Fatal Puumala Hantavirus Infection in a Patient with Common Variable Immunodeficiency (CVID)
Abstract
1. Introduction
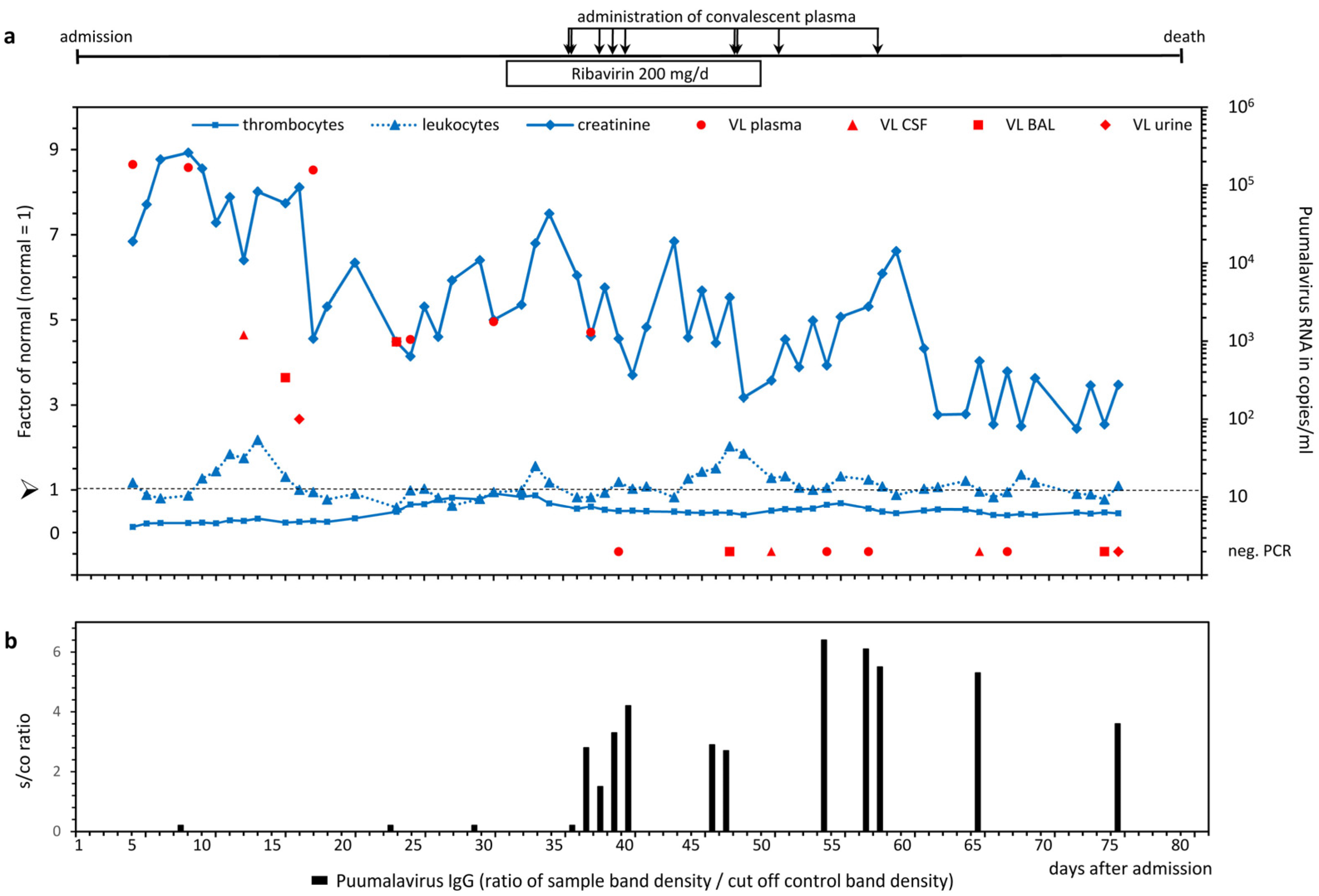
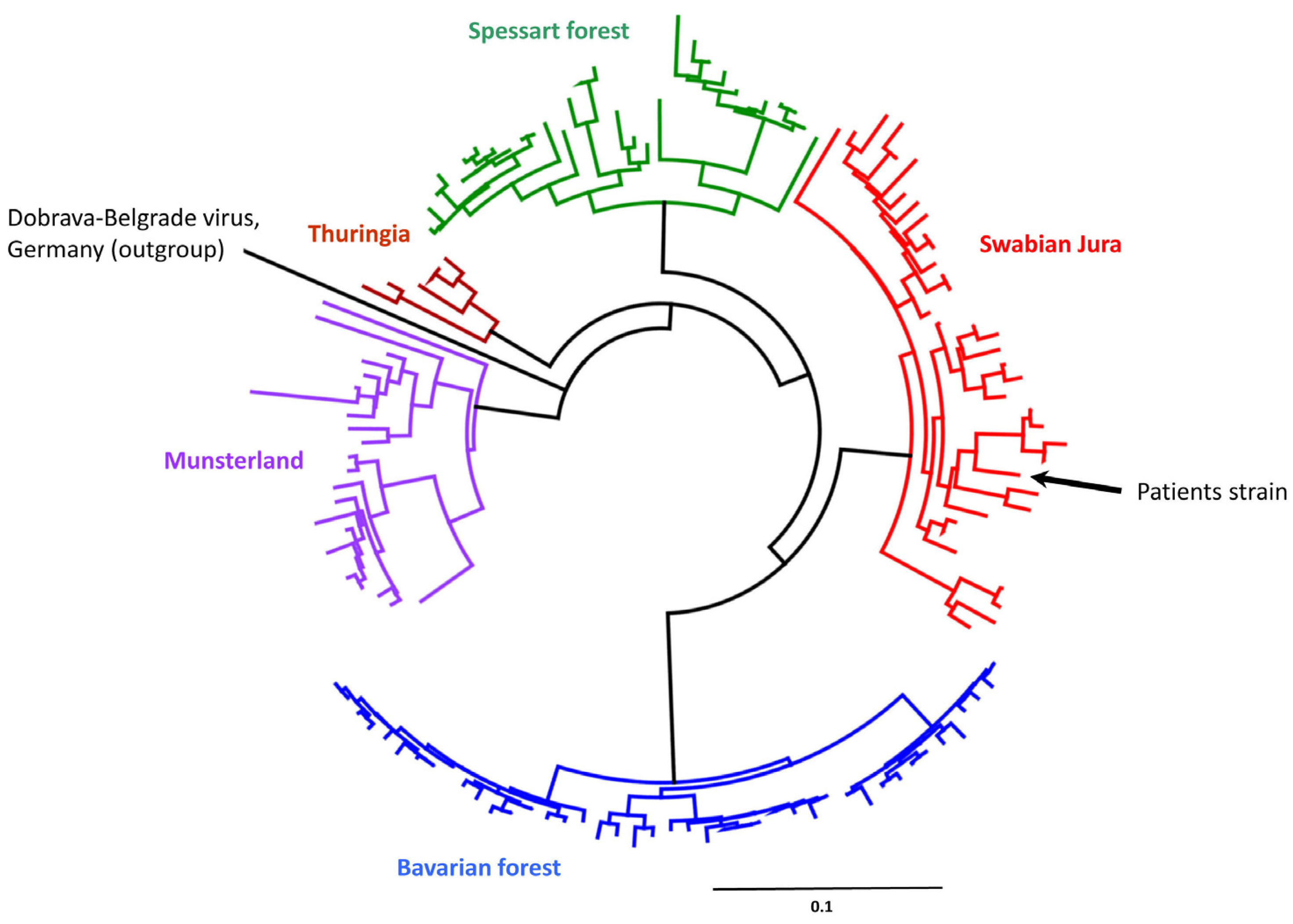
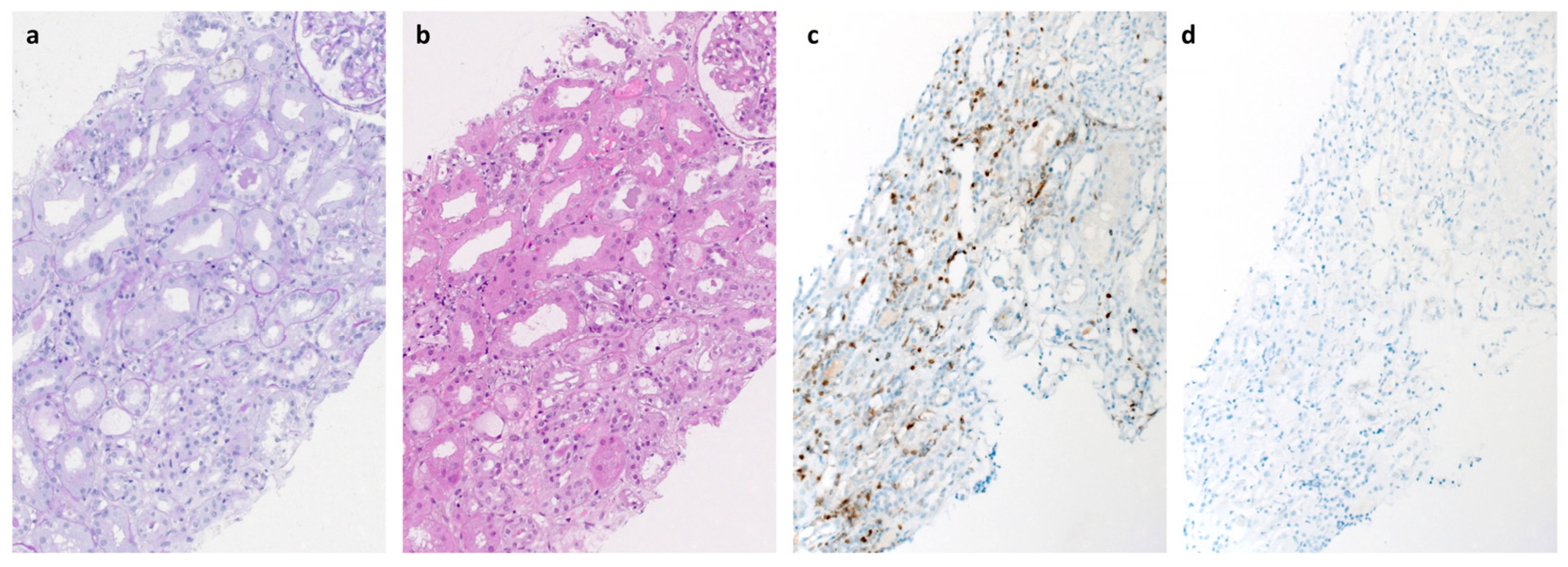
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kruger, D.H.; Figueiredo, L.T.; Song, J.W.; Klempa, B. Hantaviruses—Globally emerging pathogens. J. Clin. Virol. 2015, 64, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.; Kruger, D.H.; Auste, B.; Stark, K.; Hofmann, J.; Weiss, S. Molecular and epidemiological characteristics of human Puumala and Dobrava-Belgrade hantavirus infections, Germany, 2001 to 2017. Euro. Surveill. 2019, 24, 1800675. [Google Scholar] [CrossRef] [PubMed]
- Makary, P.; Kanerva, M.; Ollgren, J.; Virtanen, M.J.; Vapalahti, O.; Lyytikainen, O. Disease burden of Puumala virus infections, 1995–2008. Epidemiol. Infect. 2010, 138, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Hjertqvist, M.; Klein, S.L.; Ahlm, C.; Klingstrom, J. Mortality rate patterns for hemorrhagic fever with renal syndrome caused by Puumala virus. Emerg. Infect. Dis. 2010, 16, 1584–1586. [Google Scholar] [CrossRef] [PubMed]
- Park, M.A.; Li, J.T.; Hagan, J.B.; Maddox, D.E.; Abraham, R.S. Common variable immunodeficiency: A new look at an old disease. Lancet 2008, 372, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Vial, P.A.; Valdivieso, F.; Calvo, M.; Rioseco, M.L.; Riquelme, R.; Araneda, A.; Tomicic, V.; Graf, J.; Paredes, L.; Florenzano, M.; et al. A non-randomized multicentre trial of human immune plasma for treatment of hantavirus cardiopulmonary syndrome caused by Andes virus. Antivir. Ther. 2015, 20, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Kramski, M.; Meisel, H.; Klempa, B.; Kruger, D.H.; Pauli, G.; Nitsche, A. Detection and typing of human pathogenic hantaviruses by real-time reverse transcription-PCR and pyrosequencing. Clin. Chem. 2007, 53, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, J.; Hofmann, J.; Enders, M.; Tewald, F.; Oehme, R.M.; Rosenfeld, U.M.; Ali, H.S.; Schlegel, M.; Essbauer, S.; Osterberg, A.; et al. Multiple synchronous outbreaks of Puumala virus, Germany, 2010. Emerg. Infect. Dis. 2012, 18, 1461–1464. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.P.W.; Buckland, M.; Breuer, J.; Lowe, D.M. Viral infection in primary antibody deficiency syndromes. Rev. Med. Virol. 2019, 29, e2049. [Google Scholar] [CrossRef] [PubMed]
- Evander, M.; Eriksson, I.; Pettersson, L.; Juto, P.; Ahlm, C.; Olsson, G.E.; Bucht, G.; Allard, A. Puumala hantavirus viremia diagnosed by real-time reverse transcriptase PCR using samples from patients with hemorrhagic fever and renal syndrome. J. Clin. Microbiol. 2007, 45, 2491–2497. [Google Scholar] [CrossRef] [PubMed]
- Garanina, E.; Martynova, E.; Davidyuk, Y.; Kabwe, E.; Ivanov, K.; Titova, A.; Markelova, M.; Zhuravleva, M.; Cherepnev, G.; Shakirova, V.G.; et al. Cytokine Storm Combined with Humoral Immune Response Defect in Fatal Hemorrhagic Fever with Renal Syndrome Case, Tatarstan, Russia. Viruses 2019, 11, 601. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, L.; Thunberg, T.; Rocklov, J.; Klingstrom, J.; Evander, M.; Ahlm, C. Viral load and humoral immune response in association with disease severity in Puumala hantavirus-infected patients--implications for treatment. Clin. Microbiol. Infect. 2014, 20, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Korva, M.; Saksida, A.; Kejzar, N.; Schmaljohn, C.; Avsic-Zupanc, T. Viral load and immune response dynamics in patients with haemorrhagic fever with renal syndrome. Clin. Microbiol. Infect. 2013, 19, E358–E366. [Google Scholar] [CrossRef] [PubMed]
- Tuiskunen Back, A.; Rasmuson, J.; Thunberg, T.; Rankin, G.; Wigren Bystrom, J.; Andersson, C.; Sjodin, A.; Forsell, M.; Ahlm, C. Clinical and genomic characterisation of a fatal Puumala orthohantavirus case with low levels of neutralising antibodies. Infect. Dis. 2022, 54, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Klingstrom, J.; Smed-Sorensen, A.; Maleki, K.T.; Sola-Riera, C.; Ahlm, C.; Bjorkstrom, N.K.; Ljunggren, H.G. Innate and adaptive immune responses against human Puumala virus infection: Immunopathogenesis and suggestions for novel treatment strategies for severe hantavirus-associated syndromes. J. Intern. Med. 2019, 285, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Schonrich, G.; Raftery, M.J. Dendritic Cells (DCs) as “Fire Accelerants” of Hantaviral Pathogenesis. Viruses 2019, 11, 849. [Google Scholar] [CrossRef] [PubMed]
- Mustonen, J.; Outinen, T.; Laine, O.; Porsti, I.; Vaheri, A.; Makela, S. Kidney disease in Puumala hantavirus infection. Infect. Dis. 2017, 49, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Brocato, R.L.; Hooper, J.W. Progress on the Prevention and Treatment of Hantavirus Disease. Viruses 2019, 11, 610. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steininger, P.; Herbst, L.; Bihlmaier, K.; Willam, C.; Körper, S.; Schrezenmeier, H.; Klüter, H.; Pfister, F.; Amann, K.; Weiss, S.; et al. Fatal Puumala Hantavirus Infection in a Patient with Common Variable Immunodeficiency (CVID). Microorganisms 2023, 11, 283. https://doi.org/10.3390/microorganisms11020283
Steininger P, Herbst L, Bihlmaier K, Willam C, Körper S, Schrezenmeier H, Klüter H, Pfister F, Amann K, Weiss S, et al. Fatal Puumala Hantavirus Infection in a Patient with Common Variable Immunodeficiency (CVID). Microorganisms. 2023; 11(2):283. https://doi.org/10.3390/microorganisms11020283
Chicago/Turabian StyleSteininger, Philipp, Larissa Herbst, Karl Bihlmaier, Carsten Willam, Sixten Körper, Hubert Schrezenmeier, Harald Klüter, Frederick Pfister, Kerstin Amann, Sabrina Weiss, and et al. 2023. "Fatal Puumala Hantavirus Infection in a Patient with Common Variable Immunodeficiency (CVID)" Microorganisms 11, no. 2: 283. https://doi.org/10.3390/microorganisms11020283
APA StyleSteininger, P., Herbst, L., Bihlmaier, K., Willam, C., Körper, S., Schrezenmeier, H., Klüter, H., Pfister, F., Amann, K., Weiss, S., Krüger, D. H., Zimmermann, R., Korn, K., Hofmann, J., & Harrer, T. (2023). Fatal Puumala Hantavirus Infection in a Patient with Common Variable Immunodeficiency (CVID). Microorganisms, 11(2), 283. https://doi.org/10.3390/microorganisms11020283





