Development and Evaluation/Verification of a Fully Automated Test Platform for the Rapid Detection of Cyclospora cayetanensis in Produce Matrices
Abstract
1. Introduction
2. Materials and Methods
2.1. Cyclospora cayetanensis Oocysts
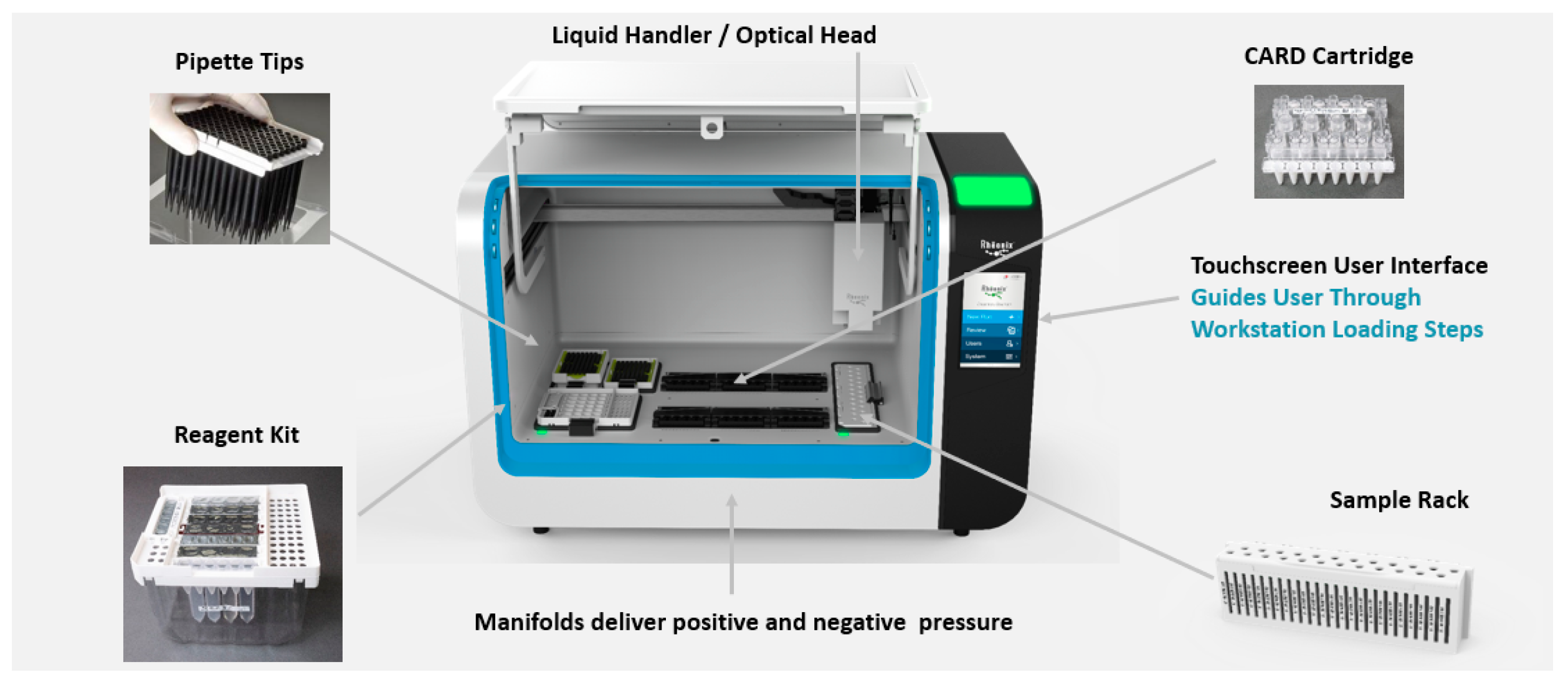
2.2. Processing of Fresh Produce Samples in the Development and Evaluation of the Fully Automated Rheonix C. cayetanensis Assay
2.3. Inclusivity and Exclusivity Panel Analysis for Specificity and Sensitivity of the Rheonix C. cayetanensis Assay
2.4. General Procedure for Performance of the Rheonix C. cayetanensis Assay
2.5. Comparison of the Performance of the Real-Time Mit1C PCR versus the Rheonix C. cayetanensis Assay
3. Results
3.1. Selection of Probe Concentrations and Hybridization Temperatures for the Rheonix C. cayetanensis Assay
3.2. Assessing Assay Sensitivity and Potential Matrix Inhibitors in the Rheonix C. cayetanensis Assay
3.3. Elimination of Potential Carry-Over Contaminations and Comparison of Master Mixes in the Rheonix C. cayetanensis Assay
3.4. Inclusivity/Exclusivity Panel Results
3.5. Verification of Rheonix Automated PCR Assay in Produce Samples Using DNA Manually Extracted (Phase I)
3.6. Phase II: Development of the Fully Automated PCR Reaction
3.7. Verification of Final Assay Format for Fully Automated PCR Assay in Produce Samples (Phase II)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ortega, Y.R.; Sanchez, R. Update on Cyclospora cayetanensis, a food-borne and waterborne parasite. Clin. Microbiol. Rev. 2010, 23, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Almeria, S.; Cinar, H.N.; Dubey, J.P. Cyclospora cayetanensis and Cyclosporiasis: An Update. Microorganisms 2019, 7, 317. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/parasites/cyclosporiasis/outbreaks/ (accessed on 2 October 2023).
- Balan, K.V.; Mammel, M.; Lipman, D.; Babu, U.; Harrison, L.M.; Almeria, S.; Durigan, M.; Leonard, S.R.; Jang, H.; Gebru, S.; et al. Development and single laboratory evaluation of a refined and specific real-time PCR detection method, using mitochondrial primers (Mit1C), for the detection of Cyclospora cayetanensis in produce. J. Food Prot. 2023, 86, 100037. [Google Scholar] [CrossRef] [PubMed]
- Almeria, S.; Chacin-Bonilla, L.; Maloney, J.G.; Santin, M. Cyclospora cayetanensis: A Perspective (2020–2023) with Emphasis on Epidemiology and Detection Methods. Microorganisms 2023, 11, 2171. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhu, H.; Malamud, D.; Barber, C.; Ongagna, Y.Y.S.; Yasmin, R.; Modak, S.; Janal, M.N.; Abrams, W.R.; Montagna, R.A. A rapid, self-confirming assay for HIV: Simultaneous detection of anti-HIV antibodies and viral RNA. J. AIDS Res. 2016, 7, 510. [Google Scholar] [CrossRef] [PubMed]
- Sabalza, M.; Yasmin, R.; Barber, C.A.; Castro, T.; Malamud, D.; Kim, B.J.; Zhu, H.; Montagna, R.A.; Abrams, W.R. Detection of ZIKA virus using reverse-transcription LAMP coupled with reverse dot blot analysis in saliva. PLoS ONE 2018, 13, e0192398. [Google Scholar] [CrossRef] [PubMed]
- Spizz, G.; Young, L.; Yasmin, R.; Chen, Z.; Lee, T.; Mahoney, D.; Zhang, X.; Mouchka, G.; Thomas, B.; Honey, W.; et al. Rheonix CARD® technology: An innovative and fully automated molecular diagnostic device. Point Care 2012, 11, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, R.; Zhu, H.; Chen, A.; Montagna, R.A. A modifiable microarray-based universal sensor: Providing sample-to results automation. Heliyon 2016, 2, e00179. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Almeria, S.; da Silva, A.J.; Blessington, T.; Cloyd, T.C.; Cinar, H.N.; Durigan, M.; Murphy, H. Evaluation of the U.S. Food and Drug Administration validated method for detection of Cyclospora cayetanensis in high-risk fresh produce matrices and a method modification for a prepared dish. Food Microbiol. 2018, 75, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Murphy, H.R.; Almeria, S.; da Silva, A.J. BAM 19b: Molecular Detection of Cyclospora Cayetanensis in Fresh Produce Using Real-Time PCR. Available online: https://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm553445.htm (accessed on 2 October 2023).
- Assurian, A.; Murphy, H.; Ewing, L.; Cinar, H.N.; da Silva, A.; Almeria, S. Evaluation of the U.S. Food and Drug Administration validated molecular method for detection of Cyclospora cayetanensis oocysts on fresh and frozen berries. Food Microbiol. 2020, 87, 103397. [Google Scholar] [CrossRef] [PubMed]
- Guidelines for the Validation of Microbiological Methods for the FDA Foods Program, 3rd ed. Available online: https://www.fda.gov/food/laboratory-methods-food/foods-program-methods-validation-processes-and-guidelines (accessed on 2 October 2023).
- Mathison, B.A.; Pritt, B.S. Cyclosporiasis—Updates on Clinical Presentation, Pathology, Clinical Diagnosis, and Treatment. Microorganisms 2021, 9, 1863. [Google Scholar] [CrossRef] [PubMed]
- Valeix, N.; Costa, D.; Basmaciyan, L.; Valot, S.; Vincent, A.; Razakandrainibe, R.; Robert-Gangneux, F.; Nourrisson, C.; Pereira, B.; Fréalle, E.; et al. Multicenter Comparative Study of Six Cryptosporidium parvum DNA Extraction Protocols Including Mechanical Pretreatment from Stool Samples. Microorganisms 2020, 8, 1450. [Google Scholar] [CrossRef] [PubMed]
- Dumètre, A.; Dubey, J.P.; Ferguson, D.J.P.; Bongrand, P.; Azas, N.; Puech, P.H. Mechanics of the Toxoplasma gondii oocyst wall. Proc. Natl. Acad. Sci. USA 2013, 110, 11535–11540. [Google Scholar] [CrossRef] [PubMed]
- Shipley, A.; Arida, J.; Almeria, S. Comparative evaluation of an easy laboratory method for the concentration of oocysts and commercial DNA isolation kits for the molecular detection of Cyclospora cayetanensis in silt loam soil samples. Microorganisms 2022, 10, 1431. [Google Scholar] [CrossRef] [PubMed]
- Tulipani, S.; Mezzetti, B.; Capocasa, F.; Bompadre, S.; Beekwilder, J.; Ric de Vos, C.H.; Capanoglu, E.; Bovy, A.; Battino, M. Antioxidants, phenolic compounds, and nutritional quality of different strawberry genotypes. J. Agric. Food Chem. 2008, 56, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]






| Mit1C | IAC | |
|---|---|---|
| Forward Primer | 5′-TCTATTTTCACCATTCTTGCTCAC-3′ | 5′-CTAACCTTCGTGATGAGCAATCG-3′ |
| Reverse Primer | 5′-TGGACTTACTAGGGTGGAGTCT-3′ | 5′-GATCAGCTACGTGAGGTCCTAC-3′ |
| Amplicon Size | 205 bp | 145 bp |
| Probe | 5′-AGGAGATAGAATGCTGGTGTATGCACC-3′ | 5′-AGCTAGTCGATGCACTCCAGTCCTCCT-3′ |
| Component | Final Concentration |
|---|---|
| Nuclease-free water | Variable |
| PerfeCTa Multiplex qPCR ToughMix Low ROX (5×) | 1× |
| Mit1C primer (Forward) | 400 nM |
| Mit1C primer (Reverse) | 400 nM |
| Mit1C Probe | 250 nM |
| IAC DNA (500 copies/µL) | 2 µL |
| IAC primer (Forward) | 400 nM |
| IAC primer (Reverse) | 400 nM |
| IAC Probe | 250 nM |
| Template | Variable |
| Total Volume | 20 µL |
| Component * | Final Concentration |
|---|---|
| Nuclease-free water | Variable |
| PerfeCTa Multiplex 5× or PCRBio 4×/dUTP/UNG | 1× |
| Mit1C primer (Forward) | 400 nM |
| Mit1C primer (Reverse) | 400 nM |
| IAC DNA (500 copies/µL) | 1.5 µL |
| IAC primer (Forward) | 400 nM |
| IAC primer (Reverse) | 400 nM |
| Template | Variable |
| Total Volume | 15 µL |
| PCR Step | Temperature/Time/Number of Cycles |
|---|---|
| Initial denaturation | 96 °C/3 min/1 cycle |
| Heat denaturation | 96 °C/15 s/40 cycles |
| Annealing/elongation | 62 °C/1 min/40 cycles |
| Mit1AA gBlock, Copies/15 µL Reaction | PerfeCTa | PCRBio with UNG and dUTP |
|---|---|---|
| 500 | 100% | 100% |
| 50 | 100% | 100% |
| 5 | 50% | 50% |
| 2.5 | NA | 50% |
| 1.25 | NA | 0 |
| 0.5 | 0 | 0 |
| Mit1AA gBlock, Copies/15 µL Reaction | PerfeCTa * No. of Positive Replicates (%) | PCRBio No. of Positive Replicates (%) |
|---|---|---|
| 6.25 | 3 (100%) | 3 (100%) |
| 3.12 | 3 (100%) | 3 ** (75%) |
| 1.56 | 3 (100%) | 2 ** (50%) |
| 0.78 | 0 (0%) | 1 ** (25%) |
| 0.39 | NA | 0 (0%) |
| Produce | No. Oocysts Seeded | Samples Examined | qPCR Results | Rheonix C. cayetanensis Assay |
|---|---|---|---|---|
| lettuce | 5 | 1 | pos | pos |
| 10 | 2 | pos | pos | |
| 200 | 2 | pos | pos | |
| Total | 5 | |||
| carrots | 0 | 1 | neg | neg |
| 10 | 1 | pos | pos (after 8 µL DNA used) | |
| 50 | 1 | pos | pos | |
| 200 | 1 | pos | pos | |
| Total | 4 | |||
| pico de gallo | 0 | 1 | neg | neg |
| 5 | 1 | pos | pos (after 8 µL DNA used) | |
| 20 | 1 | pos | pos | |
| 200 | 1 | pos | pos | |
| Total | 4 | |||
| basil | 5 | 1 | pos | pos |
| 10 | 1 | pos | pos | |
| 200 | 1 | pos | pos | |
| Total | 3 | |||
| berries | 0 | 1 | neg | neg |
| 5 | 1 | pos | pos | |
| 50 | 2 | pos | pos | |
| Total | 4 | |||
| cherry tomatoes | 0 | 1 | neg | neg |
| Total | 1 |
| Assay: Automated PCR (Rheonix) | Oocysts Numbers Seeded | No. of Samples Analyzed | No. Positive Samples (%) | Previous Results by qPCR Based on [4] (%) |
|---|---|---|---|---|
| Romaine Lettuce | 0 | 3 | 0 (0%) | Neg (100) |
| 100 | 3 | 3 (100%) | Pos (100) | |
| 5 * | 5 | 4 (80%) | Pos/Neg (50%) | |
| Cilantro | 0 | 3 | 0 (0%) | Neg (100) |
| 100 | 3 | 3 (100%) | Pos (100) | |
| 5 * | 5 | 4 (80%) | Pos/Neg (75%) |
| Matrix | Original Oocysts per mL | Matrix Dilution | Diluted Oocysts per mL | Oocysts per Reaction | Mit1C Detection |
|---|---|---|---|---|---|
| Raspberry | 100 | 1× | 100 | 4 | 1/3 (33%) |
| Raspberry | 100 | 0.5× | 50 | 2 | 7/9 (78%) |
| Raspberry | 100 | 0.2× | 20 | 0.8 | 3/3 (100%) |
| Raspberry | 100 | 0.125× | 12.5 | 0.5 | 2/2 (100%) |
| Raspberry | 100 | 0.1× | 10 | 0.4 | 2/2 (100%) |
| Raspberry | 50 | 1× | 50 | 2 | 2/3 (67%) |
| Raspberry | 50 | 0.4× | 20 | 0.8 | 1/3 (33%) |
| Raspberry | 40 | 0.5× | 20 | 0.8 | 3/3 (100%) |
| Raspberry | 20 | 0.5× | 10 | 0.4 | 3/3 (100%) |
| Raspberry | 10 | 0.5× | 5 | 0.2 | 2/4 (50%) |
| Raspberry | 10 | 0.25× | 2.5 | 0.1 | 0/3 (0%) |
| Raspberry | 10 | 0.125× | 1.25 | 0.05 | 3/3 (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Kim, B.J.; Spizz, G.; Rothrock, D.; Yasmin, R.; Arida, J.; Grocholl, J.; Montagna, R.; Schwartz, B.; Trujillo, S.; et al. Development and Evaluation/Verification of a Fully Automated Test Platform for the Rapid Detection of Cyclospora cayetanensis in Produce Matrices. Microorganisms 2023, 11, 2805. https://doi.org/10.3390/microorganisms11112805
Zhu H, Kim BJ, Spizz G, Rothrock D, Yasmin R, Arida J, Grocholl J, Montagna R, Schwartz B, Trujillo S, et al. Development and Evaluation/Verification of a Fully Automated Test Platform for the Rapid Detection of Cyclospora cayetanensis in Produce Matrices. Microorganisms. 2023; 11(11):2805. https://doi.org/10.3390/microorganisms11112805
Chicago/Turabian StyleZhu, Hui, Beum Jun Kim, Gwendolyn Spizz, Derek Rothrock, Rubina Yasmin, Joseph Arida, John Grocholl, Richard Montagna, Brooke Schwartz, Socrates Trujillo, and et al. 2023. "Development and Evaluation/Verification of a Fully Automated Test Platform for the Rapid Detection of Cyclospora cayetanensis in Produce Matrices" Microorganisms 11, no. 11: 2805. https://doi.org/10.3390/microorganisms11112805
APA StyleZhu, H., Kim, B. J., Spizz, G., Rothrock, D., Yasmin, R., Arida, J., Grocholl, J., Montagna, R., Schwartz, B., Trujillo, S., & Almeria, S. (2023). Development and Evaluation/Verification of a Fully Automated Test Platform for the Rapid Detection of Cyclospora cayetanensis in Produce Matrices. Microorganisms, 11(11), 2805. https://doi.org/10.3390/microorganisms11112805







