Arbuscular Mycorrhizal Fungi Associated with Maize (Zea mays L.) in the Formation and Stability of Aggregates in Two Types of Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Area
2.2. Methodology
2.2.1. Characterization and Classification of Experimental Soils
2.2.2. Biological Material
2.2.3. Fertilization
2.2.4. Experimental Design
2.2.5. Experiment Setup
2.2.6. Variables Evaluated
- The AS was determined in the soils before applying the treatments and at the end of the experiment (potting soil); the AS was determined by wet sieving and air-drying method (2 to 0.5 mm) [41].
- Ps was determined by the Bray and Kurtz method 1, Pp by colorimetry with nitro-vanadomolybdate and Ns in soil and plant were obtained by the Kjeldahl method [38], both at the end of the experiment.
- Co by AMF was obtained by differential staining technique with trypan blue [48] at the end of the experiment.
- Sp was obtained in 100 g of soil by direct counting of AMF spores extracted from the study soils using the wet sieving method [42] at the beginning and the end of the experiment.
- The HP was measured with a flexometer, the measurement was taken from the soil surface to the highest part of the plant while the plant diameter was measured with a vernier at 20 cm from the soil surface before harvesting.
2.2.7. Statistical Analysis
3. Results
3.1. Soils under Study
3.2. Mycorrhizal Fungi in Soils
3.3. Aggregates Stability
3.4. Yield
4. Discussion
4.1. Substrate Characteristics
4.2. Colonization and Sporulation
4.3. Aggregates Stability
4.4. Yield
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, R.; Guo, W.; Bi, N.; Guo, J.; Wang, L.; Zhao, J.; Zhang, J. Arbuscular mycorrhizal fungi affect the growth, nutrient uptake and water status of maize (Zea mays L.) grown in two types of coal mine spoils under drought stress. Appl. Soil Ecol. 2015, 88, 41–49. [Google Scholar] [CrossRef]
- Diagne, N.; Ngom, M.; Djighaly, P.I.; Fall, D.; Hocher, V.; Svistoonoff, S. Roles of arbuscular mycorrhizal fungi on plant growth and performance: Importance in biotic and abiotic stressed regulation. Diversity 2020, 12, 370. [Google Scholar] [CrossRef]
- Mathur, S.; Jajoo, A. Arbuscular mycorrhizal fungi protect maize plants from high temperature stress by regulating photosystem II heterogeneity. Ind. Crop. Prod. 2020, 143, 111934. [Google Scholar] [CrossRef]
- Zhang, S.; Meng, L.; Hou, J.; Liu, X.; Ogundeji, A.O.; Cheng, Z.; Yin, T.; Clarke, N.; Hu, B.; Li, S. Maize/soybean intercropping improves stability of soil aggregates driven by arbuscular mycorrhizal fungi in a black soil of northeast China. Plant Soil 2022, 481, 63–82. [Google Scholar] [CrossRef]
- Hartmann, A.; Schmid, M.; van Tuinen, D.; Berg, G. Plant-driven selection of microbes. Plant Soil 2009, 321, 235–257. [Google Scholar] [CrossRef]
- Yu, Z.; Zhao, X.; Su, L.; Yan, K.; Li, B.; He, Y.; Zhan, F. Effect of an arbuscular mycorrhizal fungus on maize growth and cadmium migration in a sand column. Ecotoxicol. Environ. Saf. 2021, 225, 112782. [Google Scholar] [CrossRef]
- Lu, X.; Lu, X.; Liao, Y. Effect of tillage treatment on the diversity of soil arbuscular mycorrhizal fungal and soil aggregate-associated carbon content. Front. Microbiol. 2018, 9, 2986. [Google Scholar] [CrossRef]
- Moebius-Clune, D.J.; Moebius-Clune, B.N.; van Es, H.M.; Pawlowska, T.E. Arbuscular mycorrhizal fungi associated with a single agronomic plant host across the landscape: Community differentiation along a soil textural gradient. Soil Biol. Biochem. 2013, 64, 191–199. [Google Scholar] [CrossRef]
- Davison, J.; Moora, M.; Semchenko, M.; Adenan, S.B.; Ahmed, T.; Akhmetzhanova, A.A.; Öpik, M. Temperature and pH define the realised niche space of arbuscular mycorrhizal fungi. New Phytol. 2021, 231, 763–776. [Google Scholar] [CrossRef]
- Xu, J.; Liu, S.; Song, S.; Guo, H.; Tang, J.; Yong, J.W.H.; Ma, Y.; Chen, X. Arbuscular mycorrhizal fungi influence decomposition and the associated soil microbial community under different soil phosphorus availability. Soil Biol. Biochem. 2018, 120, 181–190. [Google Scholar] [CrossRef]
- Sanchez-Lizarraga, A.L.; Arenas-Montaño, V.; Marino-Marmolejo, E.N.; Dendooven, L.; Velazquez-Fernandez, J.B.; Davila-Vazquez, G.; Rodriguez-Campos, J.; Hernández-Cuevas, L.; Contreras-Ramos, S.M. Vinasse irrigation: Effects on soil fertility and arbuscular mycorrhizal fungi population. J. Soils Sediments 2018, 18, 3256–3270. [Google Scholar] [CrossRef]
- Oehl, F.; Laczko, E.; Bogenrieder, A.; Stahr, K.; Bösch, R.; van der Heijden, M.; Sieverding, E. Soil type and land use intensity determine the composition of arbuscular mycorrhizal fungal communities. Soil Biol. Biochem. 2010, 42, 724–738. [Google Scholar] [CrossRef]
- Horn, S.; Hempel, S.; Verbruggen, E.; Rillig, M.C.; Caruso, T. Linking the community structure of arbuscular mycorrhizal fungi and plants: A story of interdependence. ISME J. 2017, 11, 1400–1411. [Google Scholar] [CrossRef] [PubMed]
- Hoeksema, J.D.; Chaudhary, V.B.; Gehring, C.A.; Johnson, N.C.; Karst, J.; Koide, R.T.; Pringle, A.; Zabinski, C.; Bever, J.D.; Moore, J.C. A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol. Lett. 2010, 13, 394–407. [Google Scholar] [CrossRef]
- Schappe, T.; Albornoz, F.E.; Turner, B.L.; Neat, A.; Condit, R.; Jones, F.A. The role of soil chemistry and plant neighbourhoods in structuring fungal communities in three Panamanian rainforests. J. Ecol. 2017, 105, 569–579. [Google Scholar] [CrossRef]
- Bueno, C.G.; Gerz, M.; Moora, M.; León, D.; Gomez-García, D.; García de León, D.; Fonte, X.; Al-Quraishy, S.; Hozzein, W.N.; Zobel, M. Distribution of plant mycorrhizal traits along an elevational gradient does not fully mirror the latitudinal gradient. Mycorrhiza 2021, 31, 141–159. [Google Scholar] [CrossRef]
- Koorem, K.; Tulva, I.; Davison, J.; Jairus, T.; Opik, M.; Vasar, M.; Zobel, M.; Moora, M. Arbuscular mycorrhizal fungal communities in forest plant roots are simultaneously shaped by host characteristics and canopy-mediated light availability. Plant Soil 2017, 410, 259–271. [Google Scholar] [CrossRef]
- Carrillo-Aguilar, D.G.; Hernández-Ortega, H.A.; Franco-Ramírez, A.; Vallejo-Jiménez, B.; Guzmán-González, S.; Manzo-Sánchez, G.; Sánchez-Rangel, J.C. Influencia de las propiedades edáficas en la abundancia de esporas y colonización de hongos micorrízicos arbusculares en banano en dos temporadas del año. Sci. Fungorum 2021, 51, e1306. [Google Scholar] [CrossRef]
- Stark, S.; Kytöviita, M.M. Evidence of antagonistic interactions between rhizosphere microorganisms and mycorrhizal fungi associated with birch (Betula pubescens). Acta Oecol. 2005, 28, 149–155. [Google Scholar] [CrossRef]
- Welc, M.; Ravnskov, S.; Kieliszewsk-Rokicka, B.; Larsen, J. Suppression of other soil microorganisms by mycelium of arbuscular mycorrhizal fungi in root-free soil. Soil Biol. Biochem. 2010, 42, 1534–1540. [Google Scholar] [CrossRef]
- Ishaq, L.; Tae, A.A.; Airthur, M.A.; Bako, P.O. Effect of single and mixed inoculation of arbuscular mycorrhizal fungi and phosphorus fertilizer application on corn growth in calcareous soil. Biodiversitas 2021, 22, 1920–1926. [Google Scholar] [CrossRef]
- Palacios-Zambrano, J.J.; García-García, V.S. Influence of dosages of biofertilizers composed of mycorrhizae and diazotrophs on the corn productivity. ESPOCH Congr. Ecuadorian J. STEAM 2021, 1, 953–961. [Google Scholar] [CrossRef]
- Sundram, S.; Meon, S.; Seman, I.A.; Othman, R. Symbiotic interaction of endophytic bacteria with arbuscular mycorrhizal fungi and its antagonistic effect on Ganoderma boninense. J. Microbiol. 2011, 49, 551–557. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, E.E.; Abdelrazik, E. Biocontrol of Fusarium root rot in squash using mycorrhizal fungi and antagonistic microorganisms. Egypt. J. Biol. Pest Control 2022, 32, 13. [Google Scholar] [CrossRef]
- Okiobé, S.T.; Abossolo-Angue, M.; Bougnom, B.P.; Onana, B.; Nwaga, D. Improvement of arbuscular mycorrhizal fungi inoculum production by nutrient solution concentration and soil texture variation. Int. J. Agron. Agric. Res. 2015, 6, 7–20. [Google Scholar]
- Adeyemi, N.O.; Atayese, M.O.; Olubode, A.A. Identification and relative abundance of native arbuscular mycorrhizal fungi associated with oil-seed crops and maize (Zea mays L.) in derived savannah of Nigeria. Acta Fytotech. Zootech. 2019, 22, 84–89. [Google Scholar] [CrossRef]
- Sukmawati, S.; Adnyana, A.; Suprapta, D.N.; Proborini, M.; Soni, P.; Adinurani, P.G. Multiplication arbuscular mycorrhizal fungi in corn (Zea mays L.) with pots culture at greenhouse. E3S Web Conf. 2021, 226, 00044. [Google Scholar] [CrossRef]
- Jiménez-Ortiz, M.M.; Gómez-Álvarez, R.; Oliva-Hernández, J.; Granados-Zurita, L.; Pat-Fernández, J.M.; Aranda-Ibáñez, E.M. Influencia del estiércol composteado y micorriza arbuscular sobre la composición química del suelo y el rendimiento productivo de maíz forrajero (Zea mays L.). Nova Sci. 2019, 11, 23. [Google Scholar] [CrossRef]
- Thapa, B.; Mowrer, J. Effects of carbon amendments, tillage and cover cropping on arbuscular mycorrhizal fungi association and root architecture in corn and cotton crop sequence. Agronomy 2022, 12, 2185. [Google Scholar] [CrossRef]
- Soudzilovskaia, N.A.; Stijn Vaessen, S.; Barcelo, M.; He, J.; Rahimlou, S.; Abarenkov, K.; Brundrett, M.C.; Gomes, S.I.F.; Merckx, V.; Tedersoo, L. FungalRoot: Global online database of plant mycorrhizal associations. New Phytol. 2020, 227, 955–966. [Google Scholar] [CrossRef]
- Ureta, C.; González, E.J.; Espinosa, A.; Trueba, A.; Piñeyro-Nelson, A.; Álvarez-Buylla, E.R. Maize yield in Mexico under climate change. Agric. Syst. 2020, 177, 102697. [Google Scholar] [CrossRef]
- Cervantes-Gámez, R.G.; Peñuelas-Rubio, O.; Araujo-Benard, N.; Alicia Fierro- Coronado, R.A.; Dora Trejo-Aguilar, D.; Maldonado-Mendoza, I.E.; Cordero-Ramírez, J.D. Diversidad de hongos micorrízicos arbusculares asociados a plantas voluntarias de maíz en suelos de transición: Ecosistema natural—Uso agrícola. Sci. Fungorum 2021, 51, e1330. [Google Scholar] [CrossRef]
- Xu, P.; Liang, L.Z.; Dong, X.Y.; Shen, R.F. Effect of arbuscular mycorrhizal fungi on aggregate stability of a clay soil inoculating with two different host plants. Acta Agric. Scand. B—Soil Plant Sci. 2015, 65, 23–29. [Google Scholar] [CrossRef]
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils, 15th ed.; Pearson Education Limited: London, UK, 2017; 1104p. [Google Scholar]
- García, E. Modificaciones al Sistema de Clasificación Climática de Köppen; Instituto de Geografía-Universidad Nacional Autónoma de México: México City, DF, México, 2004; 98p. [Google Scholar]
- SMN (Servicio Meteorológico Nacional). Normales Climatológicas Periodos 1951–2010 y 1981–2000; Servicio Meteorológico Nacional: México City, México, 2015; Available online: https://smn.conagua.gob.mx/es/ (accessed on 13 January 2023).
- Gobierno del Estado de Jalisco. Actualización del Atlas Municipal de Riesgos por Amenazas Naturales y Antrópicas en el Municipio de Tlajomulco de Zúñiga, Jalisco. Mapa Geológico. 2018. Available online: https://tlajomulco.gob.mx/ProteccionCivil/1.1.7%20MAPA%20GEOL%C3%93GICO.pdf (accessed on 20 June 2023).
- IIEG-Jalisco (Instituto de Información Estadística y Geografía de Jalisco). Recursos Cartográficos/Suelos, Vegetación y Uso del Suelo. 2018. Available online: https://iieg.gob.mx/contenido/Municipios/TlajomulcodeZuniga.pdf (accessed on 18 June 2023).
- Schoeneberger, P.J.; Wysocki, D.A.; Benham, E.C. Soil Survey Staff. In Field Book for Describing and Sampling Soils, Version 3.0; Natural Resources Conservation Service, National Soil Survey Center: Lincoln, NE, USA, 2012. [Google Scholar]
- SEMARNAT (Secretaria del Medio Ambiente y Recursos Naturales). In Norma Oficial Mexicana que establece las Especificaciones de Fertilidad, Salinidad y Clasificación de Suelos. Estudios, Muestreos y Análisis (NOM-021-RECNAT-2000); Diario Oficial de la Federación: México City, México, 2002; 85p.
- SSS (Soil Survey Staff). Keys to Soil Taxonomy, 13th ed.; USDA Natural Resources Conservation Service: Washington, DC, USA, 2022; 325p. [Google Scholar]
- Gerdemann, J.W.; Nicolson, T.H. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- INVAM. The International Collection of Vesicular Arbuscular Mycorrhizal Fungi; University of Kansas: Lawrence, KS, USA, 2023; Available online: https://invam.ku.edu (accessed on 25 June 2023).
- Glomeromycota. Agricultural University of Szczecin. 2023. Available online: http://www.zor.zut.edu.pl/Glomeromycota/Introduction.html (accessed on 18 June 2023).
- Glomeromycota PHYLOGENY. Symplanta. 2023. Available online: http://www.amf-phylogeny.com/ (accessed on 18 June 2023).
- Redecker, D.; Schüβler, A.; Stockinger, H.; Stürmer, S.; Morton, J.B.; Walker, C. An evidence-based consensus for the classification or arbuscular mycorrhizal fungi (Glomeromycota). Mycorrhiza 2013, 23, 515–531. [Google Scholar] [CrossRef]
- SADER (Secretaría de Agricultura y Desarrollo Rural). Guía para el uso de Fertilizantes en el Estado de Jalisco. 2021. Available online: https://www.gob.mx/cms/uploads/attachment/file/829014/Triptico_Jalisco.pdf (accessed on 21 June 2022).
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Minitab Inc. Minitab® State College. Minitab Inc. Pennsylvania, EEUU. 2013. Available online: https://www.minitab.com/es-mx/ (accessed on 15 January 2021).
- Southard, R.J.; Driese, S.G.; Nordt, L.C. Vertisols. In Handbook of Soli Science, 2nd ed.; Huang, P.M., Li, Y., Sumner, M.E., Eds.; Properties and processes; CRC Press Taylor & Francis: Boca Raton, FL, USA, 2012; Volume 1, pp. 33.82–33.97. [Google Scholar]
- Nordt, L.C.; Collins, M.E.; Monger, H.C.; Fanning, G.S. Entisols. In Handbook of Soli Science, 2nd ed.; Huang, P.M., Li, Y., Sumner, M.E., Eds.; Properties and processes; CRC Press Taylor & Francis: Boca Raton, FL, USA, 2012; Volume 1, pp. 33.49–33.63. [Google Scholar]
- Porta Casanellas, J.; López-Acevedo Reguerín, M.; Poch Claret, R.M. Edafología: Uso y Protección de Suelos, 4th ed.; Mundi-Prensa Libros: Mexico City, DF, México, 2019; 624p. [Google Scholar]
- Jamiołkowska, A.; Księżniak, A.; Gałązka, A.; Hetman, B.; Kopacki, M.; Skwaryło-Bednarz, B. Impact of abiotic factors on development of the community of arbuscular mycorrhizal fungi in the soil: A Review. Int. Agrophys. 2018, 32, 133–140. [Google Scholar] [CrossRef]
- Getachew, A.; Chilot, Y.; Teklu, E. Soil Acidity Management; Ethiopian Institute of Agricultural Research (EIAR): Addis Ababa, Ethiopia, 2019; 56p. [Google Scholar]
- Kome, G.K.; Enang, R.K.; Tabi, F.O.; Yerima, B.P.K. Influence of Clay Minerals on Some Soil Fertility Attributes: A Review. Open J. Soil Sci. 2019, 9, 155–188. [Google Scholar] [CrossRef]
- Gosling, P.; Proctor, M.; Jones, J.; Bending, G.D. Distribution and diversity of Paraglomus spp. in tilled agricultural soils. Mycorrhiza 2013, 24, 1–11. [Google Scholar] [CrossRef]
- Carballar-Hernández, S.; Hernández-Cuevas, L.V.; Montaño, N.M.; Larsen, J.; Ferrera-Cerrato, R.; Taboada-Gaytán, O.R.; Montiel-González, A.M.; Alarcón, A. Native communities of arbuscular mycorrhizal fungi associated with Capsicum annuum L. respond to soil properties and agronomic management under field conditions. Agric. Ecosyst. Environ. 2017, 245, 43–51. [Google Scholar] [CrossRef]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Payró de la Cruz, E.; Salgado-García, S.; De los Santos-López, G.; Castelán-Estrada, M.; Córdova-Sánchez, S.; Gómez-Leyva, J.F.; Hernández-Cuevas, L. Diversity of VAM in soils used to cultivate sugarcane (Saccharum spp.) in Tabasco, Mexico. Agro. Product. 2021, 14, 11. [Google Scholar] [CrossRef]
- Jansa, J.; Erb, A.; Oberholzer, H.R.; Šmilauer, P.; Egli, S. Soil and geography are more important determinants of indigenous arbuscular mycorrhizal communities than management practices in Swiss agricultural soils. Mol. Ecol. 2014, 23, 2118–2135. [Google Scholar] [CrossRef]
- Polo-Marcial, M.H.; Lara-Pérez, L.A.; Goto, B.T.; Margarito-Vista, X.; Andrade-Torres, A. Glomeromycota in Mexico, a country with very high richness. Sydowia 2021, 74, 33–63. [Google Scholar]
- Bahadur, A.; Batool, A.; Nasir, F.; Jiang, S.; Mingsen, Q.; Zhang, Q.; Pan, J.; Liu, Y.; Feng, H. Mechanistic insights into arbuscular mycorrhizal fungi-mediated drought stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 4199. [Google Scholar] [CrossRef] [PubMed]
- Sanclemente Reyes, O.E.; Sánchez de Prager, M.; Prager-Mosquera, M. Prácticas agroecológicas, micorrización y productividad del intercultivo maíz-soya (Zea mays L.-Glycine max L.). Idesia 2018, 36, 217–224. [Google Scholar] [CrossRef]
- Njeru, E.; Avio, L.; Sbrana, C.; Turrini, A.; Bocci, G.; Bàrberi, P.; Giovannetti, M. First evidence for a major cover crop effect on arbuscular mycorrhizal fungi and organic maize growth. ASDt 2014, 34, 841–848. [Google Scholar] [CrossRef]
- Liu, W.; Shanshan Jiang, S.; Zhang, Y.; Yue, S.; Christie, P.; Murray, P.J.; Li, X.; Zhang, J. Spatiotemporal changes in arbuscular mycorrhizal fungal communities under different nitrogen inputs over a 5-year period in intensive agricultural ecosystems on the North China Plain. FEMS Microbiol. Ecol. 2014, 90, 436–453. [Google Scholar] [CrossRef]
- Mena-Echevarría, A.M.; Portugal, V.O.; Suárez, K.F.; Mompié, E.J.; Flores, R.S. Influencia de la inoculación con Glomus hoi-like y un conglomerado de especies de HMA en el crecimiento de plantas de sorgo sometidas o no a estrés hídrico. Cultiv. Trop. 2011, 32, 11–17. Available online: https://www.redalyc.org/pdf/1932/193222352002.pdf (accessed on 25 June 2022).
- Bender, S.F.; Schlaeppi, K.; Held, A.; Van der Heijden, M.G.A. Establishment success and crop growth effects of an arbuscular mycorrhizal fungus inoculated into Swiss corn fields. Agric. Ecosyst. Environ. 2019, 273, 13–24. [Google Scholar] [CrossRef]
- Wilson, J.M. Competition for infection between vesicular-arbuscular mycorrhizal fungi. New Phytol. 1984, 97, 427–435. [Google Scholar] [CrossRef]
- Ghezzehei, T.A. Soil Structure. In Handbook of Soli Science, 2nd ed.; Huang, P.M., Li, Y., Sumner, M.E., Eds.; Properties and processes; CRC Press Taylor & Francis: Boca Raton, FL, USA, 2012; Volume 1, pp. 2.1–2.17. [Google Scholar]
- Behrends-Kraemer, F.; Morrás, H.; Fernández, P.L.; Duval, M.; Galantini, J.; Garibaldi, L. Influence of edaphic and management factors on soils aggregates stability under no-tillage in Mollisols and Vertisols of the Pampa Region, Argentina. Soil Tillage Res. 2021, 209, 104901. [Google Scholar] [CrossRef]
- Qin, H.; Niu, L.; Wu, Q.; Chen, J.; Li, Y.; Liang, C.; Xu, Q.; Fuhrmann, J.J.; Shen, Y. Bamboo forest expansion increases soil organic carbon through its effect on soil arbuscular mycorrhizal fungal community and abundance. Plant Soil 2017, 420, 407–421. [Google Scholar] [CrossRef]
- Liang, T.; Shi, X.; Guo, T.; Peng, S. Arbuscular mycorrhizal fungus mediate changes in mycorrhizosphere soil aggregates. Agric. Sci. 2015, 6, 1455–1463. [Google Scholar] [CrossRef]
- Syamsiyah, J.; Herawati, A.; Mujiyo. The potential of arbuscular mycorrhizal fungi application on aggregrate stability in alfisol soil. IOP Conf. Ser. Environ. Earth Sci. 2018, 142, 012045. [Google Scholar] [CrossRef]
- Morris, E.K.; Morris, D.J.P.; Vogt, S.; Gleber, S.C.; Bigalke, M.; Wilcke, W.; Rillig, M.C. Visualizing the dynamics of soil aggregation as affected by arbuscular mycorrhizal fungi. ISME J. 2019, 13, 1639–1646. [Google Scholar] [CrossRef]
- Heller, P.; Carrara, J.E. Multiplex qPCR assays to distinguish individual species of arbuscular mycorrhizal fungi from roots and soil. Mycorrhiza 2022, 32, 155–164. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Wang, Y.; Zhang, Q.; Luo, J.; Jiang, X.; Liu, W.; Zhao, X. Effects of arbuscular mycorrhizal fungi on improvement of degraded landscape soil in an ionized rare earth mining area, subtropical China. Soil Sci. Soc. Am. J. 2022, 86, 275–292. [Google Scholar] [CrossRef]
- Koziol, L.; Bever, J.D. The missing link in grassland restoration: Arbuscular mycorrhizal fungi inoculation increases plant diversity and accelerates succession. J. Appl. Ecol. 2017, 54, 1301–1309. [Google Scholar] [CrossRef]
- Barbosa, M.V.; Pedroso, D.F.; Curi, N.; Carbone, C.M.A. Do different arbuscular mycorrhizal fungi affect the formation and stability of soil aggregates. Cienc. Agrotecnologia 2019, 43, e003519. [Google Scholar] [CrossRef]
- Leifheit, E.F.; Veresoglu, S.D.; Lehmann, A.; Morris, E.K.; Rilling, M.C. Multiple factors influence the role of arbuscular mycorrhizal fungi in soil aggregation—A meta-analysis. Plant Soil 2014, 374, 523–537. [Google Scholar] [CrossRef]
- Cozzolino, V.; Di Meo, V.; Piccolo, A. Impact of arbuscular mycorrhizal fungi applications on maize production and soil phosphorus availability. J. Geochem. Explor. 2013, 129, 40–44. [Google Scholar] [CrossRef]
- Moreira-Salgado, F.H.; Sousa-Moreira, F.M.; Siqueira, J.O.; Barbosa, R.H.; Paulino, H.B.; Carbone-Carneiro, M.A. Arbuscular mycorrhizal fungi and colonization stimulant in cotton and maize. Ciência Rural 2017, 06, e20151535. [Google Scholar] [CrossRef]
- Wahid, F.; Fahad, S.; Danish, S.; Adnan, M.; Yue, Z.; Saud, S.; Siddiqui, M.H.; Brtnicky, M.; Hammerschmiedt, T.; Datta, R. Sustainable management with mycorrhizae and phosphate solubilizing bacteria for enhanced phosphorus uptake in calcareous soils. Agriculture 2020, 10, 334. [Google Scholar] [CrossRef]
- Saboor, A.; Ali, M.A.; Danish, S.; Ahmed, N.; Fahad, S.; Datta, R.; Ansari, M.J.; Nasif, O.; Habib ur Rahman, M.; Glick, B.R. Effect of arbuscular mycorrhizal fungi on the physiological functioning of maize under zinc-deficient soils. Sci. Rep. 2021, 11, 18468. [Google Scholar] [CrossRef] [PubMed]
- Mena-Echevarría, A.; Fernández, K.; Olalde, V.; Serrato, R. Diferencias en la respuesta del maíz (Zea mays L.) a la inoculación con Glomus cubense (Y. Rodr. & Dalpé) y con un conglomerado de especies de hongos micorrízicos arbusculares (HMA). Cult. Trop. 2013, 34, 12–15. [Google Scholar]
- Colina-Navarrete, E.; Paredes-Acosta, E.; Gutiérrez-Mora, X.; Vera-Suarez, M. Efecto de fertilización nitrogenada en maíz (Zea mays L.) sobre poblaciones de hongos micorrízicos, en Babahoyo. J. Sci. Res. 2020, 5, 135–155. [Google Scholar] [CrossRef]
- Fall, A.F.; Nakabonge, G.; Ssekandi, J.; Founoune-Mboup, H.; Apori, S.O.; Ndiaye, A.; Badji, A.; Ngom, K. Roles of arbuscular mycorrhizal fungi on soil fertility: Contribution in the improvement of physical, chemical, and biological properties of the soil. Front. Fungal Biol. 2022, 3, 723892. [Google Scholar] [CrossRef]
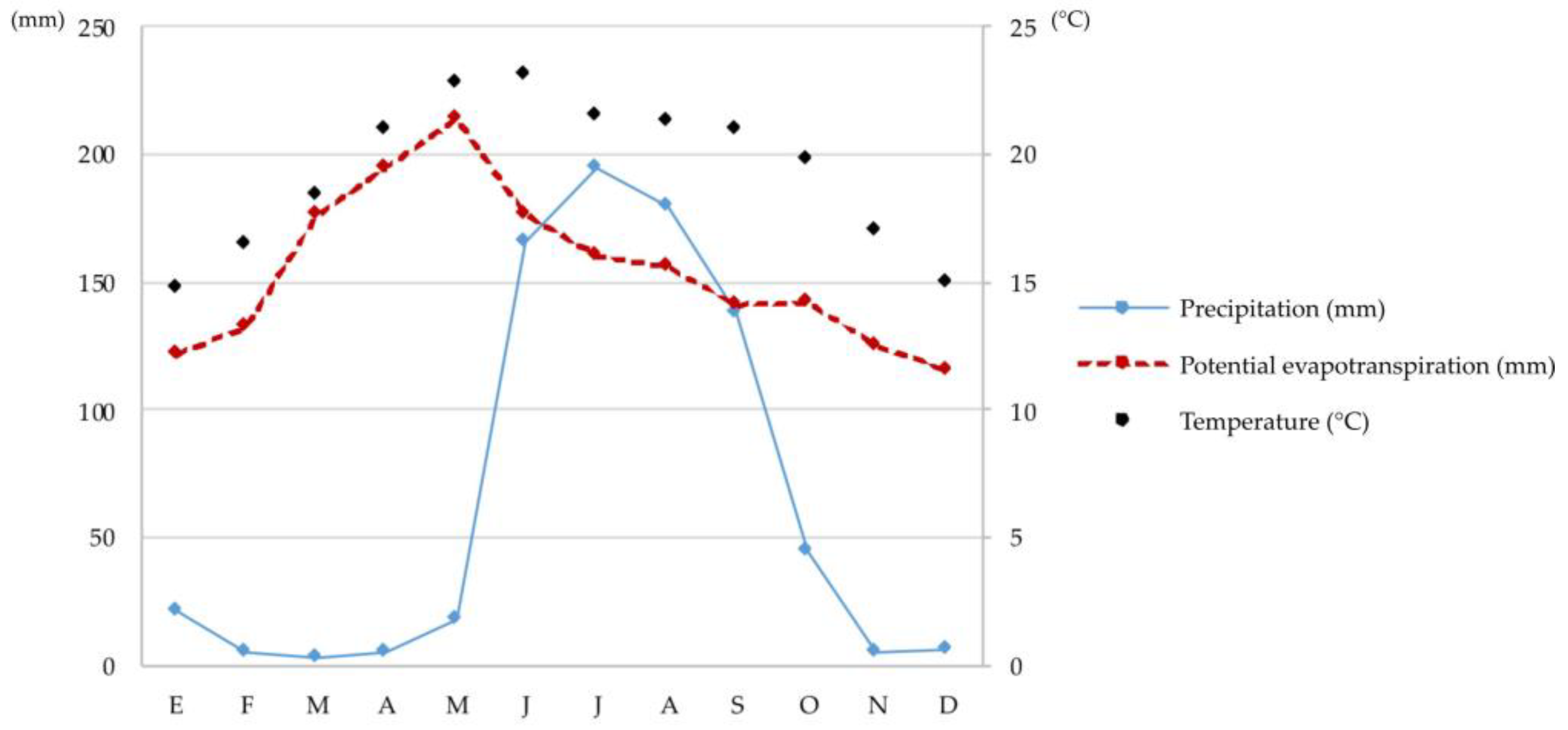

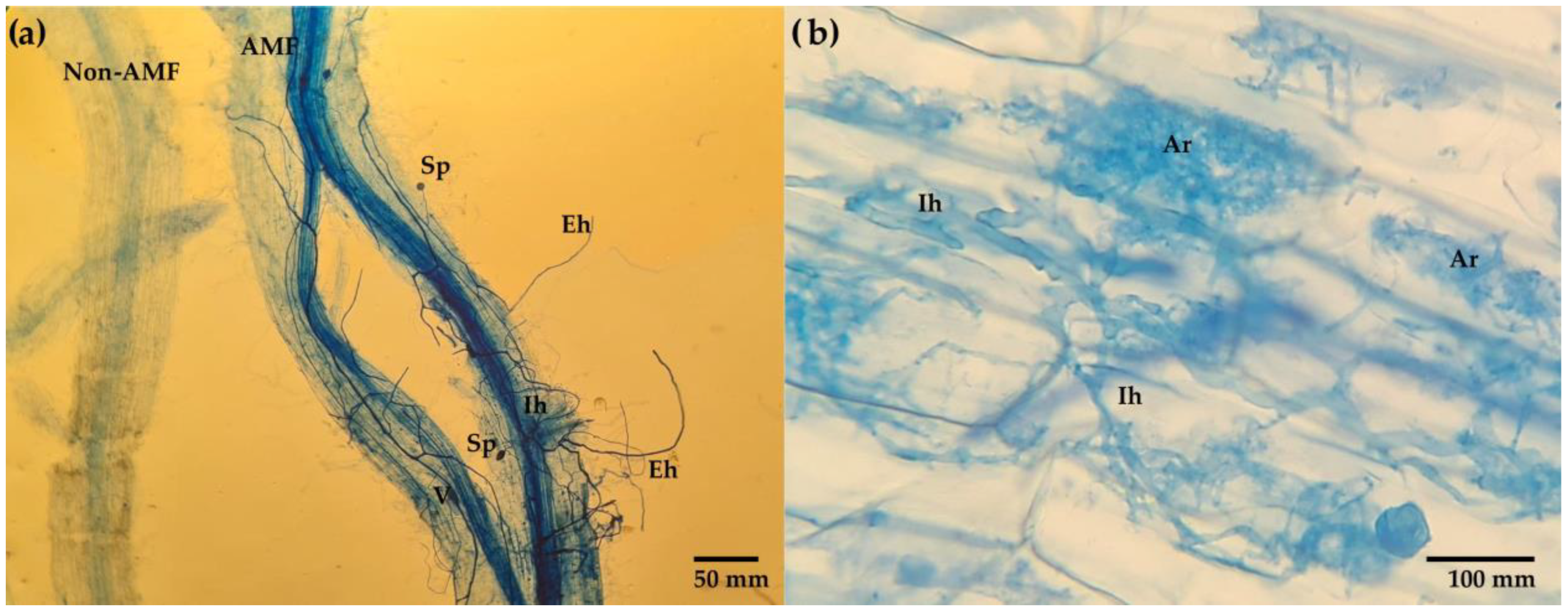


| Soil | Hs1 | Deep (cm) | OC (%) | pH | EC dS m−1 | S (%) | L (%) | R (%) | Bd (g cm−3) | CEC (cmol(+) Kg−1) | PBS (%) | P2O5 (mg Kg−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | Ap | 0–20 | 1.09 | 4.40 | 0.89 | 3.26 | 46.98 | 49.76 | 1.34 | 13.45 | 34.80 | 93.04 |
| C1 | 20–45 | 1.03 | 4.35 | 0.52 | 1.80 | 48.12 | 50.08 | 1.36 | 11.22 | 19.32 | 52.93 | |
| C2 | 45–72 | 0.59 | 6.31 | 0.47 | 1.10 | 59.86 | 39.04 | 1.53 | 12.10 | 38.83 | 6.42 | |
| C3 | >72 | 0.92 | 7.63 | 0.60 | 3.54 | 38.24 | 58.22 | 1.47 | 17.46 | 21.35 | 3.21 | |
| S2 | Ap | 0–16 | 1.64 | 6.87 | 0.98 | 44.60 | 32.04 | 23.36 | 1.34 | 15.41 | 57.12 | 140.40 |
| C1 | 16–45 | 0.83 | 5.62 | 0.16 | 40.28 | 36.36 | 23.36 | 1.32 | 16.51 | 60.30 | 116.15 | |
| C2 | 45–65 | 0.46 | 6.28 | 0.33 | 59.72 | 23.32 | 16.96 | 1.15 | 9.10 | 30.41 | 16.40 | |
| C3 | 65–82 | 0.75 | 6.80 | 0.34 | 65.52 | 17.48 | 20.00 | 1.11 | 11.40 | 41.73 | 6.12 | |
| C4 | 82–95 | 0.48 | 6.61 | 0.38 | 58.64 | 21.68 | 19.48 | 1.29 | 11.20 | 40.79 | 12.43 | |
| C5 | >95 | 0.35 | 6.80 | 0.25 | 40.84 | 32.76 | 26.40 | 1.36 | 15.12 | 55.80 | 10.68 |
| AMF | Typic Dystrustert | Typic Ustifluvent |
|---|---|---|
| Rhizophagus aggregatus (N.C. Schenck and G.S. Sm.) C. Walker | X | |
| Funneliformis geosporum (T.H. Nicolson and Gerd.) C. Walker and A. Schluessler | X | X |
| Paraglomus occultum (C. Walker) J.B. Morton and D. Redecker | X | X |
| Diversispora aurantia (Błaszk, Blanke, Renker and Buscot) C. Walker and A. Schüßler | X | X |
| Diversispora trimurales (Koske and Halvorson) C. Walker and A. Schüßler | X | |
| Gigaspora candida Bhattacharjee, Mukerji, J.P. Tewari and Skoropad | X | |
| Gigaspora gigantea (T.H. Nicolson and Gerd.) Gerd. and Trappe | X | |
| Acaulospora mellea Spain and N.C. Schenck | X | |
| Septoglomus sp. | X |
| Treat 1 | N | Sp | Co | AS | Yd | PH | SD | Pp | Np |
|---|---|---|---|---|---|---|---|---|---|
| S1A0f0 | 5 | 866.00 h 2 | 70.66 cd | 63.29 de | 56.90 b | 2.40 abcd | 3.10 a | 3.03 a | 1.06 d |
| S1A1f0 | 5 | 2000.00 d | 97.33 a | 79.76 a | 27.50 b | 2.16 cd | 2.78 b | 3.00 a | 1.08 d |
| S1A2f0 | 5 | 1570.00 f | 96.67 a | 72.58 b | 36.00 b | 2.16 cd | 3.38 a | 0.76 d | 1.06 d |
| S2A0f0 | 5 | 2284.00 c | 96.67 a | 62.93 de | 68.20 b | 2.34 bcd | 1.92 c | 1.44 bcd | 1.00 d |
| S2A1f0 | 5 | 3153.00 a | 90.67 ab | 67.86 bcd | 76.09 b | 1.95 d | 1.92 c | 1.50 abcd | 0.60 e |
| S2A2f0 | 5 | 1760.00 e | 94.00 ab | 50.35 f | 74.60 b | 2.07 d | 1.84 c | 0.91 cd | 1.13 d |
| S1A0f1 | 5 | 1492.00 f | 59.32 d | 62.27 e | 55.40 b | 2.51 abcd | 2.78 b | 2.55 ab | 2.08 b |
| S1A1f1 | 5 | 2742.00 b | 83.33 abc | 68.52 bc | 73.50 b | 2.78 abc | 3.10 ab | 2.16 abcd | 1.73 c |
| S1A2f1 | 5 | 1926.00 d | 77.99 bc | 64.89 cde | 61.50 b | 2.83 ab | 2.68 b | 1.42 bcd | 2.43 a |
| S2A0f1 | 5 | 2342.00 c | 82.67 abc | 46.21 f | 176.70 a | 3.05 a | 1.76 c | 1.66 abcd | 1.82 c |
| S2A1f1 | 5 | 1914.00 d | 84.64 abc | 50.51 f | 151.30 a | 3.08 a | 1.82 c | 2.28 abcd | 1.94 bc |
| S2A2f1 | 5 | 1760.00 e | 93.33 ab | 40.45 g | 186.90 a | 3.02 a | 1.88 c | 2.32 abc | 1.94 bc |
| Factor | Soil | AMF | Fertilizer | Interactions | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | F2 | p | F | p | F | p | S-A | S-f | f-A | S-A-f |
| Sp 1 | 674.00 | 0.000 | 2111.49 | 0.000 | 344.62 | 0.000 | * | |||
| Co | 23.38 | 0.000 | 18.15 | 0.000 | 30.48 | 0.000 | * | |||
| AS | 573.96 | 0.000 | 68.95 | 0.000 | 243.23 | 0.000 | * | |||
| Ps | 190,442.69 | 0.000 | 15,917.47 | 0.000 | 33,829.45 | 0.000 | * | |||
| Ns | 4.10 | 0.048 | 15.19 | 0.000 | 9.13 | 0.004 | * | |||
| Pp | 6.74 | 0.012 | 9.76 | 0.000 | 3.90 | 0.054 | * | |||
| Np | 50.17 | 0.000 | 59.44 | 0.000 | 1719.79 | 0.000 | * | * | ||
| Yd | 519.48 | 0.000 | 9.04 | 0.000 | 290.80 | 0.000 | * | |||
| Variable | Co 1 | Sp | AS | AP | DT | Ps | Ns | Pp | Np | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sp | r p | 0.237 0.069 | ||||||||
| AS | r p | 0.015 0.075 | 0.537 0.050 | |||||||
| AP | r p | −0.215 0.099 | 0.061 0.644 | −0.482 0.000 | ||||||
| DT | r p | −0.270 0.037 | −0.222 0.644 | 0.654 0.000 | −0.242 0.062 | |||||
| Ps | r p | 0.253 0.050 | 0.048 0.714 | −0.485 0.000 | 0.290 0.024 | −0.594 0.000 | ||||
| Ns | r p | 0.154 0.241 | −0.212 0.105 | −0.075 0.570 | 0.138 0.293 | 0.084 0.525 | 0.339 0.009 | |||
| Pp | r p | −0.232 0.075 | −0.107 0.414 | 0.023 0.861 | 0.239 0.065 | 0.148 0.260 | 0.301 0.019 | 0.201 0.123 | ||
| Np | r p | −0.448 0.000 | −0.086 0.515 | −0.371 0.003 | 0.657 0.000 | −0.008 0.954 | 0.170 0.193 | 0.148 0.258 | 0.131 0.317 | |
| Yd | r p | 0.101 0.444 | 0.433 0.049 | −0.820 0.051 | 0.516 0.000 | 0.394 0.000 | 0.515 0.000 | 0.159 0.224 | −0.141 0.282 | 0.392 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Leyva, J.F.; Segura-Castruita, M.A.; Hernández-Cuevas, L.V.; Íñiguez-Rivas, M. Arbuscular Mycorrhizal Fungi Associated with Maize (Zea mays L.) in the Formation and Stability of Aggregates in Two Types of Soil. Microorganisms 2023, 11, 2615. https://doi.org/10.3390/microorganisms11112615
Gómez-Leyva JF, Segura-Castruita MA, Hernández-Cuevas LV, Íñiguez-Rivas M. Arbuscular Mycorrhizal Fungi Associated with Maize (Zea mays L.) in the Formation and Stability of Aggregates in Two Types of Soil. Microorganisms. 2023; 11(11):2615. https://doi.org/10.3390/microorganisms11112615
Chicago/Turabian StyleGómez-Leyva, Juan Florencio, Miguel Angel Segura-Castruita, Laura Verónica Hernández-Cuevas, and Mayra Íñiguez-Rivas. 2023. "Arbuscular Mycorrhizal Fungi Associated with Maize (Zea mays L.) in the Formation and Stability of Aggregates in Two Types of Soil" Microorganisms 11, no. 11: 2615. https://doi.org/10.3390/microorganisms11112615
APA StyleGómez-Leyva, J. F., Segura-Castruita, M. A., Hernández-Cuevas, L. V., & Íñiguez-Rivas, M. (2023). Arbuscular Mycorrhizal Fungi Associated with Maize (Zea mays L.) in the Formation and Stability of Aggregates in Two Types of Soil. Microorganisms, 11(11), 2615. https://doi.org/10.3390/microorganisms11112615








