Isolation and Characterization of Bacteriophage VA5 against Vibrio alginolyticus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Samples and Strains
2.1.2. Reagents and Media
2.2. Methods
2.2.1. Phage Isolation and Screening
2.2.2. Bacteriophage Purification and Titer Determination
2.2.3. Identification of Phage Morphology
2.2.4. Genome-Wide and Phylogenetic Analysis
2.2.5. Determination of Optimal Multiple Infection of Phage
2.2.6. One-Step Growth Curve of Phage
2.2.7. Effects of Temperature, Ultraviolet Irradiation, and pH on Phage Activity
2.2.8. Bacteriophage Inhibitory Activity
2.2.9. Determination of Host Bacteria Range
2.2.10. Statistics and Analysis of Data
3. Results
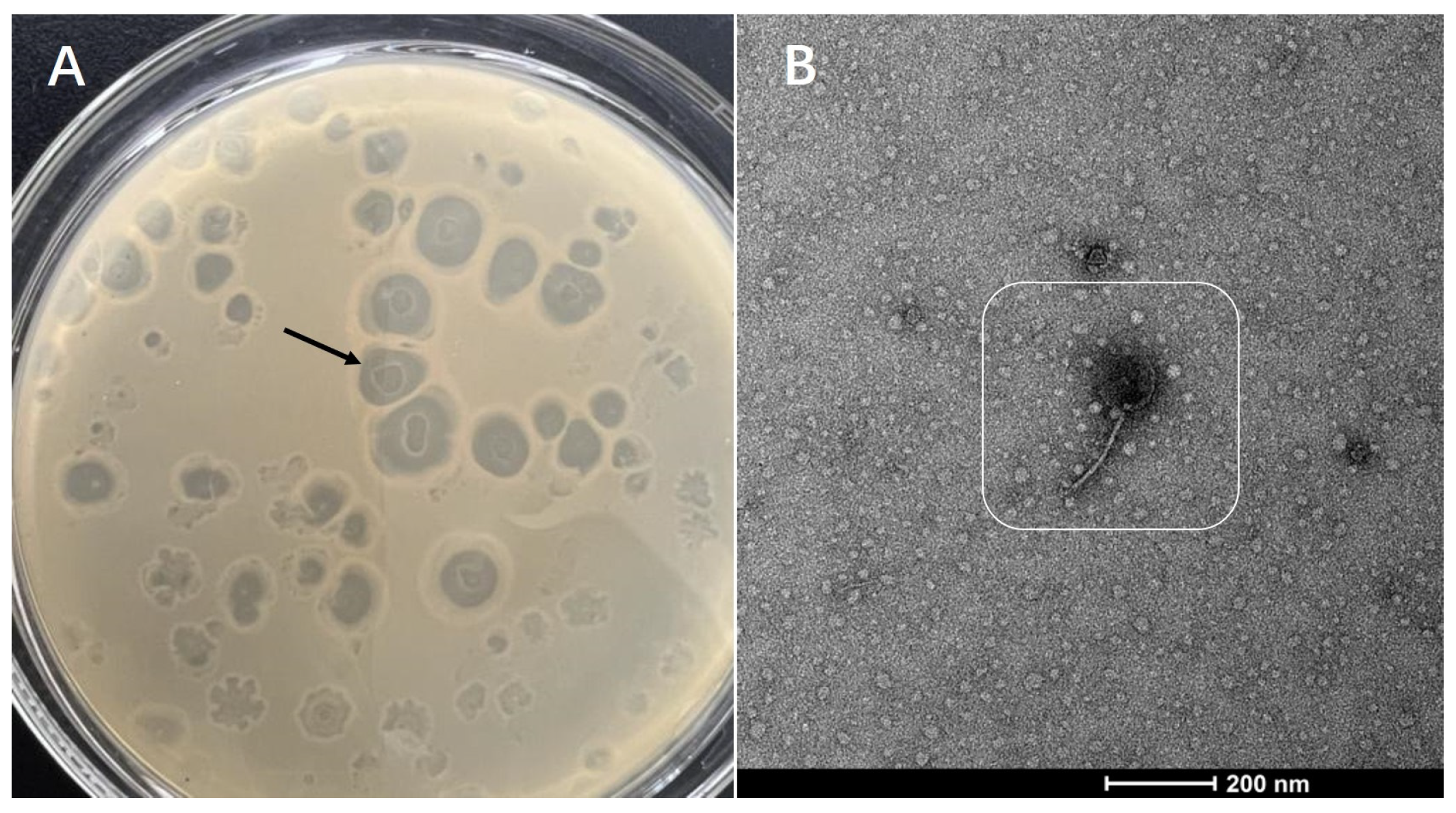
3.1. Isolation and Purification of Phages and Morphological Characteristics of Plaque
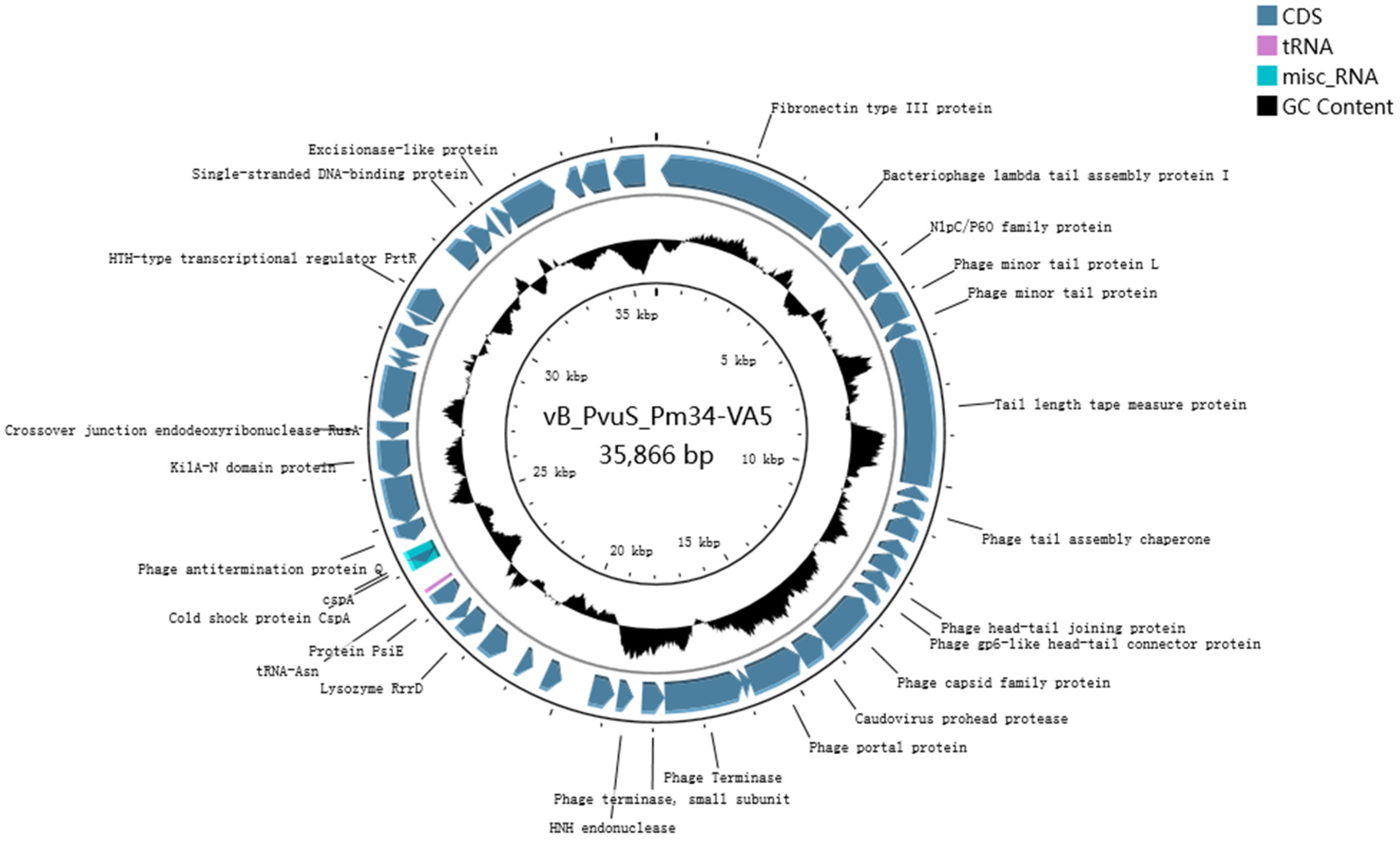
3.2. Whole-Genome Analysis of Phage
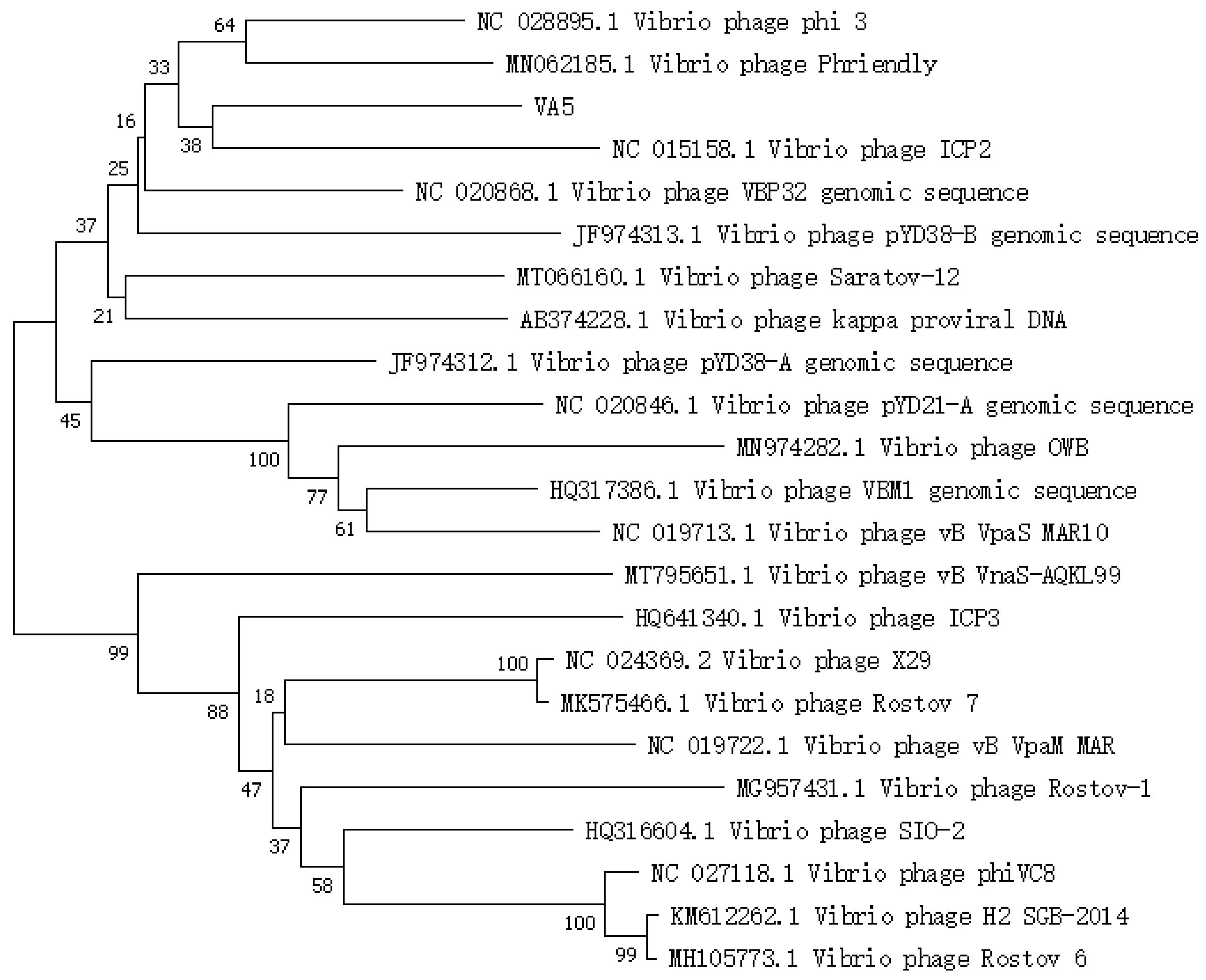
3.3. Phylogenetic Analysis
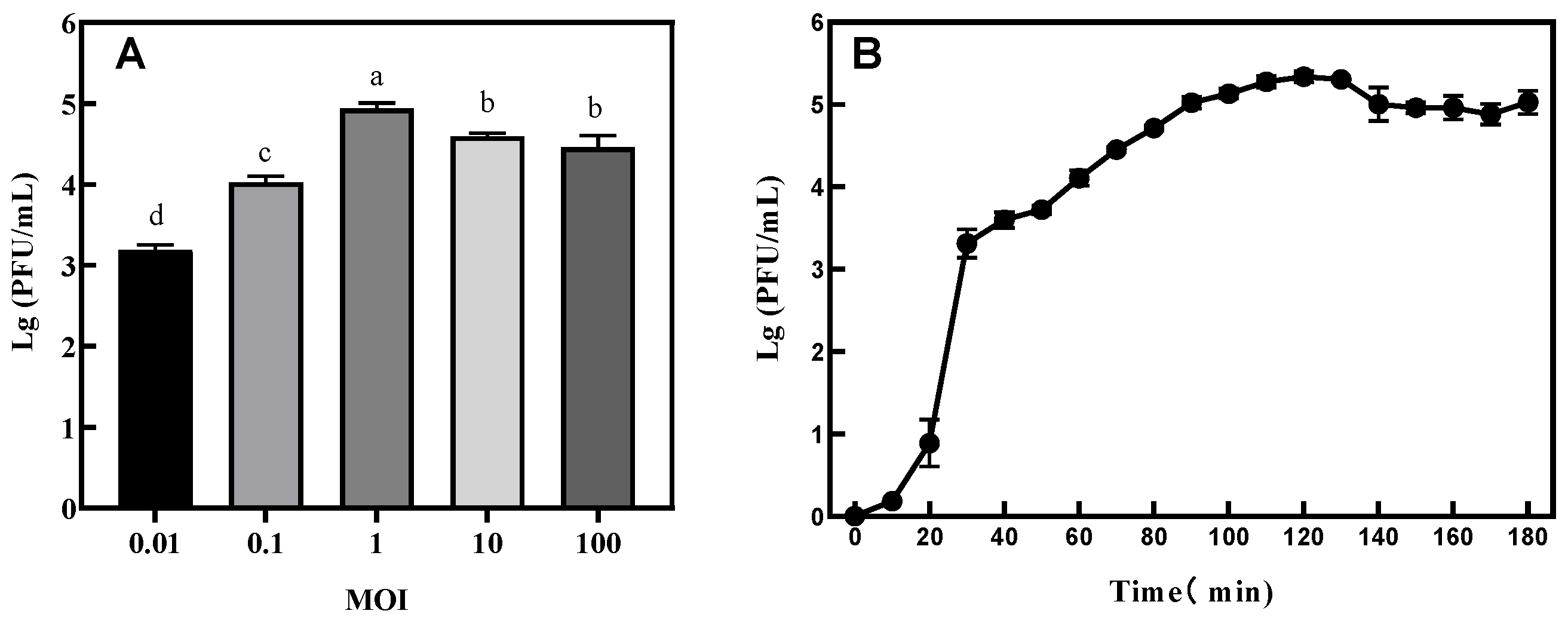
3.4. The Optimal MOI and One-Step Growth Curve Determination
3.5. Determination of Phage Stability under UV Irradiation, Temperature, and pH
3.6. Determination of the Range of Host Bacteria
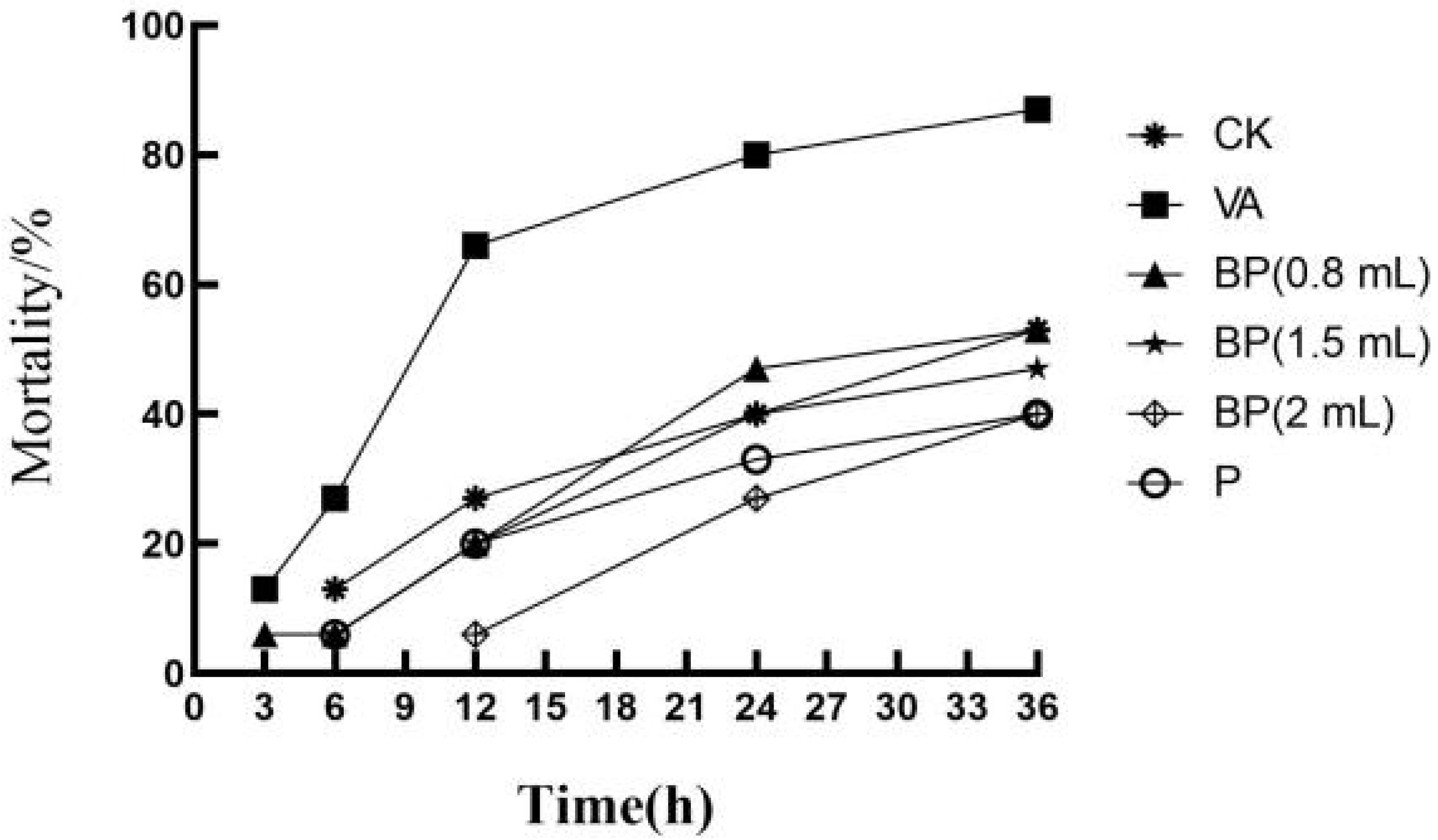
3.7. Antibacterial Properties of Phages
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, R. Biological Characterization and Genome-Wide Analysis of Two Marine Bacteriophages. Master’s Thesis, Beijing University of Chemical Technology, Beijing, China, 2022. [Google Scholar] [CrossRef]
- Diab, A.M.; Khalil, R.H.; Abu Leila, R.H.; Abotaleb, M.M.; Khallaf, M.A.; Dawood, M.A. Cross-protection of Listonella anguillarum and Vibrio alginolyticus FKC bacterins to control vibriosis in European sea bass (Dicentrarchus labrax). Aquaculture 2021, 535, 736379. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, J.; Wang, L.; Dong, J.; Ceng, X.; Guo, X.; Shen, Z.; Song, G.; Zhang, D.; Zahg, M.; et al. Isolation and characterization of Vibrio alginolyticus phage Va2001 and its application. Food Ind. Sci. Technol. 2021, 42, 102–109. [Google Scholar] [CrossRef]
- Hörmansdorfer, S.; Wentges, H.; Neugebaur-Büchler, K.; Bauer, J. Isolation of Vibrio alginolyticus from seawater aquaria. Int. J. Hyg. Environ. Health 2000, 203, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Sasikala, D.; Srinivasan, P. Characterization of potential lytic bacteriophage against Vibrio alginolyticus and its therapeutic implications on biofilm dispersal. Microb. Pathog. 2016, 101, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Hu, J.; Wei, T.; Ding, W.; Miao, Q.; Ning, Z.; Fan, S.; Wu, H.; Lu, J.; Lv, M.; et al. Fast and sensitive graphene oxide-DNAzyme-based biosensor for Vibrio alginolyticus detection. J. Fish Dis. 2022, 45, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Donati, V.; Dalsgaard, I.; Runtuvuori-Salmela, A.; Kunttu, H.M.; Jørgensen, J.; Castillo, D.; Sundberg, L.-R.; Madsen, L. Interactions between Rainbow Trout Eyed Eggs and Flavobacterium spp. Using a Bath Challenge Model: Preliminary Evaluation of Bacteriophages as Pathogen Control Agents. Microorganisms 2021, 9, 971. [Google Scholar] [CrossRef]
- Andrezal, M.; Oravcova, L.; Kadličekova, V.; Ozaee, E.; Elnwrani, S.; Bugala, J.; Markuskova, B.; Kajsik, M.; Drahovska, H. Characterization and the host specificity of Pet-CM3–4, a new phage infecting Cronobacter and Enterobacter strains. Virus Res. 2023, 324, 199025. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Yasuda, M.; Nishikawa, H.; Kuroda, M.; Ujihara, T.; Shuin, T.; Shen, Y.; Jin, Z.; Fujimoto, S.; Nasimuzzaman, M.D.; et al. Experimental protection of mice against lethal Staphylococcus aureus infection by novel bacteriophage phi MR11. J. Infect Dis. 2003, 187, 613–624. [Google Scholar] [CrossRef]
- Zheng, X. Isolation and identification of Vibrio parahaemolyticus lysogenic phage and its targeted bacterial inhibition in salmon. Master’s Thesis, Yangzhou University, Yangzhou, China, 2019. [Google Scholar] [CrossRef]
- Ali, Y.; Inusa, I.; Sanghvi, G.; Mandaliya, V.B.; Bishoyi, A.K. The current status of phage therapy and its advancement towards establishing standard antimicrobials for combating multi drug-resistant bacterial pathogens. Microb. Pathog. 2023, 181, 106199. [Google Scholar] [CrossRef]
- Hanna, N.; Tamhankar, A.J.; Lundborg, C.S. Antibiotic concentrations and antibiotic resistance in aquatic environments of the WHO Western Pacific and South-East Asia regions: A systematic review and probabilistic environmental hazard assessment. Lancet Planet. Health 2023, 7, e45–e54. [Google Scholar] [CrossRef]
- Schafhauser, B.H.; Kristofco, L.A.; de Oliveira, C.M.R.; Brooks, B.W. Global review and analysis of erythromycin in the environment: Occurrence, bioaccumulation and antibiotic resistance hazards. Environ. Pollut. 2018, 238, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Sillankorva, S.; Quinta, R.; Henriques, A.; Sereno, R.; Azeredo, J. Isolation and characterization of bacteriophages for avian pathogenic E. coli strains. J. Appl. Microbiol. 2009, 106, 1919–1927. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Ding, Y.; Nie, R.; Yao, L.; Wang, X.; Zhou, M.; Wang, J.; Wang, X. Characterization of a novel T7-like Salmonella Typhimurium (ATCC13311) bacteriophage LPST144 and its endolysin. LWT 2020, 123, 109034. [Google Scholar] [CrossRef]
- De Almeida Kumlien, A.C.M.; Pérez-Vega, C.; González-Villalobos, E.; Borrego, C.M.; Balcázar, J.L. Genome analysis of a new Escherichia phage vB_EcoM_C2-3 with lytic activity against multidrug-resistant Escherichia coli. Virus Res. 2022, 307, 198623. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Lee, H.; Park, M.-K. Isolation, characterization, and application of a novel, lytic phage vB_SalA_KFSST3 with depolymerase for the control of Salmonella and its biofilm on cantaloupe under cold temperature. Food Res. Int. 2023, 172, 113062. [Google Scholar] [CrossRef] [PubMed]
- Shahhosseini, N.; Babuadze, G.; Wong, G.; Kobinger, G.P. Mutation Signatures and In Silico Docking of Novel SARS-CoV-2 Variants of Concern. Microorganisms 2021, 9, 926. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illuminasequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Yang, M.; Chen, H.; Guo, S.; Tan, S.; Xie, Z.; Zhang, J.; Wu, Q.; Tan, Z. Characterization and genome analysis of a novel Vibrio parahaemolyticus phage vB_VpP_DE17. Virus Res. 2021, 307, 198580. [Google Scholar] [CrossRef]
- Qu, K. Isolation and Characterization of Pseudomonas aeruginosa Phage and Its Effect on Biological Periplasm. Master’s Thesis, Dalian University of Technology, Dalian, China, 2019. [Google Scholar]
- Li, J. Research on the Pathogenesis and Phage Control of Vibrio alginolyticus on Penaeus vannamei. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2020. [Google Scholar] [CrossRef]
- Hinkley, T.; Talbert, J.; Nugen, S. Phage-based biosensors for rapid testing of agricultural and process water. In Proceedings of the Abstracts of Papers from the American Chemical Society, New Orleans, LA, USA, 18–22 March 2018; p. 256. [Google Scholar]
- Garin-Fernandez, A.; Glöckner, F.O.; Wichels, A. Genomic characterization of filamentous phage vB_VpaI_VP-3218, an inducible prophage of Vibrio parahaemolyticus. Mar. Genom. 2020, 53, 100767. [Google Scholar] [CrossRef] [PubMed]
- Bailly-Bechet, M.; Vergassola, M.; Rocha, E. Causes for the intriguing presence of tRNAs in phages. Genom. Res. 2007, 17, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Delesalle, V.A.; Tanke, N.T.; Vill, A.C.; Krukonis, G.P. Testing hypotheses for the presence of tRNA genes in mycobacteriophage genomes. Bacteriophage 2016, 6, e1219441. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.F.v.D.; van der Steen, B.A.; Costa, A.R.; Brouns, S.J. Phage tRNAs evade tRNA-targeting host defenses through anticodon loop mutations. eLife 2023, 12, e85183. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, H.; Zhu, W.; Wang, J.; Wang, X. Research progress of bacteriophage-based electrochemical biosensors in the detection of foodborne pathogens. Food Sci. 2022, 43, 254–262. [Google Scholar]


| Bacteria | Plaque Formation by |
|---|---|
| Phage VA5 | |
| Vibrio parahaemolyticus | ***** |
| Vibrio cholerae | *** |
| Vibrio vulnificus | ** |
| Pseudomonas aeruginosa | \ |
| Aeromonas sobria | ** |
| Pseudomonas fluorescens | ***** |
| Aeromonas salmonicida | *** |
| Aeromonas hydrophila | * |
| Lactobacillus (rhamnosus) | * |
| Bacillus subtilis | ** |
| Edwardsiella lentus | ** |
| Escherichia coli | * |
| Time (h) | Mortality Rate (%) | |||||
|---|---|---|---|---|---|---|
| CK | VA | BP (0.8 mL) | BP (1.5 mL) | BP (2 mL) | P | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 13 | 6 | 0 | 0 | 0 |
| 6 | 13 | 27 | 6 | 6 | 0 | 6 |
| 12 | 27 | 66 | 20 | 20 | 6 | 20 |
| 24 | 40 | 80 | 47 | 40 | 27 | 33 |
| 36 | 53 | 87 | 53 | 47 | 40 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Q.; Bai, Y.; Zhou, H.; Bao, X.; Wang, H.; Zhang, L.; Lyu, M.; Wang, S. Isolation and Characterization of Bacteriophage VA5 against Vibrio alginolyticus. Microorganisms 2023, 11, 2822. https://doi.org/10.3390/microorganisms11122822
Hao Q, Bai Y, Zhou H, Bao X, Wang H, Zhang L, Lyu M, Wang S. Isolation and Characterization of Bacteriophage VA5 against Vibrio alginolyticus. Microorganisms. 2023; 11(12):2822. https://doi.org/10.3390/microorganisms11122822
Chicago/Turabian StyleHao, Qingfang, Yue Bai, Haolong Zhou, Xiuli Bao, Huanyu Wang, Lei Zhang, Mingsheng Lyu, and Shujun Wang. 2023. "Isolation and Characterization of Bacteriophage VA5 against Vibrio alginolyticus" Microorganisms 11, no. 12: 2822. https://doi.org/10.3390/microorganisms11122822
APA StyleHao, Q., Bai, Y., Zhou, H., Bao, X., Wang, H., Zhang, L., Lyu, M., & Wang, S. (2023). Isolation and Characterization of Bacteriophage VA5 against Vibrio alginolyticus. Microorganisms, 11(12), 2822. https://doi.org/10.3390/microorganisms11122822







