Isolation and Identification of Bacteria from Three Geothermal Sites of the Atacama Desert and Their Plant-Beneficial Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Sampling and Bacterial Isolation
2.3. DNA Isolation, Amplification, and Sequencing of 16S rRNA Gene
2.4. Phylogenetic Analysis
2.5. Bacterial Characterization
2.5.1. In Vitro Detection of Hydrolytic Activities
2.5.2. Determination of Plant Growth-Promoting Traits
2.5.3. Evaluation of Growth Inhibition of Phytopathogenic Fungi In Vitro
3. Results
3.1. Samples Characterization
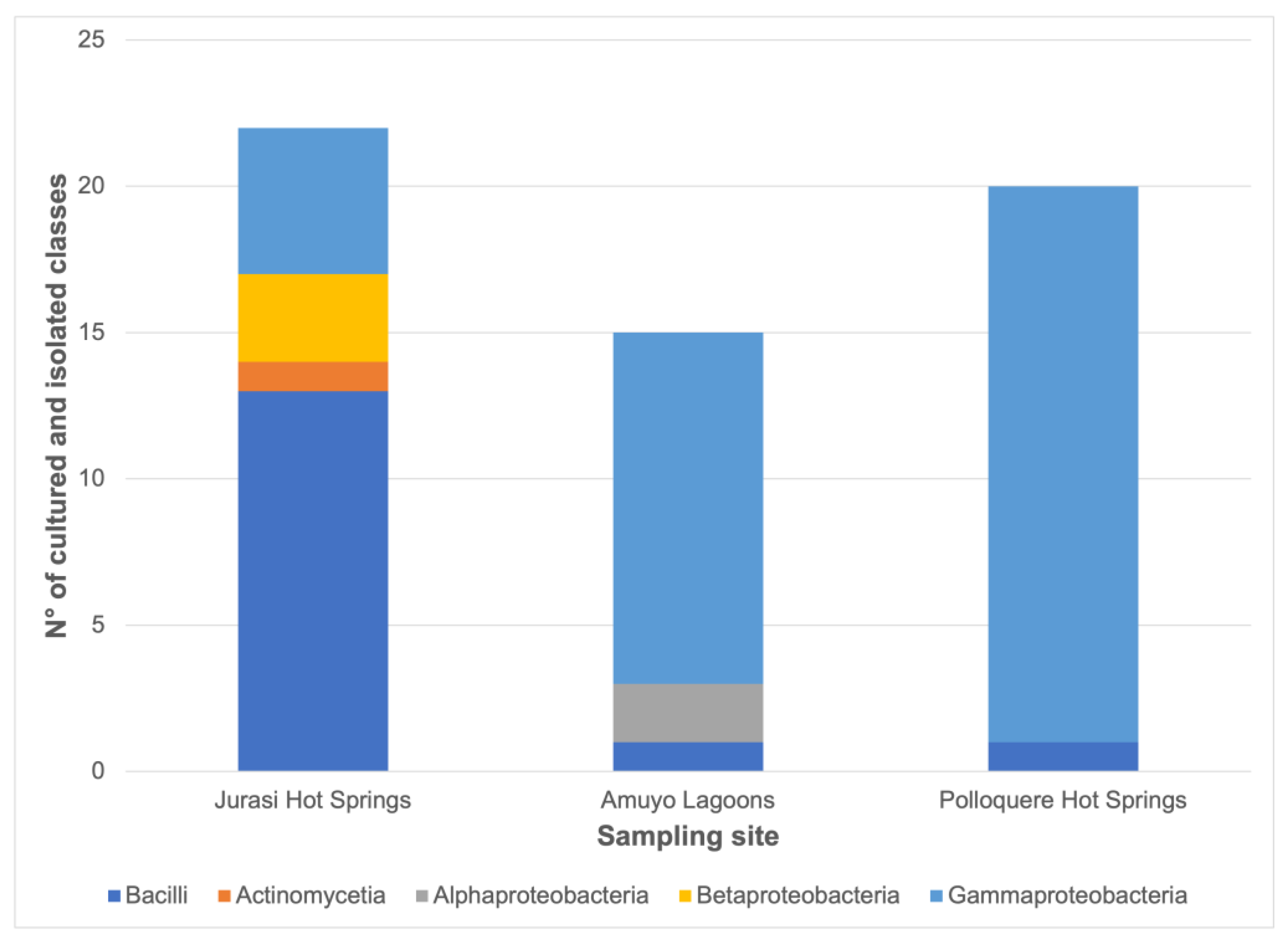
3.2. Bacterial Isolation, Identification, Distribution, and Functional Characterization
3.3. Phylogenetic Analysis
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Three Key Challenges Facing Agriculture and How to Start Solving Them. Available online: https://www.oecd.org/agriculture/key-challenges-agriculture-how-solve/ (accessed on 25 April 2022).
- Rayas-González, J.; Hernández-Abreu, E.; Valencia-Cantero, E.; López-Bucio, J. Microbial resources for improved crop productivity. In The Handbook of Microbial Bioresources, 1st ed.; Gupta, V., Sharma, G., Tuohy, M., Gaur, R., Eds.; CABI: Wallingford, UK, 2016; pp. 1–13. [Google Scholar]
- Mahilum-Tapay, L. The importance of microbial culture collections and gene banks in biotechnology. In Fundamentals in Biotechnology, 1st ed.; Doelle, H., Rokem, J., Berovic, M., Eds.; EOLSS: Oxford, UK, 2009; pp. 1–12. [Google Scholar]
- Aasfas, A.; Bargaz, A.; Yaakoubi, K.; Hilali, A.; Bennis, I.; Zeroual, Y.; Kadmiri, I. Nitrogen fixing Azotobacter species as potential soil biological enhancers for crop nutrition and yield stability. Front. Microbiol. 2021, 12, 628379. [Google Scholar]
- Tian, J.; Ge, F.; Zhang, D.; Deng, S.; Liu, X. Roles of phosphate solubilizing microorganisms from managing phosphorus deficiency to mediating biogeochemical P cycle. Biology 2021, 10, 158. [Google Scholar] [CrossRef]
- Saha, M.; Sarkar, S.; Sarkar, B.; Sharma, B.; Bhattacharjee, S.; Tribedi, P. Microbial siderophores and their potential applications: A review. Environ. Sci. Pollut. Res. Int. 2016, 23, 3984–3999. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Alqarawi, A.; Abd_Allah, E.; Hashem, A. Phytohormones and beneficial microbes: Essential components for plants to balance stress and fitness. Front. Microbiol. 2017, 8, 2104. [Google Scholar] [CrossRef]
- Muñoz-Torres, P.; Cárdenas, S.; Arismendi, M.; Huanacuni, N.; Huanca-Mamani, W.; Cifuentes, D.; Sepúlveda-Chavera, G. The endophytic Pseudomonas sp. S57 for plant-growth promotion and the biocontrol of phytopathogenic fungi and nematodes. Plants 2021, 10, 1531. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.; Zamioudis, C.; Berendsen, R.; Weller, D.; Van Wees, S.; Bakker, P. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [PubMed]
- Insights on the Agricultural Biologicals Global Market to 2026. Growing Demand for Food Owing to Booming Population Growth Is Driving the Industry. Available online: https://www.businesswire.com/news/home/20210510005337/en/Insights-on-the-Agricultural-Biologicals-Global-Market-to-2026---Growing-Demand-for-Food-Owing-to-Booming-Population-Growth-is-Driving-the-Industry---ResearchAndMarkets.com (accessed on 25 April 2022).
- Salvatierra-Martínez, R.; Sepúlveda-Chavera, G.; Huanca-Mamani, W.; Rodríguez-Molina, M. Native strains of Trichoderma from northern Chile: Adaptive tolerance in boric saline soils. Interciencia 2015, 40, 263–269. [Google Scholar]
- Rampelotto, P. Resistance of microorganisms to extreme environmental conditions and its contribution to astrobiology. Sustainability 2010, 2, 1602–1623. [Google Scholar] [CrossRef]
- Shilev, S. Plant-growth-promoting bacteria mitigating soil salinity stress in plants. Appl. Sci. 2020, 10, 7326. [Google Scholar] [CrossRef]
- Gaete, A.; Mandakovic, D.; González, M. Isolation and Identification of Soil Bacteria from Extreme Environments of Chile and Their Plant Beneficial Characteristics. Microorganisms 2020, 8, 1213. [Google Scholar] [CrossRef]
- Tuesta-Popolizio, D.; Velázquez-Fernández, J.; Rodriguez-Campos, J.; Contreras-Ramos, S. Isolation and Identification of Extremophilic Bacteria with Potential as Plant Growth Promoters (Pgpb) of A Geothermal Site: A Case Study. Geomicrobiol. J. 2021, 38, 436–450. [Google Scholar] [CrossRef]
- Huanca-Mamani, W.; Rivera-Cabello, D.; Maita-Maita, J. A simple, fast, and inexpensive CTAB-PVP-silica based method for genomic DNA isolation from single, small insect larvae and pupae. Genet. Mol. Res. 2015, 14, 8001–8007. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.; Reich, C.; Sharma, S.; Weisbaum, J.; Wilson, B.; Olsen, G. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl. Environ. Microbiol. 2008, 74, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.; Gish, W.; Miller, W.; Myers, E.; Lipman, D. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Criscuolo, A.; Gribaldo, S. BMGE (Block Mapping and Gathering with Entropy): A new software for selection of phylogenetic informative regions from multiple sequence alignments. BMC Evol. Biol. 2010, 10, 210. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, S.; Islam, S.; Ahmed, M.M.; Hossain, M.B.; Hossain, M.A. Protease producing bacteria and activity in gut of Tiger Shrimp (Penaeus monodon). J. Fish. Aquat. Sci. 2015, 10, 489–500. [Google Scholar] [CrossRef]
- Slifkin, M. Tween 80 opacity test responses of various Candida species. J. Clin. Microbiol. 2000, 38, 4626–4628. [Google Scholar] [CrossRef]
- Verma, K.; Garg, N. Detection of chitinase on chitin agar plates. Int. J. Sci. Res. 2019, 8, 1186–1189. [Google Scholar]
- Hossain, T.J.; Chowdhury, S.I.; Mozumder, H.A.; Chowdhury, M.N.A.; Ali, F.; Rahman, N.; Dey, S. Hydrolytic exoenzymes produced by bacteria isolated and identified from the gastrointestinal tract of Bombay Duck. Front. Microbiol. 2020, 11, 2097. [Google Scholar] [CrossRef] [PubMed]
- Ehmann, A. The Van Urk-Salkowski reagent-a sensitive and specific chromogenic reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives. J. Chromatogr. 1977, 132, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, E. Improved medium for isolation of Azospirillum spp. Appl. Environ. Microbiol. 1982, 44, 990–991. [Google Scholar]
- Pikovskaya, R. Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. Microbiologiya 1948, 17, 362–370. [Google Scholar]
- Schwynand, B.; Neilands, J. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Sepúlveda-Chavera, G.F.; Arismendi, M.; Muñoz-Torres, P. Endospore-Forming Bacteria Present in a Commercial Stabilized Poultry Manure Determines the Fusarium Biocontrol and the Tomato Growth Promotion. Agronomy 2020, 10, 1636. [Google Scholar] [CrossRef]
- Cornejo, L. Recursos hídricos y desarrollo socioeconómico en zonas áridas: Importancia y perspectivas de nuevas tecnologías aplicadas al tratamiento de aguas naturales y/o residuales. Ingeniare Rev. Chil. Ing. 2009, 17, 285–287. [Google Scholar] [CrossRef]
- ODEPA. Región de Arica y Parinacota: Información Regional 2018. Available online: https://www.odepa.gob.cl/wp-content/uploads/2018/02/Arica-y-Parinacota.pdf (accessed on 22 May 2023).
- Giraud, T.; Koskella, B.; Laine, A. Introduction: Microbial local adaptation: Insights from natural populations, genomics and experimental evolution. Mol. Ecol. 2017, 26, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Gadhave, K.; Gange, A. Plant-associated Bacillus spp. alter life-history traits of the specialist insect Brevicoryne brassicae L. Agric. For. Entomol. 2016, 18, 35–42. [Google Scholar] [CrossRef]
- Qessaoui, R.; Bouharroud, R.; Furze, J.; El Aalaoui, M.; Akroud, H.; Amarraque, A.; Van Vaerenberg, J.; Tahzima, R.; Mayad, E.; Chebli, B. Applications of new rhizobacteria Pseudomonas isolates in agroecology via fundamental processes complementing plant growth. Sci. Rep. 2019, 9, 12832. [Google Scholar] [CrossRef]
- Dutkiewicz, J.; Mackiewicz, B.; Lemieszek, M.; Golec, M.; Milanowski, J. Pantoea agglomerans: A mysterious bacterium of evil and good. Part IV. Beneficial effects. Ann. Agric. Environ. Med. 2016, 23, 206–222. [Google Scholar] [CrossRef]
- Marfetán, J.; Gallo, A.; Farias, M.; Vélez, M.; Pescuma, M.; Ordoñez, O. Exiguobacterium sp. as a bioinoculant for plant-growth promotion and Selenium biofortification strategies in horticultural plants. World J. Microbiol. Biotechnol. 2023, 39, 134. [Google Scholar] [CrossRef]
- Wu, G.; Chen, Z.; Jiang, D.; Jiang, N.; Jiang, H.; Chen, L. Oxidases and hydrolases mediate soil organic matter accumulation in chernozem of northeastern China. Geoderma 2021, 403, 115206. [Google Scholar] [CrossRef]
- Souza, R.; Cantão, M.; Nogueira, M.; Ribeiro Vasconcelos, A.; Hungria, M. Outstanding impact of soil tillage on the abundance of soil hydrolases revealed by a metagenomic approach. Braz. J. Microbiol. 2018, 49, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Amaresan, N.; Kumar, K.; Sureshbabu, K.; Madhuri, K. Plant growth-promoting potential of bacteria isolated from active volcano sites of Barren Island, India. Lett. Appl. Microbiol. 2013, 58, 130–137. [Google Scholar] [CrossRef]
- Mishra, P.; Mishra, J.; Dwivedi, S.; Arora, N. Microbial Enzymes in Biocontrol of Phytopathogens. In Microbial Enzymes: Roles and Applications in Industries; Arora, N.K., Mishra, J., Mishra, V., Eds.; Microorganisms for Sustainability; Springer: Singapore, 2020; pp. 259–285. [Google Scholar]
- Narasimha-Murthy, K.; Soumya, K.; Udayashankar, A.C.; Sudisha-Jogaiah, S.C. Biocontrol potential of plant growth-promoting rhizobacteria (PGPR) against Ralstonia solanacearum: Current and future prospects. In Biocontrol Agents and Secondary Metabolites; Woodhead Publishing: Sawston, UK, 2021; pp. 153–180. [Google Scholar]
- Majumdar, S.; Chakraborty, U. Optimization of protease production from plant growth promoting Bacillus amyloliquefaciens showing antagonistic activity against phytopathogens. Int. J. Pharm. Bio. Sci. 2017, 8, 635–642. [Google Scholar] [CrossRef]
- Muñoz, P.; Arismendi, M.; Cárdenas, S.; Cifuentes, D.; Venegas, F.; Sepúlveda-Chavera, G. Diversity of culturable bacteria isolated from ancestral crops of Arica and Parinacota Region, Atacama Desert. Antonie Van Leeuwenhoek 2020, 113, 2123–2137. [Google Scholar] [CrossRef] [PubMed]
- Tistechok, S.; Skvortsova, M.; Luzhetskyy, A.; Fedorenko, V.; Parnikoza, I.; Gromyko, O. Antagonistic and plant growth promoting properties of actinomycetes from rhizosphere Deschampsia antarctica È. Desv. (Galindez Island, Antarctica). Український Антарктичний Журнал 2019, 1, 1727–7485. [Google Scholar] [CrossRef]
- Yadav, A.N.; Yadav, N.; Sachan, S.G.; Saxena, A.K. Biodiversity of psychrotrophic microbes and their biotechnological applications. J. Appl. Biol. Biotechnol. 2019, 7, 99–108. [Google Scholar]
- Govindasamy, V.; George, P.; Kumar, M.; Aher, L.; Raina, S.K.; Rane, J.; Annapurna, K.; Minhas, P.S. Multi-trait PGP rhizobacterial endophytes alleviate drought stress in a senescent genotype of sorghum [Sorghum bicolor (L.) Moench]. 3 Biotech 2019, 10, 13. [Google Scholar] [CrossRef]
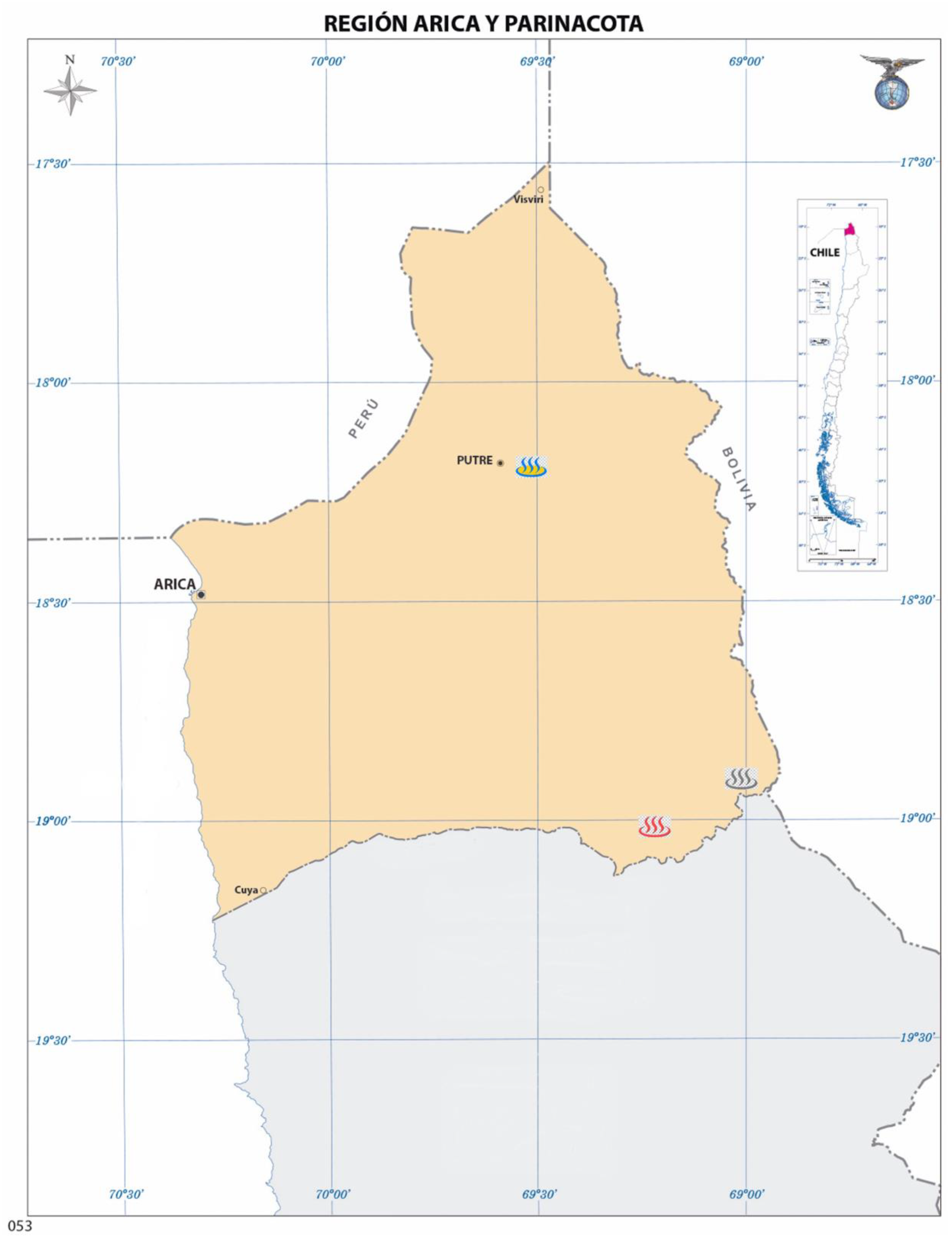
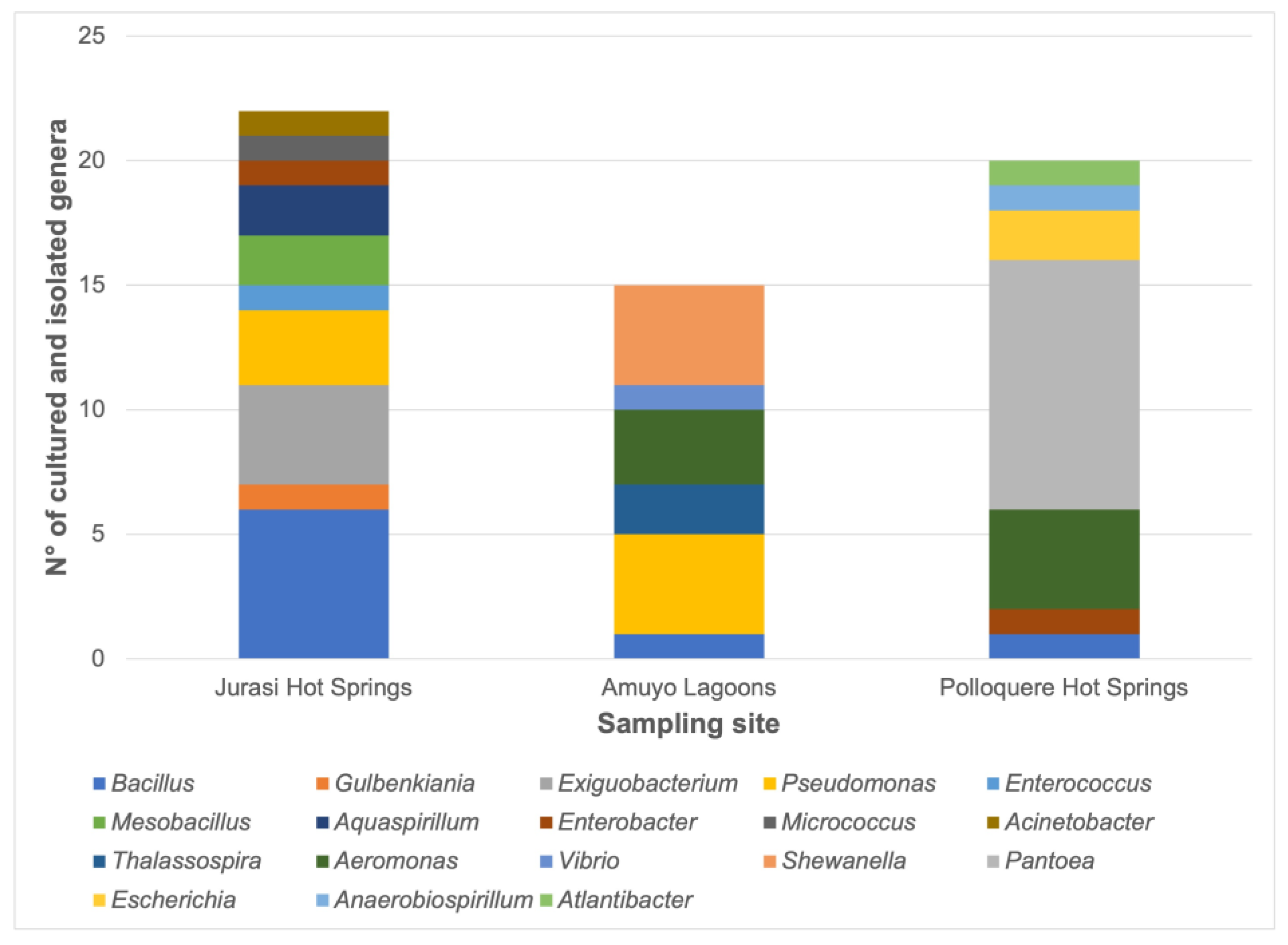
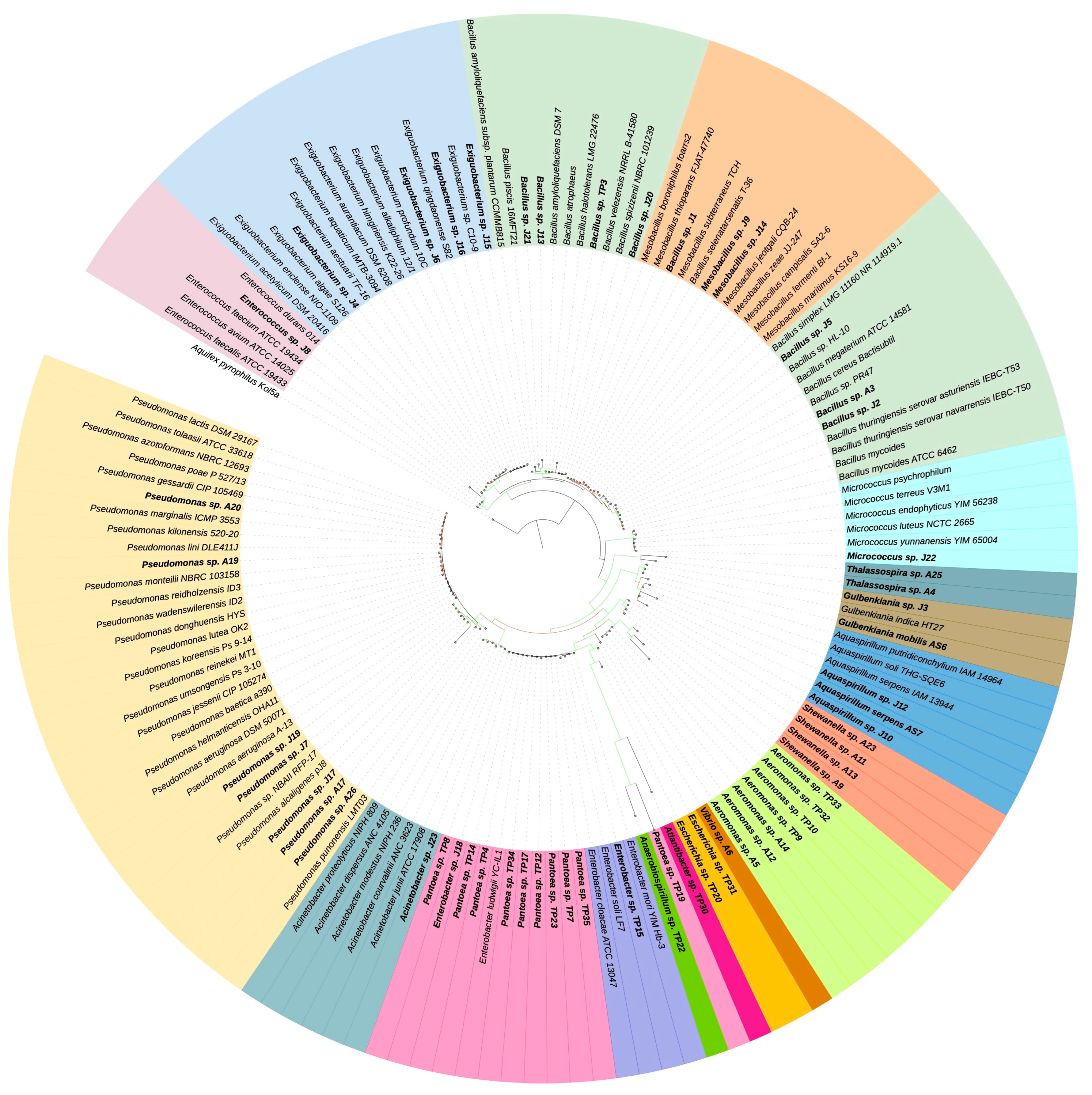
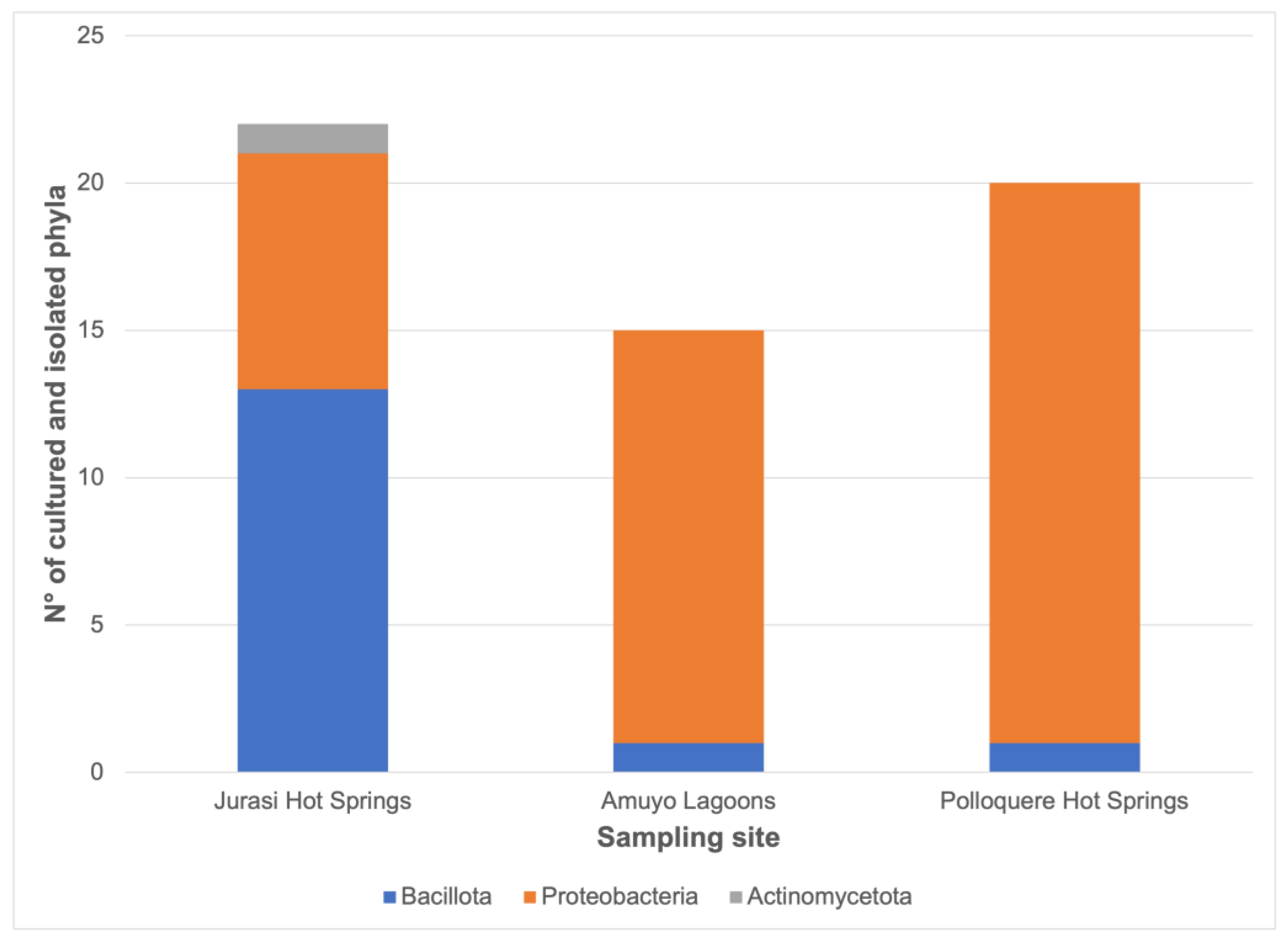
| Parameter | Jurasi Hot Springs | Jurasi Hot Springs |
|---|---|---|
| Altitude (m.a.s.l.) | 4100 | 4100 |
| Type of Sample | water | sediment |
| Temperature (°C) | 32–64 | 35–65 |
| Electrical Conductivity (mS/cm) | 2.79 ± 0.21 | 4.38 ± 0.27 |
| pH | 6.97 ± 0.09 | 7.76 ± 0.11 |
| Ca (mg/L) | 188.65 ± 17.5 | 400.95 ± 23.3 |
| Mg (mg/L) | 3.47 ± 0.22 | 10.80 ± 0.37 |
| K (mg/L) | 26.15 ± 0.16 | 87.35 ± 0.27 |
| Na (mg/L) | 158.26 ± 1.54 | 304.63 ± 3.33 |
| Cl− (mg/L) | 261.99 ± 5.48 | 747.30 ± 9.98 |
| SO42− (mg/L) | 438.37 ± 5.40 | 438.60 ± 8.53 |
| NO3− (mg/L) | 0.00 ± 0.00 | 3.80 ± 0.28 |
| HCO3− (mg/L) | 69.54 ± 3.22 | 69.54 ± 4.51 |
| CO32− (mg/L) | 0.00 ± 0.00 | 0.00 ± 0.00 |
| H2PO4− (mg/L) | 1.50 ± 0.07 | 74.65 ± 2.97 |
| B (mg/L) | 7.30 ± 0.87 | 20.38 ± 2.05 |
| Cu (mg/L) | 0.02 ± 0.00 | 12.38 ± 0.21 |
| Mn (mg/L) | 0.68 ± 0.05 | 112.95 ± 1.99 |
| Zn (mg/L) | 0.26 ± 0.09 | 7.01 ± 1.26 |
| Fe (mg/L) | 1.47 ± 0.10 | 196.59 ± 3.27 |
| As (mg/L) | 0.19 ± 0.05 | 15.08 ± 0.89 |
| Parameter | Green Lagoon | Green Lagoon | Red Lagoon | Red Lagoon | Yellow Lagoon | Yellow Lagoon |
|---|---|---|---|---|---|---|
| Altitude (m.a.s.l.) | 3700 | 3700 | 3700 | 3700 | 3700 | 3700 |
| Type of Sample | water | sediment | water | sediment | water | sediment |
| Temperature (°C) | 21–35 | 21–35 | 22–25 | 22–25 | 22–52 | 22–52 |
| Electrical Conductivity (mS/cm) | 13.42 ± 0.31 | 23.09 ± 0.46 | 14.39 ± 0.33 | 15.84 ± 0.23 | 13.63 ± 0.28 | 12.24 ± 0.32 |
| pH | 7.16 ± 0.12 | 7.91 ± 0.06 | 7.59 ± 0.12 | 7.91 ± 0.17 | 7.01 ± 0.08 | 8.21 ± 0.11 |
| Ca (mg/L) | 926.67 ± 23.77 | 1547.63 ± 58.30 | 970.50 ± 33.87 | 735.38 ± 48.22 | 936.33 ± 43.78 | 738.30 ± 42.90 |
| Mg (mg/L) | 58.96 ± 3.57 | 74.08 ± 6.76 | 65.27 ± 5.67 | 33.72 ± 3.27 | 61.23 ± 6.31 | 31.48 ± 4.27 |
| K (mg/L) | 759.98 ± 10.98 | 836.12 ± 22.10 | 841.47 ± 31.03 | 852.18 ± 29.87 | 779.49 ± 41.31 | 582.85 ± 24.84 |
| Na (mg/L) | 2960.03 ± 58.66 | 2329.90 ± 68.70 | 3079.33 ± 71.52 | 2051.62 ± 65.03 | 2999.77 ± 67.85 | 1566.87 ± 58.80 |
| Cl− (mg/L) | 4398.45 ± 66.43 | 5550.19 ± 76.65 | 4920.30 ± 55.98 | 4546.65 ± 56.87 | 4622.10 ± 79.65 | 3399.48 ± 48.68 |
| SO42− (mg/L) | 3046.39 ± 43.65 | 1954.32 ± 52.01 | 2847.65 ± 55.65 | 1096.21 ± 37.86 | 2926.32 ± 66.50 | 1159.09 ± 36.87 |
| NO3− (mg/L) | 0.00 ± 0.00 | 3.80 ± 0.63 | 1.90 ± 0.45 | 0.38 ± 0.13 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| HCO3− (mg/L) | 727.93 ± 23.40 | 799.40 ± 32.43 | 687.27 ± 43.17 | 191.24 ± 23.41 | 671.00 ± 45.21 | 185.44 ± 42.11 |
| CO32− (mg/L) | 0.00 ± 0.00 | 82.80 ± 2.80 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 22.70 ± 0.56 |
| H2PO4− (mg/L) | 8.99 ± 0.23 | 11.76 ± 0.43 | 12.52 ± 0.35 | 69.96 ± 1.02 | 9.54 ± 0.55 | 5.07 ± 0.35 |
| B (mg/L) | 27.10 ± 0.11 | 29.46 ± 0.16 | 28.73 ± 0.22 | 18.22 ± 0.34 | 27.14 ± 0.43 | 30.03 ± 0.49 |
| Cu (mg/L) | 0.04 ± 0.01 | 0.66 ± 0.10 | 0.03 ± 0.01 | 0.79 ± 0.12 | 0.04 ± 0.01 | 0.66 ± 0.14 |
| Mn (mg/L) | 4.70 ± 0.56 | 63.65 ± 3.65 | 2.94 ± 0.35 | 86.93 ± 5.45 | 3.36 ± 0.54 | 74.56 ± 3.68 |
| Zn (mg/L) | 0.59 ± 0.02 | 18.34 ± 0.32 | 0.77 ± 0.03 | 14.06 ± 0.43 | 0.52 ± 0.02 | 3.76 ± 0.66 |
| Fe (mg/L) | 4.94 ± 0.22 | 197.27 ± 2.57 | 7.97 ± 0.44 | 104.04 ± 3.84 | 6.19 ± 0.53 | 49.45 ± 4.88 |
| As (mg/L) | 18.08 ± 0.76 | 42.60 ± 2.56 | 22.24 ± 1.05 | 44.06 ± 3.48 | 16.07 ± 0.65 | 48.21 ± 4.03 |
| Parameter | Polloquere Hot Springs | Polloquere Hot Springs |
|---|---|---|
| Altitude (m.a.s.l.) | 4300 | 4300 |
| Type of Sample | water | sediment |
| Temperature (°C) | 40–75 | 40–75 |
| Electrical Conductivity (mS/cm) | 8.55 ± 0.33 | 16.34 ± 0.53 |
| pH | 6.80 ± 0.15 | 6.35 ± 0.09 |
| Ca (mg/L) | 610.24 ± 10.50 | 1082.74 ± 22.47 |
| Mg (mg/L) | 54.14 ± 4.12 | 89.80 ± 7.77 |
| K (mg/L) | 190.70 ± 5.06 | 265.52 ± 9.93 |
| Na (mg/L) | 1028.86 ± 23.55 | 2167.51 ± 54.67 |
| Cl− (mg/L) | 2236.86 ± 33.99 | 4397.10 ± 76.85 |
| SO42− (mg/L) | 808.12 ± 12.76 | 1602.57 ± 22.32 |
| NO3− (mg/L) | 0.00 ± 0.00 | 0.00 ± 0.00 |
| HCO3− (mg/L) | 0.00 ± 0.00 | 0.00 ± 0.00 |
| CO32− (mg/L) | 0.00 ± 0.00 | 0.00 ± 0.00 |
| H2PO4− (mg/L) | 0.00 ± 0.00 | 0.00 ± 0.00 |
| B (mg/L) | 5.75 ± 0.66 | 7.72 ± 0.65 |
| Cu (mg/L) | 0.44 ± 0.03 | 0.57 ± 0.02 |
| Mn (mg/L) | 69.12 ± 1.13 | 104.06 ± 3.09 |
| Zn (mg/L) | 9.02 ± 0.54 | 10.33 ± 0.63 |
| Fe (mg/L) | 110.22 ± 3.33 | 126.32 ± 4.27 |
| As (mg/L) | 22.36 ± 0.97 | 46.72 ± 1.38 |
| Activity | N° Isolates with Positive Reaction | Relative Frequency (%) |
|---|---|---|
| Protease | 35 | 61.4 |
| Lipase | 31 | 54.4 |
| Chitinase | 14 | 24.6 |
| Cellulase | 21 | 36.8 |
| Amylase | 24 | 42.1 |
| Auxin production | 34 | 59.6 |
| Siderophores production | 11 | 19.3 |
| N2 fixation | 37 | 64.9 |
| Phosphate solubilization | 43 | 75.4 |
| Botrytis cinerea * | 7 | 12.3 |
| Fusarium oxysporum * | 1 | 1.8 |
| Alternaria sp. * | 12 | 21.1 |
| Geotrichum candidum * | 10 | 17.5 |
| Phytium sp. * | 9 | 15.8 |
| Macrophomina phaseolina * | 8 | 14.0 |
| Monilinia fructicola * | 1 | 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Torres, P.; Márquez, S.L.; Sepúlveda-Chavera, G.; Cárdenas-Ninasivincha, S.; Arismendi-Macuer, M.; Huanca-Mamani, W.; Aguilar, Y.; Quezada, A.; Bugueño, F. Isolation and Identification of Bacteria from Three Geothermal Sites of the Atacama Desert and Their Plant-Beneficial Characteristics. Microorganisms 2023, 11, 2635. https://doi.org/10.3390/microorganisms11112635
Muñoz-Torres P, Márquez SL, Sepúlveda-Chavera G, Cárdenas-Ninasivincha S, Arismendi-Macuer M, Huanca-Mamani W, Aguilar Y, Quezada A, Bugueño F. Isolation and Identification of Bacteria from Three Geothermal Sites of the Atacama Desert and Their Plant-Beneficial Characteristics. Microorganisms. 2023; 11(11):2635. https://doi.org/10.3390/microorganisms11112635
Chicago/Turabian StyleMuñoz-Torres, Patricio, Sebastián L. Márquez, Germán Sepúlveda-Chavera, Steffany Cárdenas-Ninasivincha, Mabel Arismendi-Macuer, Wilson Huanca-Mamani, Yola Aguilar, Antonio Quezada, and Franco Bugueño. 2023. "Isolation and Identification of Bacteria from Three Geothermal Sites of the Atacama Desert and Their Plant-Beneficial Characteristics" Microorganisms 11, no. 11: 2635. https://doi.org/10.3390/microorganisms11112635
APA StyleMuñoz-Torres, P., Márquez, S. L., Sepúlveda-Chavera, G., Cárdenas-Ninasivincha, S., Arismendi-Macuer, M., Huanca-Mamani, W., Aguilar, Y., Quezada, A., & Bugueño, F. (2023). Isolation and Identification of Bacteria from Three Geothermal Sites of the Atacama Desert and Their Plant-Beneficial Characteristics. Microorganisms, 11(11), 2635. https://doi.org/10.3390/microorganisms11112635








