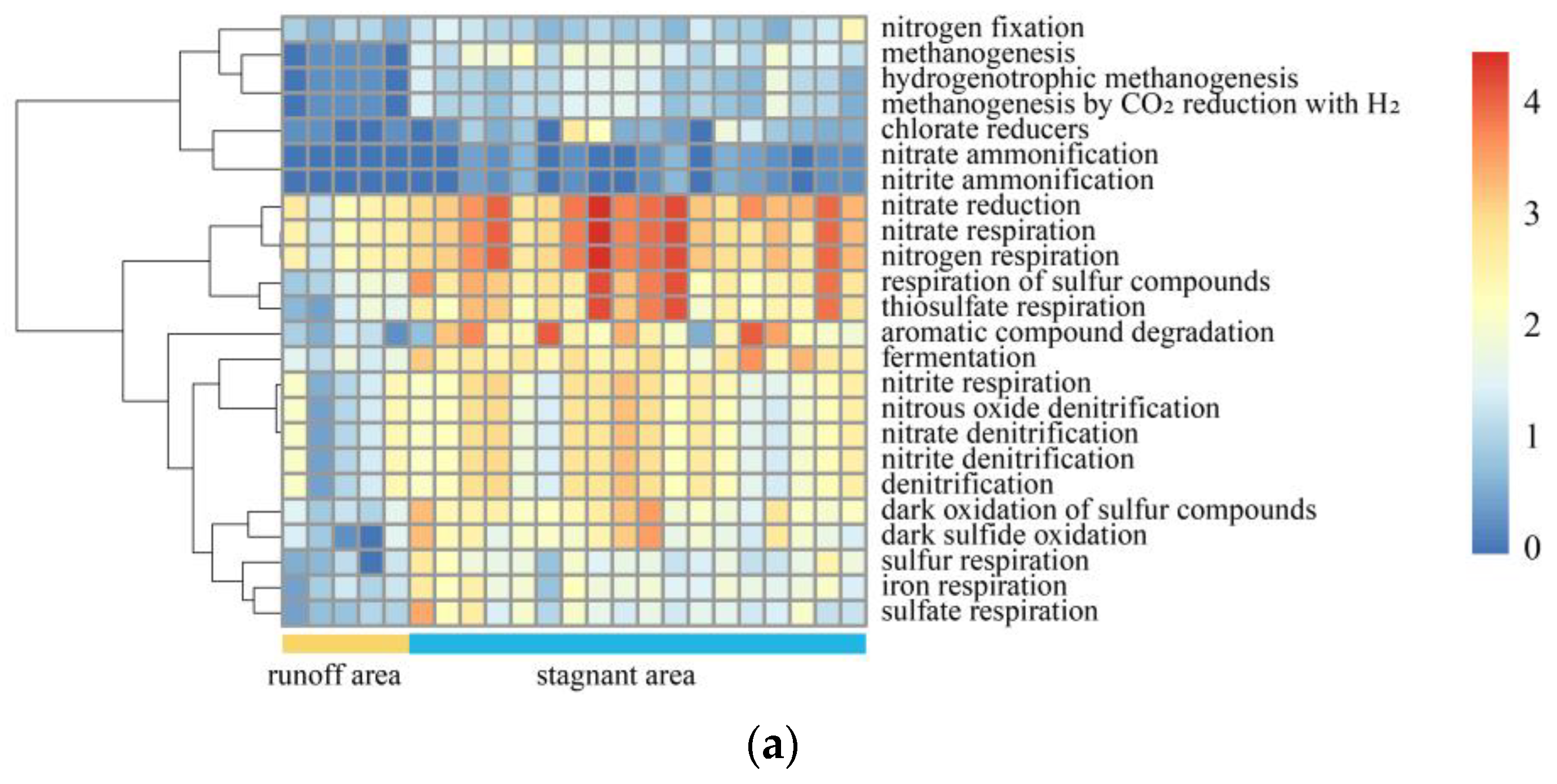
5.2. Metagenome Sequencing Results Verified the C-N-S Microbial Differences between the Stagnant Areas and Runoff Areas
5.3. Major Environmental Attributes Shaping Microbial Community Functional Structure
5.4. Degradation Mechanism of Organic Matter and Biogenic Methane
5.5. N Cycling: The Mechanism of N Cycling in Microorganisms
5.6. Relationship of C-N-S Functional Microorganisms
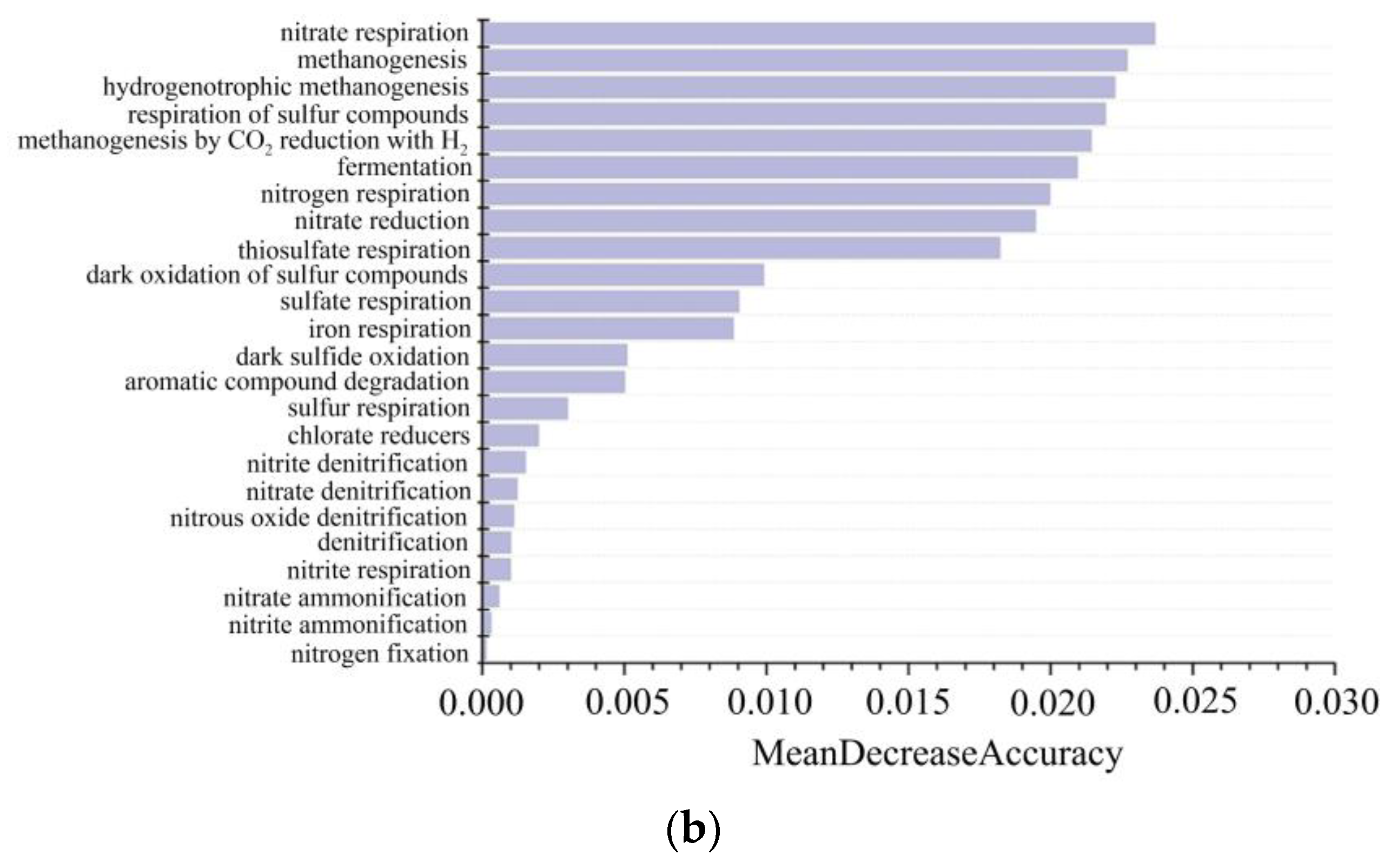
5.7. Machine Learning Classification of C-N-S Microorganisms
5.8. Indication of Microbial Sequencing in the Process of Coalbed Methane Exploration and Development
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, Z.; Qin, Y.; Wu, C.; Qin, Z.; Li, G.; Li, C. Geochemical response of produced water in the CBM well group with multiple coal seams and its geological significance-A case study of the Songhe well group in Western Guizhou. Int. J. Coal Geol. 2019, 207, 39–51. [Google Scholar] [CrossRef]
- Yang, Z.; Qin, Y.; Qin, Z.; Yi, T.; Li, C.; Zhang, Z. Characteristics of dissolved inorganic carbon in produced water from coalbed methane wells and its geological significance. Pet. Explor. Dev. 2020, 47, 1074–1083. [Google Scholar] [CrossRef]
- Chen, S.; Tao, S.; Tian, W.; Tang, D.; Zhang, B.; Liu, P. Hydrogeological control on the accumulation and production of coalbed methane in the Anze Block, southern Qinshui Basin, China. J. Pet. Sci. Eng. 2021, 198, 108138. [Google Scholar] [CrossRef]
- Guo, C.; Qin, Y.; Xia, Y.; Ma, D.; Han, D.; Chen, Y.; Chen, W.; Jian, K.; Lu, L. Geochemical characteristics of water produced from CBM wells and implications for commingling CBM production: A case study of the Bide-Santang Basin, western Guizhou, China. J. Pet. Sci. Eng. 2017, 159, 666–678. [Google Scholar] [CrossRef]
- Song, Y.; Liu, H.; Hong, F.; Qin, S.; Liu, S.; Li, G.; Zhao, M. Syncline reservoir pooling as a general model for coalbed methane (CBM) accumulations: Mechanisms and case studies. J. Pet. Sci. Eng. 2012, 88–89, 5–12. [Google Scholar] [CrossRef]
- Wang, Q.; Su, X.; Su, L.; Zhou, F. CBM geological characteristics and exploration potential in the Sunan Syncline block, southern north China basin. J. Pet. Sci. Eng. 2020, 186, 106713. [Google Scholar] [CrossRef]
- Strapoc, D.; Picardal, F.W.; Turich, C.; Schaperdoth, I.; Macalady, J.L.; Lipp, J.S.; Lin, Y.S.; Ertefai, T.F.; Schubotz, F.; Hinrichs, K.U.; et al. Methane-producing microbial community in a coal bed of the Illinois Basin. Appl. Environ. Microbiol. 2008, 74, 2424–2432. [Google Scholar] [CrossRef]
- Shimizu, S.; Akiyama, M.; Naganuma, T.; Fujioka, M.; Nako, M.; Ishijima, Y. Molecular characterization of microbial communities in deep coal seam groundwater of northern Japan. Geobiology 2007, 5, 423–433. [Google Scholar] [CrossRef]
- Guo, H.; Liu, R.; Yu, Z.; Zhang, H.; Yun, J.; Li, Y.; Liu, X.; Pan, J. Pyrosequencing reveals the dominance of methylotrophic methanogenesis in a coal bed methane reservoir associated with Eastern Ordos Basin in China. Int. J. Coal Geol. 2012, 93, 56–61. [Google Scholar] [CrossRef]
- Midgley, D.J.; Hendry, P.; Pinetown, K.L.; Fuentes, D.; Gong, S.; Mitchell, D.L.; Faiz, M. Characterisation of a microbial community associated with a deep, coal seam methane reservoir in the Gippsland Basin, Australia. Int. J. Coal Geol. 2010, 82, 232–239. [Google Scholar] [CrossRef]
- Shi, W.; Tang, S.H.; Huang, W.H.; Zhang, S.H.; Li, Z.C. Distribution Characteristics of C-N-S Microorganism Genes in Different Hydraulic Zones of High-Rank Coal Reservoirs in Southern Qinshui Basin. ACS Omega 2021, 6, 21395–21409. [Google Scholar] [CrossRef]
- Tang, Y.-Q.; Ji, P.; Lai, G.-L.; Chi, C.-Q.; Liu, Z.-S.; Wu, X.-L. Diverse microbial community from the coalbeds of the Ordos Basin, China. Int. J. Coal Geol. 2012, 90–91, 21–33. [Google Scholar] [CrossRef]
- Wei, M.; Yu, Z.; Jiang, Z.; Zhang, H. Microbial diversity and biogenic methane potential of a thermogenic-gas coal mine. Int. J. Coal Geol. 2014, 134–135, 96–107. [Google Scholar] [CrossRef]
- Guo, H.; Yu, Z.; Zhang, H. Phylogenetic diversity of microbial communities associated with coalbed methane gas from Eastern Ordos Basin, China. Int. J. Coal Geol. 2015, 150–151, 120–126. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, H.; Wang, H.; Urynowicz, M.A.; Hu, A.; Yu, C.-P.; Fallgren, P.; Jin, S.; Zheng, H.; Zeng, R.J.; et al. Enhanced production of secondary biogenic coalbed natural gas from a subbituminous coal treated by hydrogen peroxide and its geochemical and microbiological analyses. Fuel 2019, 236, 1345–1355. [Google Scholar] [CrossRef]
- Pashin, J.C.; McIntyre-Redden, M.R.; Mann, S.D.; Kopaska-Merkel, D.C.; Varonka, M.; Orem, W. Relationships between water and gas chemistry in mature coalbed methane reservoirs of the Black Warrior Basin. Int. J. Coal Geol. 2014, 126, 92–105. [Google Scholar] [CrossRef]
- Schweitzer, H.; Ritter, D.; McIntosh, J.; Barnhart, E.; Cunningham, A.; Vinson, D.; Orem, W.; Fields, M.W. Changes in microbial communities and associated water and gas geochemistry across a sulfate gradient in coal beds: Powder River Basin, USA. Geochim. Cosmochim. Acta 2019, 245, 495–513. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, Y.N.; Yau, P.M.; Pandey, R.; Harpalani, S. A metaproteomic approach for identifying proteins in anaerobic bioreactors converting coal to methane. Int. J. Coal Geol. 2015, 146, 91–103. [Google Scholar] [CrossRef]
- Wong, H.L.; White, R.A.; Visscher, P.T.; Charlesworth, J.C.; Vazquez-Campos, X.; Burns, B.P. Disentangling the drivers of functional complexity at the metagenomic level in Shark Bay microbial mat microbiomes. ISME J. 2018, 12, 2619–2639. [Google Scholar] [CrossRef]
- Wang, B.; Sun, F.J.; Tang, D.Z.; Zhao, Y.; Song, Z.H.; Tao, Y. Hydrological control rule on coalbed methane enrichment and high yield in FZ Block of Qinshui Basin. Fuel 2015, 140, 568–577. [Google Scholar] [CrossRef]
- Zhang, S.H.; Tang, S.H.; Li, Z.C.; Pan, Z.J.; Shi, W. Study of hydrochemical characteristics of CBM co-produced water of the Shizhuangnan Block in the southern Qinshui Basin, China, on its implication of CBM development. Int. J. Coal Geol. 2016, 159, 169–182. [Google Scholar] [CrossRef]
- Zhang, S.H.; Tang, S.H.; Li, Z.C.; Guo, Q.L.; Pan, Z.J. Stable isotope characteristics of CBM co-produced water and implications for CBM development: The example of the Shizhuangnan block in the southern Qinshui Basin, China. J. Nat. Gas Sci. Eng. 2015, 27, 1400–1411. [Google Scholar] [CrossRef]
- Flores, R.M.; Rice, C.A.; Stricker, G.D.; Warden, A.; Ellis, M.S. Methanogenic pathways of coal-bed gas in the Powder River Basin, United States: The geologic factor. Int. J. Coal Geol. 2008, 76, 52–75. [Google Scholar] [CrossRef]
- Bucha, M.; Detman, A.; Pleśniak, Ł.; Drzewicki, W.; Kufka, D.; Chojnacka, A.; Mielecki, D.; Krajniak, J.; Jędrysek, M.O.; Sikora, A.; et al. Microbial methane formation from different lithotypes of Miocene lignites from the Konin Basin, Poland: Geochemistry of the gases and composition of the microbial communities. Int. J. Coal Geol. 2020, 229, 103558. [Google Scholar] [CrossRef]
- Penner, T.J.; Foght, J.M.; Budwill, K. Microbial diversity of western Canadian subsurface coal beds and methanogenic coal enrichment cultures. Int. J. Coal Geol. 2010, 82, 81–93. [Google Scholar] [CrossRef]
- Berlendis, S.; Beyssac, O.; Derenne, S.; Benzerara, K.; Anquetil, C.; Guillaumet, M.; Estève, I.; Capelle, B. Comparative mineralogy, organic geochemistry and microbial diversity of the Autun black shale and Graissessac coal (France). Int. J. Coal Geol. 2014, 132, 147–157. [Google Scholar] [CrossRef]
- Zerkle, A.L.; Kamyshny, A.; Kump, L.R.; Farquhar, J.; Oduro, H.; Arthur, M.A. Sulfur cycling in a stratified euxinic lake with moderately high sulfate: Constraints from quadruple S isotopes. Geochim. Cosmochim. Acta 2010, 74, 4953–4970. [Google Scholar] [CrossRef]
- Zopfi, J.; Bttcher, M.E.; Jorgensen, B.B. Biogeochemistry of sulfur and iron in Thioploca-colonized surface sediments in the upwelling area off central chile. Geochim. Cosmochim. Acta 2008, 72, 827–843. [Google Scholar] [CrossRef]
- Li, X.N.; Gilhooly, W.P.; Zerkle, A.L.; Lyons, T.W.; Farquhar, J.; Werne, J.P.; Varela, R.; Scranton, M.I. Stable sulfur isotopes in the water column of the Cariaco Basin. Geochim. Cosmochim. Acta 2010, 74, 6764–6778. [Google Scholar] [CrossRef]
- Treude, T.; Krause, S.; Maltby, J.; Dale, A.W.; Coffin, R.; Hamdan, L.J. Sulfate reduction and methane oxidation activity below the sulfate-methane transition zone in Alaskan Beaufort Sea continental margin sediments: Implications for deep sulfur cycling. Geochim. Cosmochim. Acta 2014, 144, 217–237. [Google Scholar] [CrossRef]
- Gomes, M.L.; Johnston, D.T. Oxygen and sulfur isotopes in sulfate in modern euxinic systems with implications for evaluating the extent of euxinia in ancient oceans. Geochim. Cosmochim. Acta 2017, 205, 331–359. [Google Scholar] [CrossRef]
- Wang, B.; Yu, Z.; Zhang, Y.; Zhang, H. Microbial communities from the Huaibei Coalfield alter the physicochemical properties of coal in methanogenic bioconversion. Int. J. Coal Geol. 2019, 202, 85–94. [Google Scholar] [CrossRef]
- Barnhart, E.P.; Weeks, E.P.; Jones, E.J.P.; Ritter, D.J.; McIntosh, J.C.; Clark, A.C.; Ruppert, L.F.; Cunningham, A.B.; Vinson, D.S.; Orem, W.; et al. Hydrogeochemistry and coal-associated bacterial populations from a methanogenic coal bed. Int. J. Coal Geol. 2016, 162, 14–26. [Google Scholar] [CrossRef]
- Detman, A.; Bucha, M.; Simoneit, B.R.T.; Mielecki, D.; Piwowarczyk, C.; Chojnacka, A.; Błaszczyk, M.K.; Jędrysek, M.O.; Marynowski, L.; Sikora, A. Lignite biodegradation under conditions of acidic molasses fermentation. Int. J. Coal Geol. 2018, 196, 274–287. [Google Scholar] [CrossRef]
- Kroeger, M.E.; Meredith, L.K.; Meyer, K.M.; Webster, K.D.; de Camargo, P.B.; de Souza, L.F.; Tsai, S.M.; van Haren, J.; Saleska, S.; Bohannan, B.J.M.; et al. Rainforest-to-pasture conversion stimulates soil methanogenesis across the Brazilian Amazon. ISME J. 2021, 15, 658–672. [Google Scholar] [CrossRef]
- Dekas, A.E.; Connon, S.A.; Chadwick, G.L.; Trembath-Reichert, E.; Orphan, V.J. Activity and interactions of methane seep microorganisms assessed by parallel transcription and FISH-NanoSIMS analyses. ISME J. 2016, 10, 678–692. [Google Scholar] [CrossRef]
- Boyd, J.A.; Jungbluth, S.P.; Leu, A.O.; Evans, P.N.; Woodcroft, B.J.; Chadwick, G.L.; Orphan, V.J.; Amend, J.P.; Rappe, M.S.; Tyson, G.W. Divergent methyl-coenzyme M reductase genes in a deep-subseafloor Archaeoglobi. ISME J. 2019, 13, 1269–1279. [Google Scholar] [CrossRef]
- Orem, W.; Tatu, C.; Varonka, M.; Lerch, H.; Bates, A.; Engle, M.; Crosby, L.; McIntosh, J. Organic substances in produced and formation water from unconventional natural gas extraction in coal and shale. Int. J. Coal Geol. 2014, 126, 20–31. [Google Scholar] [CrossRef]
- Formolo, M.; Martini, A.; Petsch, S. Biodegradation of sedimentary organic matter associated with coalbed methane in the Powder River and San Juan Basins, U.S.A. Int. J. Coal Geol. 2008, 76, 86–97. [Google Scholar] [CrossRef]
- Schlegel, M.E.; McIntosh, J.C.; Bates, B.L.; Kirk, M.F.; Martini, A.M. Comparison of fluid geochemistry and microbiology of multiple organic-rich reservoirs in the Illinois Basin, USA: Evidence for controls on methanogenesis and microbial transport. Geochim. Cosmochim. Acta 2011, 75, 1903–1919. [Google Scholar] [CrossRef]
- Barnhart, E.P.; De Leon, K.B.; Ramsay, B.D.; Cunningham, A.B.; Fields, M.W. Investigation of coal-associated bacterial and archaeal populations from a diffusive microbial sampler (DMS). Int. J. Coal Geol. 2013, 115, 64–70. [Google Scholar] [CrossRef]
- Xu, H.; Tang, D.Z.; Tang, S.H.; Zhang, W.Z.; Meng, Y.J.; Gao, L.J.; Xie, S.Z.; Zhao, J.L. Geologic and hydrological controls on coal reservoir water production in marine coal-bearing strata: A case study of the Carboniferous Taiyuan Formation in the Liulin area, eastern Ordos Basin, China. Mar. Pet. Geol. 2015, 59, 517–526. [Google Scholar] [CrossRef]
- Yao, Y.B.; Liu, D.M.; Yan, T.T. Geological and hydrogeological controls on the accumulation of coalbed methane in the Weibei field, southeastern Ordos Basin. Int. J. Coal Geol. 2014, 121, 148–159. [Google Scholar] [CrossRef]
- Yang, Y.F.; Wu, L.W.; Lin, Q.Y.; Yuan, M.T.; Xu, D.P.; Yu, H.; Hu, Y.G.; Duan, J.C.; Li, X.Z.; He, Z.L.; et al. Responses of the functional structure of soil microbial community to livestock grazing in the Tibetan alpine grassland. Global Change Biol. 2013, 19, 637–648. [Google Scholar] [CrossRef]
- Li, D.J.; Zhou, X.H.; Wu, L.Y.; Zhou, J.Z.; Luo, Y.Q. Contrasting responses of heterotrophic and autotrophic respiration to experimental warming in a winter annual-dominated prairie. Global Change Biol. 2013, 19, 3553–3564. [Google Scholar] [CrossRef]
- Shi, Z.; Lin, Y.; Wilcox, K.R.; Souza, L.; Jiang, L.F.; Jiang, J.; Jung, C.G.; Xu, X.; Yuan, M.T.; Guo, X.; et al. Successional change in species composition alters climate sensitivity of grassland productivity. Glob. Change Biol. 2018, 24, 4993–5003. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.M.M.; Zhang, J.; Xue, K.; Wu, L.Y.; Deng, Y.; Deng, J.; Hale, L.R.; Zhou, X.S.; He, Z.L.; Yang, Y.F.; et al. Microbial functional diversity covaries with permafrost thaw-induced environmental heterogeneity in tundra soil. Global Change Biol. 2018, 24, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.T.; Liang, J.Y.; Hale, L.E.; Jung, C.G.; Chen, J.; Zhou, J.Z.; Xu, M.G.; Yuan, M.T.; Wu, L.Y.; Bracho, R.; et al. Enhanced decomposition of stable soil organic carbon and microbial catabolic potentials by long-term field warming. Global Change Biol. 2017, 23, 4765–4776. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.T.; Zhao, H.H.; Zhang, X.; Zhou, J.Z.; Li, G.H. Contrasting microbial functional genes in two distinct saline-alkali and slightly acidic oil-contaminated sites. Sci. Total Environ. 2014, 487, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Levy-Booth, D.J.; Hashimi, A.; Roccor, R.; Liu, L.Y.; Renneckar, S.; Eltis, L.D.; Mohn, W.W. Genomics and metatranscriptomics of biogeochemical cycling and degradation of lignin-derived aromatic compounds in thermal swamp sediment. ISME J. 2021, 15, 879–893. [Google Scholar] [CrossRef]
- Doud, D.F.R.; Bowers, R.M.; Schulz, F.; De Raad, M.; Deng, K.; Tarver, A.; Glasgow, E.; Vander Meulen, K.; Fox, B.; Deutsch, S.; et al. Function-driven single-cell genomics uncovers cellulose-degrading bacteria from the rare biosphere. ISME J. 2020, 14, 659–675. [Google Scholar] [CrossRef] [PubMed]
- Strąpoć, D.; Mastalerz, M.; Dawson, K.; Macalady, J.; Callaghan, A.V.; Wawrik, B.; Turich, C.; Ashby, M. Biogeochemistry of Microbial Coal-Bed Methane. Annu. Rev. Earth Planet. Sci. 2011, 39, 617–656. [Google Scholar] [CrossRef]
- Van Nostrand, J.D.; He, Z.L.; Zhou, J.Z. Use of functional gene arrays for elucidating in situ biodegradation. Front. Microbiol. 2012, 3, 339. [Google Scholar] [CrossRef] [PubMed]
- Hemme, C.L.; Tu, Q.C.; Shi, Z.; Qin, Y.J.; Gao, W.M.; Deng, Y.; Van Nostrand, J.D.; Wu, L.Y.; He, Z.L.; Chain, P.S.G.; et al. Comparative metagenomics reveals impact of contaminants on groundwater microbiomes. Front. Microbiol. 2015, 6, 1205. [Google Scholar] [CrossRef]
- Shen, C.C.; Shi, Y.; Ni, Y.Y.; Deng, Y.; Van Nostrand, J.D.; He, Z.L.; Zhou, J.Z.; Chu, H.Y. Dramatic Increases of Soil Microbial Functional Gene Diversity at the Treeline Ecotone of Changbai Mountain. Front. Microbiol. 2016, 7, 1184. [Google Scholar] [CrossRef]
- Yu, H.; Deng, Y.; He, Z.L.; Van Nostrand, J.D.; Wang, S.; Jin, D.C.; Wang, A.J.; Wu, L.Y.; Wang, D.H.; Tai, X.; et al. Elevated CO2 and Warming Altered Grassland Microbial Communities in Soil Top-Layers. Front. Microbiol. 2018, 9, 1970. [Google Scholar] [CrossRef]
- Zou, Y.; Ning, D.L.; Huang, Y.; Liang, Y.T.; Wang, H.; Duan, L.; Yuan, T.; He, Z.L.; Yang, Y.F.; Xue, K.; et al. Functional structures of soil microbial community relate to contrasting N2O emission patterns from a highly acidified forest. Sci. Total Environ. 2020, 725, 138504. [Google Scholar] [CrossRef]
- Penton, C.R.; Yang, C.Y.; Wu, L.Y.; Wang, Q.; Zhang, J.; Liu, F.F.; Qin, Y.J.; Deng, Y.; Hemme, C.L.; Zheng, T.L.; et al. NifH-Harboring Bacterial Community Composition across an Alaskan Permafrost Thaw Gradient. Front. Microbiol. 2016, 7, 1894. [Google Scholar] [CrossRef]
- Shen, L.D.; Zheng, P.H.; Ma, S.J. Nitrogen loss through anaerobic ammonium oxidation in agricultural drainage ditches. Biol. Fertil. Soils 2016, 52, 127–136. [Google Scholar] [CrossRef]
- Sun, X.; Frey, C.; Garcia-Robledo, E.; Jayakumar, A.; Ward, B.B. Microbial niche differentiation explains nitrite oxidation in marine oxygen minimum zones. ISME J. 2021, 15, 1317–1329. [Google Scholar] [CrossRef]
- Hu, B.L.; Shen, L.D.; Lian, X.; Zhu, Q.; Liu, S.; Huang, Q.; He, Z.F.; Geng, S.; Cheng, D.Q.; Lou, L.P.; et al. Evidence for nitrite-dependent anaerobic methane oxidation as a previously overlooked microbial methane sink in wetlands. Proc. Natl. Acad. Sci. USA 2014, 111, 4495–4500. [Google Scholar] [CrossRef] [PubMed]
- Wenk, C.B.; Zopfi, J.; Blees, J.; Veronesi, M.; Niemann, H.; Lehmann, M.F. Community N and O isotope fractionation by sulfide-dependent denitrification and anammox in a stratified lacustrine water column. Geochim. Cosmochim. Acta 2014, 125, 551–563. [Google Scholar] [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.D.; Wu, H.S.; Gao, Z.Q.; Xu, X.H.; Chen, T.X.; Liu, S.; Cheng, H.X. Occurrence and importance of anaerobic ammonium-oxidising bacteria in vegetable soils. Appl. Microbiol. Biotechnol. 2015, 99, 5709–5718. [Google Scholar] [CrossRef]
- Hemme, C.L.; Deng, Y.; Gentry, T.J.; Fields, M.W.; Wu, L.Y.; Barua, S.; Barry, K.; Tringe, S.G.; Watson, D.B.; He, Z.L.; et al. Metagenomic insights into evolution of a heavy metal-contaminated groundwater microbial community. ISME J. 2010, 4, 660–672. [Google Scholar] [CrossRef]
- Gulay, A.; Musovic, S.; Albrechtsen, H.J.; Abu Al-Soud, W.; Sorensen, S.J.; Smets, B.F. Ecological patterns, diversity and core taxa of microbial communities in groundwater-fed rapid gravity filters. ISME J. 2016, 10, 2209–2222. [Google Scholar] [CrossRef]
- Guo, X.; Gao, Q.; Yuan, M.T.; Wang, G.S.; Zhou, X.S.; Feng, J.J.; Shi, Z.; Hale, L.R.; Wu, L.W.; Zhou, A.F.; et al. Gene-informed decomposition model predicts lower soil carbon loss due to persistent microbial adaptation to warming. Nat. Commun. 2020, 11, 4897. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, X.S.; Hale, L.; Yuan, M.T.; Ning, D.L.; Feng, J.J.; Shi, Z.; Li, Z.X.; Feng, B.; Gao, Q.; et al. Climate warming accelerates temporal scaling of grassland soil microbial biodiversity. Nat. Ecol. Evol. 2019, 3, 612–619. [Google Scholar] [CrossRef]
- Huang, Z.X.; Sednek, C.; Urynowicz, M.A.; Guo, H.G.; Wang, Q.R.; Fallgren, P.; Jin, S.; Jin, Y.; Igwe, U.; Li, S.P. Low carbon renewable natural gas production from coalbeds and implications for carbon capture and storage. Nat. Commun. 2017, 8, 568. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, W.; Tang, S.; Zhang, S. Microbiome of High-Rank Coal Reservoirs in the High-Production Areas of the Southern Qinshui Basin. Microorganisms 2023, 11, 497. https://doi.org/10.3390/microorganisms11020497
Shi W, Tang S, Zhang S. Microbiome of High-Rank Coal Reservoirs in the High-Production Areas of the Southern Qinshui Basin. Microorganisms. 2023; 11(2):497. https://doi.org/10.3390/microorganisms11020497
Chicago/Turabian StyleShi, Wei, Shuheng Tang, and Songhang Zhang. 2023. "Microbiome of High-Rank Coal Reservoirs in the High-Production Areas of the Southern Qinshui Basin" Microorganisms 11, no. 2: 497. https://doi.org/10.3390/microorganisms11020497
APA StyleShi, W., Tang, S., & Zhang, S. (2023). Microbiome of High-Rank Coal Reservoirs in the High-Production Areas of the Southern Qinshui Basin. Microorganisms, 11(2), 497. https://doi.org/10.3390/microorganisms11020497





