Mpox: Clinical Outcomes and Impact of Vaccination in People with and without HIV: A Population-Wide Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Study Design and Data Sources
2.3. Study Population
3. Statistical Analysis
4. Ethics Approval
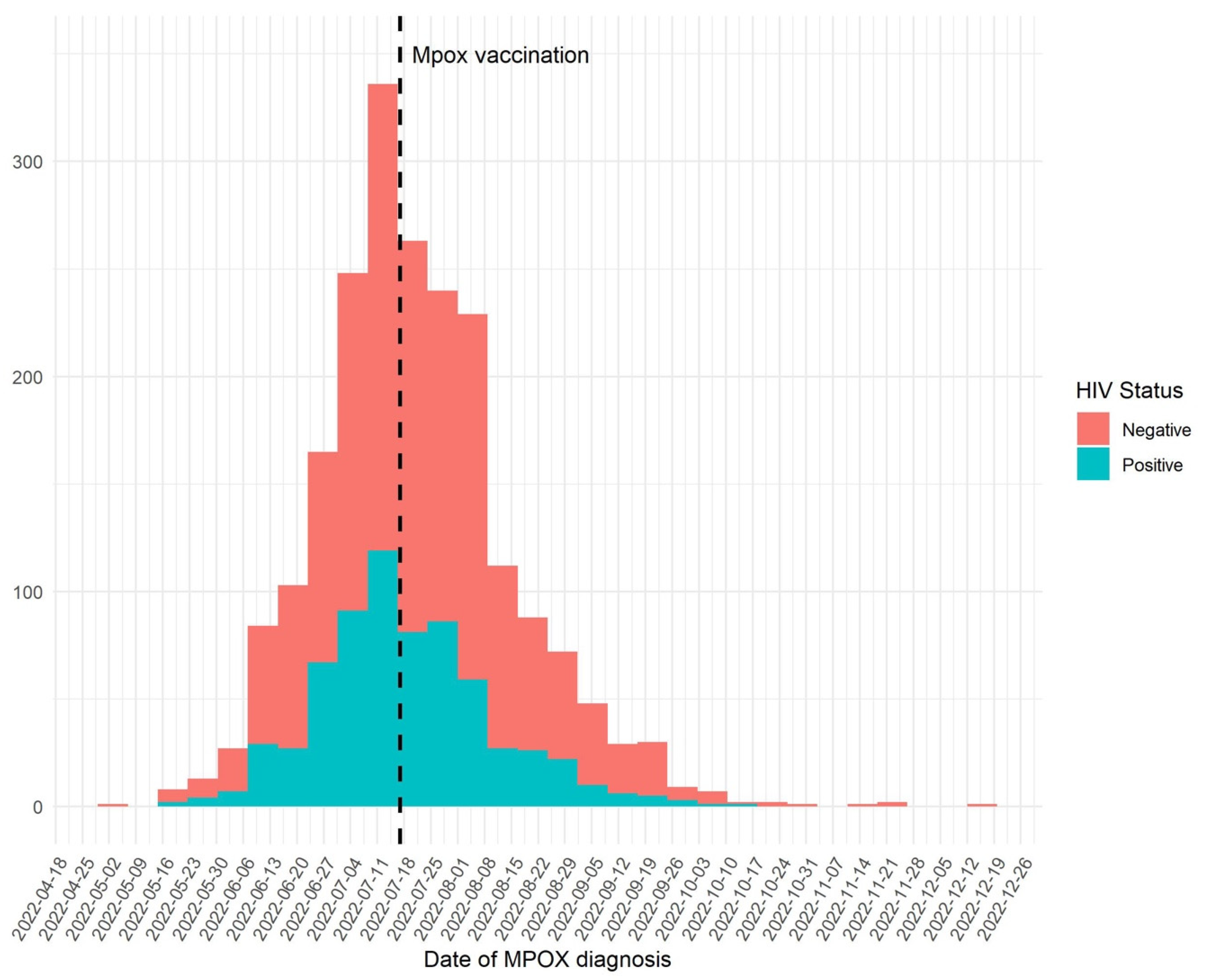
5. Results
6. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Transparency Statement
References
- Vivancos, R.; Anderson, C.; Blomquist, P.; Balasegaram, S.; Bell, A.; Bishop, L.; Brown, C.S.; Chow, Y.; Edeghere, O.; Florence, I.; et al. Community Transmission of Monkeypox in the United Kingdom, April to May 2022. Eurosurveillance 2022, 27, 2200422. [Google Scholar] [CrossRef] [PubMed]
- Duque, M.P.; Ribeiro, S.; Martins, J.V.; Casaca, P.; Leite, P.P.; Tavares, M.; Mansinho, K.; Duque, L.M.; Fernandes, C.; Cordeiro, R.; et al. Ongoing Monkeypox Virus Outbreak, Portugal, 29 April to 23 May 2022. Eurosurveillance 2022, 27, 2200424. [Google Scholar] [CrossRef]
- Martínez, J.I.; Montalbán, E.G.; Bueno, S.J.; Martínez, F.M.; Juliá, A.N.; Díaz, J.S.; Marín, N.G.; Deorador, E.C.; Forte, A.N.; García, M.A.; et al. Monkeypox Outbreak Predominantly Affecting Men Who Have Sex with Men, Madrid, Spain, 26 April to 16 June 2022. Eurosurveillance 2022, 27, 2200471. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization, Geneva. Multi-Country Monkeypox Outbreak in Non-Endemic Countries. 21 May 2022. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON385 (accessed on 30 October 2023).
- Selb, R.; Werber, D.; Falkenhorst, G.; Steffen, G.; Lachmann, R.; Ruscher, C.; McFarland, S.; Bartel, A.; Hemmers, L.; Koppe, U.; et al. A Shift from Travel-Associated Cases to Autochthonous Transmission with Berlin as Epicentre of the Monkeypox Outbreak in Germany, May to June 2022. Eurosurveillance 2022, 27, 2200499. [Google Scholar] [CrossRef]
- Antinori, A.; Mazzotta, V.; Vita, S.; Carletti, F.; Tacconi, D.; Lapini, L.E.; D’Abramo, A.; Cicalini, S.; Lapa, D.; Pittalis, S.; et al. Epidemiological, Clinical and Virological Characteristics of Four Cases of Monkeypox Support Transmission through Sexual Contact, Italy, May 2022. Eurosurveillance 2022, 27, 2200421. [Google Scholar] [CrossRef]
- Nuzzo, J.B.; Borio, L.L.; Gostin, L.O. The WHO Declaration of Monkeypox as a Global Public Health Emergency. JAMA 2022, 328, 615–617. [Google Scholar] [CrossRef] [PubMed]
- Multi-Country Outbreak of Mpox, External Situation Report #28. 19 September 2023. Available online: https://www.who.int/publications/m/item/multi-country-outbreak-of-mpox--external-situation-report-28---19-september-2023 (accessed on 30 October 2023).
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The Changing Epidemiology of Human Monkeypox—A Potential Threat? A Systematic Review. PLoS Negl. Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef]
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; et al. Monkeypox Virus Infection in Humans across 16 Countries—April–June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef]
- Thornhill, J.P.; Palich, R.; Ghosn, J.; Walmsley, S.; Moschese, D.; Cortes, C.P.; Galliez, R.M.; Garlin, A.B.; Nozza, S.; Mitja, O.; et al. Human Monkeypox Virus Infection in Women and Non-Binary Individuals during the 2022 Outbreaks: A Global Case Series. Lancet 2022, 400, 1953–1965. [Google Scholar] [CrossRef]
- Mitjà, O.; Alemany, A.; Marks, M.; Lezama Mora, J.I.; Rodríguez-Aldama, J.C.; Torres Silva, M.S.; Corral Herrera, E.A.; Crabtree-Ramirez, B.; Blanco, J.L.; Girometti, N.; et al. Mpox in People with Advanced HIV Infection: A Global Case Series. Lancet 2023, 401, 939–949. [Google Scholar] [CrossRef]
- Titanji, B.K.; Eick-Cost, A.; Partan, E.S.; Epstein, L.; Wells, N.; Stahlman, S.L.; Devineni, P.; Munyoki, B.; Pyarajan, S.; Balajee, A.; et al. Effectiveness of Smallpox Vaccination to Prevent Mpox in Military Personnel. N. Engl. J. Med. 2023, 389, 1147–1148. [Google Scholar] [CrossRef] [PubMed]
- Malone, S.M.; Mitra, A.K.; Onumah, N.A.; Brown, A.; Jones, L.M.; Tresvant, D.; Brown, C.S.; Onyia, A.U.; Iseguede, F.O. Safety and Efficacy of Post-Eradication Smallpox Vaccine as an Mpox Vaccine: A Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 2963. [Google Scholar] [CrossRef] [PubMed]
- Núm Disposición 2837 Del BOE Núm. 65 de 2015. 2015. Available online: https://www.boe.es/buscar/doc.php?id=BOE-A-2015-2837 (accessed on 30 October 2023).
- Bruguera, A.; Nomah, D.; Moreno-Fornés, S.; Díaz, Y.; Aceitón, J.; Reyes-Urueña, J.; Ambrosioni, J.; Llibre, J.M.; Falcó, V.; Imaz, A.; et al. Cohort Profile: PISCIS, a Population-Based Cohort of People Living with HIV in Catalonia and Balearic Islands. Int. J. Epidemiol. 2023, 52, e241–e252. [Google Scholar] [CrossRef]
- Government of Catalonia. Public Program of Data Analysis for Health Research and Innovation in Catalonia –PADRIS–. AQuAS. Barcelona. Available online: https://aquas.gencat.cat/web/.content/minisite/aquas/fem/avaluacio/procediments/Methodological_Guide_RWD_HTA_AQuAS_2022.pdf (accessed on 30 October 2023).
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.M.; Sundararajan, V. Updating and Validating the Charlson Comorbidity Index and Score for Risk Adjustment in Hospital Discharge Abstracts Using Data from 6 Countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Riser, A.P.; Hanley, A.; Cima, M.; Lewis, L.; Saadeh, K.; Alarcón, J.; Finn, L.; Kim, M.; Adams, J.; Holt, D.; et al. Epidemiologic and clinical features of mpox-associated deaths—United States, May 10, 2022–March 7, 2023. Morb. Mortal. Wkly. Rep. 2023, 72, 404. [Google Scholar] [CrossRef]
- Deputy, N.P.; Deckert, J.; Chard, A.N.; Sandberg, N.; Moulia, D.L.; Barkley, E.; Dalton, A.F.; Sweet, C.; Cohn, A.C.; Little, D.R.; et al. Vaccine Effectiveness of JYNNEOS against Mpox Disease in the United States. N. Engl. J. Med. 2023, 388, 2434–2443. [Google Scholar] [CrossRef]
- Dalton, A.F. Estimated effectiveness of JYNNEOS vaccine in preventing mpox: A multijurisdictional case-control study—United States, August 19, 2022–March 31, 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 553–558. [Google Scholar] [CrossRef]
- Wolff Sagy, Y.; Zucker, R.; Hammerman, A.; Markovits, H.; Arieh, N.G.; Abu Ahmad, W.; Battat, E.; Ramot, N.; Carmeli, G.; Mark-Amir, A.; et al. Real-World Effectiveness of a Single Dose of Mpox Vaccine in Males. Nat. Med. 2023, 29, 748–752. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention Rates of Mpox Cases by Vaccination Status. Available online: https://www.cdc.gov/poxvirus/mpox/cases-data/mpx-vaccine-effectiveness.html#print (accessed on 30 October 2023).
- Dimitrov, D.; Adamson, B.; Matrajt, L. Evaluation of Mpox Vaccine Dose-Sparing Strategies. PNAS Nexus 2023, 2, pgad095. [Google Scholar] [CrossRef]
- Kota, K.K.; Hong, J.; Zelaya, C.; Riser, A.P.; Rodriguez, A.; Weller, D.L.; Spicknall, I.H.; Kriss, J.L.; Lee, F.; Boersma, P.; et al. Racial and ethnic disparities in mpox cases and vaccination among adult males—United States, May–December 2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 398–403. [Google Scholar] [CrossRef]
- Birkett, M.; Neray, B.; Janulis, P.; Phillips, G.; Mustanski, B. Intersectional Identities and HIV: Race and Ethnicity Drive Patterns of Sexual Mixing. AIDS Behav. 2019, 23, 1452–1459. [Google Scholar] [CrossRef] [PubMed]


| Mpox in PWoH n = 1280 | Mpox in PWH n = 842 | p-Value | |
|---|---|---|---|
| Age | 36 (30–43) | 40 (34–46) | <0.001 |
| Male, n (%) | 1229 (96.1) | 840 (99.8) | <0.001 |
| Origin, n (%) | |||
| Spanish | 756 (59.1) | 438 (52.0) | 0.001 |
| European | 192 (15.0) | 113 (13.4) | 0.31 |
| Latin American | 245 (19.1) | 259 (30.8) | <0.001 |
| Other | 87 (6.8) | 32 (3.8) | 0.003 |
| Asymptomatic, n (%) | 62 (4.8) | 20 (2.4) | 0.004 |
| Symptoms, n (%) | |||
| Fever | 641 (50.1) | 467 (55.5) | 0.015 |
| Asthenia | 394 (30.8) | 243 (28.9) | 0.35 |
| Odynophagia | 268 (20.9) | 201 (23.9) | 0.11 |
| Myalgia | 251 (19.6) | 150 (17.8) | 0.30 |
| Headache | 303 (23.7) | 164 (19.5) | 0.023 |
| Generalized lymphadenopathy | 153 (12.0) | 94 (11.2) | 0.58 |
| Localized lymphadenopathy | 530 (41.4) | 346 (41.1) | 0.89 |
| Anogenital exanthema | 727 (56.8) | 472 (56.1) | 0.74 |
| Oro-facial exanthema | 338 (26.4) | 239 (28.4) | 0.32 |
| Exanthema in other localization | 509 (39.8) | 393 (46.7) | 0.002 |
| Type of exanthema | |||
| Maculopapular | 181 (14.1) | 129 (15.3) | 0.45 |
| Vesicular | 308 (24.1) | 185 (22.0) | 0.26 |
| Pustular | 237 (18.5) | 194 (23.0) | 0.011 |
| Umbilicated | 194 (15.2) | 95 (11.3) | 0.011 |
| Crusts | 127 (9.9) | 83 (9.9) | 0.96 |
| Hemorrhagic | 4 (0.3) | 3 (0.4) | 0.86 |
| Complications *, n (%) | 57 (4.5) | 50 (5.9) | 0.13 |
| Skin bacterial infections | 22 (1.7) | 15 (1.8) | 0.91 |
| Localizations: | |||
| Ano-genital | 9 (34.6) | 6 (42.9) | |
| Face | 9 (34.6) | 2 (14.3) | |
| Limbs | 4 (15.4) | 1 (7.1) | |
| Oral | 4 (15.4) | 5 (35.7) | |
| Corneal infections | 5 (0.4) | 3 (0.4) | 0.9 |
| Pneumonia | 0 | 2 (0.2) | 0.081 |
| Proctitis | 15 (1.2) | 14 (1.7) | 0.34 |
| Hospitalization | 22 (1.7) | 22 (2.6) | 0.16 |
| ICU | 1 (0.1) | 0 | 0.42 |
| Sepsis | 0 | 0 | |
| Encephalitis | 0 | 0 | |
| Death | 0 | 0 | |
| Other | 3 (0.2) | 0 | |
| Long term complications | 74 (9.3) | 65 (12.3) | 0.085 |
| PWH with CD4 ≥ 200 Cells/μL at Mpox Diagnosis n = 661 (98.2) | PWH with CD4 < 200 Cells/μL at Mpox Diagnosis n = 12 (1.8) | p-Value | |
|---|---|---|---|
| Demographics | |||
| Male, n (%) | 659 (99.7) | 12 (99.7) | 0.87 |
| Age (years), median (IQR) | 41 (35–46) | 41 (32–44) | 0.26 |
| Birth area, n (%) | |||
| Spain | 268 (40.5) | 0 | |
| Eastern Europe | 17 (2.6) | 0 | |
| Western Europe and Northern America | 84 (12.7) | 0 | |
| Africa | 11 (1.7) | 0 | |
| Latin America | 271 (41.0) | 12 (100) | <0.0001 |
| Other | 10 (1.5) | 0 | |
| Route of HIV transmission, n (%) | 0.48 | ||
| MSM | 610 (95.5) | 11 (91.7) | |
| Heterosexual men | 13 (2.0) | 1 (8.3) | |
| IDU | 11 (1.7) | 0 | |
| Unknown | 5 (0.8) | 0 | |
| Mpox | |||
| Asymptomatic, n (%) | 19 (2.9) | 0 | 0.55 |
| Symptoms, n (%) | |||
| Fever | 364 (55.1) | 6 (50.0) | 0.73 |
| Asthenia | 181 (27.4) | 5 (41.7) | 0.27 |
| Odynophagia | 144 (21.8) | 3 (25.0) | 0.79 |
| Muscular pain | 115 (17.4) | 3 (25.0) | 0.49 |
| Headache | 121 (18.3) | 0 | 0.10 |
| Generalized lymphadenopathy | 73 (11.0) | 2 (16.7) | 0.54 |
| Localized lymphadenopathy | 264 (39.9) | 4 (33.3) | 0.64 |
| Anogenital exanthema | 350 (53.0) | 6 (50.0) | 0.84 |
| Oro-facial exanthema | 197 (29.8) | 1 (8.3) | 0.22 |
| Generalized exanthema | 295 (44.6) | 10 (83.3) | 0.008 |
| Type of exanthema | |||
| Maculopapular | 97 (14.7) | 3 (25.0) | 0.32 |
| Vesicular | 130 (19.7) | 3 (25.0) | 0.65 |
| Pustular | 136 (20.6) | 6 (50.0) | 0.013 |
| Umbilical | 64 (9.7) | 1 (8.3) | 0.88 |
| Crusts | 52 (7.9) | 2 (16.7) | 0.26 |
| Hemorrhagic | 2 (0.30) | 1 (8.3) | <0.0001 |
| Complications, n (%) | |||
| Bacterial infections | 6 (0.9) | 0 | 0.74 |
| Cornea infections | 3 (0.5) | 0 | 0.82 |
| Pneumonia | 1 (0.2) | 0 | 0.89 |
| Proctitis | 11 (1.7) | 0 | 0.65 |
| Hospitalization | 13 (2.0) | 2 (16.7) | 0.001 |
| HIV-related | |||
| CD4 cell count at mpox, (cells/μL), median (IQR) | 724 (540–912) | 122 (72–176) | <0.0001 |
| Viral load < 50 c/mL at mpox | 553 (83.7) | 8 (66.7) | 0.12 |
| Viral load < 200 c/mL at mpox | 579 (87.6) | 10 (83.3) | 0.66 |
| AIDS-defining event prior to mpox | 51 (7.7) | 4 (33.3) | 0.001 |
| Comorbidity | |||
| Malignancy | 38 (5.8) | 3 (25.0) | 0.006 |
| Autoimmune disease | 22 (3.3) | 0 | 0.52 |
| Inflammatory bowel disease | 16 (2.4) | 1 (8.3) | 0.20 |
| Multiple sclerosis | 0 | 0 | |
| History of psychiatric disease | 207 (31.3) | 3 (25.0) | 0.64 |
| Charlson comorbidity score at mpox, | 0.23 | ||
| 0 | 523 (79.2) | 10 (83.3) | |
| 1 | 41 (6.2) | 0 | |
| 2–3 | 87 (13.2) | 1 (8.3) | |
| ≥4 | 10 (1.5) | 1 (8.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Iguacel, R.; Pericas, C.; Bruguera, A.; Rosell, G.; Martínez, E.; Díaz, Y.; Alonso, L.; Nomah, D.K.; Blanco, J.L.; Domingo, P.; et al. Mpox: Clinical Outcomes and Impact of Vaccination in People with and without HIV: A Population-Wide Study. Microorganisms 2023, 11, 2701. https://doi.org/10.3390/microorganisms11112701
Martín-Iguacel R, Pericas C, Bruguera A, Rosell G, Martínez E, Díaz Y, Alonso L, Nomah DK, Blanco JL, Domingo P, et al. Mpox: Clinical Outcomes and Impact of Vaccination in People with and without HIV: A Population-Wide Study. Microorganisms. 2023; 11(11):2701. https://doi.org/10.3390/microorganisms11112701
Chicago/Turabian StyleMartín-Iguacel, Raquel, Carles Pericas, Andreu Bruguera, Gemma Rosell, Erica Martínez, Yesika Díaz, Lucia Alonso, Daniel Kwakye Nomah, Jose Luis Blanco, Pere Domingo, and et al. 2023. "Mpox: Clinical Outcomes and Impact of Vaccination in People with and without HIV: A Population-Wide Study" Microorganisms 11, no. 11: 2701. https://doi.org/10.3390/microorganisms11112701
APA StyleMartín-Iguacel, R., Pericas, C., Bruguera, A., Rosell, G., Martínez, E., Díaz, Y., Alonso, L., Nomah, D. K., Blanco, J. L., Domingo, P., Álvarez-López, P., Linares, M. S., Vilades Laborda, C., Mera, A., Calzado Isbert, S., Johansen, I. S., Miró, J. M., Casabona, J., & Llibre, J. M., on behalf of the PISCIS Study Group. (2023). Mpox: Clinical Outcomes and Impact of Vaccination in People with and without HIV: A Population-Wide Study. Microorganisms, 11(11), 2701. https://doi.org/10.3390/microorganisms11112701








