Secondary Metabolites from the Nematode-Trapping Fungus Dactylellina haptotyla YMF1.03409
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Strain and Culture
2.2. General Experimental Procedures
2.3. Screening of Culture Conditions
2.4. Fermentation and Isolation of Compounds
2.5. Nematicidal Activity of Compounds
3. Results
3.1. Culture and Fermentation of D. haptotyla YMF1.03409
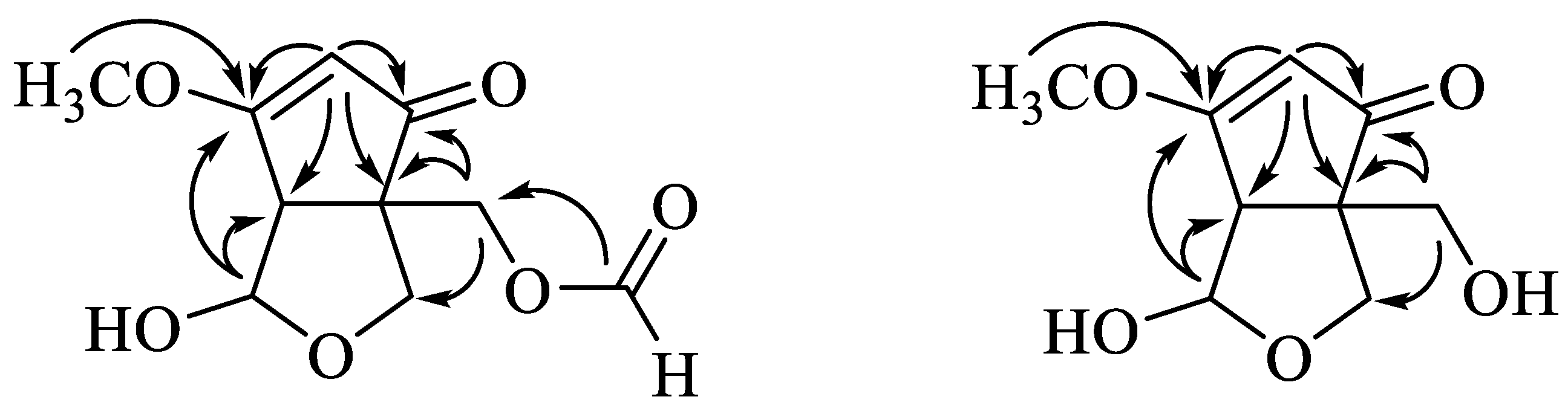
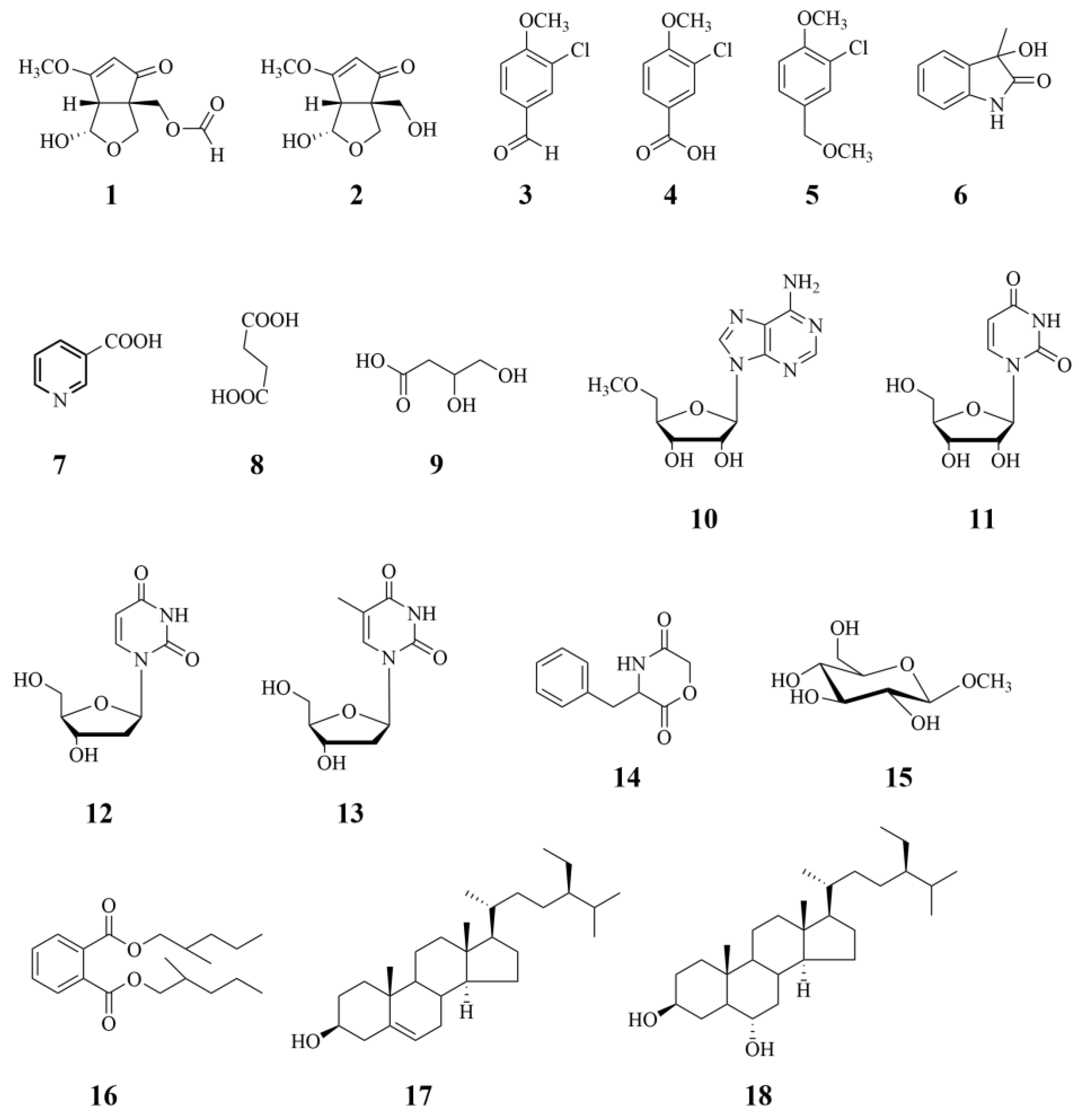
3.2. Structural Identification of Compounds
3.3. Nematicidal Activity of Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nguyen, V.T.; Yu, N.H.; Lee, Y.; Hwang, I.M.; Bui, H.X.; Kim, J.C. Nematicidal activity of cyclopiazonic acid derived from Penicillium commune against root-knot nematodes and optimization of the culture fermentation process. Front. Microbiol. 2021, 12, 726504. [Google Scholar] [CrossRef] [PubMed]
- Trudgill, D.L.; Blok, V.C. Apomictic, polyphagous root-knot nematodes: Exceptionally successful and damaging biotrophic root pathogens. Annu. Rev. Phytopathol. 2001, 39, 53–77. [Google Scholar] [CrossRef] [PubMed]
- Barron, G. The nematode-destroying fungi. Trans. Br. Mycol. Soc. 1978, 70, 488. [Google Scholar] [CrossRef]
- Li, G.; Zhang, K.; Xu, J.; Dong, J.; Liu, Y. Nematicidal substances from fungi. Recent Pat. Biotechnol. 2007, 1, 212–233. [Google Scholar] [CrossRef]
- Jansson, H.-B. Attraction of nematodes to endoparasitic nematophagous fungi. Trans. Br. Mycol. Soc. 1982, 79, 25–29. [Google Scholar] [CrossRef]
- Meerupati, T.; Andersson, K.M.; Friman, E.; Kumar, D.; Tunlid, A.; Ahrén, D. Genomic mechanisms accounting for the adaptation to parasitism in nematode-trapping fungi. PLoS Genet. 2013, 9, e1003909. [Google Scholar] [CrossRef]
- Andersson, K.M.; Meerupati, T.; Levander, F.; Friman, E.; Ahrén, D.; Tunlid, A. Proteome of the nematode-trapping cells of the fungus Monacrosporium haptotylum. Appl. Environ. Microbiol. 2013, 79, 4993–5004. [Google Scholar] [CrossRef]
- Fekete, C.; Tholander, M.; Rajashekar, B.; Ahren, D.; Friman, E.; Johansson, T.; Tunlid, A. Paralysis of nematodes: Shifts in the transcriptome of the nematode-trapping fungus Monacrosporium haptotylum during infection of Caenorhabditis elegans. Environ. Microbiol. 2008, 10, 364–375. [Google Scholar] [CrossRef]
- Huang, D.; Yu, C.; Shao, Z.; Cai, M.; Li, G.; Zheng, L.; Yu, Z.; Zhang, J. Identification and characterization of nematicidal volatile organic compounds from deep-sea Virgibacillus dokdonensis MCCC 1A00493. Molecules 2020, 25, 744. [Google Scholar] [CrossRef]
- Kozlovsky, A.G.; Zhelifonova, V.P.; Adanin, V.M.; Ozerskaya, S.M.; Gräfe, U. Nosporins A and B, new metabolites from a filamentous fungus, VKM-3750. Arch. Pharm. 2003, 58, 76–77. [Google Scholar] [CrossRef]
- Mostafa, M.A.; Bowley, R.M.; Racys, D.T.; Henry, M.C.; Sutherland, A. Iron(III)-catalyzed chlorination of activated arenes. J. Org. Chem. 2017, 82, 7529–7537. [Google Scholar] [CrossRef] [PubMed]
- Naik, R.G.; Wheeler, T.S. Reactivity of the ω-halogen atom in p-alkoxybenzyl halides: Preparation of phenylacetic acids. J. Chem. Soc. 1938, 1780–1783. [Google Scholar] [CrossRef]
- England, D.B.; Merey, G.; Padwa, A. Substitution and cyclization reactions involving the quasi-antiaromatic 2H-indol-2-one ring system. Org. Lett. 2007, 9, 3805–3807. [Google Scholar] [CrossRef] [PubMed]
- Imbriglio, J.E.; Chang, S.; Liang, R.; Raghavan, S.; Schmidt, D.; Smenton, A.; Tria, S.; Schrader, T.O.; Jung, J.K.; Esser, C.; et al. GPR109a agonists. part 1: 5-alkyl and 5-aryl-pyrazole-tetrazoles as agonists of the human orphan G-protein coupled receptor GPR109a. Bioorg. Med. Chem. Lett. 2009, 19, 2121–2124. [Google Scholar] [CrossRef]
- Gaviglio, C.; Doctorovich, F. Hydrogen-free homogeneous catalytic reduction of olefins in aqueous solutions. J. Org. Chem. 2008, 73, 5379–5384. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Lee, E.J.; Wu, T.S. Antityrosinase principles and constituents of the petals of Crocus sativus. J. Org. Chem. 2004, 67, 437–440. [Google Scholar] [CrossRef]
- van Tilburg, E.W.; van der Klein, P.A.M.; von Frijtag, J.; Künzel, D.; de Groote, M.; Stannek, C.; Lorenzen, A.; Ijzerman, A.P. 5′-O-alkyl ethers of N,2-substituted adenosine derivatives: Partial agonists for the adenosine A1 and A3 receptors. J. Med. Chem. 2001, 44, 2966–2975. [Google Scholar] [CrossRef]
- Chen, X.S.; Chen, D.H.; Si, J.Y.; Tu, G.Z. Chemical constituents of Typhonium giganteum Engl. J. Asian Nat. Prod. Res. 2001, 3, 277–283. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Xu, Y.Z. Systematic assignment of NMR spectra of 5-substituted-4-thiopyrimidine nucleosides. Magn. Reson. Chem. 2013, 51, 523–529. [Google Scholar] [CrossRef]
- Yu, X.; Yl, W.; Shi-Xiang, G.; Cheng, S.; Zhong-Yuan, Z. Chemical composition of Hydrilla verticillata (L. f.) royle in Taihu Lake. Chin. J. Chem. 2007, 25, 661–665. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, R.Q.; Liu, X.N.; Wang, M. Determination of the content of poly (lactic acid-co-phenylalanine) by infrared spectroscopy. Spectrosc. Spect. Anal. 2009, 29, 661–664. [Google Scholar] [CrossRef]
- Gorin, P.A.J.; Mazurek, M. Further studies on the assignment of signals in 13C magnetic resonance spectra of aldoses and derived methyl glycosides. Can. J. Chem. 1975, 53, 1212–1223. [Google Scholar] [CrossRef]
- Singh, N.; Mahmood, U.; Kaul, V.K.; Jirovetz, L. A new phthalic acid ester from Ajuga bracteosa. Nat. Prod. Res. 2006, 20, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Ali, M.; Alam, P. New phytoconstituents from the stem bark of Tinospora cordifolia Miers. Nat. Prod. Res. 2010, 24, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Garcez, F.; Garcez, W.; Miguel, D.; Serea, A.A.T.; Prado, F.C. Chemical constituents from Terminalia glabrescens. J. Braz. Chem. Soc. 2003, 14, 461–465. [Google Scholar] [CrossRef]
- Okamoto, K.; Narayama, S.; Katsuo, A.; Shigematsu, I.; Yanase, H. Biosynthesis of p-anisaldehyde by the white-rot basidiomycete Pleurotus ostreatus. J. Biosci. Bioeng. 2002, 93, 207–210. [Google Scholar] [CrossRef]
- Schalchli, H.H.E.; Becerra, J.; Briceño, G.; Hernández, V.; Rubilar, O.; Diez, M.C. Volatiles from white-rot fungi for controlling plant pathogenic fungi. Chem. Ecol. 2015, 31, 754–763. [Google Scholar] [CrossRef]
- Georgousaki, K.; Tsafantakis, N.; Gumeni, S.; Lambrinidis, G.; González-Menéndez, V.; Tormo, J.R.; Genilloud, O.; Trougakos, I.P.; Fokialakis, N. Biological evaluation and in silico study of benzoic acid derivatives from Bjerkandera adusta targeting proteostasis network modules. Molecules 2020, 25, 666. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Ren, S.X.; Ren, N.; Zhang, J.J.; Geng, L.N.; Wang, S.P.; Shi, S.K. Crystal structures, spectroscopic, and thermal properties of dysprosium (III) and europium (III) complexes with 3-chloro-4-methoxybenzoic and 1,10-phenanthroline. J. Therm. Anal. Calorim. 2015, 119, 1803–1810. [Google Scholar] [CrossRef]
- Maia, A.C.D.; de Lima, C.T.; Navarro, D.; Chartier, M.; Giulietti, A.M.; Machado, I.C. The floral scents of Nymphaea subg. Hydrocallis (Nymphaeaceae), the new world night-blooming water lilies, and their relation with putative pollinators. Phytochemistry 2014, 103, 67–75. [Google Scholar] [CrossRef]
- Skordos, K.W.; Skiles, G.L.; Laycock, J.D.; Lanza, D.L.; Yost, G.S. Evidence supporting the formation of 2,3-epoxy-3-methylindoline: A reactive intermediate of the pneumotoxin 3-methylindole. Chem. Res. Toxicol. 1998, 11, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.D.; Seligman, R.B. The anti-tuberculous activity of some derivatives of p-aminosalicylic acid, nicotinic acid, and isonicotinic acid. Antibiot. Chemother. 1955, 5, 129–131. [Google Scholar]
- Shershevskii, M.G. Effect of nicotinic acid on the fibrinolytic activity of the blood in atherosclerosis. Kardiologiia 1963, 22, 1154–1156. [Google Scholar]
- Cornils, B.; Lappe, P. Dicarboxylic acids, aliphatic. Ullmann’s Encycl. Ind. Chem. 2022, 15, 4807–4817. [Google Scholar] [CrossRef]
- Fukui, S.; Shimoyama, T.; Tamura, K.; Yamamura, M.; Satomi, M. Mucosal blood flow and generation of superoxide in rat experimental colitis induced by succinic acid. J. Gastroenterol. 1997, 32, 464–471. [Google Scholar] [CrossRef]
- Mollick, T.; Laín, S. Modulating pyrimidine ribonucleotide levels for the treatment of cancer. Cell Metab. 2020, 8, 12. [Google Scholar] [CrossRef]
- Oksenhendler, E. Azidothymidine. Nouv. Rev. Fr. Hematol. 1989, 31, 69–72. [Google Scholar]
- Saleem, B.; Islam, M.; Ahmed, A.; Saeed, H.; Imtiaz, F.; Muzaffar, S. Bioassay-guided isolation and in silico study of antihyperlipidemic compounds from Onosma hispidum Wall. J. Biomol. Struct. Dyn. 2023, 1–15. [Google Scholar] [CrossRef]
- Khan, A.; Rahman, M.; Islam, S. Antipyretic activity of Peperomia pellucida leaves in rabbit. Turk. J. Biol. 2008, 32, 37–41. Available online: https://journals.tubitak.gov.tr/biology/vol32/iss1/6 (accessed on 1 October 2023).
- Pateh, U.; Haruna, A.K.; Garba, M.; Iliya, I.; Sule, I.M.; Abubakar, M.; Ambi, A. Isolation of stigmasterol, β-sitosterol and 2-hydroxyhexadecanoic acid methyl ester from the rhizomes of Stylochiton lancifolius Pyer and Kotchy (Aeaceae). Niger. J. Pharm. Res. 2009, 8, 19–25. [Google Scholar]
- Khanam, S.; Sultana, R. Isolation of β-sitosterol and stigmasterol as active immunomodulatory constituent from the fruits of Solanum xanthocarpum (Solanaceae). Int. J. Sci. Prog. Res. 2012, 3, 1057–1060. [Google Scholar]
- Hsueh, Y.P.; Gronquist, M.R.; Schwarz, E.M.; Nath, R.D.; Lee, C.H.; Gharib, S.; Schroeder, F.C.; Sternberg, P.W. Nematophagous fungus Arthrobotrys oligospora mimics olfactory cues of sex and food to lure its nematode prey. eLife 2017, 6, e20023. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Hu, X.; Pop, M.; Wernet, N.; Kirschhöfer, F.; Brenner-Weiß, G.; Keller, J.; Bunzel, M.; Fischer, R. Fatal attraction of Caenorhabditis elegans to predatory fungi through 6-methyl-salicylic acid. Nat. Commun. 2021, 12, 5462. [Google Scholar] [CrossRef] [PubMed]
- Song, T.Y.; Xu, Z.F.; Chen, Y.H.; Ding, Q.Y.; Sun, Y.R.; Miao, Y.; Zhang, K.Q.; Niu, X.M. Potent nematicidal activity and new hybrid metabolite production by disruption of a cytochrome P450 gene involved in the biosynthesis of morphological regulatory arthrosporols in nematode-trapping fungus Arthrobotrys oligospora. J. Agric. Food Chem. 2017, 65, 4111–4120. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.M.; Wang, J.T.; Hu, Q.Y.; Li, C.Q.; Mo, M.H.; Zhang, K.Q.; Li, G.H.; Zhao, P.J. 2-Furoic acid associated with the infection of nematodes by Dactylellina haptotyla and its biocontrol potential on plant root-knot nematodes. Microbiol. Spectr. 2023, 11, e0189623. [Google Scholar] [CrossRef] [PubMed]

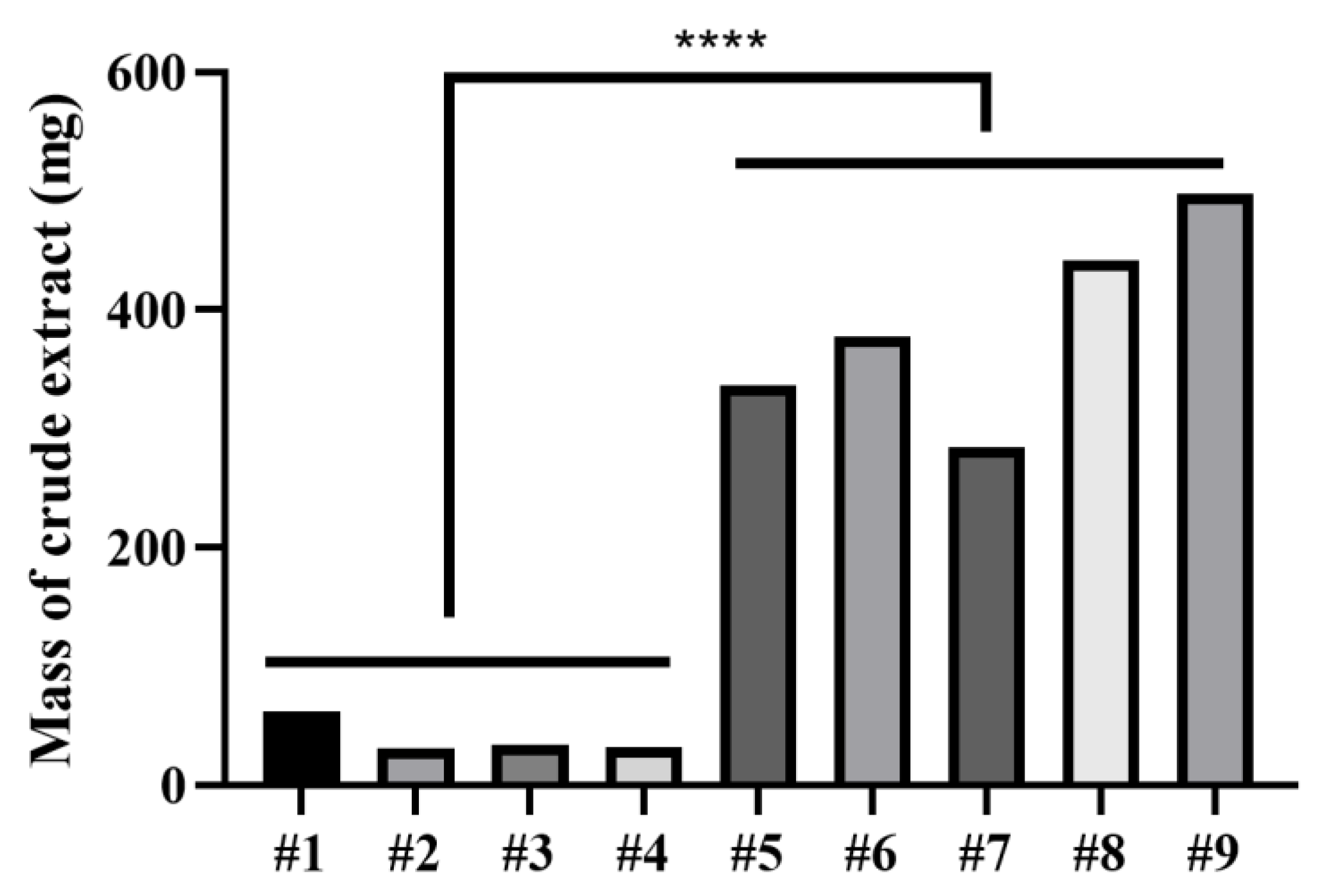
| Media Number | Media Formulation |
|---|---|
| #1 | 0.5 g KH2PO4, 0.3 g MgSO4, 3 g yeast extract, 10 g glucose, 10 g sodium glutamate, 20 g malt extract, 20 g mannitol, 1 L H2O |
| #2 | 12.2 mg 5-azacytidine, 0.3 g MgSO4, 3 g yeast extract, 0.5 g KH2PO4, 10 g glucose, 10 g sodium glutamate, 20 g malt extract, 20 g mannitol, 1 L H2O |
| #3 | 4 g yeast extract, 4 g glucose, 10 g malt extract, 1 L H2O |
| #4 | 12.2 mg 5-azacytidine, 4 g yeast extract, 4 g glucose, 10 g malt extract, 1 L H2O |
| #5 | 60 g rice, 50 mL H2O |
| #6 | 0.3 g tryptone, 60 g rice, 50 mL H2O |
| #7 | 0.3 g tryptone, 60 g rice, 50 mL H2O, 5 g pork liver |
| #8 | 0.3 g (NH4)2SO4, 60 g rice, 50 mL H2O |
| #9 | 0.3 g (NH4)2SO4, 60 g rice, 50 mL H2O, 5 g pork liver |
| Position | 1 (in CDCl3) | 2 (in CD3OD) | ||||
|---|---|---|---|---|---|---|
| 1H | 13C | HMBC | 1H | 13C | HMBC | |
| 1 | - | 203.4, s | - | - | 209.4, s | - |
| 2 | 5.35 (s) | 105.1, d | C-1, C-3, C-4, C-5 | 5.41 (s) | 106.1, d | C-1, C-3, C-4, C-5 |
| 3 | - | 187.8, s | - | - | 191.4, s | - |
| 4 | 3.28 (brs) | 56.9, d | C-1, C-2, C-3, C-9, C-8 | 3.24 (brs) | 58.5, d | C-1, C-2, C-3, C-9, C-8 |
| 5 | - | 58.3, s | - | - | 63.1, s | - |
| 6 | 3.92 (d, J = 9.2) | 69.5, t | C-1, C-5, C-9 | 3.65 (d, J = 10.7) | 70.0, t | C-1, C-9 |
| 4.06 (d, J = 9.2) | C-1, C-8, C-9 | 3.92 (d, J = 10.7) | C-1, C-9 | |||
| 8 | 5.59 (s) | 98.4, d | C-3, C-5, C-6, 3-OCH3 | 5.40 (brs) | 99.3, d | C-3, C-5, C-6, 3-OCH3 |
| 9 | 4.34 (d, J = 11.0) | 62.9, t | C-1, C-4, C-5, C-6, 9-OCHO | 3.76 (d, J = 9.0) | 62.9, t | C-1, C-4, C-5 |
| 4.54 (d, J = 11.0) | C-1, C-4, C-5, C-6, 9-OCHO | 3.81 (d, J = 9.0) | C-1, C-4, C-5 | |||
| 3-OCH3 | 3.90 (s) | 59.3, q | C-3 | 3.93 (s) | 60.2, q | C-3 |
| 9-OCHO | 8.03 (s) | 160.3, d | C-9 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, H.; Zhang, G.; Zhao, P.; Li, G. Secondary Metabolites from the Nematode-Trapping Fungus Dactylellina haptotyla YMF1.03409. Microorganisms 2023, 11, 2693. https://doi.org/10.3390/microorganisms11112693
Lei H, Zhang G, Zhao P, Li G. Secondary Metabolites from the Nematode-Trapping Fungus Dactylellina haptotyla YMF1.03409. Microorganisms. 2023; 11(11):2693. https://doi.org/10.3390/microorganisms11112693
Chicago/Turabian StyleLei, Hongmei, Guangke Zhang, Peiji Zhao, and Guohong Li. 2023. "Secondary Metabolites from the Nematode-Trapping Fungus Dactylellina haptotyla YMF1.03409" Microorganisms 11, no. 11: 2693. https://doi.org/10.3390/microorganisms11112693
APA StyleLei, H., Zhang, G., Zhao, P., & Li, G. (2023). Secondary Metabolites from the Nematode-Trapping Fungus Dactylellina haptotyla YMF1.03409. Microorganisms, 11(11), 2693. https://doi.org/10.3390/microorganisms11112693







