Potent Antiviral Activity of Vitamin B12 against Severe Acute Respiratory Syndrome Coronavirus 2, Middle East Respiratory Syndrome Coronavirus, and Human Coronavirus 229E
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Cytotoxicity and Inhibitory Activity against SARS-CoV-2 Virus
2.1.1. Vitamins and Test Viruses
2.1.2. Determination of In Vitro Cytotoxic Concentration 50% (CC50) and Antiviral Inhibitory Concentration 50% (IC50)
2.2. In Silico Analysis
2.2.1. Ligand Preparation
2.2.2. In Silico 3D Structure Protein Preparation
2.2.3. Molecular Docking
2.3. Quantitative Real-Time RT-PCR Assessment of RNA Expression after Treatment
2.4. Antiviral Activity Using Plaque Reduction Assay
virus with treatment)/(count of plaques in virus control) × 100
2.5. In Vitro Mechanism of Action
2.5.1. Viral Adsorption Mechanism
2.5.2. Viral Replication Mechanism
2.5.3. Neutralization Mechanism
2.6. Statistical Analysis
3. Results
3.1. Cytotoxicity and Inhibitory Activity of Reference Vitamins against Coronaviruses
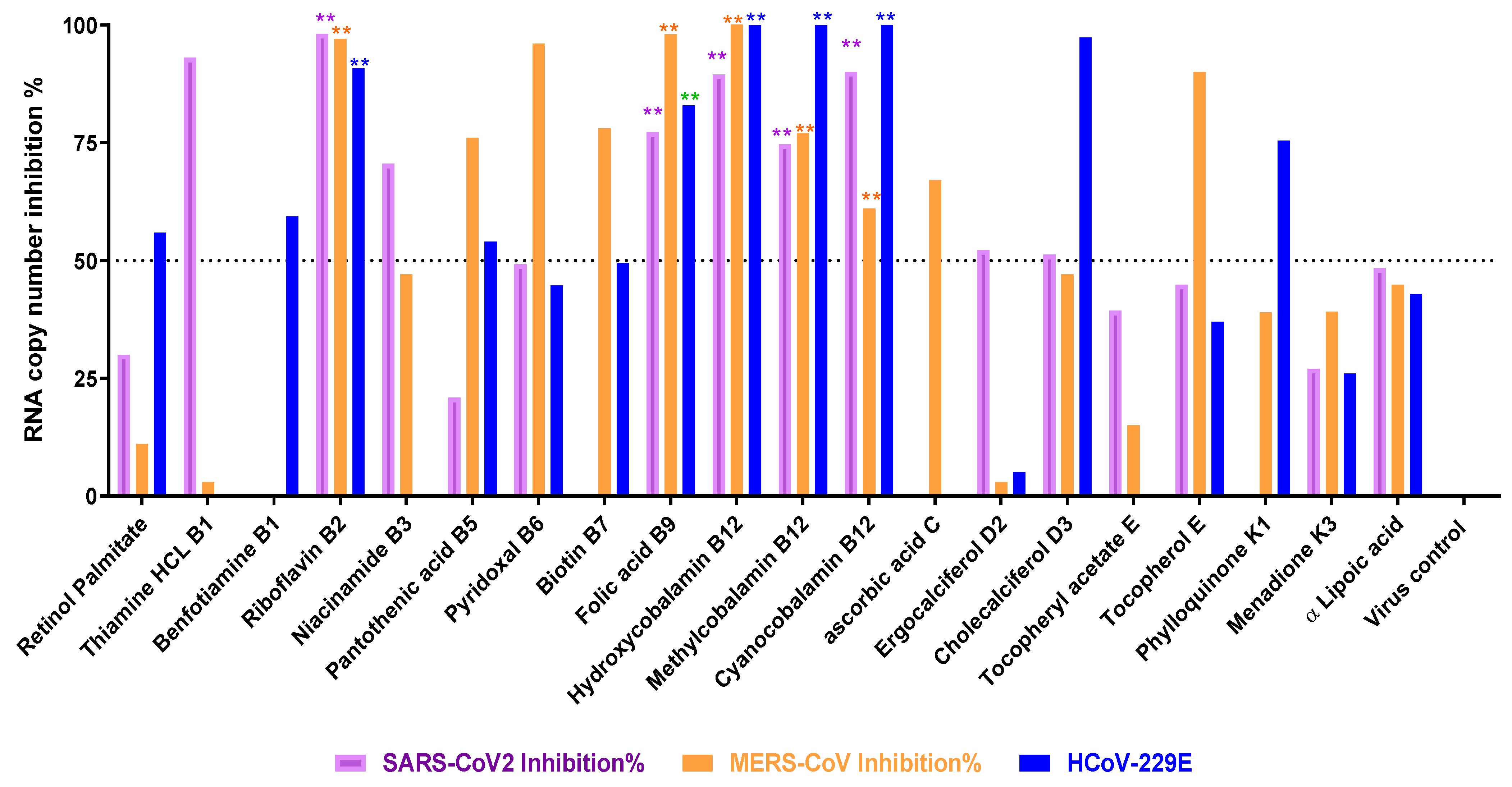
3.2. Titration of Viral RNA Inhibition Percentage Using qRT-PCR after Treatment
3.3. Protein Assembly and Preparation for Docking
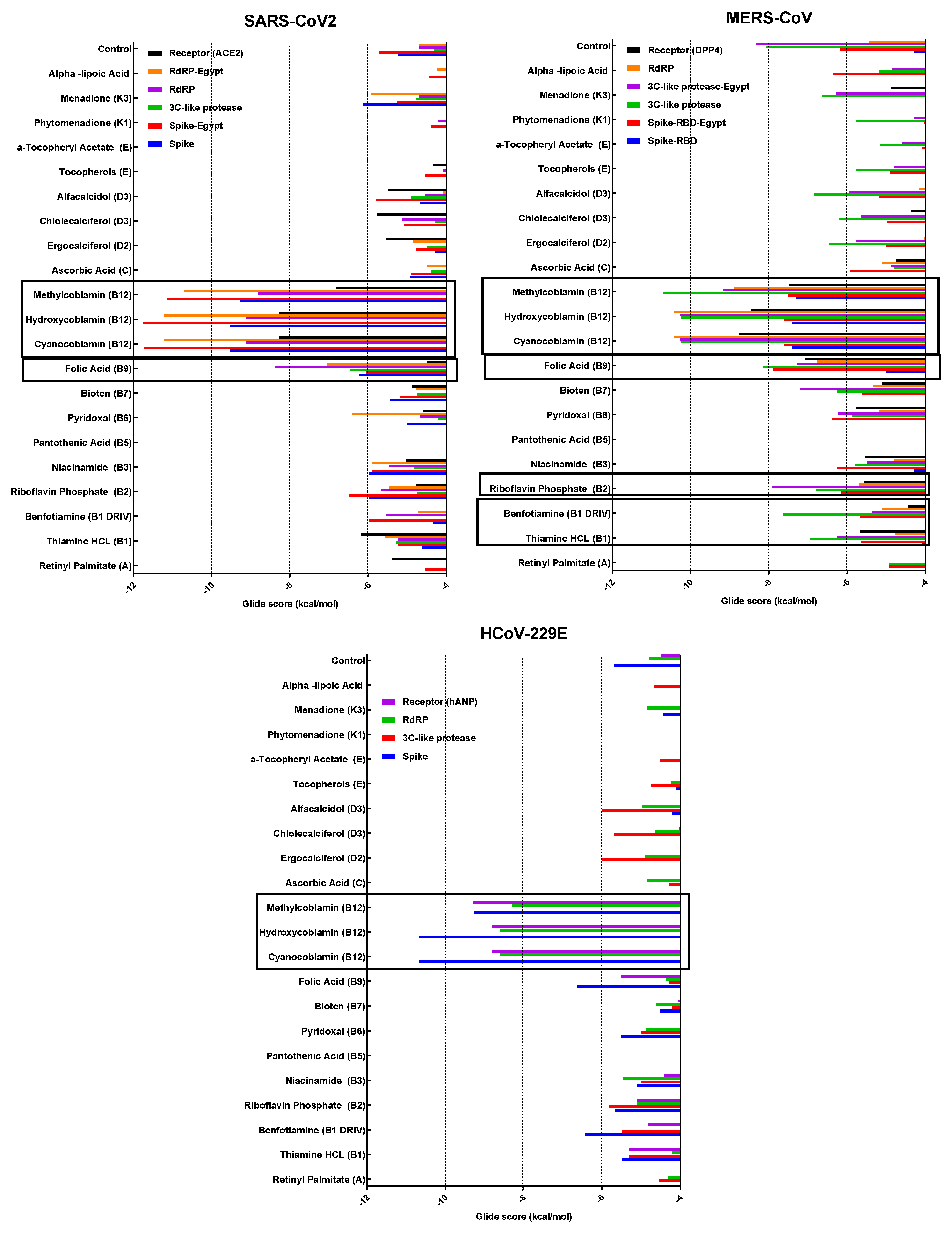
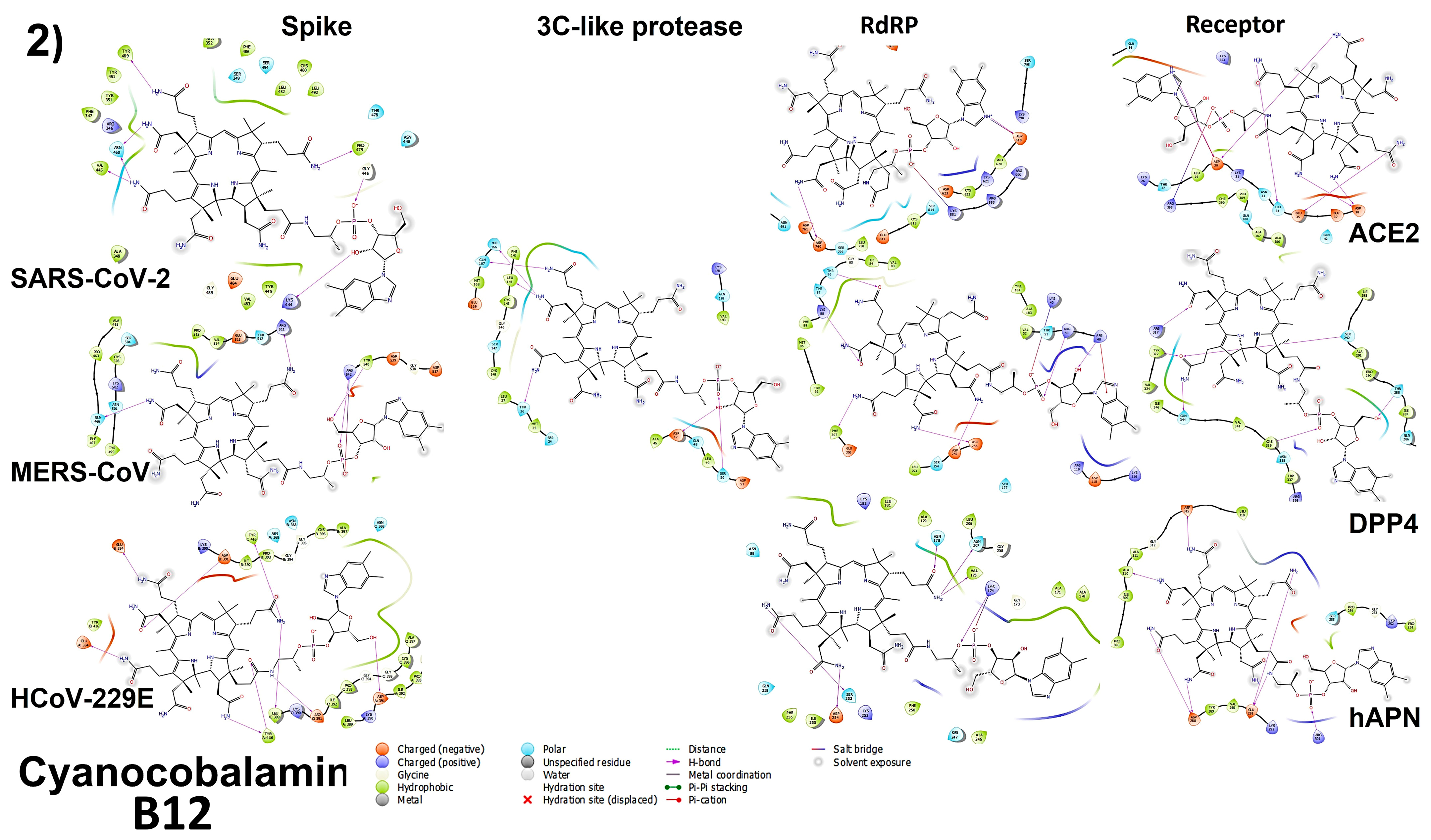
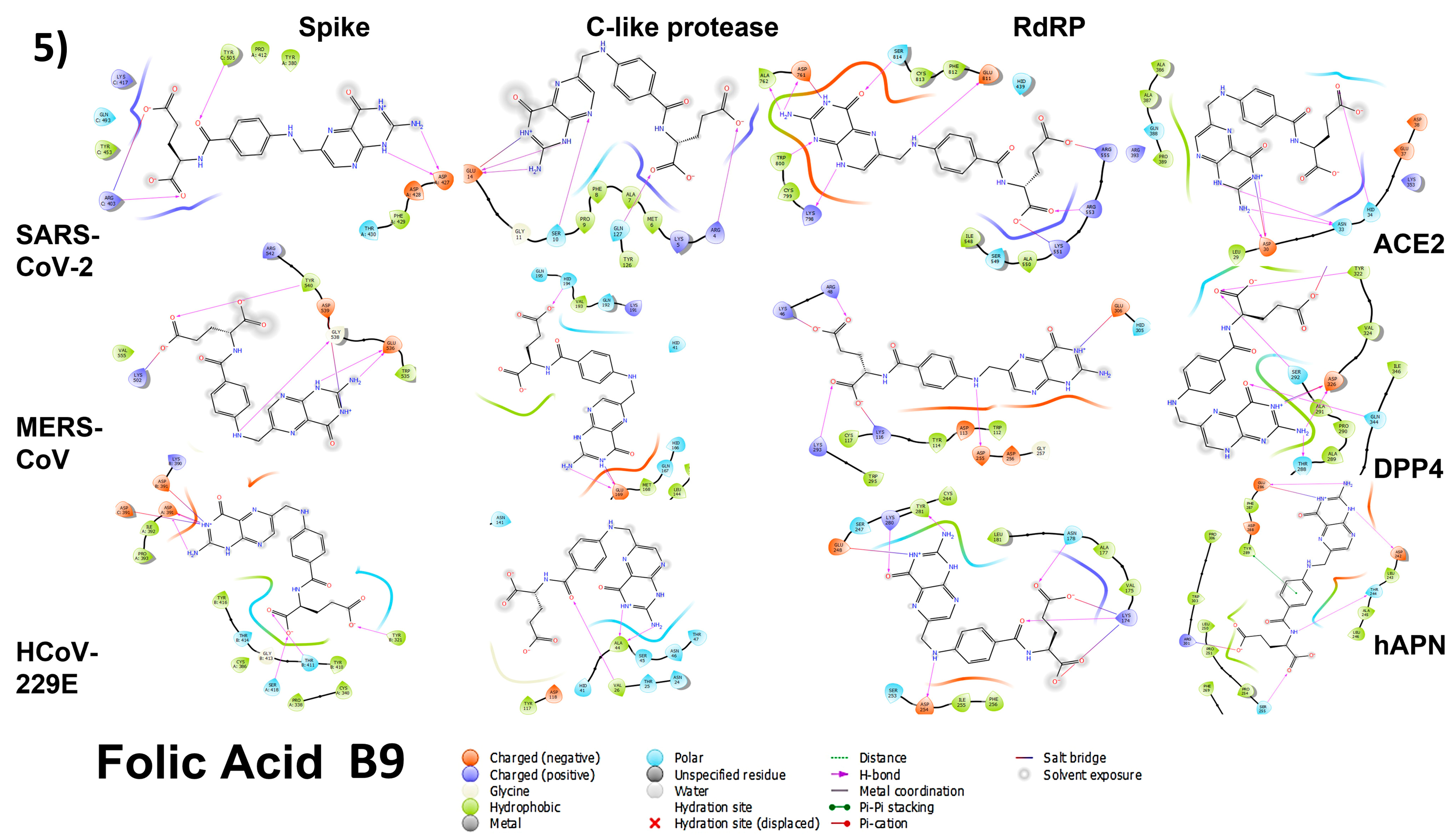
3.4. Molecular Docking
3.4.1. Spike Proteins
3.4.2. 3CL (M-Protease)
3.4.3. Cell Receptors
3.4.4. RNA-Dependent RNA Polymerase (RdRP) Proteins
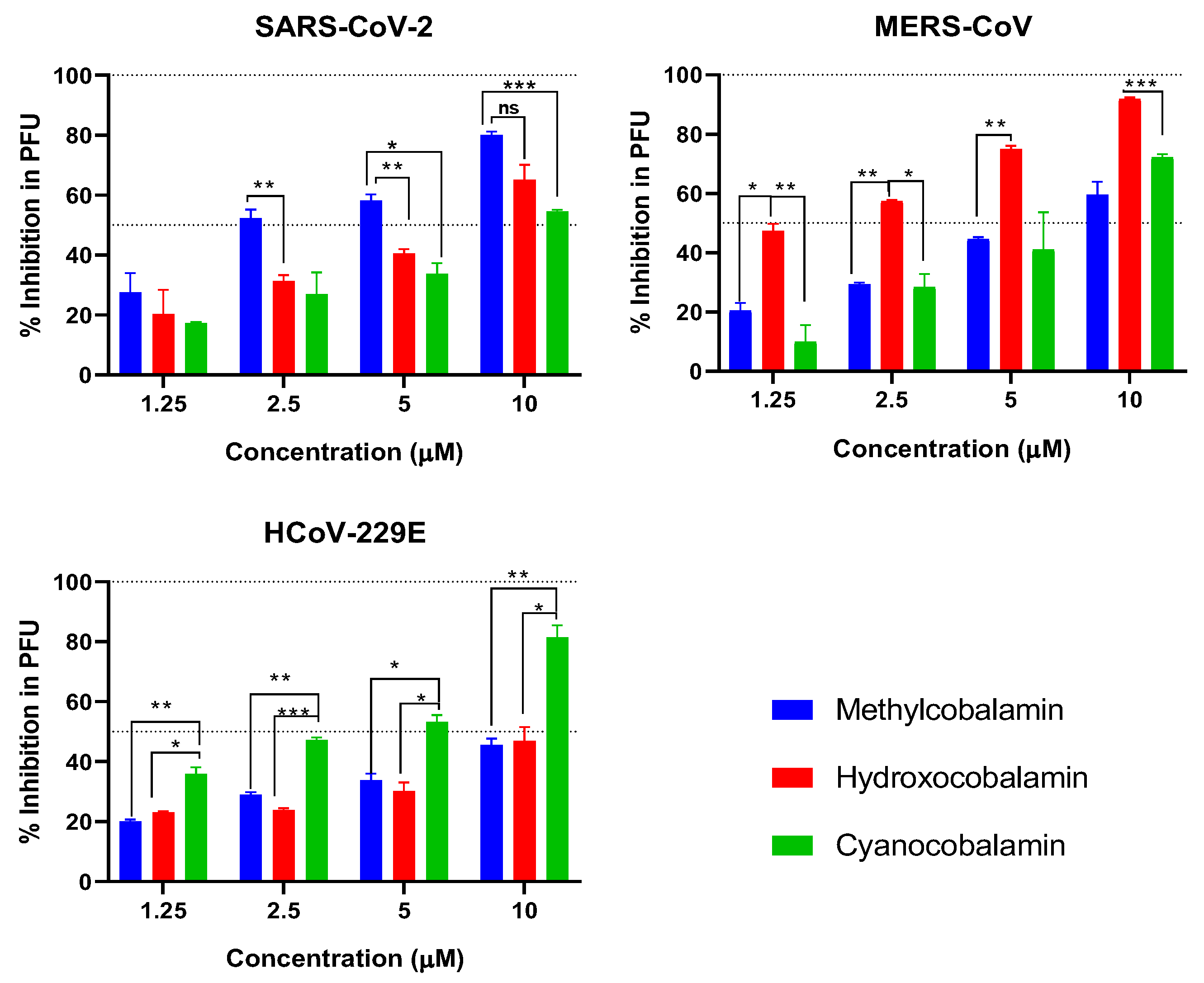
3.5. Plaque Reduction Assay
3.6. Analyzing the Binding Capacity through Docking
3.6.1. Spike RBD and Cell Receptors
3.6.2. RNA-Dependent RNA Polymerase
3.6.3. 3CL Protease
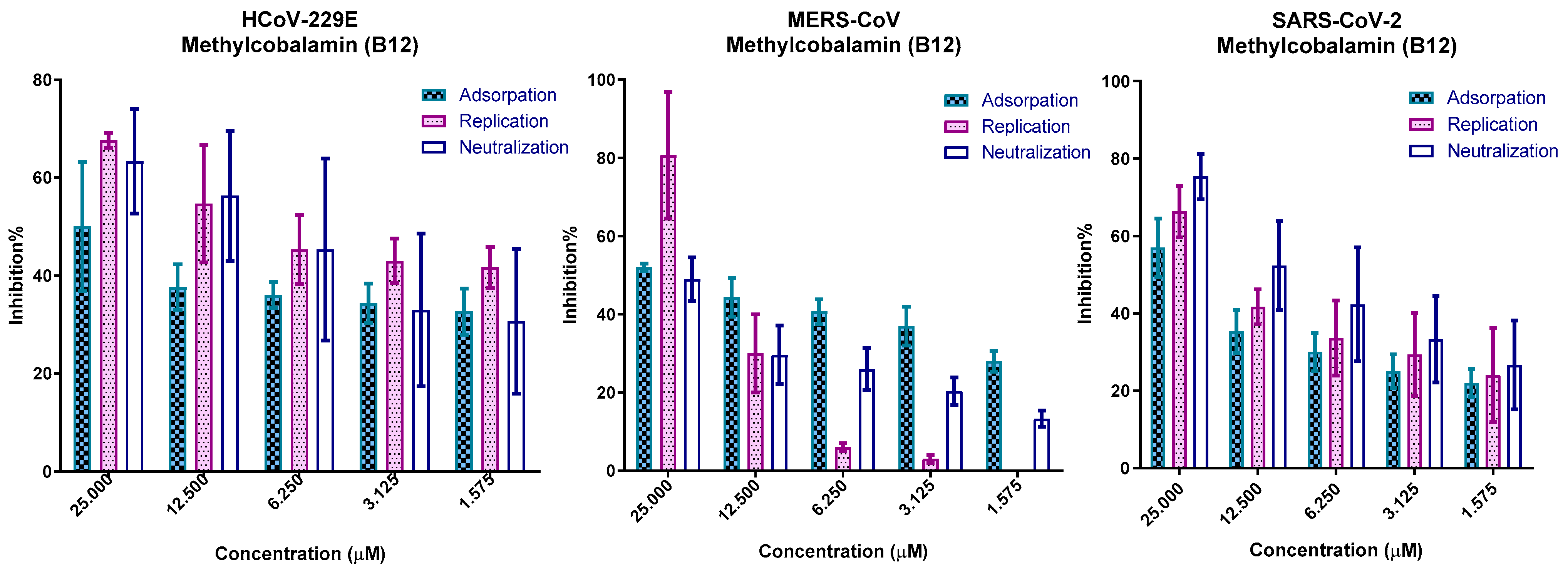
3.7. In Vitro Mechanism of Action
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Graham, R.L.; Donaldson, E.F.; Baric, R.S. A decade after SARS: Strategies for controlling emerging coronaviruses. Nat. Rev. Microbiol. 2013, 11, 836–848. [Google Scholar] [CrossRef]
- Su, S.; Wong, G.; Shi, W.; Liu, J.; Lai, A.C.K.; Zhou, J.; Liu, W.; Bi, Y.; Gao, G.F. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016, 24, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Gaunt, E.R.; Hardie, A.; Claas, E.C.; Simmonds, P.; Templeton, K.E. Epidemiology and Clinical Presentations of the Four Human Coronaviruses 229E, HKU1, NL63, and OC43 Detected over 3 Years Using a Novel Multiplex Real-Time PCR Method. J. Clin. Microbiol. 2010, 48, 2940–2947. [Google Scholar] [CrossRef]
- Lim, Y.X.; Ng, Y.L.; Tam, J.P.; Liu, D.X. Human Coronaviruses: A Review of Virus–Host Interactions. Diseases 2016, 4, 26. [Google Scholar] [CrossRef]
- Mostafa, A.; Kandeil, A.; AMM Elshaier, Y.; Kutkat, O.; Moatasim, Y.; Rashad, A.A.; Shehata, M.; Gomaa, M.R.; Mahrous, N.; Mahmoud, S.H.; et al. FDA-Approved Drugs with Potent In Vitro Antiviral Activity against Severe Acute Respiratory Syndrome Coronavirus 2. Pharmaceuticals 2020, 13, 443. [Google Scholar] [CrossRef]
- Medha Pandya, S.S.; Dhanalakshmi, M.; Tanzil, J.; Amisha, P.; Ayushman, G.; Sushma, D.; Kajari, D.; Jayashankar, D. Unravelling Vitamin B12 as a potential inhibitor against SARS-CoV-2: A computational approach. A Comput. Approach Inform. Med. Unlocked 2022, 30, 100951. [Google Scholar]
- Lykstad, J.; Sharma, S. Biochemistry, Water Soluble Vitamins; [Updated 6 March 2023]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538510/ (accessed on 25 October 2023).
- Telcian, A.G.; Zdrenghea, M.T.; Edwards, M.R.; Laza-Stanca, V.; Mallia, P.; Johnston, S.L.; Stanciu, L.A. Vitamin D increases the antiviral activity of bronchial epithelial cells in vitro. Antivir. Res. 2017, 137, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Gal-Tanamy, M.; Bachmetov, L.; Ravid, A.; Koren, R.; Erman, A.; Tur-Kaspa, R.; Zemel, R. Vitamin D: An innate antiviral agent suppressing hepatitis C virus in human hepatocytes. Hepatology 2011, 54, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
- Bishop, E.L.; Ismailova, A.; Dimeloe, S.; Hewison, M.; White, J.H. Vitamin D and Immune Regulation: Antibacterial, Antiviral, Anti-Inflammatory. JBMR Plus 2021, 5, e10405. [Google Scholar] [CrossRef]
- Hansdottir, S.; Monick, M.M.; Lovan, N.; Powers, L.; Gerke, A.; Hunninghake, G.W. Vitamin D decreases respiratory syncytial virus induction of NF-kappaB-linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J. Immunol. 2010, 184, 965–974. [Google Scholar] [CrossRef]
- Nagpal, S.; Na, S.; Rathnachalam, R. Noncalcemic actions of vitamin D receptor ligands. Endocr. Rev. 2005, 26, 662–687. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, D.A.; Camargo, C.A.; Sluyter, J.D.; Aglipay, M.; Aloia, J.F.; Ganmaa, D.; Bergman, P.; Bischoff-Ferrari, H.A.; Borzutzky, A.; Damsgaard, C.T.; et al. Vitamin D supplementation to prevent acute respiratory infections: A systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021, 9, 276–292. [Google Scholar] [CrossRef]
- van Helmond, N.; Brobyn, T.L.; LaRiccia, P.J.; Cafaro, T.; Hunter, K.; Roy, S.; Bandomer, B.; Ng, K.Q.; Goldstein, H.; Mitrev, L.V.; et al. Vitamin D3 Supplementation at 5000 IU Daily for the Prevention of Influenza-like Illness in Healthcare Workers: A Pragmatic Randomized Clinical Trial. Nutrients 2023, 15, 180. [Google Scholar] [CrossRef] [PubMed]
- Bitetto, D.; Fabris, C.; Fornasiere, E.; Pipan, C.; Fumolo, E.; Cussigh, A.; Bignulin, S.; Cmet, S.; Fontanini, E.; Falleti, E.; et al. Vitamin D supplementation improves response to antiviral treatment for recurrent hepatitis C. Transpl. Transplant. Int. 2011, 24, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, K.; Stojanovska, L.; Prakash, M.; Apostolopoulos, V. The effects of vitamin B on the immune/cytokine network and their involvement in depression. Maturitas 2017, 96, 58–71. [Google Scholar] [CrossRef]
- Yoshii, K.; Hosomi, K.; Sawane, K.; Kunisawa, J. Metabolism of dietary and microbial vitamin B family in the regulation of host immunity. Front. Nutr. 2019, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Batista, K.S.; Cintra, V.M.; Lucena, P.A.F.; Manhães-de-Castro, R.; Toscano, A.E.; Costa, L.P.; Queiroz, M.; de Andrade, S.M.; Guzman-Quevedo, O.; Aquino, J.S. The role of vitamin B12 in viral infections: A comprehensive review of its relationship with the muscle-gut-brain axis and implications for SARS-CoV-2 infection. Nutr. Rev. 2022, 80, 561–578. [Google Scholar] [CrossRef]
- Junaid, K.; Ejaz, H. Effective Immune Functions of Micronutrients against SARS-CoV-2. Nutrients 2020, 12, 2992. [Google Scholar] [CrossRef]
- Ross, A.C.; Stephensen, C.B. Vitamin A and retinoids in antiviral responses. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1996, 10, 979–985. [Google Scholar] [CrossRef]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System–Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [PubMed]
- Food and Nutrition Board, Institute of Medicine. Vitamin B12. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academies Press: Washington, DC, USA, 1998; pp. 306–356. [Google Scholar]
- World Health Organization; Food and Agricultural Organization of the United Nations. Vitamin B12. In Vitamin and Mineral Requirements in Human Nutrition: Report of a Joint FAO/WHO Expert Consultation, 2nd ed.; World Health Organization: Bangkok, Thailand, 1998; pp. 279–288. [Google Scholar]
- Kandeil, A.; Mostafa, A.; El-Shesheny, R.; Shehata, M.; Roshdy, W.H.; Ahmed, S.S.; Gomaa, M.; Taweel, A.E.; Kayed, A.E.; Mahmoud, S.H.; et al. Coding-complete genome sequences of two SARS-CoV-2 isolates from Egypt. Microbiol. Resour. Announc. 2020, 9, e00489-20. [Google Scholar] [CrossRef] [PubMed]
- Kandeil, A.; Gomaa, M.; Nageh, A.; Shehata, M.M.; Kayed, A.E.; Sabir, J.S.M.; Abiadh, A.; Jrijer, J.; Amr, Z.; Abi Said, M.; et al. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) in Dromedary Camels in Africa and Middle East. Viruses 2019, 11, 717. [Google Scholar] [CrossRef] [PubMed]
- Kutkat, O.; Moatasim, Y.; Al-Karmalawy, A.A.; Abulkhair, H.S.; Gomaa, M.R.; El-Taweel, A.N.; Abo Shama, N.M.; GabAllah, M.; Mahmoud, D.B.; Kayali, G.; et al. Robust antiviral activity of commonly prescribed antidepressants against emerging coronaviruses: In vitro and in silico drug repurposing studies. Sci. Rep. 2022, 12, 12920. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Gomaa, M.R.; El Rifay, A.S.; Shehata, M.; Kandeil, A.; Nabil Kamel, M.; Marouf, M.A.; GabAllah, M.; El Taweel, A.; Kayed, A.E.; Kutkat, O.; et al. Incidence, household transmission, and neutralizing antibody seroprevalence of Coronavirus Disease 2019 in Egypt: Results of a community-based cohort. PLOS Pathog. 2021, 17, e1009413. [Google Scholar] [CrossRef]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal Violet Assay for Determining Viability of Cultured Cells. Cold Spring Harb. Protoc. 2016, 2016, pdb.prot087379. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2019 update: Improved access to chemical data. Nucleic Acids Res. 2018, 47, D1102–D1109. [Google Scholar]
- Sali, A.; Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef]
- Lovell, S.C.; Davis, I.W.; Arendall, W.B., 3rd; de Bakker, P.I.; Word, J.M.; Prisant, M.G.; Richardson, J.S.; Richardson, D.C. Structure validation by Calpha geometry: Phi, psi and Cbeta deviation. Proteins 2003, 50, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Sastry, G.M.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 2013, 27, 221–234. [Google Scholar]
- Arbeitman, C.R.; Auge, G.; Blaustein, M.; Bredeston, L.; Corapi, E.S.; Craig, P.O.; Cossio, L.A.; Dain, L.; D’Alessio, C.; Elias, F.; et al. Structural and functional comparison of SARS-CoV-2-spike receptor binding domain produced in Pichia pastoris and mammalian cells. Sci. Rep. 2020, 10, 21779. [Google Scholar]
- Huang, Y.; Yang, C.; Xu, X.-f.; Xu, W.; Liu, S.-w. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar]
- Wong, A.H.M.; Tomlinson, A.C.A.; Zhou, D.; Satkunarajah, M.; Chen, K.; Sharon, C.; Desforges, M.; Talbot, P.J.; Rini, J.M. Receptor-binding loops in alphacoronavirus adaptation and evolution. Nat. Commun. 2017, 8, 1735. [Google Scholar]
- Ahmad, J.; Ikram, S.; Ahmad, F.; Rehman, I.U.; Mushtaq, M. SARS-CoV-2 RNA Dependent RNA polymerase (RdRp)—A drug repurposing study. Heliyon 2020, 6, e04502. [Google Scholar]
- Elfiky, A.A.; Azzam, E.B. Novel guanosine derivatives against MERS CoV polymerase: An in silico perspective. J. Biomol. Struct. Dyn. 2021, 39, 2923–2931. [Google Scholar] [PubMed]
- Wang, N.; Shi, X.; Jiang, L.; Zhang, S.; Wang, D.; Tong, P.; Guo, D.; Fu, L.; Cui, Y.; Liu, X.; et al. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res. 2013, 23, 986–993. [Google Scholar]
- Guy, J.L.; Jackson, R.M.; Jensen, H.A.; Hooper, N.M.; Turner, A.J. Identification of critical active-site residues in angiotensin-converting enzyme-2 (ACE2) by site-directed mutagenesis. Febs J. 2005, 272, 3512–3520. [Google Scholar] [PubMed]
- St John, S.E.; Anson, B.J.; Mesecar, A.D. X-ray Structure and Inhibition of 3C-like Protease from Porcine Epidemic Diarrhea Virus. Sci. Rep. 2016, 6, 25961. [Google Scholar] [PubMed]
- Kiemer, L.; Lund, O.; Brunak, S.; Blom, N. Coronavirus 3CLpro proteinase cleavage sites: Possible relevance to SARS virus pathology. BMC Bioinform. 2004, 5, 72. [Google Scholar]
- Bonavia, A.; Zelus, B.D.; Wentworth, D.E.; Talbot, P.J.; Holmes, K.V. Identification of a receptor-binding domain of the spike glycoprotein of human coronavirus HCoV-229E. J. Virol. 2003, 77, 2530–2538. [Google Scholar] [CrossRef]
- Kandeil, A.; Mostafa, A.; Kutkat, O.; Moatasim, Y.; Al-Karmalawy, A.A.; Rashad, A.A.; Kayed, A.E.; Kayed, A.E.; El-Shesheny, R.; Kayali, G. Bioactive polyphenolic compounds showing strong antiviral activities against severe acute respiratory syndrome coronavirus 2. Pathogens 2021, 10, 758. [Google Scholar] [PubMed]
- Loutfy, S.A.; Abdel-Salam, A.I.; Moatasim, Y.; Gomaa, M.R.; Abdel Fattah, N.F.; Emam, M.H.; Ali, F.; ElShehaby, H.A.; Ragab, E.A.; Alam El-Din, H.M.; et al. Antiviral activity of chitosan nanoparticles encapsulating silymarin (Sil–CNPs) against SARS-CoV-2 (in silico and in vitro study). RSC Adv. 2022, 12, 15775–15786. [Google Scholar]
- Li, X.; Geng, M.; Peng, Y.; Meng, L.; Lu, S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020, 10, 102–108. [Google Scholar] [PubMed]
- Masood, M.U.; Haider, Z.; Khan, Z.; Ahmad, A.; Munawar, N.; Aftab, S.O.; Ghouri, M.Z. Analysis of SARS-CoV-2 RNA-dependent RNA polymerase as a potential therapeutic drug target using a computational approach. J. Transl. Med. 2020, 18, 275. [Google Scholar]
- Giordano, D.; De Masi, L.; Argenio, M.A.; Facchiano, A. Structural Dissection of Viral Spike-Protein Binding of SARS-CoV-2 and SARS-CoV-1 to the Human Angiotensin-Converting Enzyme 2 (ACE2) as Cellular Receptor. Biomedicines 2021, 9, 1038. [Google Scholar]
- Li, Z.; Tomlinson, A.C.A.; Wong, A.H.M.; Zhou, D.; Desforges, M.; Talbot, P.J.; Benlekbir, S.; Rubinstein, J.L.; Rini, J.M. The human coronavirus HCoV-229E S-protein structure and receptor binding. eLife 2019, 8, e51230. [Google Scholar]
- Jovic, T.H.; Ali, S.R.; Ibrahim, N.; Jessop, Z.M.; Tarassoli, S.P.; Dobbs, T.D.; Holford, P.; Thornton, C.A.; Whitaker, I.S. Could Vitamins Help in the Fight Against COVID-19? Nutrients 2020, 12, 2550. [Google Scholar] [PubMed]
- Vlieg-Boerstra, B.; de Jong, N.; Meyer, R.; Agostoni, C.; De Cosmi, V.; Grimshaw, K.; Milani, G.P.; Muraro, A.; Oude Elberink, H.; Pali-Schöll, I.; et al. Nutrient supplementation for prevention of viral respiratory tract infections in healthy subjects: A systematic review and meta-analysis. Allergy 2022, 77, 1373–1388. [Google Scholar]
- Rawat, D.; Roy, A.; Maitra, S.; Gulati, A.; Khanna, P.; Baidya, D.K. Vitamin C and COVID-19 treatment: A systematic review and meta-analysis of randomized controlled trials. Diabetes Metab. Syndr. 2021, 15, 102324. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Patel, D.; Bittel, B.; Wolski, K.; Wang, Q.; Kumar, A.; Il’Giovine, Z.J.; Mehra, R.; McWilliams, C.; Nissen, S.E.; et al. Effect of High-Dose Zinc and Ascorbic Acid Supplementation vs Usual Care on Symptom Length and Reduction Among Ambulatory Patients With SARS-CoV-2 Infection: The COVID A to Z Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e210369. [Google Scholar] [CrossRef]
- Ahmed, S.; Hossain, M.; Chakrabortty, D.; Arafat, K.I.; Hosen, M.J.; Khan, M.M.R. Impacts of vitamin C and D supplement on COVID-19 treatment: Possible patho-mechanisms and evidence from different countries. Egypt. J. Bronchol. 2023, 17, 13. [Google Scholar]
- Lotfi, F.; Akbarzadeh-Khiavi, M.; Lotfi, Z.; Rahbarnia, L.; Safary, A.; Zarredar, H.; Baghbanzadeh, A.; Naghili, B.; Baradaran, B. Micronutrient therapy and effective immune response: A promising approach for management of COVID-19. Infection 2021, 49, 1133–1147. [Google Scholar]
- The Office of Dietary Supplements (ODS); the National Institutes of Health (NIH). Vitamin B12 Fact Sheet for Health Professionals. 2017. Available online: https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/ (accessed on 25 October 2023).
- Kandeel, M.; Al-Nazawi, M. Virtual screening and repurposing of FDA approved drugs against COVID-19 main protease. Life Sci. 2020, 251, 117627. [Google Scholar] [CrossRef]
- Su, C.-M.; Wang, L.; Yoo, D. Activation of NF-κB and induction of proinflammatory cytokine expressions mediated by ORF7a protein of SARS-CoV-2. Sci. Rep. 2021, 11, 13464. [Google Scholar] [CrossRef] [PubMed]
- Kircheis, R.; Haasbach, E.; Lueftenegger, D.; Heyken, W.T.; Ocker, M.; Planz, O. NF-κB Pathway as a Potential Target for Treatment of Critical Stage COVID-19 Patients. Front. Immunol. 2020, 11, 598444. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Mou, L.; Long, Q.; Deng, D.; Hu, R.; Cheng, J.; Wu, J. SARS-CoV-2 ORF3a positively regulates NF-κB activity by enhancing IKKβ-NEMO interaction. Virus Res. 2023, 328, 199086. [Google Scholar] [CrossRef] [PubMed]
- De Diego, M.L.; Nieto-Torres, J.L.; Regla-Nava, J.A.; Jimenez-Guardeño, J.M.; Fernandez-Delgado, R.; Fett, C.; Castaño-Rodriguez, C.; Perlman, S.; Enjuanes, L. Inhibition of NF-κB-Mediated Inflammation in Severe Acute Respiratory Syndrome Coronavirus-Infected Mice Increases Survival. J. Virol. 2014, 88, 913–924. [Google Scholar]
- Manzanares, W.H.G. Vitamin B12: The forgotten micronutrient for critical care. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 662–668. [Google Scholar] [CrossRef] [PubMed]
- van de Lagemaat, E.E.d.G.L.; van den Heuvel, E.G.H.M. Vitamin B12 in relation to oxidative stress: A systematic review. Nutrients 2019, 11, 482. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.; Rajput, U.; Dawre, R.; Sonkawade, N.; Pawar, S.; Sonteke, S.; Varvatte, B.; Aathira, K.; Gadekar, K.; Varma, S. Severe malnutrition and anemia are associated with severe COVID in infants. J. Trop. Pediatr. 2021, 67, fmaa084. [Google Scholar] [CrossRef] [PubMed]
- Wolffenbuttel, B.H.; Wouters, H.J.; Heiner-Fokkema, M.R.; van der Klauw, M.M. The many faces of cobalamin (vitamin B12) deficiency. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, Y.; Liu, S.; Lin, Y.; Lu, P. Vitamin B12 for herpetic neuralgia: A meta-analysis of randomised controlled trials. Complement. Ther. Med. 2018, 41, 277–282. [Google Scholar] [CrossRef]
- Syed, E.U.; Wasay, M.; Awan, S. Vitamin B12 supplementation in treating major depressive disorder: A randomized controlled trial. Open Neurol. J. 2013, 7, 44. [Google Scholar] [CrossRef]
- Baldewicz, T.T.; Goodkin, K.; Blaney, N.T.; Shor-Posner, G.; Kumar, M.; Wilkie, F.L.; Baum, M.K.; Eisdorfer, C. Cobalamin level is related to self-reported and clinically rated mood and to syndromal depression in bereaved HIV-1+ and HIV-1− homosexual men. J. Psychosom. Res. 2000, 48, 177–185. [Google Scholar] [CrossRef]
- dos Santos, L.M.J. Can vitamin B12 be an adjuvant to COVID-19 treatment? GSC Biol. Pharm. Sci. 2020, 376, 66040–66170. [Google Scholar]
- Bashandy, S.A.E.; Ebaid, H.; Abdelmottaleb Moussa, S.A.; Alhazza, I.M.; Hassan, I.; Alaamer, A.; Al Tamimi, J. Potential effects of the combination of nicotinamide, vitamin B2 and vitamin C on oxidative-mediated hepatotoxicity induced by thioacetamide. Lipids Health Dis. 2018, 17, 29. [Google Scholar] [CrossRef]
- Farah, N.; Chin, V.K.; Chong, P.P.; Lim, W.F.; Lim, C.W.; Basir, R.; Chang, S.K.; Lee, T.Y. Riboflavin as a promising antimicrobial agent? A multi-perspective review. Curr. Res. Microb. Sci. 2022, 3, 100111. [Google Scholar] [PubMed]
- Keil, S.D.; Ragan, I.; Yonemura, S.; Hartson, L.; Dart, N.K.; Bowen, R. Inactivation of severe acute respiratory syndrome coronavirus 2 in plasma and platelet products using a riboflavin and ultraviolet light-based photochemical treatment. Vox Sang. 2020, 115, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Akasov, R.A.; Khaydukov, E.V.; Andreyuk, D.S.; Sholina, N.V.; Sheremeta, A.N.; Romanov, D.V.; Kostyuk, G.P.; Panchenko, V.Y.; Kovalchuk, M.V. Riboflavin for COVID-19 Adjuvant Treatment in Patients With Mental Health Disorders: Observational Study. Front. Pharmacol. 2022, 13, 755745. [Google Scholar] [CrossRef]
- Darand, M.; Hassanizadeh, S.; Martami, F.; Shams, S.; Mirzaei, M.; Hosseinzadeh, M. The association between B vitamins and the risk of COVID-19. Br. J. Nutr. 2022, 130, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Colunga Biancatelli, R.M.L.; Berrill, M.; Marik, P.E. The antiviral properties of vitamin C. Expert. Rev. Anti-Infect. Ther. 2020, 18, 99–101. [Google Scholar] [CrossRef]
- Gao, D.; Xu, M.; Wang, G.; Lv, J.; Ma, X.; Guo, Y.; Zhang, D.; Yang, H.; Jiang, W.; Deng, F.; et al. The efficiency and safety of high-dose vitamin C in patients with COVID-19: A retrospective cohort study. Aging 2021, 13, 7020–7034. [Google Scholar] [CrossRef] [PubMed]
- Jude, E.B.; Tentolouris, N.; Rastogi, A.; Yap, M.H.; Pedrosa, H.C.; Ling, S.F. Vitamin D prescribing practices among clinical practitioners during the COVID-19 pandemic. Health Sci. Rep. 2022, 5, e691. [Google Scholar] [CrossRef] [PubMed]
- Sabico, S.; Enani, M.A.; Sheshah, E.; Aljohani, N.J.; Aldisi, D.A.; Alotaibi, N.H.; Alshingetti, N.; Alomar, S.Y.; Alnaami, A.M.; Amer, O.E.; et al. Effects of a 2-Week 5000 IU versus 1000 IU Vitamin D3 Supplementation on Recovery of Symptoms in Patients with Mild to Moderate Covid-19: A Randomized Clinical Trial. Nutrients 2021, 13, 2170. [Google Scholar] [CrossRef]
- Murai, I.H.; Fernandes, A.L.; Sales, L.P.; Pinto, A.J.; Goessler, K.F.; Duran, C.S.C.; Silva, C.B.R.; Franco, A.S.; Macedo, M.B.; Dalmolin, H.H.H.; et al. Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients With Moderate to Severe COVID-19: A Randomized Clinical Trial. JAMA 2021, 325, 1053–1060. [Google Scholar] [CrossRef]
- Varikasuvu, S.R.; Thangappazham, B.; Vykunta, A.; Duggina, P.; Manne, M.; Raj, H.; Aloori, S. COVID-19 and vitamin D (Co-VIVID study): A systematic review and meta-analysis of randomized controlled trials. Expert. Rev. Anti-Infect. Ther. 2022, 20, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, D.A.; Holt, H.; Greenig, M.; Talaei, M.; Perdek, N.; Pfeffer, P.; Vivaldi, G.; Maltby, S.; Symons, J.; Barlow, N.L.; et al. Effect of a test-and-treat approach to vitamin D supplementation on risk of all cause acute respiratory tract infection and covid-19: Phase 3 randomised controlled trial (CORONAVIT). BMJ 2022, 378, e071230. [Google Scholar] [CrossRef] [PubMed]
- Mariani, J.; Antonietti, L.; Tajer, C.; Ferder, L.; Inserra, F.; Sanchez Cunto, M.; Brosio, D.; Ross, F.; Zylberman, M.; López, D.E.; et al. High-dose vitamin D versus placebo to prevent complications in COVID-19 patients: Multicentre randomized controlled clinical trial. PLoS ONE 2022, 17, e0267918. [Google Scholar] [CrossRef] [PubMed]
- Murai, I.H.; Fernandes, A.L.; Sales, L.P.; Pinto, A.J.; Goessler, K.F.; Duran, C.S.C.; Silva, C.B.R.; Franco, A.S.; Macedo, M.B.; Dalmolin, H.H.H.; et al. Effect of a Single High-Dose Vitamin D3 on the Length of Hospital Stay of Severely 25-Hydroxyvitamin D-Deficient Patients with COVID-19. Clinics 2021, 76, 1053–1060. [Google Scholar] [CrossRef]




| Chemical Name | Generic Name | CC50 | SARS-CoV-2 | MERS-CoV | HCoV-229E | |||
|---|---|---|---|---|---|---|---|---|
| IC50 | SI | IC50 | SI | IC50 | SI | |||
| Retinyl Palmitate | A | 466.3 | 41.0 | 11.4 | 125.0 | 3.7 | 345.9 | 1.3 |
| Thiamine HCL | B1 | 580.3 | 52.0 | 11.2 | 128.7 | 4.5 | 79.5 | 7.3 |
| Benfotiamine (Thiamine) | B1 | 509.1 | 18.6 | 27.3 | 387.1 | 1.3 | 525.5 | 1.0 |
| Riboflavin Phosphate | B2 | 848.2 | 37.4 | 22.7 | 208.0 | 4.1 | 14.8 | 57.5 |
| Niacinamide (niacin) | B3 | 1052.0 | 26.9 | 39.2 | 219.7 | 4.8 | 35.6 | 29.5 |
| Pantothenic Acid | B5 | 2316.0 | 917.8 | 2.5 | 55.1 | 42.0 | 158.3 | 14.6 |
| Pyridoxal | B6 | 523.1 | 110.6 | 4.7 | 267.2 | 2.0 | 9.6 | 54.5 |
| Biotin | B7 | 575.4 | 129.1 | 4.5 | 317.3 | 1.8 | >CC50 | 0 |
| Folic acid | B9 | 349.5 | 110.5 | 3.2 | >CC50 | 0 | 330.6 | 1.1 |
| Hydroxycobalamin | B12 | 631.1 | 17.0 | 37.1 | 21.5 | 29.3 | 26.1 | 24.2 |
| Methylcobalamin | B12 | 393.7 | 12.0 | 32.9 | 6.0 | 65.4 | 29.7 | 13.3 |
| Cyanocobalamin | B12 | 246.9 | 13.1 | 18.9 | 29.6 | 8.3 | 104.2 | 2.4 |
| Ascorbic Acid | C | 570.5 | 168.4 | 3.4 | 165.4 | 3.4 | 81.8 | 7.0 |
| Ergocalciferol | D2 | 73.4 | 5.4 | 13.5 | 14.6 | 5.0 | 42.0 | 1.7 |
| Chlolecalciferol | D3 | 170.8 | 29.8 | 5.7 | 38.6 | 4.4 | 33.9 | 5.0 |
| a-Tocopheryl Acetate | E | 570.2 | 122.3 | 4.7 | 104.5 | 5.5 | 127.9 | 4.5 |
| Tocopherols | E | 566.8 | 106.0 | 5.3 | 36.0 | 15.7 | 286.4 | 2.0 |
| Alpha lipoic Acid | a-lipoic Acid | 574.6 | 41.2 | 13.9 | 35.4 | 16.2 | 288.3 | 2.0 |
| Phytomenadione | K1 | 1088.0 | 297.3 | 3.7 | 474.9 | 2.3 | 473.7 | 2.3 |
| Menadione | K3 | 147.8 | 10.3 | 14.4 | 45.2 | 3.3 | 156.8 | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moatasim, Y.; Kutkat, O.; Osman, A.M.; Gomaa, M.R.; Okda, F.; El Sayes, M.; Kamel, M.N.; Gaballah, M.; Mostafa, A.; El-Shesheny, R.; et al. Potent Antiviral Activity of Vitamin B12 against Severe Acute Respiratory Syndrome Coronavirus 2, Middle East Respiratory Syndrome Coronavirus, and Human Coronavirus 229E. Microorganisms 2023, 11, 2777. https://doi.org/10.3390/microorganisms11112777
Moatasim Y, Kutkat O, Osman AM, Gomaa MR, Okda F, El Sayes M, Kamel MN, Gaballah M, Mostafa A, El-Shesheny R, et al. Potent Antiviral Activity of Vitamin B12 against Severe Acute Respiratory Syndrome Coronavirus 2, Middle East Respiratory Syndrome Coronavirus, and Human Coronavirus 229E. Microorganisms. 2023; 11(11):2777. https://doi.org/10.3390/microorganisms11112777
Chicago/Turabian StyleMoatasim, Yassmin, Omnia Kutkat, Ahmed M. Osman, Mokhtar R. Gomaa, Faten Okda, Mohamed El Sayes, Mina Nabil Kamel, Mohamed Gaballah, Ahmed Mostafa, Rabeh El-Shesheny, and et al. 2023. "Potent Antiviral Activity of Vitamin B12 against Severe Acute Respiratory Syndrome Coronavirus 2, Middle East Respiratory Syndrome Coronavirus, and Human Coronavirus 229E" Microorganisms 11, no. 11: 2777. https://doi.org/10.3390/microorganisms11112777
APA StyleMoatasim, Y., Kutkat, O., Osman, A. M., Gomaa, M. R., Okda, F., El Sayes, M., Kamel, M. N., Gaballah, M., Mostafa, A., El-Shesheny, R., Kayali, G., Ali, M. A., & Kandeil, A. (2023). Potent Antiviral Activity of Vitamin B12 against Severe Acute Respiratory Syndrome Coronavirus 2, Middle East Respiratory Syndrome Coronavirus, and Human Coronavirus 229E. Microorganisms, 11(11), 2777. https://doi.org/10.3390/microorganisms11112777













