Abstract
The Triatoma brasiliensis species complex is a monophyletic group encompassing two subspecies and six species. Recently, a hybrid zone of members of this complex was recorded in the state of Pernambuco. Questions concerning the capability of the hybrids to become infected with Trypanosoma cruzi have been raised. This study aimed to compare the susceptibility of Triatoma b. brasiliensis, Triatoma juazeirensis, and their experimental hybrids to infection with T. cruzi. We infected the parentals and their experimental hybrids (obtained through reciprocal crosses) through artificial feeding with citrated rabbit blood, to which the TcI 0354 strain of T. cruzi had been added. The insects were weighed before and after feeding on the rabbit blood, and then they were dissected on the 10th, 20th, and 30th day after infection. Both the hybrids and the parentals remained infected throughout the experiment. The parasite was mostly found in the epimastigote form. The number of epimastigotes was significantly lower in the stomach and small intestine of T. juazeirensis than in the hybrids or in T. b. brasiliensis. A significantly higher percentage of metacyclic trypomastigotes was detected in the small intestine and rectum of the hybrids. Hybrids demonstrated higher susceptibility to the TcI 0354 strain than their parentals, opening up new avenues to be investigated.
1. Introduction
Chagas disease remains one of the most important and yet neglected diseases in the world, and until now, there have been no drugs available to cure the illness in its chronic phase [1]. Therefore, strategies that aim to monitor and control insect vectors are the most effective measures to prevent the transmission of the protozoan Trypanosoma cruzi, an etiologic agent, to humans [2]. American trypanosomiasis, better known as Chagas disease, affects more than six million people around the world, and changes in its epidemiology pose new challenges for controlling the illness [2,3]. Currently, there are more than 155 triatomine species recognized as potential vectors of T. cruzi that occur mainly in Central and South America; however, only a dozen of them offer a real risk for the transmission of this etiological agent to human populations [4,5,6,7,8].
Trypanosoma cruzi is a flagellated protozoan parasite of mammals that is transmitted via bloodsucking insects of the Triatominae subfamily. It causes Chagas disease, an important human disease in Neotropical America [4,9,10]. Distinct methods of transmission of the protozoan have also been reported among human populations, for instance, through the consumption of contaminated wild animals [11,12,13]. The disease is now considered a global public health problem due to human migration from endemic to non-endemic areas, where countries such as Australia, Canada, Japan, Spain, and the United States of America have the highest number of immigrants infected by T. cruzi [14].
The parasite develops inside the triatomine insect’s intestinal tract, and its first transformation from trypomastigotes to epimastigotes takes place in the stomach, where the blood is stored nearly undigested. When the parasite reaches the small intestine, a multiplication boost occurs, and the parasite population density increases. Some of the parasites migrate to the rectum, while others continue to multiply in this portion of the intestinal tract. The different properties of the environment of the rectum provide better attachment conditions for the parasite, which leads to a transformation in its infective form, i.e., the metacyclic trypomastigote form [15]. Subsequently, the insects eliminate the parasite’s infective form in their feces and urine, which can be deposited on the skin of the mammal host species, eventually transmitting T. cruzi [12,16,17]. The triatomines’ vector capacity is related to several factors: their geographic distribution, feeding behavior, and physiological, genetic, and environmental parameters [17,18,19,20].
The Triatoma brasiliensis species complex is a monophyletic group of the Triatominae subfamily, and multidisciplinary studies have been carried out to reveal the phylogenetic relationships among the species [21,22,23,24,25,26]. Currently, this complex encompasses two subspecies and six species: T. b. brasiliensis Neiva, 1911; T. b. macromelasoma Galvão, 1956; T. bahiensis Sherlock & Serafim, 1987; T. juazeirensis Costa & Felix, 2006; T. lenti Sherlock & Serafim, 1967; T. melanica Neiva e Lent, 1941; T. petrocchiae Pinto & Barreto, 1925; and T. sherlocki Papa et al., 2002. The members of this species complex are of distinct epidemiologic importance, have a clear geographic distribution, and can be distinguished by their morphological characteristics [22,27,28,29,30]. Triatoma b. brasiliensis is one of the most important triatomine species in northeastern Brazil because of its wide geographic distribution (in five Brazilian states), its high rate of domiciliation, and its spread through natural infection [27,29,31,32]. Moreover, the programs to control Triatoma infestans (Klug, 1834), which was previously considered the main vector in Latin America, were not so effective for T. b. brasiliensis, a native vector, which colonized not just human domiciles but also infested several artificial and natural ecotopes [6,27,31,32,33,34,35,36,37].
Morphometric studies on the T. brasiliensis species complex have identified T. b. macromelasoma as a possible hybridization product between T. juazeirensis and T. b. brasiliensis, suggesting, for the first time, a homoploidal hybrid speciation in the triatomine group [38]. In addition, thirteen different phenotypes of T. b. brasiliensis were found in peridomiciliary areas in the state of Pernambuco, and their intermediate patterns were confirmed using molecular tools, establishing them as hybrids of members of this species complex [39].
The triatomine’s susceptibility to infection depends on several factors, such as the vector species becoming infected by the parasite; the parasite density, which is modulated by the insect’s physiological barriers [15,40]; the multiplication rates of the parasite; the capacity of the parasite to reach the insect’s rectum [41]; and adaptation of the parasite strain to the triatomine species [40,42,43]. On the other hand, T. cruzi strains have biological, biochemical, molecular, and genetic diversity, along with eco-epidemiological complexity [44,45]. Therefore, interactions between the parasite and insect vectors raise complex questions that are yet to be understood.
Studies on the capacity of the triatomine species to become infected with the parasite T. cruzi and how this is associated with its capacity to colonize human domiciles are of great importance for public health and governmental services since this information is crucial for the application of more precise monitoring and measures to control the Chagas disease vectors [2,27,46].
The objective of this study was to explore the susceptibility of the hybrids from T. b. brasiliensis and T. juazeirensis to T. cruzi in comparison to the susceptibility of their parental subspecies and species by performing experimental infection.
2. Materials and Methods
2.1. Insects
Triatoma b. brasiliensis and T. juazeirensis fifth-instar nymphs from laboratory colonies kept under standardized conditions (52–70% relative humidity and 23–24.8 °C) were randomly selected (Table 1).

Table 1.
Data on the localities of the founder species of the colonies.
Initially, 10 fifth-instar nymphs (5 males and 5 females) from each species were sexed and separated into containers (14 × 14 × 15 cm) to obtain virgin adults. The insects were fed once a week on Swiss Webster mice (license: LW-18/11 from the Ethics Committee on the Use of Animals of the Oswaldo Cruz Institute (CEUA-IOC)) until reaching the imaginal molt. When some of the specimens died, they were replaced by other virgin adults. Thirty other fifth-instar nymphs of T. b. brasiliensis and T. juazeirensis were kept starving for 30 days and were then used in the infection experiment.
2.2. Experiments of Species Crossing
After the imaginal molt, interspecies crosses of T. b. brasiliensis females × T. juazeirensis males and T. juazeirensis females × T. b. brasiliensis males were performed in separate containers, and they were fed mice once a week. The hybrids were named “Hbj” and “Hjb”.
The F1 hybrids, thus obtained, were placed in separate containers and were reared until at least 30 fifth-instar nymphs had been obtained from each crossing. In total, 30 fifth-instar specimens of T. b. brasiliensis and T. juazeirensis plus 60 F1 hybrids (30) of each crossing combination were used in the infection experiment with strain 0354 of T. cruzi (Figure 1).
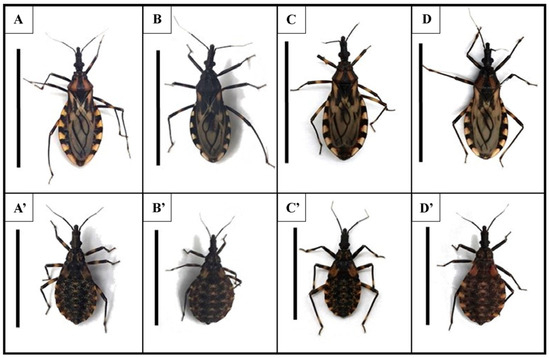
Figure 1.
General aspect of adults (top) and fifth-instar nymphs (bottom) of Triatoma brasiliensis (A,A’), Triatoma juazeirensis (B,B’), and their reciprocal hybrids “Hbj” (♀ T. b. brasiliensis × ♂ T. juazeirensis) (C,C’) and “Hjb” (♀ T. juazeirensis × ♂ T. b. brasiliensis) (D,D’), scale= 25mm for adults and 20 mm for nymphs.
2.3. Trypanosoma cruzi
The parasite isolate used in this study was obtained from the institutional trypanosome collection consisting of wild and domestic mammals and vectors (Coleção de Trypanosoma de Mamíferos Silvestres, Domésticos e Vetores, COLTRYP). Trypanosoma cruzi strain 0354 (TcI) was originally isolated from T. b. brasiliensis from Caicó, its type locality, and it has been maintained through cryopreservation at −195 °C in liquid nitrogen since 2006. This isolate was previously characterized by means of multiplex PCR on the mini-exon gene, as described by another study [47].
Epimastigotes were grown in MacNeal, Novy, and Nicolle (NNN) medium with a liver infusion tryptose (LIT) overlay and supplemented with 10% fetal bovine serum, as previously described in [40,48].
2.4. Infective Feeding of the Insects
Thirty fifth-instar nymphs of the parental species (T. b. brasiliensis and T. juazeirensis) and their hybrids (Hbj and Hjb) were artificially fed through latex membranes 10 mL of citrated rabbit blood (0.1 mL of sodium citrate/mL), which had been centrifuged at a speed of 3500 rpm for 10 min to separate the plasma from the erythrocytes. We removed the plasma and washed the erythrocytes three times with phosphate-buffered saline (PBS; pH 7.2).
After washing, the erythrocytes were resuspended in LIT (the same volume as that of the plasma removed) containing 1.5 × 107/mL epimastigotes in the exponential growth phase (v/v), and they were counted using a hemocytometer. This mixture was then transferred to a glass bottle where peripheral circulation of heated water was carried out at a temperature of around 37 °C. A latex membrane coated the open bottom of the bottle, on which the insects fed. Fifteen insects were divided between two bottles so that all of the insects could move and reach the food source. Thus, only fully engorged insects were used in the experiment.
For this experiment, the insects were previously and individually marked with different gouache colors (non-toxic) on the legs and pronotum, allowing for the differentiation of each of the specimens. Then, we weighed the insects individually on a precision scale (Libor AEG, Shimadzu, Kyoto, Japan) before and after the infective meal. Thus, it was possible to measure their blood volume ingestion by calculating the weight difference, which was based on the weight before and after the blood meal (Supplementary Table S1).
Fifteen days after the first feeding, the insects were fed again with chicken blood without parasites because the percentage of flagella declines if the insects are subjected to long periods of fasting [15].
2.5. Analysis of T. cruzi Forms in Insects
Ten triatomines were dissected 10, 20, and 30 days after the infective blood meal, totaling thirty insects in each group. The outer edge of the abdomen of the triatomine (conexivum) was cut in the posterior–anterior direction and, with the dorsal region exposed, the entire digestive tract was removed and transferred to a Petri dish where it was divided into three segments: stomach, intestine, and rectum. These segments were then macerated in 200 μL of PBS (pH 7.2).
To examine the insect’s biological content (feces, urine, and digestive tract tissues), each segment of the digestive tract was individually displayed on a hemocytometer to count the distinct parasite forms: epimastigotes, metacyclic forms, and transitional forms [40,42,48,49,50,51] (Supplementary Table S2). For a better understanding and visualization of the percentages in the distinct compartments of the insect’s gut, two tables are presented wherein the first shows the percentages of the different T. cruzi forms across the experiment and the second compares the percentages of the parasite forms in each gut compartment.
2.6. Statistical Analyses
The effects of the number of days after the infective blood meal and groups on the number of parasite forms in the stomach, intestine, and rectum were analyzed by fitting generalized linear models (GLM) with the Poisson error distribution, which was corrected for overdispersion. The choice of the best statistical model applied in the analysis was made through comparisons between the adjustments of the complete models and models with reduced variables, where a maximum likelihood test was used to compare changes in deviances before and after removing variables. Interactions between variables were considered only when significant. The goodness of fit was determined using “half-normal-plot” plots with simulated envelopes at a 95% significance level. For the analysis, the statistical software R version 4.1.3 (The R Foundation for Statistical Computing; http://www.R-project.org, accessed 4 May 2023) was used. When the effect of the variables was significant, the averages of the number of parasite forms were compared using the cld function of the multcomp package of the R software (R Core Team 2020).
3. Results
3.1. Percentage of the T. cruzi Forms across the Days of Analysis
The forms found in the digestive tube were classified as epimastigotes, trypomastigotes, and transition forms. The latter includes any parasite intermediate forms, as already described in the literature [40]. Table 2 shows the variation in the relative percentage of T. cruzi forms (per specimen, for each time) of each form found in each compartment of the digestive tube over the course of the analysis. It was verified that there was a higher percentage of epimastigote forms in all compartments of the digestive tube in both parentals and hybrids, despite their numbers decreasing between the stomach and the rectum until the end of the experiment. The highest percentages of metacyclic forms (the infective ones) in the rectum were observed in the hybrids Hbj and Hjb.

Table 2.
Percentages of different forms of Trypanosoma cruzi found in different compartments of the digestive tube (stomach, intestine, and rectum) on the 10th, 20th, and 30th days after infection in Triatoma b. brasiliensis, Triatoma juazeirensis, and their hybrids Hbj (♀ T. b. brasiliensis × ♂ T. juazeirensis) and Hjb (♀ T. juazeirensis × ♂ T. b. brasiliensis) throughout the experiment.
The percentage of parasite numbers in the stomach declined over the course of the analysis for both hybrids and for T. b. brasiliensis. Triatoma juazeirensis also declined, but the kinetics were different (Table 2).
In the small intestine, the parasite percentage increased in the hybrids over the days of observation. The parentals also experienced increases in parasite percentages, although T. juazeirensis presented with the highest percentage on the 20th day, and for T. b. brasiliensis, the values were very close on the 20th and 30th days (Table 2).
In general, the percentages of parasites in the rectum were lower when compared to the other parts of the digestive tube. There was also an increase in percentages of the parasites in the rectum over the course of the analysis, mainly for Hbj and T. b. brasiliensis (Table 2).
3.2. Developmental Stages in Each Compartment of the Digestive Tube
Epimastigotes were the predominant form in all compartments and in all groups, demonstrating the highest percentages across all of the days of observation (Table 3). Transitional forms, when compared to the epimastigotes, were recorded in lower percentages in all compartments of the digestive tract in all of the insects, with oscillating values in both the hybrids and the parentals (Table 3). Trypomastigotes were observed in all parts of the digestive tract of the insects, although they had lower percentages compared to the epimastigotes and demonstrated oscillating values when compared to the transitional form. Among the specimens studied, T. b brasiliensis and T. juazeirensis had the lowest numbers of trypomastigotes in their intestines (Table 3).

Table 3.
Relative percentages of different forms of Trypanosoma cruzi found in different compartments of the digestive tube (stomach, intestine, and rectum) on the 10th, 20th, and 30th days after infection in Triatoma b. brasiliensis, Triatoma juazeirensis, and their hybrids Hbj (♀ T. b. brasiliensis × ♂ T. juazeirensis) and Hjb (♀ T. juazeirensis × ♂ T. b. brasiliensis).
The number of epimastigotes (F1,85 = 65.6, p < 0.001), transition forms (F1,85 = 16.7, p < 0.001), and trypomastigotes (F1,85 = 11.63, p < 0.001) of T. cruzi in the insects’ stomachs differed after long periods of time. On the other hand, the number of epimastigotes (F3,86 = 1.43, p = 0.23) and transition forms (F3,86 = 0.67, p = 0.56) of T. cruzi was not different among the triatomine groups (parentals and hybrids). Only the number of trypomastigotes was significantly distinct among the groups (F3,86 = 5.83, p < 0.01).
The number of epimastigotes and transition forms in the small intestine varied either with time (F1,113 = 5.32, p < 0.05 and F1,113 = 5.62, p < 0.05, respectively) or based on the triatomine group (F3,113 = 5.53 p < 0.05 and F1,113 = 11.38, p < 0.05, respectively). The variation in the number of trypomastigotes in the small intestine was affected by the interaction effects of the triatomine group and time (time: F1,113 = 6.93 p < 0.05; triatomine group: F3,114 = 3.43, p < 0.05; time× triatomine group: F3,110 = 4.75, p < 0.05). In this case, the highest percentages of trypomastigotes were found in hybrids Hbj and Hjb with oscillating values across the days of observation in both groups. In the parentals, the highest values were recorded on the 30th day, being 5% and 4% for T. juazeirensis and T. b. brasiliensis, respectively (Table 3).
The number of epimastigotes and transient forms in the rectum varied only with the observation time (F1,93 = 6.55, p < 0.05 and F1,93 = 4.62, p < 0.05, respectively). However, the different groups of triatomines had no significant effect on these T. cruzi forms (F3,94 = 0.279, p = 0.83; F3,94 = 6.55, p = 0.348). In general, the number of these forms of T. cruzi increased with observation time.
In the rectum, the hybrids showed the highest percentages of trypomastigotes (16% on the 20th day in the Hbj and 31% on the 20th day in the Hjb), when compared to the parentals. In T. juazeirensis and T. b. brasiliensis, trypomastigotes were not recorded on the 10th day, while their highest percentages (6.5% and 7%, respectively) were observed on the 30th day (Table 3).
3.3. Blood Meal Volume
As shown in Supplementary Table S1, some specimens with low infectivity ingested the same amount of blood as those that had a higher infection rate. The correlation analyses between the amount of blood ingested and parasitic infection were not significant for all tests (additional files 2–5; p > 0.05), with the exception being the correlation between T. b. brasiliensis and the amount of blood ingested on the 20th day after the blood meal.
4. Discussion
In the literature, few studies have addressed the susceptibility of natural or experimental hybrids of triatomines to infection by T. cruzi. Therefore, it is extremely important to understand how a particular strain of this etiologic agent can interact with the triatomine vector species and their hybrids. In this unprecedented study, susceptibility to the T. cruzi strain 0354 (TcI) infection was evaluated by comparing T. b. brasiliensis, T. juazeirensis, and their reciprocal experimental hybrids. In our experiment, under laboratory conditions, it was revealed that the hybrids of the T. brasiliensis complex are able to be infected by T. cruzi, and they developed the infective forms in the rectum in higher percentages than their parentals. It is important to stress that in some cases, hybrids may be sterile, which would reduce their epidemiological importance. However, this is not the case for the hybrids of the T. brasiliensis complex, specifically those between T. b. brasiliensis and T. juazeirensis, since their fertility was already demonstrated until the F3 phase under laboratory conditions [21]. In addition to this, it was suggested more recently that the species’ sexual choice is not always conspecific but can increase genetic variability, which emphasizes the importance of better understanding the hybrids’ vector competence and capacity [52]. We also want to stress that the parentals, T. b brasiliensis and T. juazeirensis, play a relevant role as vectors of the etiologic agent of Chagas disease in several states of the northeast region of Brazil [27,28,29,30,31,32,36,37,53].
The host–parasite relationship drives triatomines to transmit T. cruzi to susceptible hosts, which involves several factors such as the morphogenesis process of the parasite [54]; the susceptibility of the insect vector to the parasite strain [55]; the average time between blood-feeding and infective defecation, which occurs when the triatomine is still in direct contact with the host’s skin; and the number of blood meals at each stage during the insect developmental cycle [56]. These characteristics provide important information that enables us to evaluate the vector capacity of the triatomine species [57], which covers several biological, ecological, and behavioral parameters of the insect; also, we can evaluate their vector competence, which is estimated as the proportion of individuals susceptible to the etiological agent within the population [58].
In our experiment, it was found that the epimastigote form was prevalent in all compartments of the digestive tract in both the hybrids and the parentals. This can be corroborated with previous findings where T. infestans was infected with the Y strain, in which epimastigotes, and occasionally amastigotes and metacyclic forms, were found in the intestine [59].
According to a previous analysis of the interaction of a T. cruzi strain with a particular vector species, the proportionality of the numbers of epimastigotes, spheromastigotes, and metacyclic trypomastigotes in the vector digestive tract can be modified according to several aspects, including the particularities of each specimen [50]. Our results showed that the populations of epimastigotes and transitional forms in the stomach of T. juazeirensis were significantly lower either in the hybrids or in T. b. brasiliensis. This difference in the proportion of these T. cruzi populations may affect the metacyclogenesis, which appears to be vector-dependent [40].
The decrease in the epimastigote population in the rectum and the increase in the metacyclic population in the gut are expected events in vector species susceptible to T. cruzi infection, such as Rhodnius neglectus Lent, 1954, Rhodnius Prolixus Stal, 1859, and Panstrongylus megistus (Burmeister, 1835), when infected with the Y strain [40]. In the present experiment, a higher prevalence of epimastigotes was observed in all insects’ gut compartments. The trypomastigotes had lower numbers over the experiment compared to the other forms; however, this form was recorded in significantly higher percentages in the hybrids’ rectums. An experimental infection carried out with members of the T. brasiliensis complex using the 913 strain demonstrated that the development of the parasite was similar in all vectors. However, mice infected with the 913 strain from T. b. macromelasoma (a subspecies with a hybrid origin) [23,38] had higher rates on the 20th day after infection [43]. In the present study, it was observed the experimental hybrids showed a decrease in the parasite population (in the case of Hjb) after that period (the 20th day).
Transitional forms were also distributed throughout the insects’ guts and were present in all observation periods, but their peak density oscillated over different days and groups. The presence of transitional forms is necessary for parasite development because the transformation from the epimastigote into the metacyclic form leads to an indeterminate form [40]. The speed at which this transformation occurs depends on the nature of the T. cruzi strain since the faster the strain can perform this process, the better the multiplication conditions will be for establishing an infection, and higher production of metacyclic trypomastigotes will also occur [54].
An experiment performed with T. infestans showed a tendency to overcome infection with the Y strain, whereas P. megistus was the species most susceptible to infection. This latter species had a better interaction with the Y strain, such that it continued to present with infection over a period of time [60]. In our experiment, the analysis of strain 0354 demonstrated that it is able to interact with T. b. brasiliensis and T. juazeirensis, especially with the hybrids. Sometimes, the strains fail to complete their life cycle, i.e., they fail to infect the digestive tract of the insect or present with low levels of infection [48]. In a previous study, for example, the infectivity of strain 0354 showed different behavior in T. infestans. The epimastigote form was prevalent throughout the digestive tract until the 20th day after infection, but on the 30th day, the trypomastigote metacyclic form was most frequent, especially in the rectum; this was different in the hybrids and parentals, in which the epimastigote form was more prevalent during all observation periods. This suggests that the development of strain 0354 in the T. brasiliensis species complex and hybrids was slower than in T. infestans. In analyzing the development of T. cruzi in the insect digestive tract, we should consider the digestive tract as a series of micro-habitats in which the parasite interacts with different factors that can modulate its development, such as the amount of blood ingested, hemolytic factors, the perimicrovillar membrane, and the insect’s innate immune system [40,41,42,61].
Triatoma b. brasiliensis presents with high rates of infection in nature [31,32]. On the contrary, the present study, it demonstrated a low percentage of metacyclic forms. Other studies showed that other species that are also often naturally found to have high rates of infection presented with low percentages under laboratory conditions [40].
The results from T. juazeirensis showed a slower process of metacyclogenesis since the metacyclic trypomastigote percentages were the lowest ones. Like the hybrids and T. b. brasiliensis, this species showed an ability to host and maintain the parasite. Nonetheless, T. juazeirensis is often found to have a low rate of natural infection when compared to the other species, as already mentioned in the literature [31,32,34,53].
For the insects to become infected with the parasite, they need to ingest an infective blood meal. Some studies have suggested that for high levels of infection, the insects would need to ingest large amounts of blood infected with the parasite. Nevertheless, it is also known that the persistence of the parasite in the insect will depend on several factors relating to the insect’s immune system [15]. In our study, insects with high levels of infection (~49 × 105) ingested the same or lower amounts of blood (~220 mg) than the specimens with lower levels of infection (~4 × 105). Moreover, specimens that ingested very small amounts of blood (e.g., 91 mg) had a sufficient quantity of parasites to initiate and maintain the infection in their digestive tract for up to 30 days after feeding. Another study evaluating the susceptibility of four species of triatomines to infection with the Y strain also found the amount of blood ingested did not have any influence on the level of infection presented by the insects [55].
All of the insect groups analyzed were susceptible to infection with T. cruzi strain 0354, but the hybrids maintained greater stability of infectivity, presenting with higher numbers of metacyclic trypomastigotes in the rectum throughout the experiment and across all observation periods. This means that the hybrids could be more susceptible to infection than the parentals. This difference between parentals and hybrids might be the result of the distinct efficiency of qualitative interactions between the strain and the vector. In comparison with T. infestans, P. megistus also became infected with the T. cruzi Y strain more efficiently, thus demonstrating higher numbers of positive insects, increased positivity over time, and a higher maintenance rate of infection [60].
Small intraspecific differences in the susceptibility among triatomines infected with the same strain of T. cruzi have been proven to occur. A group of T. infestans reared in the same colony showed different rates of infection in comparison with a group of specimens newly captured from the natural environment [62]. This may explain why T. b. brasiliensis initially presented with infection with strain 0354 at a lower rate than that of the hybrids. Additionally, this vector is known for its high infection rates in natural environments, and it was expected to have higher levels than the other groups [27,31,32].
The natural ecotope of the insects also needs to be considered since the specimens used in these experiments were all reared under laboratory conditions. Some studies have emphasized the superiority of wild vectors over domestic ones for studying T. cruzi infection [61], such as T. infestans and R. neglectus infected with the Y strain, which had a lower infection rate than Dipetalogaster maxima (Uhler, 1894) and Triatoma matogrossensis Leite & Barbosa, 1953, and which are typically wild species that invade domiciles [55].
The results reported here open up new avenues to be explored and can be applied for triatomine control. The interaction between T. cruzi and triatomines is a complex issue; therefore, the higher susceptibility to T. cruzi observed in the hybrid specimens could be associated with specific factors that must be deeply analyzed and experimentally tested. Among the possibilities for future related studies that compare triatomine hybrids and parentals, studying the gut components and microbiota, as stressed by Fuentes–Vicente [20], could help to improve the triatomine control program and reduce the use of insecticides.
5. Conclusions
In the present study, the 0354 T. cruzi strain was shown to be able to develop and maintain its cycle in the digestive tract of T. b. brasiliensis and T. juazeirensis as well as in that of their experimental hybrids. The obtained results, in addition to recent findings that showed the presence of natural hybrids in areas where different species of the T. brasiliensis complex are circulating, contribute to the consideration of the potential participation of these hybrids in the natural transmission cycle of T. cruzi. The hybrids showed greater parasite distributional homogeneity for up to 30 days after infection. However, further analysis is required to determine whether T. b. brasiliensis might present with more infective forms in the rectum, even though its development initially was slower. The same analyses would be necessary for T. juazeirensis; nevertheless, this species showed greater numbers of negative specimens than the other analyzed groups, and therefore, its vector competence seems to be less effective than that observed for T. b. brasiliensis and the hybrids.
The present study points out that the hybrids, as potential vectors of T. cruzi, are able to transmit the etiological agent in the natural hybrid zones where T. b. brasiliensis and T. juazeirensis can be found and enable us to understand the dynamics of some of the mechanisms that occur in the development of strain 0354. Further analysis of the susceptibility of these insects to different T. cruzi strains, as well as the infection of these hybrids in the natural environment, needs to be conducted.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms11122850/s1, Supplementary Table S1. Volume of blood (mg) ingested and parasitic population density per specimen on the 10th, 20th, and 30th days after the blood meal. Supplementary Table S2. Number of T. cruzi parasites in each segment of the digestive tract individually displayed on a hemocytometer to count the distinct forms: epimastigotes, transitional forms, and trypomastigotes.
Author Contributions
J.C. and C.J.d.C.M. formulated the idea of the study. N.C., C.J.d.C.M. and J.C. designed the experiment. N.C. and C.J.d.C.M. carried out the experiments. C.R. carried out the static analysis and validated the results. N.C. wrote the first draft of the manuscript. C.J.d.C.M., L.P., C.R., T.C.M.G. and J.C. contributed to further versions. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), and the National Council for Scientific and Technological Development—CNPq for J.C.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article and its additional files.
Acknowledgments
We are grateful to the technicians Marcos Antônio Lima dos Santos and Carlos Alberto Ardé Ruiz and to Ana Maria Jansen from the Trypanosomatid Biology Laboratory for kindly providing the isolates and for their initial assistance with the isolate cultures.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
Hbj—F1 hybrids from crossing ♀ T. b. brasiliensis × ♂ T. juazeirensis; Hjb—F1 hybrids from crossing ♀ T. juazeirensis × ♂ T. b. brasiliensis; BA_Bahia; RN_Rio Grande do Norte; COLTRYP—Collection of Trypanosoma of Wild and Domestic Mammals and Vectors, from the Oswaldo Cruz Institute; PCR—polymerase chain reaction; NNN—biphasic culture medium (MacNeal, Novy, and Nicolle); LIT—liver infusion tryptose culture medium; v/v—volume/volume; PBS—phosphate-buffered saline.
References
- Stanaway, J.D.; Roth, G. The burden of Chagas disease estimates and challenges. Glob. Heart 2015, 10, 139–144. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organization. Chagas Disease (American Trypanosomiasis). 2023. Available online: https://www.who.int/news-room/facts-in-pictures/detail/chagas-disease (accessed on 20 April 2023).
- Cordovez, J.M.; Sanabria, C. Environmental changes can produce shifts in Chagas disease infection risk. Environ. Health Insights 2014, 2, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Lent, H.; Wygodzinsky, P. Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas’ disease. Bull. Am. Mus. Nat. Hist. 1979, 163, 125–520. [Google Scholar]
- Monteiro, F.A.; Weirauch, C.; Felix, M.; Lazoski, C.; Abad-Franch, F. Evolution, Systematics, and Biogeography of the Triatominae, Vectors of Chagas Disease. Adv. Parasitol. 2018, 99, 265–344. [Google Scholar]
- Alevi, K.C.C.; de Oliveira, J.; da Silva Rocha, D.; Galvão, C. Trends in Taxonomy of Chagas Disease Vectors (Hemiptera, Reduviidae, Triatominae): From Linnaean to Integrative Taxonomy. Pathogens 2021, 10, 1627. [Google Scholar] [CrossRef]
- Costa, J.; Dale, C.; Galvão, C.; Almeida, C.E.; Dujardin, J.P. Do the new triatomine species pose new challenges or strategies for monitoring Chagas disease? An overview from 1979–2021. Mem. Inst. Oswaldo Cruz 2021, 116, 1–10. [Google Scholar] [CrossRef]
- Gil-Santana, H.R.; Chavez, T.; Pita, S.; Panzera, F.; Galvão, C. Panstrongylus noireaui, a remarkable new species of Triatominae (Hemiptera, Reduviidae) from Bolivia. ZooKeys 2022, 1104, 203–225. [Google Scholar] [CrossRef]
- Chagas, C. Nova tripanozomiaze humana. Estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp. ajente etiolojico de nova entidade morbida do homem. Mem. Inst. Oswaldo Cruz 1909, 1, 159–218. [Google Scholar] [CrossRef]
- Jansen, A.M.; de Souza, R.T.; Roque, A.L.R.; das Chagas Xavier, S.C. Trypanosoma cruzi: An Ancient and Successful Enzootic Parasite. In Infectious Tropical Diseases and One Health in Latin America; Parasitology Research Monographs; Mehlhorn, H., Heukelbach, J., Eds.; Springer: Cham, Switzerland, 2022; Volume 16. [Google Scholar]
- Castro, E. Chagas’ disease: Lessons from routine donation testing. Transfus. Med. 2009, 19, 16–23. [Google Scholar] [CrossRef]
- Dias, J.C.P.; Borges-Pereira, J.; Macedo, V.L. Doença de Chagas. In Dinâmica das Doenças Infecciosas e Parasitárias; Coura, J.R., Ed.; Editora Guanabara Koogan: Rio De Janeiro, Brazil, 2013; pp. 606–641. [Google Scholar]
- Kransdorf, E.P.; Zakowski, P.C.; Kobashigawa, J.A. Chagas disease in solid organ and heart transplantation. Curr. Opin. Infect. Dis. 2014, 27, 418–424. [Google Scholar] [CrossRef]
- Coura, J.R.; Vĩñas, P.A. Chagas disease: A new worldwide challenge. Nature 2010, 465 (Suppl. S7301), S6–S7. [Google Scholar] [CrossRef]
- Kollien, A.H.; Schaub, G.A. The Development of Trypanosoma cruzi in Triatominae. Parasitol. Today 2000, 16, 381–387. [Google Scholar] [CrossRef]
- Guarneri, A.A.; Silva-Cardoso, L.; Atella, G. Interação Parasito-Vetor (Tripanossomatídeos). In Tópicos Avançados em Entomologia Molecular; Silva-Neto, M.A.C., Winter, C.E., Termignoni, C., Eds.; INCT—Entomologia Molecular; Tabajara da Silva Vaz Junior: Rio de Janeiro, Brazil, 2012; Volume 1, pp. 1–44. [Google Scholar]
- Silva, F.S. A importância hematofágica e parasitológica da saliva dos insetos Hematófagos. Rev. Tróp. –Ciênc. Agrár. Biol. 2009, 3, 3–19. [Google Scholar]
- Dias, E. Observações sobre eliminação de dejeções e tempo de sucção em alguns triatomíneos sul-americanos. Mem. Inst. Oswaldo Cruz 1956, 54, 115–124. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gurgel-Gonçalves, R.; Galvao, C.; Costa, J.; Peterson, A.T. Geographic distribution of Chagas disease vectors in Brazil based on ecological niche modeling. J. Trop. Med. 2012, 2012, 705326. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Vicente, J.A.; Gutiérrez-Cabrera, A.E.; Flores-Villegas, A.L.; Lowenberger, C.; Benelli, G.; Salazar-Schettino, P.M.; Córdoba-Aguilar, A. What makes an effective Chagas disease vector? Factors underlying Trypanosoma cruzi-triatomine interactions. Acta Trop. 2018, 183, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Almeida, C.E.; Dujardin, J.P.; Beard, C.B. Crossing experiments detect genetic incompatibility among populations of Triatoma brasiliensis Neiva, 1911 (Heteroptera, Reduviidae, Triatominae). Mem. Inst. Oswaldo Cruz 2003, 98, 637–639. [Google Scholar] [CrossRef]
- Costa, J.; Lima-Neiva, V.; Almeida, C.E. O complexo Triatoma brasiliensis (Hemiptera, Reduviidae, Triatominae) como modelo de estudo: Uma abordagem multidisciplinar e ecoepidemiológica. Med. Trop. Vetores 2020, 6, 99–121. [Google Scholar]
- Monteiro, F.A.; Donnelly, M.J.; Beard, C.B.; Costa, J. Nested clade and phylogeographic analyses of the Chagas disease vector Triatoma brasiliensis in Northeast Brazil. Mol. Phylogenet. Evol. 2004, 32, 46–56. [Google Scholar] [CrossRef]
- Mendonça, V.J.; da Silva, M.T.; de Araujo, R.F.; Martins, J., Jr.; Bacci, M., Jr.; Almeida, C.E. Phylogeny of Triatoma sherlocki (Hemiptera: Reduviidae: Triatominae) inferred from two mitochondrial genes suggests its location within the Triatoma brasiliensis complex. Am. J. Trop. Med. Hyg. 2009, 81, 858–864. [Google Scholar] [CrossRef]
- Mendonça, V.J.; Alevi, K.C.C.; Pinotti, H.; Gurgel-Gonçalves, R.; Pita, S.; Guerra, A.L.; Panzera, F.; Araújo, R.F.; Azeredo-Oliveira, M.T.V.; Rosa, J.A. Revalidation of Triatoma bahiensis Sherlock & Serafim, 1967 (Hemiptera: Reduviidae) and phylogeny of the T. brasiliensis species complex. Zootaxa 2016, 4107, 239–254. [Google Scholar] [PubMed]
- Oliveira, J.; Marcet, P.L.; Takiya, D.M.; Mendonça, V.J.; Belintani, T.; Bargues, M.D.; Mateo, L.; Chagas, V.; Folly-Ramos, E.; Cordeiro-Estrela, P.; et al. Combined phylogenetic and morphometric information to delimit and unify the Triatoma brasiliensis species complex and the brasiliensis subcomplex. Acta Trop. 2017, 170, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Almeida, C.E.; Dontson, E.; Lins, A.; Vinhaes, M.C.; Silveira, A.C.; Beard, C.B. The epidemiologic importance of Triatoma brasiliensis as a Chagas disease vector in Brazil: A revision of domiciliary captures during 1993–1999. Mem. Inst. Oswaldo Cruz 2003, 98, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Correia, N.C.; Neiva, V.L.; Goncalves, T.C.M.; Felix, M. Revalidation and redescription of Triatoma brasiliensis macromelasoma Galvão, 1956 and an identification key for the Triatoma brasiliensis complex (Hemiptera: Reduviidae: Triatominae). Mem. Inst. Oswaldo Cruz 2013, 108, 785–789. [Google Scholar] [CrossRef]
- Costa, J.; Dornak, L.L.; Almeida, C.D.; Townsend-Peterson, A. Distributional potential of the Triatoma brasiliensis species complex at present and under scenarios of future climate conditions. Parasite Vectors 2014, 7, 238. [Google Scholar] [CrossRef]
- Dale, C.; Almeida, C.E.; Mendonça, V.J.; Oliveira, J.; da Rosa, J.A.; Galvão, C.; Costa, J. An updated and illustrated dichotomous key for the Chagas disease vectors of Triatoma brasiliensis species complex and their epidemiologic importance. ZooKeys 2018, 805, 33–43. [Google Scholar] [CrossRef]
- Lillioso, M.; Ramos, E.F.; Rocha, F.L.; Rabinovich, J.; Capdevielle-Dulac, C.; Harry, M.; Marcet, P.L.; Costa, J.; Almeida, C.E. High Triatoma brasiliensis Densities and Trypanosoma cruzi Prevalence in Domestic And Peridomestic habitats in the state of Rio Grande Do Norte, Brazil: The Source For Chagas Disease Outbreaks? Am. J. Trop. Med. Hyg. 2017, 6, 1456. [Google Scholar] [CrossRef]
- Lima-Neiva, V.; Toma, H.K.; Aguiar, L.M.A.; Lopes, C.M.; Dias, L.P.; Gonçalves, T.C.M.; Costa, J. The connection between Trypanosoma cruzi transmission cycles by Triatoma brasiliensis brasiliensis: A threat to human health in an area susceptible to desertification in the Seridó, Rio Grande do Norte, Brazil. PLoS Negl. Trop. Dis. 2021, 15, e0009919. [Google Scholar] [CrossRef]
- Silveira, A.C.; Vinhaes, M.C. Elimination of vector-borne transmission of Chagas disease. Mem. Inst. Oswaldo Cruz 1999, 94, 405–411. [Google Scholar] [CrossRef]
- Costa, J.; Almeida, J.R.; Britto, C.; Duarte, R.; Marchon-Silva, V.; Pacheco, R. Ecotopes, natural infection and trophic resources of Triatoma brasiliensis (Hemiptera, Reduviidae, Triatominae). Mem. Inst. Oswaldo Cruz 1998, 93, 7–13. [Google Scholar] [CrossRef]
- Dias, J.C.P. Southern Cone Initiative for the elimination of domestic populations of Triatoma infestans and the interruption of transfusional Chagas disease. Mem. Inst. Oswaldo Cruz 2007, 102, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, C.M.; Cavalcanti, L.P.G.; Souza, R.C.M.; Barbosa, S.E.; Xavier, S.C.C.; Jansen, A.M.; Ramalho, R.D.; Diotaiuti, L. Domestic, peridomestic and wild hosts in the transmission of Trypanosoma cruzi in the Caatinga area colonised by Triatoma brasiliensis. Mem. Inst. Oswaldo Cruz 2014, 109, 7. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, C.M.; Belisário, C.J.; Pessoa, G.C.D.; Barezani, C.P.; Rosa, A.C.L.; Ferreira, F.C.; Ramos, A.N., Jr.; Gurtler, R.E.; Diotaiuti, L. Microsatellite variation revealed panmictic pattern for Triatoma brasiliensis (Triatominae: Reduviidae) in rural northeastern Brazil: The control measures implications. BMC Genom. 2020, 21, 92. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Peterson, A.T.; Dujardin, J.P. Morphological evidence suggests homoploid hybridization as a possible mode of speciation in the Triatominae (Hemiptera, Heteroptera, Reduviidae). Infect. Genet. Evol. 2009, 9, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Bargues, M.D.; Neiva, V.L.; Lawrence, G.G.; Gumiel, M.; Oliveira, G.; Cabello, P.; Lima, M.M.; Dotson, E.; Provance, D.W.; et al. Phenotypic variability confirmed by nuclear ribosomal DNA suggests a possible natural hybrid zone of Triatoma brasiliensis species complex. Infect. Genet. Evol. 2016, 37, 77–87. [Google Scholar] [CrossRef][Green Version]
- Perlowagora-Szumlewicz, A.; Moreira, C.J.C. In vivo differentiation of Trypanosoma cruzi–1. Experimental evidence of the influence of vector species on metacyclogenesis. Mem. Inst. Oswaldo Cruz 1994, 89, 603–618. [Google Scholar] [CrossRef]
- Garcia, E.S.; Genta, F.A.; Azambuja, P.; Schaub, G.A. Interactions between intestinal compounds of triatomines and Trypanosoma cruzi. Trends Parasitol. 2010, 26, 499–505. [Google Scholar] [CrossRef]
- Carvalho-Moreira, C.J.; Spata, M.C.; Coura, J.R.; Garcia, E.S.; Azambuja, P.; Gonzalez, M.S.; Mello, C.B. In vivo and in vitro metacyclogenesis tests of two strains of Trypanosoma cruzi in the triatomine vectors Triatoma pseudomaculata and Rhodnius neglectus: Short/long-term and comparative study. Exp. Parasitol. 2003, 103, 102–111. [Google Scholar] [CrossRef]
- Costa, J.; Araújo, C.A.C.; Freitas, C.A.V.; Borges-Pereira, J. Are members of the Triatoma brasiliensis (Hemiptera, Reduviidae) species complex able to alter the biology and virulence of a Trypanosoma cruzi Strain? Neotrop. Entomol. 2015, 44, 186–193. [Google Scholar] [CrossRef]
- Zingales, B.; Andrade, S.G.; Briones, M.R.S.; Campbell, D.A.; Chiari, E.; Fernandes, O.; Schijman, A.G. A new consensus for Trypanosoma cruzi intraspecific nomenclature: Second revision meeting recommends TcI to TcVI. Mem. Inst. Oswaldo Cruz 2009, 104, 1051–1054. [Google Scholar] [CrossRef]
- Zingales, B.; Bartholomeu, D.C. Trypanosoma cruzi genetic diversity: Impact on transmission cycles and Chagas disease. Mem. Inst. Oswaldo Cruz 2022, 117, e210193. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hernandez, F.; Martínez-Ibarra, J.A.; Catalá, S.; Villalobos, G.; de la Torre, P.; Laclette, J.P.; Alejandre-Aguilar, R.; Espinoza, B. Natural crossbreeding between sympatric species of the Phyllosoma complex (Insecta: Hemiptera: Reduviidae) indicate the existence of only one species with morphologic and genetic variations. Am. J. Trop. Med. Hyg. 2010, 82, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, O.; Santos, S.S.; Cupolillo, E.; Mendonça, B.; Derre, R.; Junqueira, A.C.; Santos, L.C.; Sturm, N.R.; Naiff, R.D.; Barrett, T.V.; et al. A mini-exon multiplex polymerase chain reaction to distinguish the major groups of Trypanosoma cruzi and T. rangeli in the Brazilian Amazon. Trans. R. Soc. Trop. Med. Hyg. 2001, 95, 97–99. [Google Scholar] [CrossRef]
- Mello, C.B.; Azambuja, P.; Garcia, E.S.; Ratcliffe, N.A. Differential in Vitro and in Vivo Behavior of Three Strains of Trypanosoma cruzi in the Gut and Hemolymph of Rhodnius prolixus. Exp. Parasitol. 1996, 82, 112–121. [Google Scholar] [CrossRef]
- Kollien, A.H.; Schaub, G.A. The Development of Trypanosoma cruzi (Trypanosomatidae) in the Reduviid Bug Triatoma infestans (Insecta): Influence of Starvation. J. EukMicrobiol. 1998, 45, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, N.J.; Bronfen, E. Metaciclogênese do Trypanosoma cruzi como parâmetro de interação do parasita com o triatomíneo vetor. Rev. Soc. Bras. Med. Trop. 1997, 30, 247–250. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Araújo, C.A.C.; Waniek, P.J.; Jansen, A.M. Development of Trypanosoma cruzi (TcI) isolate in the digestive tract of an unfamiliar vector, Triatoma brasiliensis (Hemiptera, Reduviidae). Acta Trop. 2008, 107, 195–199. [Google Scholar] [CrossRef]
- Antunes, C.; Dias, L.P.; Guimarães, G.A.; Oliveira, J.; Rosa, J.A.; Almeida, C.E.; Lopes, C.M.; Gonçalves, T.C.M.; Costa, J. Sexual Choice in Males of the Triatoma brasiliensis Complex: A Matter of Maintenance of the Species or Genetic Variability? Open Parasitol. J. 2020, 8, 1–9. [Google Scholar] [CrossRef]
- Ribeiro, G.; dos Santos, C.G.S.; Lanza, F.; Reis, J.; Vaccarezza, F.; Diniz, C.; Miranda, D.L.P.; de Araújo, R.F.; Cunha, G.M.; de Carvalho, C.M.M.; et al. Wide distribution of Trypanosoma cruzi-infected triatomines in the State of Bahia, Brazil. Parasites Vectors 2019, 12, 604. [Google Scholar] [CrossRef]
- Garcia, E.S.; Vieira, E.; Gomes, J.E.P.L.; Gonçalves, A.M. Molecular biology of the interaction Trypanosoma cruzi invertebrate host. Mem. Inst. Oswaldo Cruz 1984, 79, 33–37. [Google Scholar] [CrossRef]
- Silva, I.G.; Salha, L.A. Aspectos da suscetibilidade dos triatomíneos ao Trypanosoma cruzi na busca de um modelo experimental. Rev. Pat. Trop. 1994, 23, 93–100. [Google Scholar] [CrossRef]
- Zeledón, R.; Alvarado, R.; Jirón, L.F. Observations on the feeding and defecation patterns of three triatomine species (Hemiptera: Reduviidae). Acta Trop. 1977, 34, 65–77. [Google Scholar] [PubMed]
- Galvão, C.; Jurberg, J.; Cunha, V.; De Mello, R.P. Biologia do Triatoma nitida Usinger 1939 em Laboratório (Hemiptera: Reduviidae). Mem. Inst. Oswaldo Cruz 1995, 90, 657–663. [Google Scholar] [CrossRef]
- Oliveira, R.L. Principais insetos vetores e mecanismos de transmissão das doenças infecciosas e parasitárias. In Dinâmica das Doenças Infecciosas e Parasitárias; Coura, J.R., Ed.; Editora Guanabara Koogan: Rio De Janeiro, Brazil, 2013; pp. 108–130. [Google Scholar]
- Schaub, G.A. Trypanosoma cruzi: Quantitative studies of development of two strains in small intestine and rectum of the vector Triatoma infestans. Exp. Parasitol. 1989, 68, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Bronfen, E.; Dias, J.C.P.; Gouveia, S.C. Infecção experimental de Triatoma infestans e Panstrongylus megistus pela cepa Y do Trypanosomacruzi (Silva e Nussenzweig, 1953). Rev. Patol. Trop. 1984, 13, 1–7. [Google Scholar]
- Perlowagora-Szumlewicz, A.; Muller, C.A.; Moreira, C.J.C. Studies in search of a suitable experimental insect model for xenodiagnosis of hosts with Chagas’ disease. 4- The reflection of parasite stock in the responsiveness of different vector species to chronic infection with different Trypanosoma cruzi stocks. Rev. Saúde Públ. 1990, 24, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Phillips, N.R.; Bertram, D.S. Laboratory studies of Trypanosoma cruzi infections. J. Med. Entomol. 1967, 4, 168–174. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).