Abstract
Internal parasitic diseases of swine constitute a major welfare and health concern in low-input livestock farming. Due to an increase in chemical resistance, phytotherapeutic remedies have become an alternative for the prophylaxis and therapy of digestive parasitosis, albeit few remedies have been subjected to scientific validation. Low-input swine farming in Romania has adopted the traditional use of phytotherapy for controlling pathogens in livestock. The current study aimed to assess the antiparasitic potential of Calendula officinalis and Satureja hortensis against digestive parasites of swine in two low-input farms. The fecal samples were collected from sows, fatteners, and weaners, and were tested using the following coproparasitological methods: centrifugal sedimentation, flotation (Willis, McMaster egg counting technique), Ziehl–Neelsen stain modified by Henricksen, modified Blagg method, and in vitro nematode larvae/protozoan oocyst cultures. Six species of digestive parasites were diagnosed, namely Ascaris suum, Trichuris suis, Oesophagostomum spp., Balantioides coli, Eimeria spp., and Cryptosporidium spp., in various combinations, dependent on the swine category. A dose of 140 mg/kg bw/day of C. officinalis and 100 mg/kg bw/day of S. hortensis powders administered for 10 consecutive days revealed a strong antiprotozoal and anthelmintic activity on the aforementioned parasites. The curative efficacy can be attributed to the presence of polyphenols, sterols, tocopherols, and methoxylated flavones. In conclusion, our results indicate that S. hortensis and C. officinalis are promising alternatives to the commercially available antiparasitics, enabling their use as natural antiparasitic products against gastrointestinal parasites in pigs.
1. Introduction
The control of parasite infections in livestock farming is becoming increasingly important worldwide. Due to relatively high costs, most anthelmintic drugs are unavailable to rural subsistence livestock keepers. Furthermore, the large-scale use of anthelmintic drugs has led to various chemical resistance mutations [1,2]. Currently, benzimidazoles, imidazothiazoles, and macrocyclic lactones are commonly used for treating parasitic infections. A varying degree of resistance against these anthelmintics has been widely reported worldwide [3,4]. Residues of some such chemicals in the environment has the potential to disrupt the ecosystem, therefore, posing a significant threat to human health [4]. Consequently, a need to reduce the use of antibiotics and antiparasitics in livestock has shifted the spotlight onto phytotherapy [5,6]. Medicinal plants could present as an alternative to chemical molecules [4]. Phytotherapeutics are extracted from medicinal plants, while their bioactive compounds could be used to treat infectious and parasitic diseases [6,7]. The use of medicinal plants has seen a recent surge because of their lower toxicity and better biodegradability [1,6,8].
Pigs raised in conventional free-range systems appear to experience a higher standard of welfare (expressing a natural behavior, with access to outdoor areas, pasture, and enrichments) compared to pigs raised in conventional, indoor conditions [9]. On the other hand, the health risks associated with these systems (tail lesions, arthritis, skin lesions, bone fractures) can also be of concern [10]. Free-range access also contributes to the appearance of infectious and parasitic diseases [11]. Digestive parasites in swine affect reproductive output and swine performance (feed conversion, growth rate, and weight gain), with parasitized pigs being more prone to infectious and non-infectious diseases. This, in turn, undermines their gains in health and welfare [12]. Several reports attested the presence of gastrointestinal parasites in low-input farms, including protozoa (Balantioides coli, Isospora suis/Eimeria spp., Cryptosporidium spp., Giardia spp.) and nematodes (Ascaris suum, Trichuris suis, Oesophagostomum spp, and Strongyloides ransomi) [13,14,15,16]. Due to some anatomical and physiological similarities between pigs and humans, the former can serve as reservoirs for zoonotic pathogens, thus raising public health concerns [14,17]. Among such pathogens, Trichinella spiralis, A. suum, Taenia solium, B. coli, C. parvum, Toxoplasma gondii, Sarcocystis suihominis, Entamoeba polecki, and Giardia duodenalis are of particular interest [15,17,18,19,20,21].
Marigold, Calendula officinalis L., is an aromatic herb that belongs to the family Asteraceae and is used worldwide for its medicinal properties [22,23]. It also possesses several pharmacological activities such as the following: accelerating healing, neuroprotective, anti-inflammatory, hepatoprotective, antioxidant, immunostimulant, nephroprotective, hypoglycemic, gastroprotective, antibacterial, antifungal, antiviral, and insecticidal [22,23,24,25,26]. Due to the high concentration of saponins, C. officinalis showed antiparasitic activity [27,28]. The therapeutic properties of marigold are attributed to the presence of various classes of compounds, including, volatile oils (sesquiterpenoids monoterpenes), phenolic compounds (flavonoids and phenolic acids), coumarins, quinones, saponins, carotenoids, triterpenic alcohols, polyunsaturated fatty acids (calendic acid), polycarbohydrates, and other substances (proteins, amino acids, lipids saturated hydrocarbons, vitamin C, and mineral substances) [23,25,26,28,29]. C. officinalis triterpenoids are used as phytogenic feed additives which are sensory and flavoring compounds increasing growth performance, nutrient digestibility, and gut health in poultry [30,31].
Satureja hortensis L., known as summer savory, is an aromatic and medicinal plant belonging to the Lamiaceae family [32]. The major biomolecules found in extracts and essential oils of S. hortensis are volatile oils, phenolic compounds, flavonoids, tannins, steroids, acids, gums, mucilage, and pyrocatechols. The main components isolated from essential oils are carvacrol, thymol, cymene, terpinene, while alcoholic extracts are dominated by rosmarinic acid, caffeic acid, naringenin, isoferulic acid, luteolin, quercetin, and apigenin [33,34,35,36]. These bioactive compounds have been found to have a variety of biological activities including antioxidant, antispasmodic, anti-inflammatory, analgesic, antidiabetic, hepatoprotective, immunostimulant, reproduction stimulatory, vasodilatory, antimicrobial, and antiparasitic [32,34,37,38]. Carvacrol and thymol are believed to be responsible for the antiprotozoal and anthelmintic effects of S. hortensis.
These two plants were included in empirical therapies because of their antiparasitic effect. The current study aimed to evaluate the in vivo antiparasitic activity of C. officinalis and S. hortensis powders against digestive parasites in swine, in two low-input farms from the Transylvania area.
2. Materials and Methods
2.1. Chemical Analysis of Satureja hortensis and Calendula officinalis
The aerial parts of both S. hortensis and C. officinalis were utilized. Analysis of the bioactive compounds present in studied plants was conducted using high performance liquid chromatography coupled with mass spectrometry (HPLC-MS). All experimental procedures were performed at the Iuliu Haţieganu University of Medicine and Pharmacy, in Cluj-Napoca. Detailed information about the machines, methods, and techniques employed for analyzing the ethanolic extracts of marigold and summer savory can be found in a previous study [39].
2.2. Experimental Design
Before initiating the experiment, a preliminary study was carried out on a limited number of animals. During this pilot study, various doses (in accordance with existing literature), of S. hortensis and C. officinalis were administered, and their effects evaluated. The animals’ feeding behavior, the plants’ antiparasitic effectiveness, and any possible adverse reactions were meticulously observed.
The same two low-input farms (F1 and F2), as previously described in [16], were used to provide samples. F1 had a pig herd of 420 animals while F2 had 305 animals. The study was initiated in April 2022, and concluded in July 2022. The farms included in the experiment were homogeneous in terms of rearing system, geographic location, pig breeds raised, feed used, and identified parasites.
S. hortensis and C. officinalis were obtained from Romanian flora (local sources) by an authorized company who provided the plants. The aerial parts of both plants were ground, resulting in a feed containing either marigold or summer savory. Each type was then mixed with cereal flour. The study was conducted on both farms, with Bazna and Mangalitza breed pigs equally distributed in each group. A total of 240 pigs were included in the study, with 120 pigs assigned to each plant-based experiment variant on both farms. Three control groups (10 weaners, 10 fatteners, and 10 sows) and another three experimental groups (10 weaners, 10 fatteners and 10 sows) were established for each farm and plant in the experiment. Consequently, 60 pigs were used for the marigold experiment, with the same number used for the summer savory experiment, amounting to a total of 120 individuals per farm. The sows (S) were aged from 1 to 4 years with a body weight of 135 to 140 kg, fatteners (F) were aged 5 to 6 months and weighing 55 to 60 kg, and weaners (W) were aged 11 to 12 weeks and weighing 12 to 14 kg. Ten individuals, of the same age and weight, were confined in a pen, constituting an experimental group (EG). Welfare standards were met while administering the feed, diets being tailored to the animals based on their respective age categories (Table 1). Daily feed intake averages per pig were 0.8 kg for weaners, 2.5 kg for fatteners, and 3.5 kg for sows. The EG received a dose of 100 mg/kg bw/day of S. hortensis powder divided into two portions for a total period of ten consecutive days, while C. officinalis powder was administered in a dose of 140 mg/kg bw/day, identically to summer savory. The study started by testing S. hortensis for a period of 28 days, followed by C. officinalis for the same amount of time. A one-and-a–half-month period between the experiments conducted on different individuals was also included in the protocol. For each farm, swine category, and plant, three coproparasitological examinations (day 0 = before therapy, day 14 and day 28 = after therapy) were performed.

Table 1.
The diet for the EG tailored according to specific age groups.
The fecal samples were collected individually (weighing approximately 15–20 g each), placed in sterile containers, examined for the presence of macroscopic parasites, then numbered and stored at a temperature of 2–8 °C for up to 48 h, until further examination. The collected samples were tested using the following coproparasitological methods: centrifugal sedimentation, flotation (Willis, McMaster egg counting technique), Ziehl–Neelsen stain modified by Henricksen, modified Blagg method, and in vitro nematode larvae/protozoan oocyst cultures [16,40,41].
2.3. Ontologies, Ethics Statement, and Assessment of Antiparasitic Efficacy
Table S1 provides a comprehensive description of the ontologies related to medicinal plants, chemical compounds, parasites, and diseases, used in the present study.
The behaviour, welfare, and clinical condition of the pigs were continuously monitored before and during the experiment. The conducted study adhered to both national regulations (Law No. 43 of 2014) and European (EU Directive No. 63 of 2010) legislation concerning bioethical rules of experimentation on animals.
To evaluate the antiparasitic efficacy of C. officinalis and S. hortensis, a fecal egg count reduction test (FECRT) was performed. The methodology employed for this test was described by McKenna (2006) [42] and was also utilized in a previous study [43].
2.4. Statistical Analysis
The descriptive analysis was performed using Python 3.9.17 with the SciPy 1.11.1 package [44] and the visualizations using the Seaborn 0.12.2 library [45]. The inferential analysis was conducted using Python/SciPy and IBM SPSS v26 [46].
The first step was to analyze the data from a descriptive point of view. First, the evolution of parasites over time was evaluated for each parasite type and treatment regardless of pig type. Missing values were excluded from the analysis.
Considering that values were collected for two treatment groups for three time points, a repeated measures two-way ANOVA was considered to be the appropriate test. This test makes the following assumptions about the data:
- -
- Sphericity: The variances of the differences between all combinations of related groups (levels) are equal.
- -
- Normality: The distribution of the differences in the dependent variable between the two related groups should be approximately normally distributed for each level of the independent variable.
- -
- Lack of multivariate outliers.
Sphericity was tested using the Mauchly test of sphericity and the normality with the Shapiro–Wilk test. Both assumptions were violated even after data was transformed (using logarithmic or square root transforms) or outliers removed. As a result, non-parametric tests were used as they do not make assumptions about the shape of the distribution. Because the non-parametric alternative to repeated measures ANOVA (the Friedman test) does not accommodate a between-subjects factor directly, the following alternative approach was used:
- For the within-subjects factor (time) the Friedman test was used to compare the repeated measures (measurements at day 0, 14 and 28) for each group separately (control and experimental group). If the Friedman test returned significant differences, the Wilcoxon signed-rank test for pairwise comparisons was used to identify the groups with the significant differences. The Bonferroni correction for multiple comparisons was used to reduce the risk of false positives.
- For the between-subjects factor (treatment group) a Mann–Whitney U test was performed for each time point to compare the control and experimental groups.
When looking at the sample counts, values varied from 10 results for T. suis (TS) with CO for both farms (F1, F2) and all types of pigs (S, F, W) to 60 results for Eimeria spp. (ES), and B. coli (BC). Only pairs that contain more than 30 values for both farms were considered for the analysis to increase the chances of reproductible results. The analysis was conducted on results from pigs regardless of farm and type. The analyzed parasites were as follows: ES, BC, A. suum (AS), and Cryptosporidium spp. (CR).
3. Results
3.1. Chemical Analysis of S. hortensis and C. officinalis
Four major compounds were identified following the chemical analysis of the S. hortensis and C. officinalis ethanolic extracts: polyphenols (chlorogenic acid, caffeic acid, p-coumaric acid, ferulic acid, isoquercitrin, rutoside, quercitrin, quercetol, luteolin, apigenin, syringic acid, protocatechuic acid, vanilic acid), tocopherols (α-tocopherol, γ-tocopherol, Δ-tocopherol), sterols (ergosterol, stigmasterol, Β-sitosterol, campesterol) and methoxylated flavones (jaceosidin, hispidulin, acacetin) for summer savory and polyphenols (chlorogenic acid, isoquercitrin, rutoside, quercitrin, syringic acid, protocatechuic acid, vanilic acid), (α-tocopherol, γ-tocopherol, Δ-tocopherol), and sterols (ergosterol, stigmasterol, Β-sitosterol, campesterol) for marigold.
3.2. Analysis of Antiparasitic Effects of Studied Plants
The animals consumed the feed without hesitation and without any side effects or toxicity observed. In fatteners and sows treated with summer savory, a vermifuge effect was noticed, indicated by the elimination of A. suum adults through feces. Throughout the entire experiment, the welfare and health of the pigs were well maintained.
The coproparasitological examination showed co-infections with protozoa and nematodes. Six species of digestive parasites were diagnosed: Ascaris suum, Trichuris suis, Oesophagostomum spp. (OE), Balantioides coli, Eimeria spp., and Cryptosporidium spp., in variable combinations depending on the category of swine. The prevalence and average intensity of parasitic infections varied depending on each farm, age category, and studied plant. In sows, A. suum, Oesophagostomum spp., B. coli, and Eimeria spp. were encountered in both farms, while in F2, Cryptosporidium spp. was additionally found. In fatteners, A. suum, T. suis, B. coli, and Eimeria spp. were identified in both farms. In weaners from both farms, Cryptosporidium spp., B. coli, and Eimeria spp. were diagnosed, while Oesophagostomum spp. was additionally identified in F2 (Tables S2–S4).
Both summer savory and marigold were effective against all diagnosed parasites, with the exception of Cryptosporidium spp. Neither plant showed any antiprotozoal activity on Cryptosporidium. However, S. hortensis demonstrated a pronounced anthelmintic and antiprotozoal effect comparative with C. officinalis. Overall, the maximum therapeutic effects of summer savory and marigold varied according on the farm, age category, and day of examination.
The therapeutic efficacy (reduction%) of S. hortensis (SH) and C. officinalis (CO) on identified parasites across all age groups is detailed in Table 2. For Cryptosporidium only the prevalence was calculated because the classic coproparasitological methods used were not able to quantify the number of oocysts.

Table 2.
The percentage of reduction in fecal egg/oocyst/cyst count (%) registered on days 14, and 28 post-treatment in F1 and F2 farms (applying FECR formula).
3.2.1. Descriptive Statistics
The evolution of the numbers of oocysts, cysts, and eggs per gram of fecal matter was visualized for each analyzed time point (Figure 1) containing the 95% confidence intervals. In the case of ES and BC, both treatments (CO and SH) had very distinct evolution on days 14 and 28. This is also true for the AS and TS in the treatment with SH but when treated with CO, the confidence intervals had a wide overlap indicating less distinct results. In the case of OE, both treatments seemed to have less of an effect.
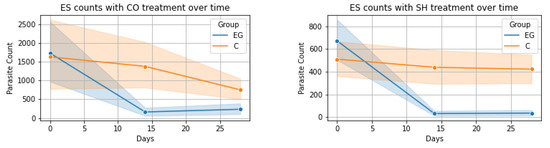
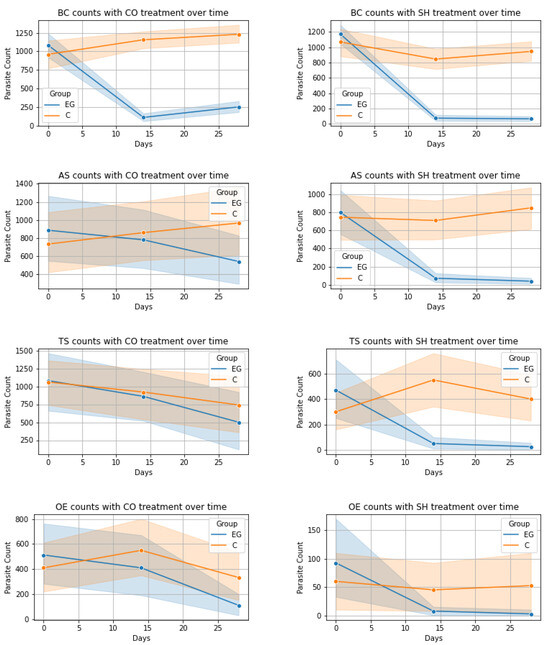
Figure 1.
Evolution of parasite count over time for each parasite-treatment pair: EG—experimental group, C—control group; ES = Eimeria spp., BC = B. coli, AS = A. suum, TS = T. suis, OE = Oesophagostomum spp., CO = Calendula officinalis, SH = Satureja hortensis.
3.2.2. Inferential Statistics
In this study, we assessed the impact of different treatments on parasite levels using a range of non-parametric statistical methods.
ES-CO Treatment: The Friedman test applied to the control group for ES parasite levels under CO treatment showed no significant variations over time (χ2(2) = 2.65, p = 0.265). However, the experimental group exhibited a notable change (χ2(2) = 24.61, p < 0.001). Wilcoxon signed-rank test comparisons in the control group revealed a significant decrease only between Day 14 and Day 28 (W = 202.5, p = 0.040), with no significant changes in other comparisons. In contrast, the experimental group saw significant decreases from Day 0 to Day 14 and Day 0 to Day 28 (W = 47.5 and W = 53.5, both p < 0.001), but not between Day 14 and Day 28. The Mann–Whitney U test indicated significant differences between the groups on Day 14 and Day 28 (U = 734.5 and U = 796.5 respectively, both p < 0.05).
ES-SH Treatment: Similar analysis for the SH treatment showed no significant changes in the control group (χ2(2) = 0.68, p = 0.711), unlike the experimental group which demonstrated significant changes (χ2(2) = 55.50, p < 0.001). Wilcoxon tests in the experimental group revealed significant decreases in ES levels from Day 0 to Day 14 (W = 7.00, p < 0.001) and from Day 0 to Day 28 (W = 10.50, p < 0.001), with no change between Day 14 and Day 28 (W = 46.00, p = 0.680). The Mann–Whitney U test showed significant disparities between the groups on Day 14 and Day 28 (U = 1058.5 and U = 982.5 respectively, both p < 0.001).
BC-CO and BC-SH Treatments: For both CO and SH treatments on BC parasite levels, Friedman tests in control groups indicated no significant changes. However, experimental groups showed pronounced shifts (CO: χ2(2) = 70.09; SH: χ2(2) = 94.54, both p < 0.001). Wilcoxon tests revealed various significant changes at different time points in experimental groups. In the experimental group, there were significant reductions in BC levels from Day 0 to Day 14 (W = 4.00, p < 0.001) and from Day 0 to Day 28 (W = 58.50, p < 0.001) with a slight but significant increase from Day 14 to Day 28 (W = 129.50, p = 0.004) in the CO treatment. Similarly, a significant decrease was observed in the SH experimental group between Day 0 and Day 14 and Day 0 and Day 28 (W = 2.00 and W = 1.00 respectively, both p < 0.001). Mann–Whitney U tests highlighted significant differences between control and experimental groups at later stages, Day 14 (U = 233.00, p < 0.001 for CO and U = 3017.50, p < 0.05 for SH) and Day 28 (U = 221.5, p < 0.001 for CO and U = 3324.50, p < 0.05 for SH).
AS-CO and AS-SH Treatments: The impact of CO and SH treatments on AS parasite levels was also analyzed. Friedman tests showed no significant changes in control groups, in contrast to significant alterations in experimental groups (χ2(2) = 7.80, p = 0.020 for CO and χ2(2) = 29.35, p < 0.001 for SH). Wilcoxon tests within the experimental groups revealed significant reductions at various time points, more specifically from Day 14 to Day 28 (W = 11.00, p = 0.004) and Day 0 to Day 28 (W = 21.00, p = 0.008) for CO, and from Day 0 to Day 14 (W = 3.00, p < 0.001) and Day 0 to Day 28 (W = 11.50, p < 0.001) for SH. Mann–Whitney U tests indicated no significant differences between the groups at most time points with the exception of Day 14 (U = 375.00, p < 0.001) and Day 28 (U = 282.0, p < 0.001) for the SH treatment.
CR-CO and CR-SH Treatments: For dichotomous CR parasite data, Friedman tests in both CO and SH treatments revealed no significant changes over time in either control or experimental groups. Chi-squared and Fisher’s exact tests for both treatments showed no significant differences between control and experimental groups at all time points.
4. Discussion
Over the past decades the use of local medicinal plants and non-chemical molecules instead of chemical drugs to treat parasitic diseases in humans and animals has experienced a resurgence [47]. Phytotherapeutic remedies are considered sustainable and adaptable to rural farming communities due to their availability and simplicity of preparation and administration to animals [48]. The therapeutic use of medicinal plants is reportedly widespread in humans, while limited in animals [49]. The aim of the current study was to assess the antiparasitic potential of two Romanian plants (S. hortensis and C. officinalis) against digestive parasites of swine. The major compounds of summer savory and marigold (polyphenols, sterols, tocopherols, and flavones) possess both in vivo and in vitro anthelmintic and antiprotozoal effects [50,51,52,53].
No studies about the dose of C. officinalis in pigs have been reported. Therefore, we extrapolated results from other animal species, including broiler chickens (150–450 mg/kg), rats (50–6000 mg/kg), mice (250–5000 mg/kg), rabbits (6000 mg/kg), and guinea pigs (250–500 mg/kg) [54,55,56,57,58]. In the current study, we elected to use a dose of 140 mg/kg bw/day, divided into two portions, administered for ten consecutive days. C. officinalis was found to be non-toxic, non-mutagenic, and non-genotoxic lacking reports of mortality [23,59]. In rare cases, marigold can cause allergic reactions on the skin, low hepatic toxicity on chronic exposure in rats, and in a rare report, anaphylactic shock [28,60,61]. The use of summer savory in swine diet and the proper dosage have been understudied. Therefore, we focused our attention onto the existing studies on humans (250–500 mg/individual/day) and various other animal species. These included rabbits (250 mg/kg/day), rats (500–5000 mg/kg/day), and broiler chicks (100–400 mg/kg/day) [32,62,63,64,65,66]. In the present study, the elected dose of S. hortensis powders was 100 mg/kg bw/day, administered under the same protocol as for marigold. Summer savory is considered a safe plant both for humans and animals, with very few side-effects, but it should be used with caution by patients with diabetes, hypoglycemia, hypertension or bleeding disorders, and is not recommended for children and pregnant women due to a lack of sufficient evidence for its use [32,33].
Ascaris suum is one of the most prevalent gastrointestinal parasites in domestic pigs widespread worldwide and causes significant economic losses in the swine industry [67,68]. In the present study, A. suum was diagnosed in fatteners and sows, in both farms. C. officinalis was variably effective against this parasite (10.3–79.9%), dependent on age group, while S. hortensis possesses a relatively strong anthelmintic activity range between 59.7% and 91.1%.
Oesophagostomum spp. is a large intestinal geohelminth—infections tend to be subclinical inducing weight loss of sows, low birth rates, and reduced growth of piglets [69]. Oesophagostomum was identified in weaners and sows. Both plants were effective against it, but summer savory (69.2–100%) was superior to marigold (28.6–60.5%).
Trichuris suis, the swine whipworm, is a widespread geohelminth with common manifestations including diarrhea, anorexia, retarded growth, and performance losses [70]. T. suis was diagnosed only in fatteners, in both farms. S. hortensis was very effective (80.5–90.3%) against T. suis, while C. officinalis had a weak anthelmintic activity range between 8.2% and 20.3%.
Cryptosporidium is a zoonotic protozoa of pigs, which causes diarrhea, particularly in immunodeficient individuals and children [71]. Cryptosporidium spp. was identified in weaners and sows, in both farms. Neither marigold nor summer savory were effective against this parasitic infection.
Balantioides coli is a ciliat-comensal protozoan which can be transmitted from pigs to humans and act as an occasional pathogen [72]. B. coli was diagnosed in both farms, in all swine categories. Both plants were very effective against B. coli. Efficacy ranged between 53.6% and 90.9% for marigold and between 63.5% and 88.4% for summer savory. No other studies about the efficacy of these two plants against B. coli have been reported.
Eimeria species are common in pigs worldwide, occasionally clinically affecting weaners and fatteners when diarrhea and weight loss can be observed upon infection with the more pathogenic species [73]. Eimeria was identified in all age categories, in both farms. Both plants demonstrated a strong antiprotozoal activity, ranging between 30.0% and 95.5% for C. officinalis and between 25.1% and 94.1% for S. hortensis, respectively.
The antiparasitic activity of marigold is very poorly understood. There are several reports on the effectiveness of this plant on protozoa such as: Plasmodium falciparum, Hexamita muris, Trichomonas spp., Chilomastix bettencourti, Leishmania spp., and also on helminths including Heligmosomoides polygyrus and Heligmosomoides bakeri [51,74,75,76,77]. Acaricidal and insecticidal activity was demonstrated against Sarcoptes scabiei, Rhipicephalus microplus, and Oncopeltus fasciatus [78,79,80]. Triterpenoid saponins, oleanic acid, and its glycosides are bioactive compounds isolated from marigold which are responsible for the antiparasitic activity [50,51]. A diet rich in carotenoids seems to promote resistance against oocysts of Eimeria and the excretion of a massive number of oocysts is attenuated or delayed. Effects included a reduction in the severity of coccidiosis symptoms as well as a delay in the parasite’s life cycle, reducing the load of oocysts in feces [1,81].
Satureja hortensis alcoholic extracts and essential oils have demonstrated antiprotozoal and anthelmintic effects against Ascaris spp., Trichuris muris, Cryptosporidium spp., Eimeria spp. and digestive strongyles of ruminants. A detailed list about the antiparasitic activities of S. hortensis is presented in Table 3. Other studies have highlighted the antiparasitic properties of Satureja species against Echinococcus granulosus, Leishmania spp., Plasmodium spp., Trypanosoma spp., Giardia lamblia, Trichomonas vaginalis, Toxoplasma gondii, and Acanthamoeba castellanii [82,83,84,85,86,87,88,89,90].

Table 3.
The antiparasitic activity of Satureja species (literature reports).
Improving the feeding value of swine diets through supplementation of bioactive and high nutritional medicinal plants such as summer savory and marigold is a viable approach to enhance the supplement utilization effectiveness for increasing productivity. Furthermore, it increases the quality of the animal products obtained via sustainable livestock production systems. Data regarding effects of different medicinal plants against gastrointestinal parasites of swine are scarce. The mechanism behind the antiprotozoal and anthelmintic effects of studied plants is still unknown. The current study and other reports revealed appropriate antiparasitic activity of S. hortensis and C. officinalis which indicate that both plants offer a viable and sustainable alternative to classic antiparasitics, enabling their use as an alternative to, or in addition to chemical drugs in parasite control programs.
5. Conclusions
The current study demonstrated the efficacy of powdered C. officinalis and S. hortensis aerial parts against digestive parasites in pigs when administered at doses of 140 mg/kg/day and 100 mg/kg/day, respectively, over a period of 10 consecutive days.
Considering the data presented in this study, summer savory and marigold powders showed promising in vivo antiparasitic activity and, therefore, might be used as antiparasitic natural products after scientific validation. C. officinalis had a strong antiprotozoal activity and mildly anthelmintic effects while S. hortensis was very effective against both helminths and protozoa infections.
Nevertheless, our discovery attests that summer savory and marigold are accessible antiparasitic remedies and can be used as an alternative therapy to chemical drugs against parasitic infections in swine, prompting the development of phytomedicine. Moreover, the current study is the first report about the antiparasitic effects of C. officinalis and S. hortensis against digestive parasites of swine, from Romania.
However, further studies are required to determine the bioactive compounds responsible for the antiparasitic activity, the potential toxic reactions, the minimum effective dosage, and the frequency of the administration for each plant.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms11122980/s1, Table S1: Ontologies/medicinal plants, chemical compounds, pathogens, and diseases, used in experiment; Table S2: The prevalence (P), at 0, 14, and 28 days in weaners; Table S3: The prevalence (P), at 0, 14, and 28 days in fatteners; Table S4: The prevalence (P), at 0, 14, and 28 days in sows.
Author Contributions
Conceptualization, M.-H.B., M.S. and V.C.; methodology, M.-H.B., V.-D.C. and V.C.; software, M.-H.B. and V.I.B.; validation, M.-H.B., A.C.-P. and M.S.; formal analysis, A.C.-P., V.I.B. and M.S.; investigation, M.-H.B., V.-D.C. and A.M.; resources, M.S. and V.C.; data curation, M.-H.B., V.I.B. and V.-D.C.; writing—original draft preparation, M.-H.B., V.-D.C., A.C.-P., V.I.B. and M.S.; writing—review and editing, M.-H.B., V.-D.C., A.C.-P., M.S. and V.C.; visualization, M.-H.B. and M.S.; supervision, M.S. and V.C.; project administration, M.S. and V.C.; funding acquisition, M.S. and V.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca (USAMV Cluj-Napoca), and by the project PPILOW. The project PPILOW has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement N°816172.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, Romania (Permit no. 313/19.04.2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Material.
Acknowledgments
Anne Collin (INRAE, Université de Tours, BOA, 37380 Nouzilly, France) is acknowledged for advice on ontologies/pathogens, diseases, medicinal plants, and chemical compounds (ATOL, AHOL, OPL, IPNI, and ChEBI references).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, S.; Zhang, L.; Li, J.; Cong, J.; Gao, F.; Zhou, G. Effects of dietary marigold extract supplementation on growth performance, pigmentation, antioxidant capacity and meat quality in broiler chickens. Asian-Australas. J. Anim. Sci. 2017, 30, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, A.; Iqbal, Z.; Kerboeuf, D.; Muhammad, G.; Khan, M.N.; Afaq, M. Anthelmintic resistance: The state of play revisited. Life Sci. 2006, 79, 2413–2431. [Google Scholar] [CrossRef] [PubMed]
- Coles, G.C.; Jackson, F.; Pomroy, W.E.; Prichard, R.K.; von Samson-Himmelstjerna, G.; Silvestre, A.; Taylor, M.A.; Vercruysse, J. The detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 2006, 136, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Ayrle, H.; Mevissen, M.; Kaske, M.; Nathues, H.; Gruetzner, N.; Melzig, M.; Walkenhorst, M. Medicinal plants–prophylactic and therapeutic options for gastrointestinal and respiratory diseases in calves and piglets? A systematic review. BMC Vet. Res. 2016, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Penedo, I.; Fernández González, C.; Tamminen, L.M.; Sundrum, A.; Emanuelson, U. Priorities and Future actions for an effective Use of Phytotherapy in livestock—Outputs from an expert Workshop. Front. Vet. Sci. 2018, 4, 248. [Google Scholar] [CrossRef] [PubMed]
- Castaño Osorio, J.C.; Giraldo García, A.M. Antiparasitic phytotherapy perspectives, scope and current development. Infectio 2019, 23, 189–204. [Google Scholar] [CrossRef]
- Ferreira, L.E.; Benincasa, B.I.; Fachin, A.L.; Franca, S.C.; Continia, S.S.H.T.; Chagas, A.C.S.; Beleboni, R.O. Thymus vulgaris L. Essential Oil and Its Main Component Thymol: Anthelmintic Effects against Haemonchus contortus from Sheep. Vet. Parasitol. 2016, 228, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Singh, R. A review on anthelmintic drugs and their future scope. Int. J. Pharm. Pharm. Sci. 2011, 3, 17–21. [Google Scholar]
- Alban, L.; Petersen, J.V.; Busch, M.E. A comparison between lesions found during meat inspection of finishing pigs raised under organic/free-range conditions and conventional, indoor conditions. Porc. Health Manag. 2015, 1, 4. [Google Scholar] [CrossRef]
- Kongsted, H.; Sørensen, J.T. Lesions found at routine meat inspection on finishing pigs are associated with production system. Vet. J. 2017, 223, 21–26. [Google Scholar] [CrossRef]
- Bonnefous, C.; Collin, A.; Guilloteau, L.A.; Guesdon, V.; Filliat, C.; Réhault-Godbert, S.; Rodenburg, T.B.; Tuyttens, F.A.M.; Warin, L.; Steenfeldt, S.; et al. Welfare issues and potential solutions for laying hens in free range and organic production systems: A review based on literature and interviews. Front. Vet. Sci. 2022, 9, 1148. [Google Scholar] [CrossRef] [PubMed]
- Sowemimo, O.A.; Asaolu, S.O.; Adegoke, F.O.; Ayanniyi, O.O. Epidemiological survey of gastrointestinal parasites of pigs in Ibadan, Southwest Nigeria. J. Public Health Epidemiol. 2012, 4, 294–298. [Google Scholar] [CrossRef]
- Eijck, I.A.J.M.; Borgsteede, F.H.M. A survey of gastrointestinal pig parasites on free-range, organic and conventional pig farms in The Netherlands. Vet. Res. Commun. 2005, 29, 407–414. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, B.B.; Sharma, R.; Gill, J.P.S. Prevalence of gastro intestinal parasites in pigs in Punjab, India. J. Parasit. Dis. 2017, 41, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Tumusiime, M.; Ntampaka, P.; Niragire, F.; Sindikubwabo, T.; Habineza, F. Prevalence of Swine Gastrointestinal Parasites in Nyagatare District, Rwanda. J. Parasitol. Res. 2020, 2020, 8814136. [Google Scholar] [CrossRef] [PubMed]
- Băieş, M.H.; Boros, Z.; Gherman, C.M.; Spînu, M.; Mathe, A.; Pataky, S.; Lefkaditis, M.; Cozma, V. Prevalence of swine gastrointestinal parasites in two free-range farms from nord-west region of Romania. Pathogens 2022, 11, 954. [Google Scholar] [CrossRef] [PubMed]
- Uddin Khan, S.; Atanasova, K.R.; Krueger, W.S.; Ramirez, A.; Gray, G.C. Epidemiology, geographical distribution, and economic consequences of swine zoonoses: A narrative review. Emerg. Microbes Infect. 2013, 2, e92. [Google Scholar] [CrossRef] [PubMed]
- Thapaliya, D.; Hanson, B.M.; Kates, A.; Klostermann, C.A.; Nair, R.; Wardyn, S.E.; Smith, T.C. Zoonotic Diseases of Swine: Food-borne and Occupational Aspects of Infection. In Zoonoses: Infections Affecting Humans and Animals; Sing, A., Ed.; Springer: Cham, Switzerland, 2023; pp. 113–162. [Google Scholar] [CrossRef]
- Solaymani-Mohammadi, S.; Petri, W.A., Jr. Zoonotic implications of the swine-transmitted protozoal infections. Vet. Parasitol. 2006, 140, 189–203. [Google Scholar] [CrossRef]
- Ziemer, C.J.; Bonner, J.M.; Cole, D.; Vinjé, J.; Constantini, V.; Goyal, S.; Gramer, M.; Mackie, R.; Meng, X.J.; Myers, G.; et al. Fate and transport of zoonotic, bacterial, viral, and parasitic pathogens during swine manure treatment, storage, and land application. J. Anim. Sci. 2010, 88, E84–E94. [Google Scholar] [CrossRef]
- Pinilla, J.C.; Morales, E.; Muñoz, A.A.F. A survey for potentially zoonotic parasites in backyard pigs in the Bucaramanga metropolitan area, Northeast Colombia. Vet. World 2021, 14, 372–379. [Google Scholar] [CrossRef]
- Muley, B.P.; Khadabadi, S.S.; Banarase, N.B. Phytochemical constituents and pharmacological activities of Calendula officinalis Linn (Asteraceae): A review. Trop. J. Pharm. Res. 2009, 8, 455–465. [Google Scholar] [CrossRef]
- Jan, N.; Andrabi, K.I.; John, R. Calendula officinalis—An important medicinal plant with potential biological properties. Proc. Indian Natl. Sci. Acad. 2017, 83, 769–787. [Google Scholar] [CrossRef]
- Singh, M.K.; Sahu, P.; Nagori, K.; Dewangan, D.; Kumar, T.; Alexander, A.; Badwaik, H.; Tripathi, D.K. Organoleptic properties in-vitro and in-vivo pharmacological activities of Calendula officinalis Linn: An over review. J. Chem. Pharm. Res. 2011, 3, 655–663. [Google Scholar]
- Khalid, K.A.; Da Silva, J.T. Biology of Calendula officinalis Linn.: Focus on pharmacology, biological activities and agronomic practices. Med. Aromat. Plant Sci. Biotechnol. 2012, 6, 12–27. [Google Scholar]
- Ashwlayanvd, K.A.; Verma, M. Therapeutic potential of Calendula officinalis. Pharm. Pharmacol. Int. J. 2018, 6, 149–155. [Google Scholar]
- Lipi, P.; Vivek, S.; Makode, K.K.; Jain, U.K. Anthelmintic activity of aqueous extracts of some saponin containing medicinal plants. Pharm. Lett. 2010, 2, 476–481. [Google Scholar]
- Verma, P.K.; Raina, R.; Agarwal, S.; Kaur, H. Phytochemical ingredients and Pharmacological potential of Calendula officinalis Linn. Pharm. Biomed. Res. 2018, 4, 1–17. [Google Scholar] [CrossRef]
- Butnariu, M.; Coradini, C.Z. Evaluation of biologically active compounds from Calendula officinalis flowers using spectrophotometry. Chem. Cent. J. 2012, 6, 35. [Google Scholar] [CrossRef]
- Shehata, A.A.; Yalçın, S.; Latorre, J.D.; Basiouni, S.; Attia, Y.A.; El-Wahab, A.; Visscher, C.; El-Seedi, H.R.; Huber, C.; Hafez, H.M.; et al. Probiotics, prebiotics, and phytogenic substances for optimizing gut health in poultry. Microorganisms 2022, 10, 395. [Google Scholar] [CrossRef]
- Kostadinović, L.; Lević, J. Effects of phytoadditives in poultry and pigs diseases. J. Agron. 2018, 1, 1–7. [Google Scholar]
- Hamidpour, R.; Hamidpour, S.; Hamidpour, M.; Shahlari, M.; Sohraby, M. Summer savory: From the selection of traditional applications to the novel effect in relief, prevention, and treatment of a number of serious illnesses such as diabetes, cardiovascular disease, Alzheimer’s disease, and cancer. J. Tradit. Complement. Med. 2014, 4, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Fierascu, I.; Dinu-Pirvu, C.E.; Fierascu, R.C.; Velescu, B.S.; Anuta, V.; Ortan, A.; Jinga, V. Phytochemical profile and biological activities of Satureja hortensis L.: A review of the last decade. Molecules 2018, 23, 2458. [Google Scholar] [CrossRef] [PubMed]
- Mohammadhosseini, M.; Beiranvand, M. Chemical composition of the essential oil from the aerial parts of Satureja hortensis as a potent medical plant using traditional hydrodistillation. J. Chem. Health Risks 2013, 3, 43–54. [Google Scholar]
- Mahboubi, M.; Kazempour, N. Chemical composition and antimicrobial activity of Satureja hortensis and Trachyspermum copticum essential oil. Iran. J. Microbiol. 2011, 3, 194–200. [Google Scholar] [PubMed]
- Bimbiraitė-Survilienė, K.; Stankevičius, M.; Šuštauskaitė, S.; Gęgotek, A.; Maruška, A.; Skrzydlewska, E.; Barsteigienė, Z.; Akuņeca, I.; Ragažinskienė, O.; Lukošius, A. Evaluation of chemical composition, radical scavenging and antitumor activities of Satureja hortensis L. herb extracts. Antioxidants 2021, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Jafari, F.; Ghavidel, F.; Zarshenas, M.M. A critical overview on the pharmacological and clinical aspects of popular Satureja species. J. Acupunct. Meridian Stud. 2016, 9, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Tepe, B.; Cilkiz, M. A pharmacological and phytochemical overview on Satureja. Pharm. Biol. 2016, 54, 375–412. [Google Scholar] [CrossRef] [PubMed]
- Bǎieş, M.H.; Gherman, C.; Boros, Z.; Olah, D.; Vlase, A.M.; Cozma-Petrut, A.; Györke, A.; Miere, D.; Vlase, L.; Crişan, G.; et al. The Effects of Allium sativum L., Artemisia absinthium L., Cucurbita pepo L., Coriandrum sativum L., Satureja hortensis L. and Calendula officinalis L. on the Embryogenesis of Ascaris suum Eggs during an In Vitro Experimental Study. Pathogens 2022, 11, 1065. [Google Scholar] [CrossRef]
- Mircean, V.; Cozma, V.; Gyorke, A. Diagnostic Coproscopic in Bolile Parazitare la Animale, (Coproparasitological Diagnostic in Parasitic Diseases in Animals); Risoprint: Cluj-Napoca, Romania, 2011; pp. 23–35. [Google Scholar]
- Manser, M.M.; Saez, A.C.; Chiodini, P.L. Faecal Parasitology: Concentration Methodology Needs to be Better Standardised. PLoS Negl. Trop. Dis. 2016, 10, e0004579. [Google Scholar] [CrossRef]
- McKenna, P.B. 2006. Further comparison of faecal egg count reduction test procedures: Sensitivity and specificity. N. Z. Vet. J. 2006, 54, 365–366. [Google Scholar] [CrossRef]
- Băieş, M.H.; Cotuţiu, V.D.; Spînu, M.; Mathe, A.; Cozma-Petruț, A.; Miere, D.; Bolboacǎ, S.D.; Cozma, V. The Effects of Coriandrum sativum L. and Cucurbita pepo L. against Gastrointestinal Parasites in Swine: An In Vivo Study. Microorganisms 2023, 11, 1230. [Google Scholar] [CrossRef] [PubMed]
- SciPy. Available online: https://scipy.org/ (accessed on 1 October 2023).
- Seaborn: Statistical Data Visualization. Available online: https://seaborn.pydata.org/ (accessed on 1 October 2023).
- IBM SPSS Statistics. Available online: https://www.ibm.com/products/spss-statistics (accessed on 1 October 2023).
- Amirmohammadi, M.; Khajoenia, S.; Bahmani, M.; Rafieian-Kopaei, M.; Eftekhari, Z.; Qorbani, M. In vivo evaluation of antiparasitic effects of Artemisia abrotanum and Salvia officinalis extracts on Syphacia obvelata, Aspiculoris tetrapetra and Hymenolepis nana parasites. Asian Pac. J. Trop. Dis. 2014, 4, S250–S254. [Google Scholar] [CrossRef]
- Bakare, A.G.; Shah, S.; Bautista-Jimenez, V.; Bhat, J.A.; Dayal, S.R.; Madzimure, J. Potential of ethno-veterinary medicine in animal health care practices in the South Pacific Island countries: A review. Trop. Anim. Health Prod. 2020, 52, 2193–2203. [Google Scholar] [CrossRef] [PubMed]
- Hart, B.L. The evolution of herbal medicine: Behavioural perspectives. Anim. Behav. 2005, 70, 975–989. [Google Scholar] [CrossRef]
- Szakiel, A.; Ruszkowski, D.; Grudniak, A.; Kurek, A.; Wolska, K.I.; Doligalska, M.; Janiszowska, W. Antibacterial and antiparasitic activity of oleanolic acid and its glycosides isolated from marigold (Calendula officinalis). Planta Med. 2008, 74, 1709–1715. [Google Scholar] [CrossRef] [PubMed]
- Doligalska, M.; Jóźwicka, K.; Kiersnowska, M.; Mroczek, A.; Pączkowski, C.; Janiszowska, W. Triterpenoid saponins affect the function of P-glycoprotein and reduce the survival of the free-living stages of Heligmosomoides bakeri. Vet. Parasitol. 2011, 179, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.M.; Storey, B.E.; Vidyashankar, A.N.; Bissinger, B.W.; Mitchell, S.M.; Howell, S.B.; Mason, M.E.; Lee, M.D.; Pedroso, A.A.; Akashe, A.; et al. Antiparasitic efficacy of a novel plant-based functional food using an Ascaris suum model in pigs. Acta Trop. 2014, 139, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.S.; Moreira, C.Z.; Cária, N.Z.; Victoriano, G.; Silva, W.F., Jr.; Magalhães, J.C. Phytotherapy: An introduction to its history, use and application. Rev. Bras. Plantas Med. 2014, 16, 290–298. [Google Scholar] [CrossRef]
- Moghaddam, S.; Kermanshahi, H.; Vahed, R.; Nasiri Moghaddam, H. The protective effects of marigold (Calendula officinalis) extract in liver damage by CCl4 in broiler chicken. Vet. Res. Biol. Prod. 2015, 28, 60–69. [Google Scholar] [CrossRef]
- Lagarto, A.; Bueno, V.; Guerra, I.; Valdés, O.; Vega, Y.; Torres, L. Acute and subchronic oral toxicities of Calendula officinalis extract in Wistar rats. Exp. Toxicol. Pathol. 2011, 63, 387–391. [Google Scholar] [CrossRef]
- Strychalski, J.; Gugołek, A.; Antoszkiewicz, Z.; Fopp-Bayat, D.; Kaczorek-Łukowska, E.; Snarska, A.; Zwierzchowski, G.; Król-Grzymała, A.; Matusevičius, P. The Effect of the BCO2 Genotype on the Expression of Genes Related to Carotenoid, Retinol, and α-Tocopherol Metabolism in Rabbits Fed a Diet with Aztec Marigold Flower Extract. Int. J. Mol. Sci. 2022, 23, 10552. [Google Scholar] [CrossRef] [PubMed]
- Sagar, R.; Sahoo, H.B.; Kar, B.; Mishra, N.K.; Mohapatra, R.; Sarangi, S.P. Pharmacological evaluation of Calendula officinalis L. on bronchial asthma in various experimental animals. Int. J. Nutr. Pharmacol. Neurol. Dis. 2014, 4, 95–103. [Google Scholar] [CrossRef]
- Leffa, D.D.; da Rosa, R.; Munhoz, B.P.; Mello, A.D.A.M.; Mandelli, F.D.; Amaral, P.d.A.; Rossatto, Â.E.; de Andrade, V.M. Genotoxic and antigenotoxic properties of Calendula officinalis extracts in mice treated with methyl methanesulfonate. Adv. Life Sci. 2012, 2, 21–28. [Google Scholar] [CrossRef]
- Silva, E.J.; Gonçalves, E.S.; Aguiar, F.; Evêncio, L.B.; Lyra, M.M.; Coelho, M.C.O.C.; Fraga, M.d.C.C.A.; Wanderley, A.G. Toxicological studies on hydroalcohol extract of Calendula officinalis L. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2007, 21, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Gol’dman, I.I. Anaphylactic shock after gargling with an infusion of Calendula. Klin. Meditsina 1974, 52, 142–143. [Google Scholar]
- Patil, K.; Sanjay, C.J.; Doggalli, N.; Devi, K.R.; Harshitha, N. A Review of Calendula officinalis—Magic in Science. J. Clin. Diagn. Res. 2022, 16, ZE23–ZE27. [Google Scholar] [CrossRef]
- Montazeri, S.; Jafari, M.; Khojasteh, S. The effect of powder and essential oil of savory medicinal plant me (Satureja hortensis) on performance and antioxidant status of broiler chicks under heat stress. Iran. J. Appl. Anim. Sci. 2014, 4, 573–577. [Google Scholar]
- Uslu, C.; Karasen, R.M.; Sahin, F.; Taysi, S.; Akcay, F. Effects of aqueous extracts of Satureja hortensis L. on rhinosinusitis treatment in rabbit. J. Ethnopharmacol. 2013, 88, 225–228. [Google Scholar] [CrossRef]
- Shanaida, M.I.; Oleshchuk, O.M. Acute toxicity determination of summer savory liquid extract. Ukr. Biopharm. J. 2017, 4, 22–26. [Google Scholar] [CrossRef][Green Version]
- Movahhedkhah, S.; Rasouli, B.; Seidavi, A.; Mazzei, D.; Laudadio, V.; Tufarelli, V. Summer savory (Satureja hortensis L.) extract as natural feed additive in broilers: Effects on growth, plasma constituents, immune response, and ileal microflora. Animals 2019, 9, 87. [Google Scholar] [CrossRef]
- Rastegarpanah, M.; Omidzohour, N.; Vahedi, H.; Malekzadeh, R. Management of Human Ulcerative Colitis by Saturex”: A Randomized Controlled Trial. Int. J. Pharmacol. 2011, 37, 3. [Google Scholar]
- Liu, G.; Wu, C.; Song, H.; Wei, S.; Xu, M.; Lin, R.; Zhao, G.; Huang, S.; Zhu, X. Comparative analyses of the complete mitochondrial genomes of Ascaris lumbricoides and Ascaris suum from humans and pigs. Gene 2012, 492, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Thamsborg, S.M.; Nejsum, P.; Mejer, H. Chapter 14. Impact of Ascaris suum in livestock. In Ascaris: The Neglected Parasite; Holland, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 363–381. [Google Scholar] [CrossRef]
- Pettersson, E.; Halvarsson, P.; Sjölund, M.; Grandi, G.; Wallgren, P.; Höglund, J. First report on reduced efficacy of ivermectin on Oesophagostomum spp. on Swedish pig farms. Vet. Parasitol. Reg. Stud. Rep. 2021, 25, 100598. [Google Scholar] [CrossRef] [PubMed]
- Li, R.W.; Wu, S.; Li, W.; Navarro, K.; Couch, R.D.; Hill, D.; Urban, J.F., Jr. Alterations in the porcine colon microbiota induced by the gastrointestinal nematode Trichuris suis. Infect. Immun. 2012, 80, 2150–2157. [Google Scholar] [CrossRef]
- Symeonidou, I.; Tassis, P.; Gelasakis, A.Ι.; Tzika, E.D.; Papadopoulos, E. Prevalence and risk factors of intestinal parasite infections in Greek swine farrow-to-finish farms. Pathogens 2020, 9, 556. [Google Scholar] [CrossRef] [PubMed]
- Giarratana, F.; Nalbone, L.; Napoli, E.; Lanzo, V.; Panebianco, A. Prevalence of Balantidium coli (Malmsten, 1857) infection in swine reared in South Italy: A widespread neglected zoonosis. Vet. World 2021, 14, 1044. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Singh, N.K.; Singh, H.; Joachim, A.; Rath, S.S.; Blake, D.P. Discrimination, molecular characterisation and phylogenetic comparison of porcine Eimeria spp. in India. Vet. Parasitol. 2018, 255, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Cernea, C.; Cernea, M.; Ognean, L.; Trîncă, S. The effect of certain essential oils on laboratory mice intestinal protozoa. Bull. Univ. Agric. Sci. Vet. Med. Cluj Napoca 2008, 65, 281–284. [Google Scholar]
- Doligalska, M.; Joźwicka, K.; Laskowska, M.; Donskow-Łysoniewska, K.; Pączkowski, C.; Janiszowska, W. Changes in Heligmosomoides polygyrus glycoprotein pattern by saponins impact the BALB/c mice immune response. Exp. Parasitol. 2013, 135, 524–531. [Google Scholar] [CrossRef]
- Nikmehr, B.; Ghaznavi, H.; Rahbar, A.; Sadr, S.; Mehrzadi, S. In vitro anti-leishmanial activity of methanolic extracts of Calendula officinalis flowers, Datura stramonium seeds, and Salvia officinalis leaves. Chin. J. Nat. Med. 2014, 12, 423–427. [Google Scholar] [CrossRef]
- Ali Ahmad, N. The effect of Marigold flower extracts on growth of Leishmania. Kirkuk J. Sci. 2018, 13, 34–42. [Google Scholar] [CrossRef]
- Alexenizer, M.; Dorn, A. Screening of medicinal and ornamental plants for insecticidal and growth regulating activity. J. Pest. Sci. 2007, 80, 205–215. [Google Scholar] [CrossRef]
- Godara, R.; Katoch, R.; Yadav, A.; Ahanger, R.R.; Bhutyal, A.D.S.; Verma, P.K.; Katoch, M.; Dutta, S.; Nisa, F. In vitro acaricidal activity of ethanolic and aqueous floral extracts of Calendula officinalis against synthetic pyrethroid resistant Rhipicephalus (Boophilus) microplus. Exp. Appl. Acarol. 2015, 67, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Dimri, U. Amelioration of sarcoptic mange-induced oxidative stress and apoptosis in dogs by using Calendula officinalis flower extracts. Int. Sch. Res. Not. 2013, 2013, 657672. [Google Scholar] [CrossRef]
- Nogareda, C.; Moreno, J.A.; Angulo, E.; Sandmann, G.; Portero, M.; Capell, T.; Christou, P. Carotenoid-enriched transgenic corn delivers bioavailable carotenoids to poultry and protects them against coccidiosis. Plant Biotechnol. J. 2016, 14, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, J.; Maggiore, M.A.; Pensel, P.E.; Denegri, G.M.; Gende, L.B.; Elissondo, M.C. In vitro and in vivo efficacy of carvacrol against Echinococcus granulosus. Acta Trop. 2016, 164, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Soosaraei, M.; Fakhar, M.; Teshnizi, S.H.; Hezarjaribi, H.Z.; Banimostafavi, E.S. Medicinal plants with promising antileishmanial activity in Iran: A systematic review and meta-analysis. Ann. Med. Surg. 2017, 21, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Van Baren, C.; Anao, I.; Lira, P.D.L.; Debenedetti, S.; Houghton, P.; Croft, S.; Martino, V. Triterpenic acids and flavonoids from Satureja parvifolia. Evaluation of their antiprotozoal activity. Z. Naturforsch. C 2006, 61, 189–192. [Google Scholar] [CrossRef]
- Davoodi, J.; Abbasi Maleki, S. Comparison anti-giardia activity of Satureja hortensis alcoholic extract and metronidazole in vitro. Adv. Herb. Med. 2016, 2, 15–21. [Google Scholar]
- Tariku, Y.; Hymete, A.; Hailu, A.; Rohloff, J. Essential-oil composition, antileishmanial, and toxicity study of Artemisia abyssinica and Satureja punctata ssp. punctata from Ethiopia. Chem. Biodivers. 2010, 7, 1009–1018. [Google Scholar] [CrossRef]
- Arbabi, M.; Fakhrieh-Kashan, Z.; Delavari, M.; Taghizadeh, M.; Hooshyar, H. The effect of alcoholic extracts of Arctium lappa L. and Satureja hortensis L. against Trichomonas vaginalis in vitro. Feyz. Med. Sci. J. 2017, 21, 298–304. [Google Scholar]
- Sülsen, V.; Güida, C.; Coussio, J.; Paveto, C.; Muschietti, L.; Martino, V. In vitro evaluation of trypanocidal activity in plants used in Argentine traditional medicine. Parasitol. Res. 2006, 98, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Malatyali, E.; Tepe, B.; Degerli, S.E.R.P.İ.L.; Berk, S. In vitro amoebicidal activities of Satureja cuneifolia and Melissa officinalis on Acanthamoeba castellanii cysts and trophozoites. Parasitol. Res. 2012, 110, 2175–2180. [Google Scholar] [CrossRef] [PubMed]
- Tepe, B. Inhibitory Effect of Satureja on Certain Types of Organisms. Rec. Nat. Prod. 2015, 9, 1–18. [Google Scholar]
- Urban, J.; Kokoska, L.; Langrova, I.; Matejkova, J. In vitro anthelmintic effects of medicinal plants used in Czech Republic. Pharm. Biol. 2008, 46, 808–813. [Google Scholar] [CrossRef]
- Trailović, S.M.; Marjanović, D.S.; Nedeljković Trailović, J.; Robertson, A.P.; Martin, R.J. Interaction of carvacrol with the Ascaris suum nicotinic acetylcholine receptors and gamma-aminobutyric acid receptors, potential mechanism of antinematodal action. Parasitol. Res. 2015, 114, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Dimah, A.S. Egg Hatching Protocol and an In Vitro Scoring System in Parascaris univalens Larvae after Exposure to Anthelmintic Drugs. Master’s Thesis, Uppsala University, Uppsala, Sweden, 2020. [Google Scholar]
- Trailovic, S.M.; Rajkovic, M.; Marjanovic, D.S.; Neveu, C.; Charvet, C.L. Action of carvacrol on Parascaris sp. and antagonistic effect on nicotinic acetylcholine receptors. Pharmaceuticals 2021, 14, 505. [Google Scholar] [CrossRef]
- Mirza, Z.; Soto, E.R.; Hu, Y.; Nguyen, T.T.; Koch, D.; Aroian, R.V.; Ostroff, G.R. Anthelmintic activity of yeast particle-encapsulated terpenes. Molecules 2020, 25, 2958. [Google Scholar] [CrossRef]
- Felici, M.; Tugnoli, B.; Ghiselli, F.; Massi, P.; Tosi, G.; Fiorentini, L.; Grilli, E. In vitro anticoccidial activity of thymol, carvacrol, and saponins. Poult. Sci. 2020, 99, 5350–5355. [Google Scholar] [CrossRef]
- Remmal, A.; Achahbar, S.; Bouddine, L.; Chami, F.; Chami, N. Oocysticidal effect of essential oil components against chicken Eimeria oocysts. Int. J. Vet. Med. 2013, 2, 133–139. [Google Scholar] [CrossRef]
- Teichmann, K.; Kostelbauer, A.; Steiner, T.; Giannenas, I.; Tontis, D.; Papadopoulos, E.; Schatzmayr, G. Phytogenics to Prevent Chicken Coccidiosis. Planta Med. 2012, 78, PF72. [Google Scholar] [CrossRef]
- Gaur, S.; Kuhlenschmidt, T.B.; Kuhlenschmidt, M.S.; Andrade, J.E. Effect of oregano essential oil and carvacrol on Cryptosporidium parvum infectivity in HCT-8 cells. Parasitol. Int. 2018, 67, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Uscanga, A.; Aycart, D.F.; Li, K.; Witola, W.H.; Laborde, J.E.A. Anti-protozoal activity of Thymol and a Thymol ester against Cryptosporidium parvum in cell culture. Int. J. Parasitol. Drugs Drug Resist. 2021, 15, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Sennouni, C.I.; Oukouia, M.; Jabeur, I.; Hamdani, H.; Chami, F.; Remmal, A. In vitro and in vivo study of the antiparasitic effect of thymol on poultry drinking water. Acta Sci. Biol. Sci. 2022, 44, e58571. [Google Scholar] [CrossRef]
- Tanghort, M.; Chefchaou, H.; Mzabi, A.; Moussa, H.; Chami, N.; Chami, F.; Remmal, A. Oocysticidal Effect of Essential Oils (EOs) and Their Major Components on Cryptosporidium baileyi and Cryptosporidium galli. Int. J. Poult. Sci. 2019, 18, 475–482. [Google Scholar] [CrossRef]
- Andre, W.P.P.; Cavalcante, G.S.; Ribeiro, W.L.C.; Dos Santos, J.M.L.; Macedo, I.T.F.; De Paula, H.C.B.; De Morais, S.M.; De Melo, J.V.; Bevilaqua, C.M.L. Anthelmintic Effect of Thymol and Thymol Acetate on Sheep Gastrointestinal Nematodes and Their Toxicity in Mice. Rev. Bras. Parasitol. Vet. 2017, 26, 323–330. [Google Scholar] [CrossRef]
- Štrbac, F.; Bosco, A.; Maurelli, M.P.; Ratajac, R.; Stojanović, D.; Simin, N.; Orčić, D.; Pušić, I.; Krnjajić, S.; Sotiraki, S.; et al. Anthelmintic properties of essential oils to control gastrointestinal nematodes in sheep—In vitro and in vivo studies. Vet. Sci. 2022, 9, 93. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).