Discovery, Identification, and Insecticidal Activity of an Aspergillus flavus Strain Isolated from a Saline–Alkali Soil Sample
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Isolation and Identification of the Strain
2.2. Extraction and Separation of Active Substances and Preparation of Reagents
2.2.1. Preparation of Spore Suspension
2.2.2. Preparation of Fermentation Solution
2.2.3. Preparation of Mycelium Reagents
2.2.4. Preparation of Methanol Extract Reagent
2.2.5. Preparation of Fermentation Filtrate
2.3. Evaluation of Biocontrol Efficacy
2.4. Evaluation of Histopathologic Changes in Infected Aphids
2.5. Evaluation of the Effect of the Strain Infection on Aphid Body Wall Extracellular Enzyme Degradation
2.5.1. Formulation of Culture Medium from Aphid Cuticles
2.5.2. Extraction of Crude Enzyme Solution
2.5.3. Determination of Lipase Activity
2.5.4. Determination of Protease Activity
2.5.5. Determination of Chitinase Activity
2.6. Statistical Analyses
3. Results
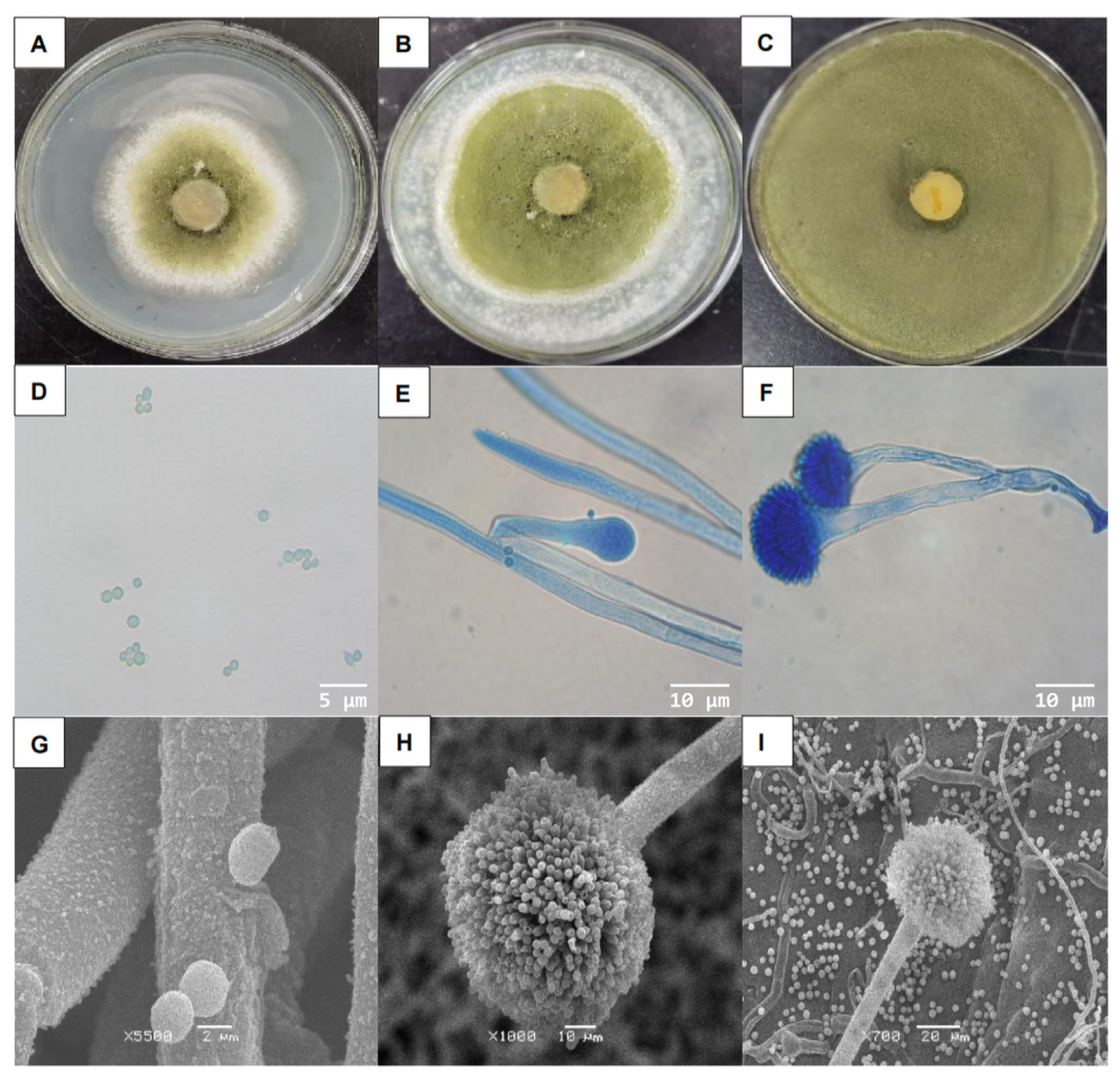
3.1. Isolation and Identification of the Strain
3.2. Biocontrol Efficacy of A. flavus
3.3. Histopathological Changes of Infected Aphids
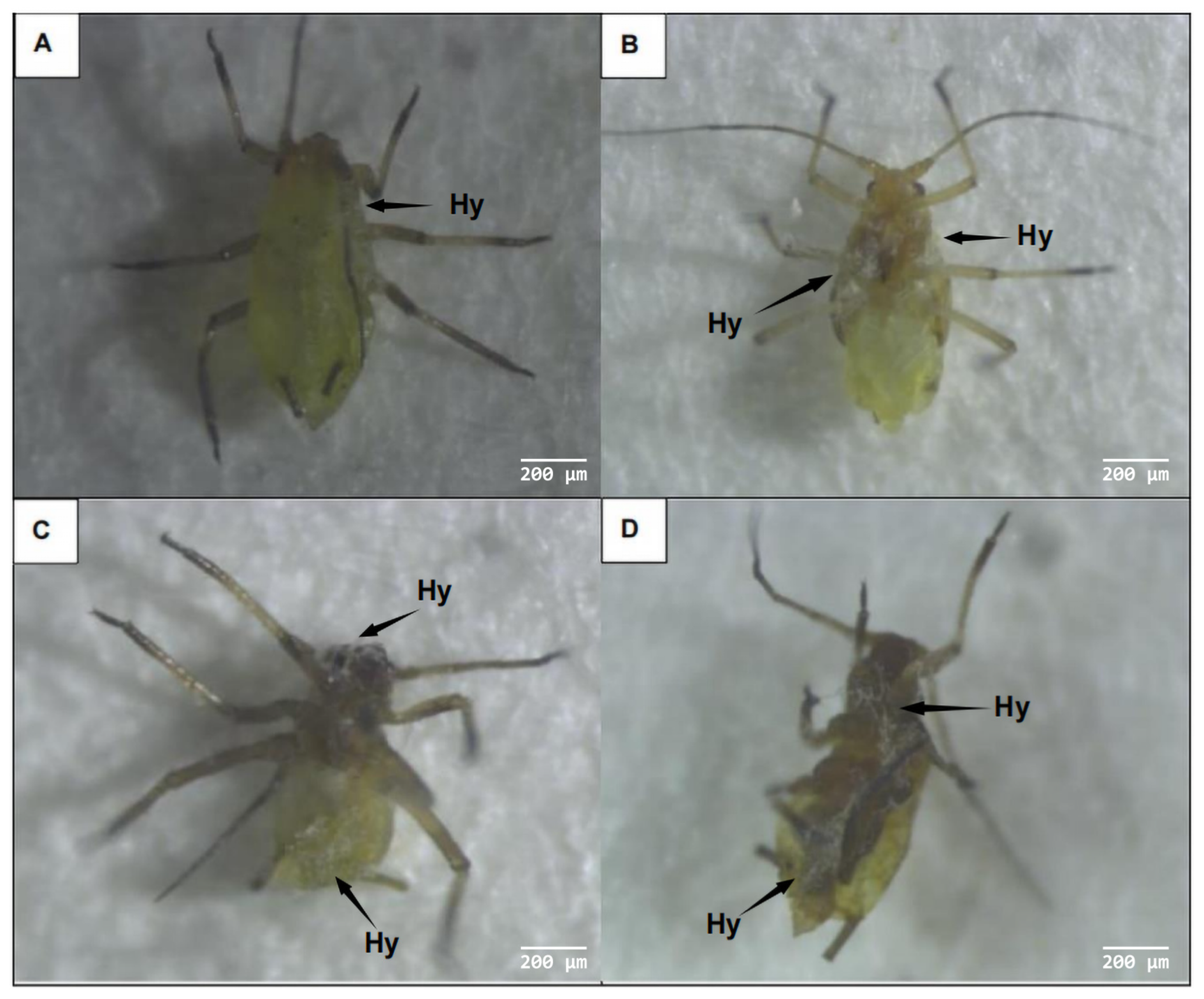
3.3.1. External Symptoms of Aphids Infected with A. flavus ‘YJNfs21.11’
3.3.2. Adhesion and Germination of A. flavus ‘YJNfs21.11’ on the Surface of Aphids
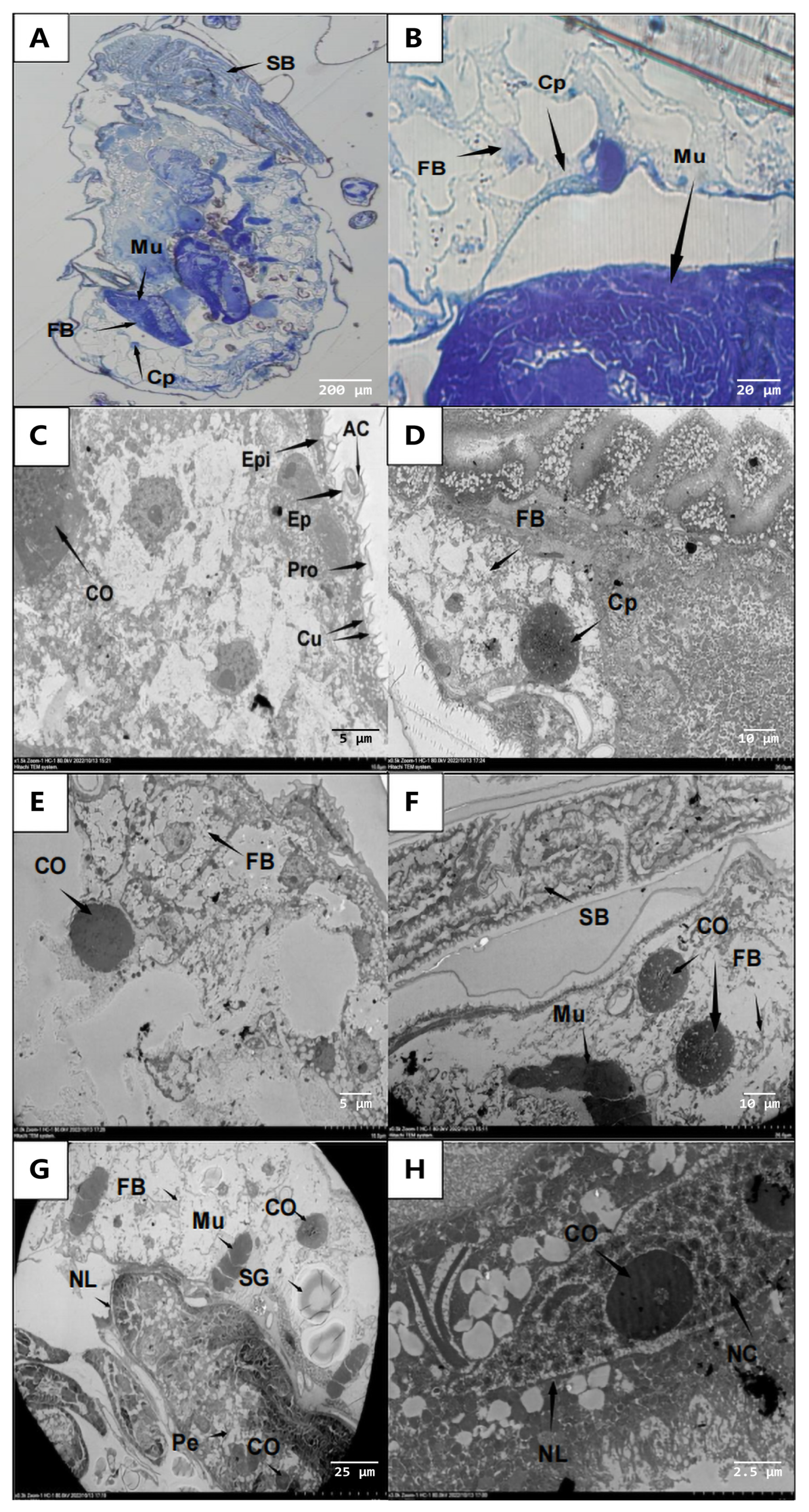
3.3.3. Invasion of the Aphid Internal Organs by A. flavus ‘YJNfs21.11’
3.4. Effect of Strain YJNfs21.11 on Extracellular Enzyme Activity during Aphid Body Wall Degradation
3.5. Relationship between Extracellular Enzyme Activity and A. flavus Pathogenesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, C.M.; Chuang, W.-P. Plant Resistance to Aphid Feeding: Behavioral, Physiological, Genetic and Molecular Cues Regulate Aphid Host Selection and Feeding: Plant Resistance to Aphid Feeding. Pest. Manag. Sci. 2014, 70, 528–540. [Google Scholar] [CrossRef]
- Elliott, N.C.; Giles, K.L.; Baum, K.A.; Elzay, S.D. Quantitative Assessment of Aphid Parasitoids and Predators in Central Oklahoma Wheat Fields during Five Growing Seasons. Southwest. Entomol. 2021, 46, 833–842. [Google Scholar] [CrossRef]
- Ismail, H.M.; Freed, S.; Naeem, A.; Malik, S.; Ali, N. The Effect of Entomopathogenic Fungi on Enzymatic Activity in Chlorpyrifos-Resistant Mosquitoes, Culex Quinquefasciatus (Diptera: Culicidae). J. Med. Entomol. 2020, 57, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Hu, Q.; Xu, Q.; Qi, X.; Zhou, F.; Chen, X. Genetic Inheritance Analysis of Melon Aphid (Aphis gossypii Glover) Resistance in Cucumber (Cucumis sativus L.). Euphytica 2015, 205, 361–367. [Google Scholar] [CrossRef]
- Jordan, M.; Sauge, M.; Vercambre, G. Chemical and Growth Traits of the Peach Tree May Induce Higher Infestation Rates of the Green Peach Aphid, Myzus persicae (Sulzer). Pest. Manag. Sci. 2020, 76, 797–806. [Google Scholar] [CrossRef]
- Davis, J.A.; Radcliffe, E.B.; Ragsdale, D.W. Effects of High and Fluctuating Temperatures on Myzus persica (Hemiptera: Aphididae). Environ. Entomol. 2006, 35, 1461–1468. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Luo, J.-Y.; Wang, C.-Y.; Lv, L.-M.; Zhu, X.-Z.; Li, C.-H.; Cui, J.-J. Identification of Aphis gossypii Glover (Hemiptera: Aphididae) Biotypes from Different Host Plants in North China. PLoS ONE 2016, 11, e0146345. [Google Scholar] [CrossRef]
- Kaleem Ullah, R.M.; Gao, F.; Sikandar, A.; Wu, H. Insights into the Effects of Insecticides on Aphids (Hemiptera: Aphididae): Resistance Mechanisms and Molecular Basis. IJMS 2023, 24, 6750. [Google Scholar] [CrossRef]
- Zaki, F.N. Efficiency of the Entomopathogenic Fungus, Beauveria bassiana (Bals), against Aphis crassivora Koch and Bemesia tabaci, Gennandius. J. Appl. Entomol. 1998, 122, 397–399. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Daskalaki, E.; Kitsiou, F.; Papantzikos, V.; Servis, D.; Bitivanos, S.; Patakioutas, G.; Eliopoulos, P.A. Dual Action of Beauveria bassiana (Hypocreales; Cordycipitaceae) Endophytic Stains as Biocontrol Agents against Sucking Pests and Plant Growth Biostimulants on Melon and Strawberry Field Plants. Microorganisms 2022, 10, 2306. [Google Scholar] [CrossRef]
- Homayoonzadeh, M.; Esmaeily, M.; Talebi, K.; Allahyari, H.; Reitz, S.; Michaud, J.P. Inoculation of Cucumber Plants with Beauveria Bassiana Enhances Resistance to Aphis gossypii (Hemiptera: Aphididae) and Increases Aphid Susceptibility to Pirimicarb. Eur. J. Entomol. 2022, 119, 1–11. [Google Scholar] [CrossRef]
- Poprawski, T.J.; Parker, P.E.; Tsai, J.H. Laboratory and Field Evaluation of Hyphomycete Insect Pathogenic Fungi for Control of Brown Citrus Aphid (Homoptera: Aphididae). Environ. Entomol. 1999, 28, 315–321. [Google Scholar] [CrossRef]
- Reingold, V.; Kottakota, C.; Birnbaum, N.; Goldenberg, M.; Lebedev, G.; Ghanim, M.; Ment, D. Intraspecies Variation of Metarhizium brunneum against the Green Peach Aphid, Myzus persicae, Provides Insight into the Complexity of Disease Progression. Pest Manag. Sci. 2021, 77, 2557–2567. [Google Scholar] [CrossRef]
- Qubbaj, T.; Samara, R. Efficacy of Three Entomopathogenic Fungi Beauveria bassiana, Metarhizium anisopliae and Lecanicillium lecanii Isolates against Black Bean Aphid, Aphis fabae (Scop.) (Hemiptera: Aphididae) on Faba Bean (Vicia faba L.). LR 2022, 45, 1572–1579. [Google Scholar] [CrossRef]
- Zare, R.; Gams, W. A Revision of Verticillium Section Prostrata. IV. The Genera Lecanicillium and Simplicillium Gen. Nov. Nova Hedwig. 2000, 71, 1–50. [Google Scholar] [CrossRef]
- Ritika; Joshi, N.; Sharma, N. Chitinase Enzyme Activity and Pathogenicity of Lecanicillium lecanii against Mustard Aphid Lipaphis erysimi (Kalt.). IJE 2021, 84, 833–836. [Google Scholar] [CrossRef]
- Manfrino, R.G.; Gutierrez, A.C.; Rueda Páramo, M.E.; Salto, C.E.; López Lastra, C.C. Prevalence of Entomophthoralean Fungi (Entomophthoromycota) of Aphids in Relation to Developmental Stages: Prevalence of Entomophthoralean Fungi on Aphids. Pest. Manag. Sci. 2016, 72, 1566–1571. [Google Scholar] [CrossRef]
- Feng, M.G.; Poprawski, T.J.; Khachatourians, G.G. Production, Formulation and Application of the Entomopathogenic Fungus Beauveria Bassiana for Insect Control: Current Status. Biocontrol Sci. Technol. 1994, 4, 3–34. [Google Scholar] [CrossRef]
- Mamo, F.T.; Shang, B.; Selvaraj, J.N.; Wang, Y.; Liu, Y. Study on Distribution and Characterization of Aspergillus flavus Strains to Screen Potential Biocontrol Agents from Different Geographical Locations of China. In Proceedings of the 2016 Academic Annual meeting of Chinese Bacteriology Society, Fuzhou, China, 19–21 August 2016; p. 1. [Google Scholar]
- Naz, I.; Khan, R.A.A.; Masood, T.; Baig, A.; Siddique, I.; Haq, S. Biological Control of Root Knot Nematode, Meloidogyne Incognita, in Vitro, Greenhouse and Field in Cucumber. Biol. Control 2021, 152, 104429. [Google Scholar] [CrossRef]
- Seye, F.; Bawin, T.; Boukraa, S.; Zimmer, J.-Y.; Ndiaye, M.; Delvigne, F.; Francis, F. Effect of Entomopathogenic Aspergillus Strains against the Pea Aphid, Acyrthosiphon pisum (Hemiptera: Aphididae). Appl. Entomol. Zool. 2014, 49, 453–458. [Google Scholar] [CrossRef]
- Chen, B.; Li, Z.Y.; Feng, M.G. Occurrence of Entomopathogenic Fungi in Migratory Alate Aphids in Yunnan Province of China. BioControl 2008, 53, 317–326. [Google Scholar] [CrossRef]
- Masi, C.; Tebiso, A.; Selva Kumar, K.V. Isolation and Characterization of Potential Multiple Extracellular Enzyme-Producing Bacteria from Waste Dumping Area in Addis Ababa. Heliyon 2023, 9, e12645. [Google Scholar] [CrossRef]
- Khusnul; Nafisa, G.; Hidana, R.; Virgianti, D.P. Influence of the Growth of Candida Albicans on Several Alternative Medium. In Proceedings of the 2nd Bakti Tunas Husada-Health Science International Conference (BTH-HSIC 2019), Tasikmalaya, Indonesia, 5–6 October 2019. [Google Scholar]
- Besas, J.R.; Dizon, E.I. Biochemical Changes of Salt-Fermented Tuna Viscera(Dayok) and Its Effect on Histamine Content during Fermentation. Int. Conf. Food Secur. Nutr. 2014, 67, 101–106. [Google Scholar]
- De Aldana, B.R.V.; Arellano, J.B.; Cuesta, M.J.; Mellado-Ortega, E.; González, V.; Zabalgogeazcoa, I. Screening Fungal Endophytes from a Wild Grass for Growth Promotion in Tritordeum, an Agricultural Cereal. Plant Sci. 2021, 303, 110762. [Google Scholar] [CrossRef]
- Sun, H.F.; Yang, H.Y.; Mu, X.J.; Wang, H.; Li, N.; Yan, Y.; Wei, M.Y. First Report of Alternaria alternata Causing Leaf Blight on Actaea dahurica in China. Plant Dis. 2022, 106, 3207. [Google Scholar] [CrossRef]
- Li, Y.; Shi, M.; Mo, Z.; Zhai, M.; Shang, C.; Xuan, J.; Hu, L. First Report of Leaf Spot on Pecan (Carya illinoinensis) Caused by Stemphylium eturmiunum in China. Plant Dis. 2023, 107, 2544. [Google Scholar] [CrossRef] [PubMed]
- Padaliya, P.J.; Desai, H.R.; Bhanderi, G.R.; Patel, R.D. Toxicity of Selected Insecticides against Cotton Mealybug (Phenacoccus Solenopsis Tinsley) in Laboratory Bioassays. IJEP 2022, 9, 164–169. [Google Scholar] [CrossRef]
- Kichu, N.; Imtinaro, L.; Neog, P.; Ozukum, L. Bioassay on Toxicity of Plant Extracts against Aphis craccivora Koch (Hemiptera: Aphididae) in French Bean (Phaseolus vulgaris L.). IJECC 2022, 12, 1216–1221. [Google Scholar] [CrossRef]
- Wei, X.; Langston, D.B.; Mehl, H.L. Comparison of Current Peanut Fungicides against Athelia rolfsii through a Laboratory Bioassay of Detached Plant Tissues. Plant Dis. 2022, 106, 2046–2052. [Google Scholar] [CrossRef]
- Wei, Q.; Yu, H.-Y.; Niu, C.-D.; Yao, R.; Wu, S.-F.; Chen, Z.; Gao, C.-F. Comparison of Insecticide Susceptibilities of Empoasca vitis (Hemiptera: Cicadellidae) from Three Main Tea-Growing Regions in China. J. Econ. Entomol. 2015, 108, 1251–1259. [Google Scholar] [CrossRef]
- Golizadeh, A.; Kamali, K.; Fathipour, Y.; Abbasipour, H. Life Table and Temperature-Dependent Development of Diadegma anurum (Hymenoptera: Ichneumonidae) on Its Host Plutella xylostella (Lepidoptera: Plutellidae). Environ. Entomol. 2008, 37, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yuan, B.; Duan, J.; Qin, Y.; Shen, H.; Ren, J.; Francis, F.; Chen, M.; Li, G. Identification of Fcl-29 as an Effective Antifungal Natural Product against Fusarium graminearum and Combinatorial Engineering Strategy for Improving Its Yield. J. Agric. Food Chem. 2023, 71, 5554–5564. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xie, Y.P.; Xiong, Q.; Liu, W.M.; Xue, J.L. Ultrastructural Exploration on the Histopathological Change in Phenacoccus fraxinus Infected with Lecanicillium lecanii. PLoS ONE 2015, 10, e0117428. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.C.; Plata-Rueda, A.; Ramírez, A.; Serrão, J.E. Susceptibility of Demotispa neivai (Coleoptera: Chrysomelidae) to Beauveria bassiana and Metarhizium anisopliae Entomopathogenic Fungal Isolates. Pest Manag. Sci. 2022, 78, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Khun, K.K.; Ash, G.J.; Stevens, M.M.; Huwer, R.K.; Wilson, B.A.L. Transmission of Metarhizium anisopliae and Beauveria bassiana to Adults of Kuschelorhynchus macadamiae (Coleoptera: Curculionidae) from Infected Adults and Conidiated Cadavers. Sci. Rep. 2021, 11, 2188. [Google Scholar] [CrossRef]
- Brey, P.T.; Latge, J.P.; Prevost, M.C. Integumental Penetration of the Pea Aphid, Acyrthosiphon pisum, by Conidiobolus obscurus (Entomophthoracease). J. Invertebr. Pathol. 1986, 48, 34–41. [Google Scholar] [CrossRef]
- Mahboub, H.H.; Shaheen, A.A. Mycological and Histopathological Identification of Potential Fish Pathogens in Nile Tilapia. Aquaculture 2021, 530, 735849. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, T.; Zhang, X.; Zhang, F.; Linhardt, R.J. Structural and Immunological Studies on the Polysaccharide from Spores of a Medicinal Entomogenous Fungus Paecilomyces cicadae. Carbohydr. Polym. 2021, 254, 117462. [Google Scholar] [CrossRef]
- Steenberg, T.; Ravn, H.P. Effect of Beauveria bassiana against Overwintering Pea Leaf Weevil, Sitona lineatus. Bull. OILB/SROP 1996, 19, 183–185. [Google Scholar]
- Sanad, S.M.; Gamaal, M.A.A.; Hemmaid, D.K. Histopathological Changes in the Liver of the Nile Fish Oreochromis niloticus Fed on the Blue-Green Algae Microcystis aeruginosa under Laboratory Conditions. In Proceedings of the International Conference on Biological, Civil and Environmental Engineering (BCEE-2015), Bali, Indonesia, 3–4 February 2015; pp. 75–90. [Google Scholar]
- Khoja, S.; Eltayef, K.M.; Baxter, I.; Myrta, A.; Bull, J.C.; Butt, T. Volatiles of the Entomopathogenic Fungus, Metarhizium Brunneum, Attract and Kill Plant Parasitic Nematodes. Biol. Control 2021, 152, 104472. [Google Scholar] [CrossRef]
- Waqar, I.; Muhammad, A.; Asad, S.; Hassan, N.; Saddeeq, A.Y.; Muhammad, Q.; Muhammad, T.; Ali, N.; Shahid, N.M.; Ali, K.K.; et al. Insect-Fungal-Interactions: A Detailed Review on Entomopathogenic Fungi Pathogenicity to Combat Insect Pests. Microb. Pathog. 2021, 159, 105122. [Google Scholar]
- Chenyin, L.; Tianyan, G.; Rui, M.; Wei, Y.; Yuyang, H.; Jingang, G.; Guogang, Z. Biochemical Characterization of a GH19 Chitinase from Streptomyces Alfalfae and Its Applications in Crystalline Chitin Conversion and Biocontrol. Int. J. Biol. Macromol. 2021, 167, 193–201. [Google Scholar]
- Lai, Y.; Chen, H.; Wei, G.; Wang, G.; Li, F.; Wang, S. In vivo gene expression profiling of the entomopathogenic fungus Beauveria bassiana elucidates its infection stratagems in Anopheles mosquito. Sci. China Life Sci. 2017, 60, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Keyhani, N.O. Lipid Biology in Fungal Stress and Virulence: Entomopathogenic Fungi. Fungal Biol. 2018, 122, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Motomura, M.; Toyomasu, T.; Mizuno, K.; Shinozawa, T. Purification and Characterization of an Aflatoxin Degradation Enzyme from Pleurotus ostreatus. Microbiol. Res. 2003, 158, 237–242. [Google Scholar] [CrossRef]
- Fernandes, E.G.; Valério, H.M.; Feltrin, T.; Sand, S.T.V.D. Variability in the Production of Extracellular Enzymes by Entomopathogenic Fungi Grown on Different Substrates. Braz. J. Microbiol. 2012, 43, 827–833. [Google Scholar] [CrossRef]
- Ajinath, D.; Sangeeta, P. Biological Control of Fusarium Wilt and Growth Promotion in Pigeon Pea (Cajanus cajan) by Antagonistic Rhizobacteria, Displaying Multiple Modes of Pathogen Inhibition. Rhizosphere 2021, 17, 100278. [Google Scholar]
- Sehrawat, A.; Sindhu, S.S.; Glick, B.R. Hydrogen Cyanide Production by Soil Bacteria: Biological Control of Pests and Promotion of Plant Growth in Sustainable Agriculture. Pedosphere 2022, 32, 15–38. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, S.; Zhong, H.; Yang, Q.; Zhang, F.; Zhuang, Z.; Yuan, J.; Nie, X.; Wang, S. Integrative analyses reveals the transcriptome-proteome correlation in biological pathways and secondary metabolism clusters in A. flavus in response to temperature. Sci. Rep. 2015, 2, 14582. [Google Scholar] [CrossRef]
- Reverberi, M.; Ricelli, A.; Zjalic, S.; Fabbri, A.A.; Fanelli, C. Natural functions of mycotoxins and control of their biosynthesis in fungi. Appl. Microbiol. Biotechnol. 2010, 87, 899–911. [Google Scholar] [CrossRef]
- Klich, M.A. Aspergillus flavus: The major producer of aflatoxin. Mol. Plant Pathol. 2007, 8, 713–722. [Google Scholar] [CrossRef]
- John, D.; Groopman, D.J.A.T.K. Aflatoxin and hepatitis B virus biomarkers: A paradigm for complex environmental exposures and cancer risk. Cancer Biomark. 2005, 1, 5–14. [Google Scholar]
- Kjærbølling, I.; Vesth, T.; Frisvad, J.C.; Nybo, J.L.; Theobald, S.; Kildgaard, S.; Petersen, T.I.; Kuo, A.; Sato, A.; Lyhne, E.K.; et al. A comparative genomics study of 23 Aspergillus species from section Flavi. Nat. Commun. 2020, 11, 1106. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, H.; Wang, F.; Adade, S.Y.S.S.; Peng, T.; Chen, Q. Discrimination of toxigenic and non-toxigenic Aspergillus flavus in wheat based on nanocomposite colorimetric sensor array. Food Chem. 2023, 430, 137048. [Google Scholar] [CrossRef] [PubMed]
- Nsabiyumva, G.; Mutegi, C.K.; Wagacha, J.M.; Mohamed, A.B.; Njeru, N.K.; Ndayihanzamaso, P.; Niyuhire, M.C.; Atehnkeng, J.; Njukwe, E.; Callicott, K.A.; et al. Aflatoxin contamination of maize and groundnut in Burundi: Distribution of contamination, identification of causal agents and potential biocontrol genotypes of Aspergillus flavus. Front. Microbiol. 2023, 14, 1106543. [Google Scholar] [CrossRef]
- Kim, G.; Lee, S.-E. Antifungal and Antiaflatoxigenic Properties of Naphthoquinones toward Aspergillus Flavus and Their Mode of Inhibitory Action on Aflatoxin Biosynthesis. Food Control 2021, 119, 107506. [Google Scholar] [CrossRef]
- Hojnik, N.; Modic, M.; Walsh, J.L.; Žigon, D.; Javornik, U.; Plavec, J.; Žegura, B.; Filipič, M.; Cvelbar, U. Unravelling the Pathways of Air Plasma Induced Aflatoxin B 1 Degradation and Detoxification. J. Hazard. Mater. 2021, 403, 123593. [Google Scholar] [CrossRef] [PubMed]
- Tianyu, Q.; Haiming, W.; Yang, Y.; Jian, Y.; Jian, J.; Jiadi, S.; Shuang, Z.; Xiulan, S. Exploration of Biodegradation Mechanism by AFB1-Degrading Strain Aspergillus Niger FS10 and Its Metabolic Feedback. Food Control 2021, 121, 107609. [Google Scholar]
- Reis, T.A.; Oliveira, T.D.D.; Zorzete, P.; Faria, P.; Corrêa, B. A non-toxigenic Aspergillus flavus strain prevents the spreading of Fusarium verticillioides and fumonisins in maize. Toxicon, 2020; 181, prepublish. [Google Scholar] [CrossRef]
- Ehrlich, K.C. Non-aflatoxigenic Aspergillus flavus to prevent aflatoxin contamination in crops: Advantages and limitations. Front. Microbiol. 2014, 5, 50. [Google Scholar] [CrossRef]
- Sun, H.; Liu, S.; Zhang, J.; Zhang, S.; Mao, J.; Xu, Y.; Zhou, J.; Mao, J. Safety evaluation and comparative genomics analysis of the industrial strain Aspergillus flavus SU-16 used for huangjiu brewing. Int. J. Food Microbiol. 2022, 380, 109859. [Google Scholar] [CrossRef]
- Tran-Dinh, N.; Pitt, J.I.; Markwell, P.J. Selection of non-toxigenic strains of Aspergillus flavus for biocontrol of aflatoxins in maize in Thailand. Biocontrol Sci. Technol. 2014, 24, 652–661. [Google Scholar] [CrossRef]
- Maxwell, L.A.; Callicott, K.A.; Bandyopadhyay, R.; Mehl, H.L.; Orbach, M.J.; Cotty, P.J. Degradation of Aflatoxins B1 by Atoxigenic Aspergillus flavus Biocontrol Agents. Plant Dis. 2021, 105, 2343–2350. [Google Scholar] [CrossRef] [PubMed]
- Lattab, N.; Kalai, S.; Bensoussan, M.; Dantigny, P. Effect of storage conditions (relative humidity, duration, and temperature) on the germination time of Aspergillus carbonarius and Penicillium chrysogenum. Int. J. Food Microbiol. 2012, 160, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Robertson, D.; Yates, B.; Nielsen, D.M.; Brown, D.; Dean, R.A.; Payne, G.A. The effect of temperature on Natural Antisense Transcript (NAT) expression in Aspergillus flavus. Curr. Genet 2008, 54, 241–269. [Google Scholar] [CrossRef]
- Wang, J.; Ogata, M.; Hirai, H.; Kawagishi, H. Detoxification of Aflatoxin B1 by Manganese Peroxidase from the White-rot Fungus Phanerochaete sordida YK-624. FEMS Microbiol. Lett. 2010, 314, 164–169. [Google Scholar] [CrossRef]
- Eshelli, M.; Harvey, L.; Edrada-Ebel, R.; McNeil, B. Metabolomics of the Bio-Degradation Process of Aflatoxin B1 by Actinomycetes at an Initial PH of 6.0. Toxins 2015, 7, 439–456. [Google Scholar] [CrossRef]
- Yin, J.; Bai, R.; Yuan, L.; Huang, J. Application of Ceriporia Lacerata HG2011 as Biocontrol Agent against Multiple Phytopathogenic Fungi and Oomycetes. Pestic. Biochem. Physiol. 2023, 190, 105316. [Google Scholar] [CrossRef]
- Mitra, D.; De Los Santos-Villalobos, S.; Parra-Cota, F.I.; Montelongo, A.M.G.; Blanco, E.L.; Lira, V.L.; Olatunbosun, A.N.; Khoshru, B.; Mondal, R.; Chidambaranathan, P.; et al. Rice (Oryza sativa L.) Plant Protection Using Dual Biological Control and Plant Growth-Promoting Agents: Current Scenarios and Future Prospects. Pedosphere 2023, 33, 268–286. [Google Scholar] [CrossRef]
- Modupeade, C.A.; Lubanza, N.; Olusegun, O.A.; Mulunda, M. A polyphasic method for the identification of aflatoxigenic Aspergilla from cashew nuts. World J. Microbiol. Biotechnol. 2019, 35, 15. [Google Scholar]
- Kumari, P.; Gogoi, R.; Srinivasa, N.; Shekhar, M. Characterization of aflatoxigenic Aspergillus flavus associated with aflatoxin B1 (AFB1) production in maize kernel in India. Indian Phytopathol. 2020, 74, 103–112. [Google Scholar] [CrossRef]




| Process | 16 h | 24 h | 48 h | |||
|---|---|---|---|---|---|---|
| Number | Mortality (%) | Corrected Efficacy (%) | Mortality (%) | Corrected Efficacy (%) | Mortality (%) | Corrected Efficacy (%) |
| spore suspension diluted 100× | (48.04 ± 6.13) b | (47.385 ± 6.21) b | (62.93 ± 3.87) ab | (54.62 ± 4.74) ab | (91.89 ± 3.07) a | (85.69 ± 5.41) a |
| spore suspension diluted 200× | (36.78 ± 6.97) bc | (35.99 ± 7.05) bc | (47.30 ± 1.54) bc | (35.49 ± 1.88) bc | (84.56 ± 2.19) abc | (72.76 ± 3.86) abc |
| spore suspension diluted 300× | (23.11 ± 5.37) cd | (22.15 ± 5.43) cd | (34.03 ± 4.77) cd | (19.24 ± 5.83) cd | (78.21 ± 0.40) bc | (61.57 ± 0.71) bc |
| mycelium reagent diluted 100× | (31.85 ± 16.27) bc | (31.00 ± 16.48) bc | (44.21 ± 4.16) bc | (41.39 ± 20.42) bc | (76.55 ± 3.94) c | (58.63 ± 6.96) c |
| methanol extract | (3.98 ± 9.34) de | (2.78 ± 9.45) de | (8.25 ± 17.78) e | (−13.80 ± 21.16) e | (45.66 ± 15.95) d | (4.13 ± 28.13) d |
| fermentation filtrate | (71.07 ± 9.90) a | (70.71 ± 10.03) a | (82.64 ± 10.12) a | (79.59 ± 13.13) a | (90.62 ± 3.47) ab | (83.46 ± 6.12) ab |
| negative control | (0.24 ± 18.09) e | (−1.01 ± 18.32) e | (17.37 ± 17.45) de | (−1.16 ± 21.36) de | (36.89 ± 4.35) d | (−11.34 ± 7.67) d |
| F | F = 14.384 | F = 14.384 | F = 17.635 | F = 14.029 | F = 32.053 | F = 32.054 |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| Process | 1 d | 3 d | 5 d | |||
|---|---|---|---|---|---|---|
| Number | Mortality (%) | Corrected Efficacy (%) | Mortality (%) | Corrected Efficacy (%) | Mortality (%) | Corrected Efficacy (%) |
| stock solution | (73.63 ± 6.03) a | (75.19 ± 5.68) a | (80.61 ± 1.89) a | (80.58 ± 1.89) a | (98.32 ± 1.01) a | (96.87 ± 1.88) a |
| stock solution diluted 2× | (68.69 ± 4.15) a | (70.54 ± 3.90) a | (77.61 ± 2.24) ab | (77.58 ± 2.24) ab | (92.51 ± 3.53) ab | (90.30 ± 2.33) ab |
| stock solution diluted 10× | (60.64 ± 6.18) a | (62.97 ± 5.82) a | (78.30 ± 0.93) ab | (78.27 ± 0.93) ab | (90.70 ± 0.65) b | (82.72 ± 1.20) bc |
| stock solution diluted 50× | (30.40 ± 9.47) b | (34.22 ± 9.31) b | (72.17 ± 4.78) b | (72.13 ± 4.79) b | (89.50 ± 5.57) b | (75.43 ± 1.68) c |
| negative control | (−5.05 ± 14.46) c | (1.17 ± 13.60) c | (9.23 ± 7.40) c | (9.10 ± 7.41) c | (49.42 ± 4.27) c | (6.02 ± 7.93) d |
| F | F = 41.702 | F = 40.932 | F = 160.826 | F = 160.828 | F = 91.950 | F = 266.98 |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| Process Number | 1 d | 3 d | 5 d | |||
|---|---|---|---|---|---|---|
| Mortality (%) | Corrected Efficacy (%) | Mortality (%) | Corrected Efficacy (%) | Mortality (%) | Corrected Efficacy (%) | |
| Spore suspension | (30.49 ± 2.17) | (26.29 ± 2.30) | (67.76 ± 4.44) | (64.90 ± 4.84) | (92.47 ± 1.75) | (91.26 ± 2.03) |
| Blank control | (6.81 ± 5.02) | - | (9.52 ± 4.14) | - | (16.24 ± 5.51) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Liu, X.; Feng, S.; Zhao, K.; Qi, Z.; Wu, W.; Xiao, J.; Xu, H.; Ran, M.; Qin, B. Discovery, Identification, and Insecticidal Activity of an Aspergillus flavus Strain Isolated from a Saline–Alkali Soil Sample. Microorganisms 2023, 11, 2788. https://doi.org/10.3390/microorganisms11112788
Song Y, Liu X, Feng S, Zhao K, Qi Z, Wu W, Xiao J, Xu H, Ran M, Qin B. Discovery, Identification, and Insecticidal Activity of an Aspergillus flavus Strain Isolated from a Saline–Alkali Soil Sample. Microorganisms. 2023; 11(11):2788. https://doi.org/10.3390/microorganisms11112788
Chicago/Turabian StyleSong, Yuxin, Xiaoli Liu, Shirong Feng, Kangbo Zhao, Zhijun Qi, Wenjun Wu, Jie Xiao, Hong Xu, Mingwei Ran, and Baofu Qin. 2023. "Discovery, Identification, and Insecticidal Activity of an Aspergillus flavus Strain Isolated from a Saline–Alkali Soil Sample" Microorganisms 11, no. 11: 2788. https://doi.org/10.3390/microorganisms11112788
APA StyleSong, Y., Liu, X., Feng, S., Zhao, K., Qi, Z., Wu, W., Xiao, J., Xu, H., Ran, M., & Qin, B. (2023). Discovery, Identification, and Insecticidal Activity of an Aspergillus flavus Strain Isolated from a Saline–Alkali Soil Sample. Microorganisms, 11(11), 2788. https://doi.org/10.3390/microorganisms11112788








