The Relationship between Acid Production and the Microbial Community of Newly Produced Coal Gangue in the Early Oxidation Stage
Abstract
:1. Introduction
2. Materials and Methods
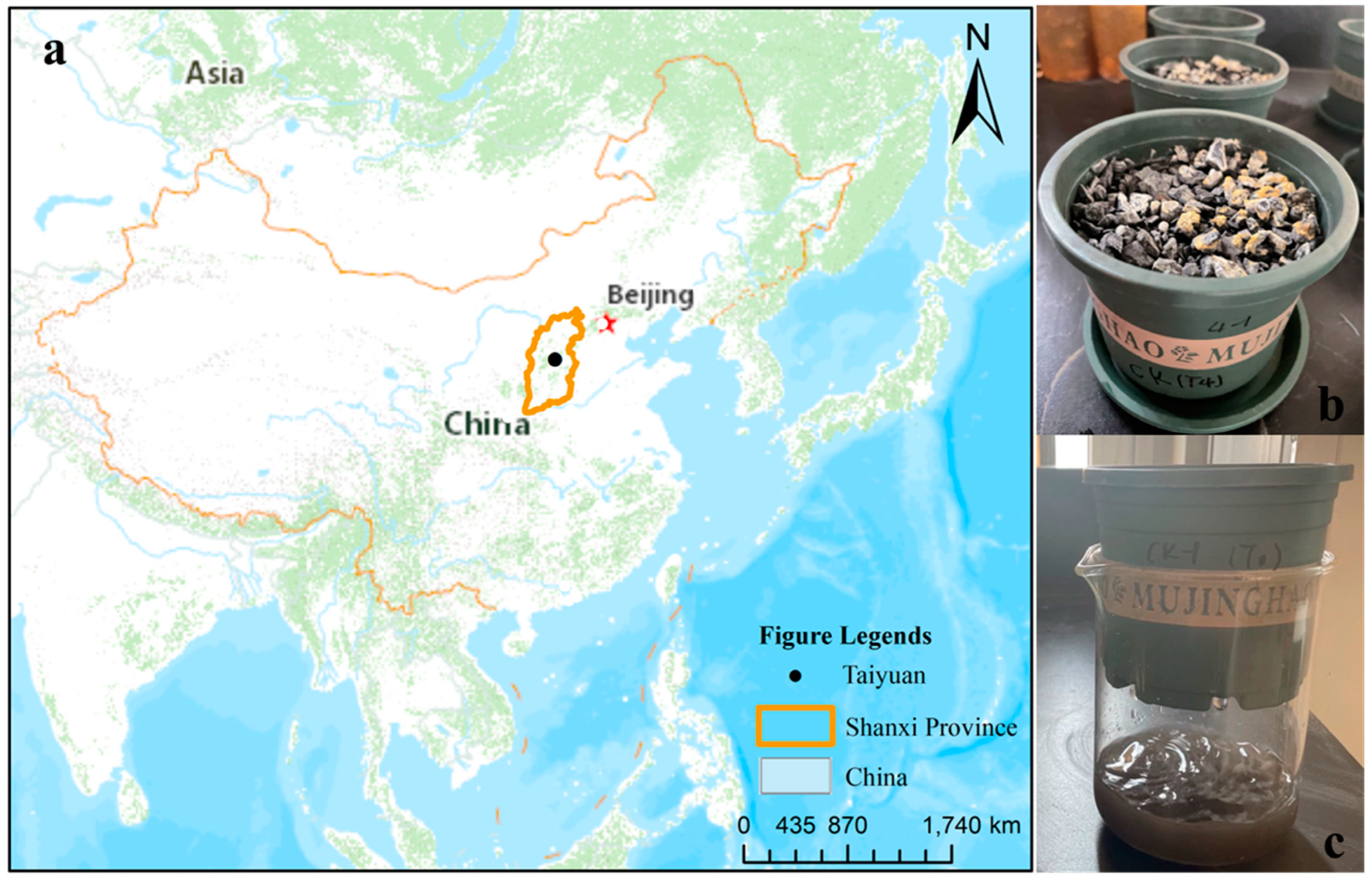
2.1. Sample Collection
2.2. Experimental Design
2.3. 16S rRNA High-Throughput Sequencing
2.4. Data Analysis
3. Results
3.1. Chemical Properties of Gangue Leaching Solutions
3.2. Species Diversity of the Gangue Microbial Community
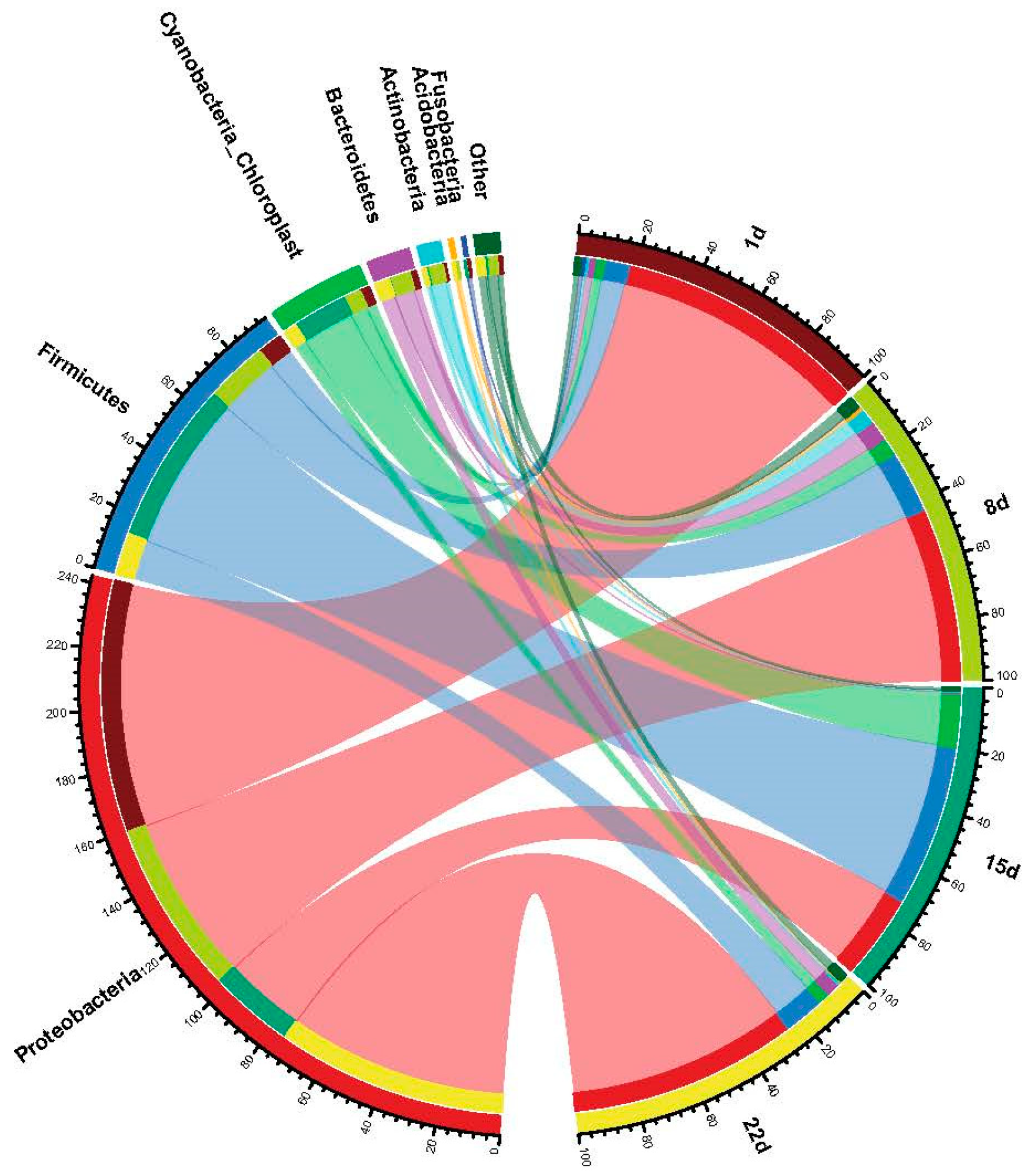
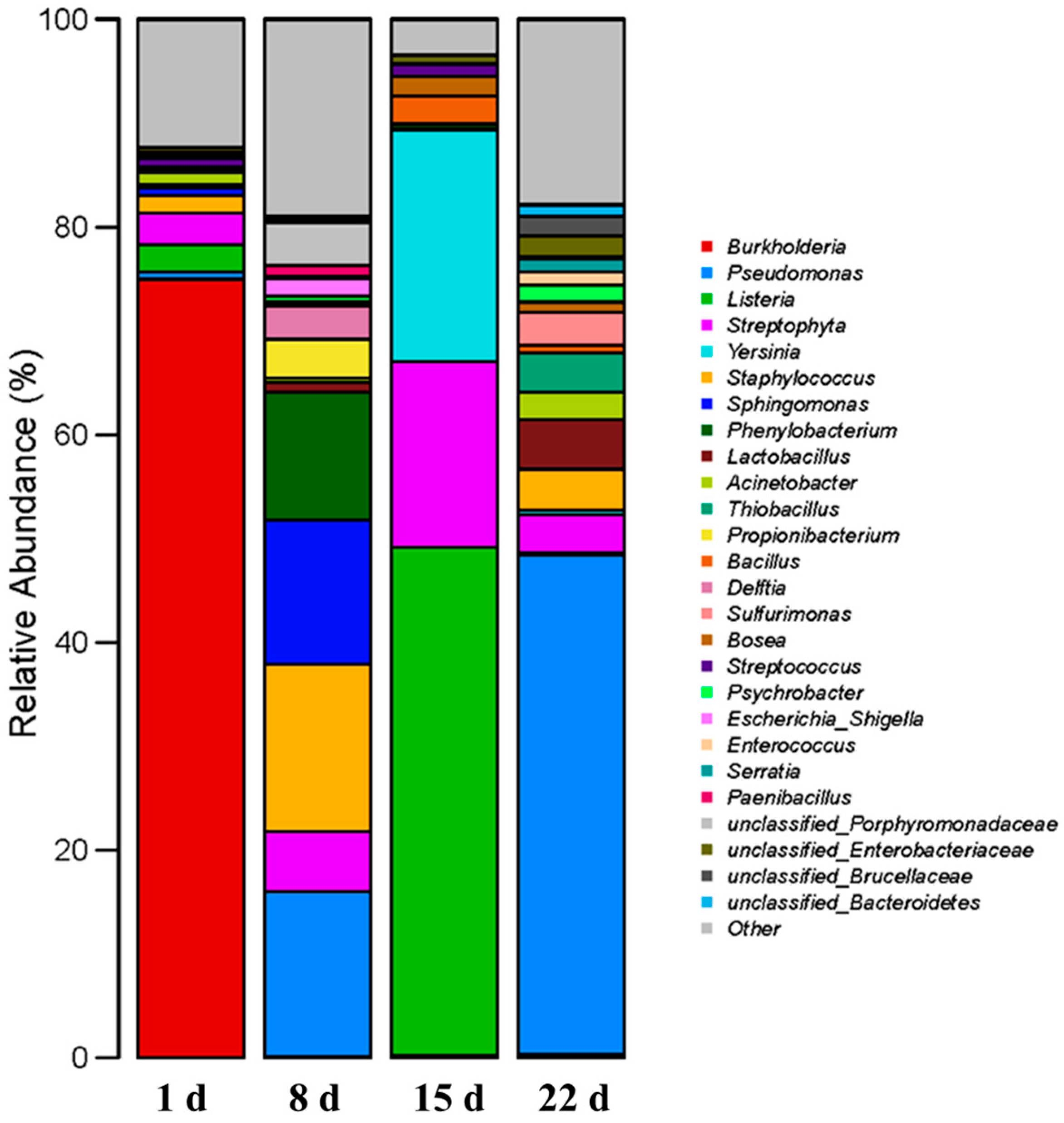
3.3. Microbial Community Structure of Coal Gangue
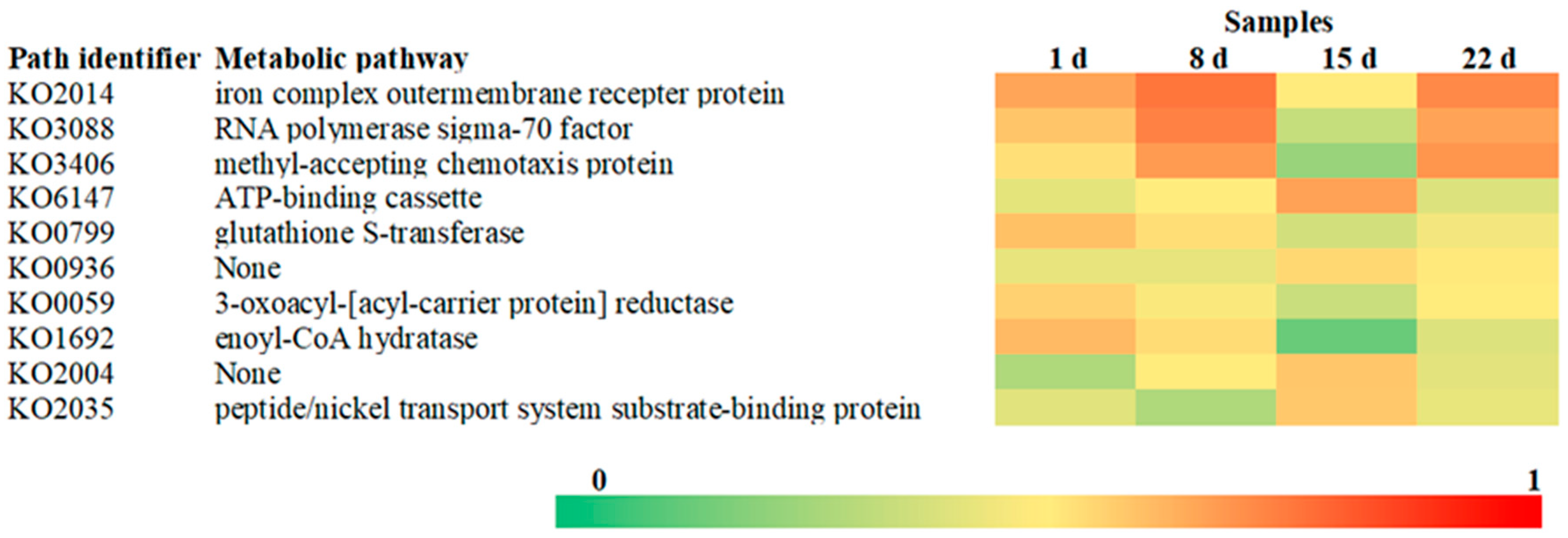
3.4. Gene Function of the Gangue Microbial Community
4. Discussion
5. Conclusions
- (1)
- The critical period for inhibiting the oxidation of newly produced gangue is from 0~15 d after it has been exposed to moisture. During the oxidation process, the gangue continues to release sulfate, while the Fe3+ concentration and iron oxidation rate in the leaching solution are always kept at low levels. This phenomenon suggests that the early acidification of newly produced gangue may be related to sulfur oxidation, but that iron oxidation catalyzed by Thiobacillus ferrooxidans is not the main driving factor.
- (2)
- The microbial community on the surface of gangue was altered after it was sufficiently saturated with moisture, and Pseudomonas proliferated and dominated the community by virtue of their excellent adaptation to environments with high Fe–Mn concentrations, thus inhibiting the continued production of Fe3+. Thiobacillus maintained a low relative abundance during the critical acidification period from 0~15 d and dominated only after the pH of gangue was reduced.
- (3)
- The microbial communities in the acidified gangue had an increased abundance of genes for nitrogen and sulfur cycle functions and were more stress resistant; but the abundance of carbon cycle genes was significantly reduced, resulting in a reduced potential to decompose environmental pollutants.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- BP, P.L.C. Statistical Review of World Energy (69nd). 2020. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/energy-outlook/bp-energy-outlook-2020.pdf (accessed on 17 June 2023).
- National Bureau of Statistics of China. Statistical bulletin on national economic and social development of the People’s Republic of China in 2021. China Stat. 2022, 3, 9–26. [Google Scholar]
- Wang, X.M.; Wang, Y.M.; Chu, Z.X.; Lu, X.W.; Chen, G.Z.; Zha, F.G.; Cui, H.B.; Cheng, Y.S.; Zhang, R.L. Effects of coal gangue addition on the chemical fraction and bioavailability of heavy metals (Zn, Pb, Cd, Cr and Cu) in copper mine tailings. J. China Coal Soc. 2017, 42, 2688–2697. [Google Scholar]
- Li, J.Y.; Wang, J.M. Comprehensive utilization and environmental risks of coal gangue: A review. J. Clean. Prod. 2019, 239, 117946. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Baker, B.J.; Banfield, J.F. Microbial communities in acid mine drainage. FEMS Microbiol. Ecol. 2003, 44, 139–152. [Google Scholar] [CrossRef]
- Acharya, B.S.; Kharel, G. Acid mine drainage from coal mining in the United States—An overview. J. Hydrol. 2020, 588, 125061. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Diep, P.; Mahadevan, R. Genomic Analysis of a Newly Isolated Acidithiobacillus ferridurans JAGS Strain Reveals Its Adaptation to Acid Mine Drainage. Minerals 2021, 11, 74. [Google Scholar] [CrossRef]
- Naidu, G.; Ryu, S.; Thiruvenkatachari, R.; Choi, Y.; Jeong, S.; Vigneswaran, S. A critical review on remediation, reuse, and resource recovery from acid mine drainage. Environ. Pollut. 2019, 247, 1110–1124. [Google Scholar] [CrossRef]
- Guo, Y.X.; Zhang, Y.Y.; Cheng, F.Q. Industrial development and prospect about comprehensive utilization of coal gangue. CIESC J. 2014, 65, 2444–2453. [Google Scholar]
- Fan, J.S.; Sun, Y.Z.; Li, X.Y.; Zhao, C.; Tian, D.; Shao, L.; Wang, J. Pollution of organic compounds and heavy metals in a coal gangue dump of the Gequan Coal Mine. Chin. J. Geochem. 2013, 32, 241–247. [Google Scholar] [CrossRef]
- Sand, W.; Jozsa, P.G.; Kovacs, Z.M.; Săsăran, N.; Schippers, A. Long-term evaluation of acid rock drainage mitigation measures in large lysimeters. J. Geochem. Explor. 2007, 92, 205–211. [Google Scholar] [CrossRef]
- Zhu, Q.; Ruan, M.Y.; Hu, Z.Q.; Ye, C. Addition of carbon sources and nutrient salts can inhibit gangue acidification by changing microbial community structure. Environ. Sci. Pollut. Res. 2022, 29, 90046–90057. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.F.; Cai, W.L.; Li, Y.Q. Bacterial Oxidation of Pyrite. Acta Microbiol. Sin. 1987, 27, 264–270. [Google Scholar]
- Egiebor, N.O.; Ben, O. Acid rock drainage formation and treatment: A review. Asia-Pac. J. Chem. Eng. 2007, 2, 47–62. [Google Scholar] [CrossRef]
- Fowler, T.A.; Holmes, P.R.; Crundwell, F.K. On the kinetics and mechanism of the dissolution of pyrite in the presence of Thiobacillus ferrooxidans. Hydrometallurgy 2001, 59, 257–270. [Google Scholar] [CrossRef]
- Rojas-Chapana, J.A.; Brtels, C.C.; Pohlmann, L.; Tributsch, H. Co-operative leaching and chemotaxis of thiobacilli studied with spherical sulphur/sulphide substrates. Process Biochem. 1998, 33, 239–248. [Google Scholar] [CrossRef]
- Editorial Board of the Determination Methods for Examination of Water and Wastewater. Determination Methods for Examination of Water and Wastewater, 4th ed.; China Environmental Science Press: Beijing, China, 2002. [Google Scholar]
- LY/T 1270-1999; Chinese Academy of Forestry. Determination of Total Silica, Iron, Aluminium, Calcium, Magnesium, Potassium, Sodium, Phosphorus, Sulphur, Manganese, Copper and Zinc in Forest Plant and Forest Floor. China Standards Press: Beijing, China, 1999.
- Zhao, L.K.; Zhou, L.Y.; Li, G.; Zhang, F.; Li, T.X. Distribution and genetic analysis of iron and manganese microbial community in soil and groundwater of mining area. Environ. Chem. 2021, 40, 1464–1479. [Google Scholar]
- Maurice, P.A.; Lee, Y.J.; Hersman, L.E. Dissolution of Al-substituted goethites by an aerobic Pseudomonas mendocina var. bacteria. Geochim. Et Cosmochim. Acta 2000, 64, 1363–1374. [Google Scholar] [CrossRef]
- Saikia, R.; Sarma, R.K.; Yadav, A.; Bora, T.C. Genetic and Functional Diversity Among the Antagonistic Potential Fluorescent Pseudomonads Isolated from Tea Rhizosphere. Curr. Microbiol. 2011, 62, 434–444. [Google Scholar] [CrossRef]
- Chambers, C.E.; Sokol, P.A. Comparison of siderophore production and utilization in pathogenic and environmental isolates of Yersinia enterocolitica. J. Clin. Microbiol. 1994, 32, 32–39. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kim, S.A.; Shim, W.B.; Seo, D.C.; Choi, S.; Lee, S.Y.; Kim, S. Colonization of Listeria monocytogenes in potting soils as affected by bacterial community composition, storage temperature, and natural amendment. Food Sci. Biotechnol. 2021, 30, 869–880. [Google Scholar] [CrossRef]
- Belzile, N.; Maki, S.; Chen, Y.W.; Goldsack, D. Inhibition of pyrite oxidation by surface treatment. Sci. Total. Environ. 1997, 196, 177–186. [Google Scholar] [CrossRef]
- Sinha, S.; Mukherjee, S. Cadmium–Induced Siderophore Production by a High Cd-Resistant Bacterial Strain Relieved Cd Toxicity in Plants Through Root Colonization. Curr. Microbiol. 2008, 56, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.X.; Liang, X.Y.; Wang, J.H.; Wang, S.; Li, X.; Ning, Y. Study on the regulatory mechanism of the earthworm microbial community in vitro and in vivo under cadmium stress. Environ. Pollut. 2021, 279, 116891. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.D. Role of Extracytoplasmic RNA Polymerase Sigma 70 Factor, PG0214, in the Survival of Porphyromonas Gingivalis and in Adaptation to Environmental Stress. Master’s Thesis, Virginia Commonwealth University, Richmond, VA, USA, 2015. Volume 4046. [Google Scholar]
- Zhang, Y.Y.; Zeng, L.B.; Guo, X.K.; Zhao, J.P. Signal transduction system in bacterial chemotaxis: A review. Chin. J. Microecol. 2011, 23, 93–97. [Google Scholar]
- Bibikov, S.I.; Miller, A.C.; Gosink, K.K.; Parkinson, J.S. Methylation-independent aerotaxis mediated by the Escherichia coli aer protein. J. Bacteriol. 2004, 186, 3730–3737. [Google Scholar] [CrossRef] [PubMed]
- Marc, S.P.; Matthew, G.; David, J.K. Chemotaxis in the human gastric pathogen Helicobacter pylori: Different roles for CheW and the three CheV paralogues, and evidence for CheV2 phosphorylation. Microbiology 2001, 147, 2493–2504. [Google Scholar]
- Galperin, M.Y. Sensory Transduction in Bacteria. In Encyclopedia of Microbiology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 447–463. [Google Scholar]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef]
- Colmer, A.R.; Hinkle, M.E. The role of microorganisms in acid mining drainage. Science 1947, 106, 253–256. [Google Scholar] [CrossRef]
- Sasaki, K.; Tsunekawa, M.; Ohtsuka, T.; Konno, H. The role of sulfur Thiobacillus thiooxidans in pyrite weathering. Colloids Surf. A Physicochem. Eng. Asp. 1998, 133, 269–278. [Google Scholar] [CrossRef]
- He, H.; Han, Y.T.; Shi, K.Y.; Hong, F.F.; Zhu, H.W.; Leng, Y.W.; Tao, X.X.; Zheng, L.; Ma, C.Y.; Zhao, Y.D. Variation of sulfur speciation of coal gangue leaching by acidophilic ferrous/sulfur oxidizing microbes. J. China Coal Soc. 2017, 42, 1304–1310. [Google Scholar]
- Leathen, W.W.; Braley, S.A.; Mcintyre, L.D. The Role of Bacteria in the Formation of Acid from Certain Sulfuritic Constituents Associated with Bituminous Coal. Appl. Microbiol. 1953, 1, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Hilton, M.; Shaygan, M.; McIntyre, N.; Baumgartl, T.; Edraki, M. The Effect of Weathering on Salt Release from Coal Mine Spoils. Minerals 2019, 9, 760. [Google Scholar] [CrossRef]
- Zhu, Q.; Hu, Z.; Ruan, M.Y. Characteristics of sulfate-reducing bacteria and organic bactericides and their potential to mitigate pollution caused by coal gangue acidification. Environ. Technol. Innov. 2020, 20, 101142. [Google Scholar] [CrossRef]
- Nostrand, J.D.V.; Wu, W.M.; Wu, L.Y.; Deng, Y.; Carley, J.; Carroll, S.; He, Z.; Gu, B.; Luo, J.; Criddle, C.S.; et al. GeoChip-based analysis of functional microbial communities during the reoxidation of a biored uceduranium-contaminated aquifer. Environ. Microbiol. 2009, 11, 2611–2626. [Google Scholar] [CrossRef] [PubMed]
- Korehi, H.; Blöthe, M.; Schippers, A. Microbial diversity at the moderate acidic stage in three different sulfidic mine tailings dumps generating acid mine drainage. Res. Microbiol. 2014, 165, 713–718. [Google Scholar] [CrossRef]



| Simple | Sequences | OTUs | Phyla | Genera |
|---|---|---|---|---|
| 1 d | 57,365 | 105 | 14 | 86 |
| 8 d | 43,923 | 575 | 18 | 270 |
| 15 d | 120,471 | 226 | 18 | 144 |
| 22 d | 46,991 | 459 | 25 | 229 |
| Time | Chao | Ace | Shannon | Simpson |
|---|---|---|---|---|
| 1 d | 102.00 | 102.00 | 1.58 | 0.56 |
| 8 d | 466.50 | 466.46 | 3.37 | 0.09 |
| 15 d | 211.00 | 210.96 | 1.56 | 0.32 |
| 22 d | 356.67 | 353.50 | 3.13 | 0.18 |
| Groups | 1 d | 8 d | 15 d | 22 d | |
|---|---|---|---|---|---|
| Nitrogen cycle | Nitrification | 0 | 72 | 10 | 159 |
| Nitrogen fixation | 107 | 231 | 967 | 65 | |
| Nitrite respiration | 133 | 134 | 6 | 404 | |
| Nitrate respiration | 195 | 171 | 30 | 790 | |
| Nitrate reduction | 321 | 1011 | 110 | 1505 | |
| Nitrogen respiration | 195 | 215 | 30 | 790 | |
| Aerobic nitrite oxidation | 0 | 68 | 2 | 115 | |
| Denitrification | 133 | 6 | 4 | 257 | |
| Carbon cycle | Methanotrophy | 0 | 12 | 18 | 22 |
| Methanol oxidation | 182 | 53 | 11 | 49 | |
| Methylotrophy | 182 | 65 | 29 | 71 | |
| Fermentation | 3818 | 3081 | 79,046 | 3859 | |
| Chemoheterotrophy | 42,154 | 15,861 | 82,641 | 26,589 | |
| Aerobic chemoheterotrophy | 38,392 | 12,904 | 3604 | 22,655 | |
| Aromatic compound degradation | 575 | 148 | 104 | 1045 | |
| Hydrocarbon degradation | 0 | 19 | 18 | 29 | |
| Sulfur cycle | Sulfate respiration | 0 | 17 | 18 | 0 |
| Sulfur respiration | 0 | 0 | 1 | 98 | |
| Thiosulfate respiration | 62 | 13 | 0 | 98 | |
| Respiration of sulfur compounds | 62 | 30 | 19 | 98 | |
| Dark sulfide oxidation | 163 | 11 | 0 | 1479 | |
| Dark oxidation of sulfur compounds | 163 | 65 | 2041 | 3085 | |
| Other | Iron respiration | 0 | 32 | 13 | 30 |
| Dark hydrogen oxidation | 207 | 39 | 0 | 76 | |
| Chlorate reducers | 0 | 0 | 6 | 199 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Q.; Ruan, M.; Hu, Z.; Miao, K.; Ye, C. The Relationship between Acid Production and the Microbial Community of Newly Produced Coal Gangue in the Early Oxidation Stage. Microorganisms 2023, 11, 2626. https://doi.org/10.3390/microorganisms11112626
Zhu Q, Ruan M, Hu Z, Miao K, Ye C. The Relationship between Acid Production and the Microbial Community of Newly Produced Coal Gangue in the Early Oxidation Stage. Microorganisms. 2023; 11(11):2626. https://doi.org/10.3390/microorganisms11112626
Chicago/Turabian StyleZhu, Qi, Mengying Ruan, Zhenqi Hu, Kexin Miao, and Chun Ye. 2023. "The Relationship between Acid Production and the Microbial Community of Newly Produced Coal Gangue in the Early Oxidation Stage" Microorganisms 11, no. 11: 2626. https://doi.org/10.3390/microorganisms11112626
APA StyleZhu, Q., Ruan, M., Hu, Z., Miao, K., & Ye, C. (2023). The Relationship between Acid Production and the Microbial Community of Newly Produced Coal Gangue in the Early Oxidation Stage. Microorganisms, 11(11), 2626. https://doi.org/10.3390/microorganisms11112626






