Abstract
Peribacillus simplex is a Gram-positive, spore-forming bacterium derived from a vast range of different origins. Notably, it is part of the plant-growth-promoting rhizobacterial community of many crops. Although members of the Bacillaceae family have been widely used in agriculture, P. simplex has, so far, remained in the shadow of its more famous relatives, e.g., Bacillus subtilis or Bacillus thuringiensis. Recent studies have, however, started to uncover the bacterium’s highly promising and versatile properties, in particular in agricultural and environmental applications. Hence, here, we review the plant-growth-promoting features of P. simplex, as well as its biocontrol activity against a variety of detrimental plant pests in different crops. We further highlight the bacterium’s potential as a bioremediation agent for environmental contaminants, such as metals, pesticide residues, or (crude) oil. Finally, we examine the recent developments in the European regulatory landscape to facilitate the use of microorganisms in plant protection products. Undoubtedly, further studies on P. simplex will reveal additional benefits for agricultural and environmentally friendly applications.
1. Introduction
Sustainable agriculture is key in ensuring a continuous food supply for the growing world population, while at the same time minimizing negative effects on the environment [1]. This is also reflected in policy developments such as the European Green Deal and its ambitious Farm to Fork strategy, aiming at halving the use and risk of chemical pesticides and increasing organic farming practices [2].
One promising approach to replacing chemical products is the use of soil microbial inoculants, which are predominantly based on plant-growth-promoting (PGP) fungi and bacteria [3,4]. When applied to soil and/or plants, these microorganisms can exert several beneficial effects on their surroundings, such as (i) acting as biopesticides, (ii) enhancing plant growth, or (iii) improving soil conditions (e.g., through bioremediation or bioadsorption) [3]. Thus, bacterial inoculants can increase agronomic efficiency by reducing production costs and environmental pollution, as well as (partially) eliminating the use of chemical fertilizers and pesticides [5].
Plant-growth-promoting bacteria (PGPB) and plant-growth-promoting rhizobacteria (PGPR) are essential parts of the soil microbiome, sustaining plant health and growth. These microorganisms colonize the soil, plant rhizosphere, and root surface or interior and fulfil a variety of useful functions, such as increasing nutrient availability, counteracting abiotic stress, or improving the stress tolerance of the plant [5,6,7,8]. Here, members of the genus Bacillus—and recently reclassified closely related genera [9]—are one of the predominant microbial communities and play an important role in maintaining healthy soils conducive for plant growth and nutrition [8,10]. These Gram-positive bacteria are characterized by their ability to form dormant endospores, enabling them to withstand harsh conditions otherwise fatal to vegetative cells [11,12,13]. In addition, their ability to produce a wide arsenal of biologically active compounds with inhibitory and/or plant-growth-promoting effects has been well documented [14,15,16].
The biocontrol activity of a microorganism can generally be classified into two mechanisms. Direct antimicrobial activity includes the synthesis of phytohormones, as well as the production of antibiotics, hydrolytic enzymes, or lipopeptides [17]. In this regard, Bacillus spp. have been recognized as promising sustainable plant protection agents presenting a viable alternative to chemical pesticides [18], with, e.g., B. thuringiensis-, B. subtilis-, and B. amyloliquefaciens-containing formulations already commercially available [17,19,20].
Indirect mechanisms of biocontrol activity include (amongst others) inducing systemic resistance (ISR) in plants [17], which activates/increases plants’ resistance towards phytopathogenic infections and indirectly stimulates plant growth [21,22]. Here, Bacillus spp. can induce systemic resistance through different mechanisms, such as the secretion of enzymes, cyclic lipopeptides, or volatile organic compounds (VOC) [23]. That said, it is important to note that there is no clear separation of ISR and antimicrobial activity, as several antimicrobial lipopeptides, e.g., fengycin and surfactin, or VOCs can simultaneously induce systemic resistance [24].
Although positive environmental impacts of members of Bacillus spp. and related genera have been widely demonstrated, studies on Peribacillus simplex have only recently started to uncover the bacterium’s wide range of highly promising PGP features, including the ability to promote plant growth through nutrient fixation, the production of antimicrobial compounds, or acting as biosorbent for environmental contaminants. Hence, here, we provide a comprehensive overview of these findings and highlight P. simplex’s potential for its use in sustainable agricultural. Finally, with a view towards the future applications of P. simplex as a biocontrol agent, we will briefly summarize the requirements and changes in the European Regulation in the light of the European Green Deal and Farm to Fork strategy, which aim to facilitate the use of microorganisms in plant protection products.
2. Genus Peribacillus
Members of the genus Peribacillus belong to the family of Bacillaceae and are rod-shaped, Gram-positive, endospore-forming bacteria. Aerobic or facultative anaerobic bacteria were previous members of the genus Bacillus, however, after an extensive taxonomic reclassification in 2020 using phylogenomics and comparative genomic analyses, the species have been rearranged based on molecular markers to form a separate monophylogenetic group of the genus Peribacillus [9,25]. Today, the genus includes 21 species, with Peribacillus simplex as the type strain (Table 1) [26].
Many of the species have been originally isolated from soil and plant samples, although they can be derived from a wide variety of origins, such as near the Viking spacecraft at Kennedy Space Center [27] or stratospheric air samples at a 41 km altitude [28].

Table 1.
Members of the Peribacillus genus. Original sources of isolation are indicated.
Table 1.
Members of the Peribacillus genus. Original sources of isolation are indicated.
| Peribacillus Species [25,26] | Original Isolation Source | Ref. |
|---|---|---|
| Peribacillus acanthi | Rhizosphere soil of a mangrove plant Acanthus ilicifolius | [29] |
| Peribacillus alkalitolerans | Marine sediment near a hydrothermal vent | [30] |
| Peribacillus asahii | Soil | [31] |
| Peribacillus butanolivorans | Soil | [32] |
| Peribacillus castrilensis | River otter | [33] |
| Peribacillus cavernae | Cave soil | [34] |
| Peribacillus deserti | Desert soil | [35] |
| Peribacillus endoradicis | Soybean root | [36] |
| Peribacillus faecalis | Cow feces | [37] |
| Peribacillus frigoritolerans | Arid soil | [38,39] |
| Peribacillus glennii | Vehicle assembly building at Kennedy Space Center | [27] |
| Peribacillus gossypii | Stem of Gossypium hirsutum | [40] |
| Peribacillus huizhouensis | Paddy field soil | [41] |
| Peribacillus kribbensis | Soil | [42] |
| Peribacillus loiseleuriae | Soil from a loiseleuria plant | [43] |
| “Peribacillus massiliglaciei”1 | Siberian permafrost | [44] |
| Peribacillus muralis | Deteriorated mural paintings | [45] |
| Peribacillus psychrosaccharolyticus | Soil or lowland marsh. | [46] |
| Peribacillus saganii | Vehicle assembly building at Kennedy Space Center | [27] |
| Peribacillus simplex | Soil | [46] |
| Peribacillus tepidiphilus | Tepid spring | [47] |
1 Nomenclature status not validly published.
3. Plant-Growth-Promoting Properties
Members of the Bacillus genus (as traditionally defined) are among the most widespread Gram-positive soil microorganisms and are predominant in the plant-growth-promoting bacteria (PGPB) community [10]. The beneficial effects of the family members have been well documented [8,10,18].
In this regard, a number of studies have highlighted P. simplex’s potential to act as a plant-growth-promoting microorganism (Table 2).

Table 2.
Examples of uses of Peribacillus simplex as plant-growth-promoting bacteria.
3.1. Plant Growth Promotion through Compound Secretion
With the aim of searching for sustainable plant supplements or alternatives to chemical fertilizers, the use of PGPB has shown great potential, minimizing environmental impacts [51]. P. simplex demonstrates a broad range of activity, stimulating growth in a large variety of commercially relevant crops, such as tomato, wheat, soybean, or corn (Table 2), and has sometimes achieved over a quarter of crop yield increase [57]. In some cases, growth stimulation can notably reach levels similar to chemical fertilizers, making the bacterium a sustainable alternative to potentially harmful chemicals in food production [50]. Growth stimulation has most commonly been attributed to direct growth promotion via auxin production (indole-3- acetic acid, IAA) or siderophore secretion [22,50,56,58].
Another way of stimulating plant growth is the emission of volatile organic compounds (VOC), e.g., acetoin and 2,3-butanediol. When emitted by PGPB bacteria, these compounds can act as plant growth promotion triggers [52]. Gutiérrez-Luna et al. suggested that the VOCs secreted by P. simplex isolated from lemon plants improved the root growth and development in Arabidopsis thaliana under greenhouse conditions [52]. These compounds, mostly ketones and aldehydes also with antimicrobial attributes, included 2-nonenal, benzaldehyde, acetophenone, 6,10,14-trimethyl-2-pentadecanone, and 1-butanol, amongst others. However, there was no direct, experimental support for the effect of specific VOCs on plant growth promotion [52].
Finally, recent studies have shown that these growth promotion effects can be maximized when using combined inoculations with other PGPBs [51] or inorganic material [58]. This effect was particularly visible when combining PGP bacteria (P. simplex) and nitrogen (N)-fixating rhizobacteria (B. subtilis, Rhizobium leguminosarum bv. Viciae) in peas [53], while P. simplex-based bioformulations showed hydrogen cyanide (HCN), siderophore, and ammonia production in wheat [49]
In contrast, studies investigating the addition of inorganic acids such as salicylic acid together with P. simplex did not show any effect on plant growth [61].
3.2. Improved Nutrient Availability
Recent research attempts have aimed at increasing the concentrations of specific nutrients or micronutrients, thus improving plant health and nutritional value [4]. Although many techniques are based on plant-breeding techniques or transgenics, the use of PGP bacteria could also boost the uptake of specific nutrients in crops.
Studies have shown that siderophore-producing P. simplex can increase the uptake of iron in potatoes, while at the same time improving overall plant growth and yield [4].
P. simplex isolates have also demonstrated a high phosphate and zinc solubilization index in wheat [49], whereas high phosphate solubilization was detected in experiments with tomato plants. The latter, however, was distinctly strain-dependent [50].
Given that, in the soil, microorganisms occur in communities presumably acting synergistically, the combination of several PGPBs has shown better plant growth promotion effects than when used in isolation [3]. For example, co-culturing canola plants with P. simplex improved the shoot and root weight, in addition to enhancing the molybdenum micronutrient uptake [48]. Higher soluble nutrient concentrations (phosphate, magnesium, manganese, and sulfur), as well as increased phosphate uptake, could be obtained in winter wheat upon co-inoculation of the soil fungus Penicillium bilaiae with P. simplex (isolated from P. biliaiae) [55]. Equally, co-culturing P. simplex with inorganic silicon (Si) could improve the phosphate (P) uptake from P-rich and P-deficient soils. This was attributed to reduced oxidative stress as a result of increased antioxidant enzyme production, ultimately lowering the environmental stress for the plant and preventing root deterioration.
3.3. Root Colonization
PGPRs colonize the soil closely surrounding plant roots (rhizosphere), where they exert beneficial effects on plants. Hence, the success of microorganisms used as inoculants in agricultural crops greatly depends on the ability to colonize the host plant roots and body and prevail against other competing microorganisms [5,62]. The successful association of the bacteria with the plant roots is achieved by chemotaxis, attachment, and distribution along the roots. Once established, the bacterial colony size will determine and improve the root coverage and antagonism [62].
P. simplex has demonstrated a good root colonization potential and persistence in several commercial plants, such as wheat, tomato, and pine tree roots [51,55,62]. In some cases, P. simplex showed a higher rate of colonization than other Bacillus species (e.g., B. subtilis) [49]. Fluorescent localization studies with the transgenic P. simplex strain S11R41 isolated from pine tree rhizosphere have, in particular, confirmed that the bacterium is able to rapidly associate with tree roots, forming clusters at emerging lateral roots and elongation zones [62].
Regarding biofilm formation, GFP-report localization studies have not evidenced any biofilm formation of P. simplex associated with tree roots [62].
4. Biocontrol Activity
P. simplex strains isolated from different environments showed biocontrol activity against a large range of phytopathogens, mostly fungi, but also nematodes and bacteria, which was detected in several commercially highly relevant plants, such as potato, wheat, or tobacco (Table 3).

Table 3.
Applications of Peribacillus simplex as biocontrol agent in selected crops/diseases and associated phytopathogens. Studies on the species’ antimicrobial activity, as well as the induction of the plant systemic response, are considered.
4.1. Antimicrobial Activity
The antifungal activity of P. simplex has been demonstrated in a number of studies, most of them conducted on the phytopathogenic fungus Fusarium spp. In vitro assays showed up to a 70% growth inhibition of the plant pest and fungal hyphal thinning [17,53,63], however, compared to B. subtilis, the effects were slightly lower [17]. In planta experiments further confirmed these antifungal properties, greatly reducing disease severity after P. simplex application to the root seedlings of row crops or in black cumin [54,63]. The authors cautioned, however, that the results obtained from in vivo and in vitro antagonistic assays were not always aligned [63], and thus appropriate care should be taken for the screening of biocontrol agents under field conditions. Schwartz et al. [53] also confirmed P. simplex’s antagonistic activity against Fusarium spp., which was, however, dependent on growth conditions. This study was of particular interest, as it demonstrated the combined antimicrobial and plant-growth-promoting effects of P. simplex isolate 30N-5 in pea (Table 2 and Table 3), suggesting that such a combined activity could be more effective under field conditions [53]. Similar results were observed for isolates PHYB1 and PHYB9 in black cumin treatment [54]. Regarding the mode of action, in silico genomic studies indicated the presence of genes involved in the chitin degradation pathway and hydrolytic enzyme production, as well as cell-wall-degrading enzymes such as cellulase, pectinase, and xylanase, all of which are indicators for P. simplex’s antimicrobial activity [17]. Scanning electron microscopy studying the interaction between P. simplex and F. camptoceras demonstrated the bacterial adhesion to the fungus and the colonization of hyphae, causing tissue maceration [54].
P. simplex also reduced fungi-associated diseases in potato (pink rot) and wheat (Septoria Tritici Blotch) [65,66], while other studies demonstrated its antagonistic activity against the phytopathogens Pectobacterium sp. and Xylella fastidiosa [67,68]. Finally, in silico studies of the strain BA2H3 suggested the production of the antimicrobial compounds bacitracin and anthrachelin [67,74].
Regarding VOCs, several studies have highlighted P. simplex’s ability to produce a variety of microbial volatile organic compounds, including 2-ethyl-3,5-dimethylpyrazine, phenol, 1-decanol, 2-propanone, and benzaldehyde [17,64]. In this regard, Gu et al. [64] showed that soil-derived P. simplex strains secreted a mix of volatile organic compounds from the phenol, alcohol, aldehyde ketone alkyl, alkene, acid, ether, or heterocyclic groups, with strong antagonistic activity against the parasitic nematodes Panagrellus redivivus and Bursaphelenchus xylophilus. One important consideration with regard to the use of bacterial VOCs is that this mix is potentially less likely to select for resistance upon fumigation treatment.
4.2. Systemic Resistance
Recent studies have indicated that, besides antifungal activity in tobacco plants, pre-treatment with the P. simplex strain HS-2 increased reactive oxygen species (ROS) production and lowered plant cell wall permeability through increased callose production in response to a pathogen challenge [23]. Both reactions are indicators of the plant immune response. In addition, priming with this strain enhanced the expression of plant-related defense genes (e.g., lipoxygenase), as well as MAPK (mitogen-activated protein kinases) signals [23].
Fungal antagonism was also demonstrated in vitro and in planta against the forest fungal pathogens Heterobasidion annosum s.s., Armillaria mellea, and Fusarium circinatum. Notably, the treatment of pine seedlings with P. simplex considerably reduced lesions and plant mortality after pathogen exposure, which was tentatively attributed to antibiosis/systemic response [69,70]. Here, a dual application of the bacterium together with essential oils able to reduce seedling lesions was suggested as a plant prophylactic treatment [70].
Several studies by Yu-xi Duan and colleagues furthermore demonstrated the antagonistic effects of P. simplex Sne545 against nematodes through the activation of induced systemic resistance in soybean using a wide range of different analytical approaches [71,72,73]. First, metabolomic and transcriptomic analyses showed that the bacterium induces ISR by modulating the accumulation of nematocidal compounds (4-vinylphenol, L-methionine, piperine, and palmitic acid) after root infection, hence improving soybean resistance against pathogenic attacks [72]. Then, additional ISR-active compounds were determined using 1H-NMR and 13C-NMR as cyclic (Pro-Tyr), phenylalanine, cyclic (Leu-Pro), uracil, cyclic (Val-Pro), and tryptophan. The latter three notably activated the root resistance pathways (SA and JA pathways) in the plant [71]. Finally, metabolomics studies identified 15 metabolites involved in nematode resistance as a result of P. simplex Sne545 priming. These metabolites were involved in the provision of nematode nutrient sources (glucose, fructose, sucrose, and trehalose), the production of nematocidal compounds (melibiose and gluconic acid, lactic acid, phytosphingosine, and noradrenaline), and improved disease resistance (oxoproline, maltose, and galactose) [73].
Studies on wild rice have furthermore highlighted that pretreatment with strain 499G2 can promote plant growth (mostly through IAA production), while at the same time inducing plant resistance [60]. Overall, this is a good illustration that systemic resistance in plants (and bacterial antagonistic activity) mostly consists of an elaborate interplay of different pathways and compounds warding off the phytopathogen and often simultaneously improving plant resistance, survival, and health [60,71,72,73].
5. Biosorption and Bioremediation
The use of microorganisms as a remedy for contaminated zones is widely accepted. Several microorganisms have shown good potential as biosorbents for binding metals, environmental contaminants, or even mineral oil, immobilizing the contaminating substances and hindering their entry into the plant, food chain, or ground water [75,76,77]. In addition, bioremediation by microorganisms can indirectly promote plant growth by reducing stress conditions. In this regard, studies throughout the years have shown the bioremediation activity of P. simplex (Figure 1).
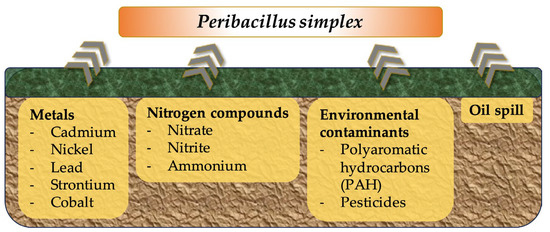
Figure 1.
Schematic representation of bioadsorption and bioremediation activity of P. simplex.
Early studies performed in the 1990s revealed that P. simplex could remove metals from contaminated soils and thus act as an environmental decontamination agent [75]. The bacterial uptake of cationic metal is usually attributed to interactions with the negatively charged cell wall. In particular, P. simplex’s ability to adsorb heavy metals showed a pH dependency with an optimum performance close to a neutral to alkaline pH. Researchers thus concluded that metal uptake was dependent on variably charged protonation sites (e.g., amino groups, phosphate, or carboxylate) [75]. As an example, Valentine and colleagues showed how the P. simplex strain ZAN-44 can adsorb divalent cadmium, nickel, cobalt, and strontium ions with a higher efficiency than B. subtilis 168 or Escherichia coli K-12. Notably, the latter two of the tested ions (60Co and 90Sr) were radionuclides, making P. simplex an interesting biosorbent for the cleaning of radioactively contaminated sites [75]. The ability of P. simplex to adsorb lead has been demonstrated in the literature, while authors have suggested that the bacterium could be exploited for bioremediation purposes [76]. Elevated levels of cadmium have been a major concern also in cocoa plants, with many initiatives aiming at reducing cadmium levels. Here, P. simplex has been proven as a highly promising sustainable biosorbent material for removing cadmium from contaminated soils and preventing its entry into plants and food chains [78].
Bioremediation activity has also been shown for other environmental contaminants such as low-molecular-weight polyaromatic hydrocarbon fluorene and phenanthrene, as well as nitrate, nitrite, and ammonium [79,80,81]. In particular, nitrogen removal capacity was favored by the strains’ (P. simplex H-b) tolerance of low temperatures [81].
The pesticidal burden of soils has become an increasing concern in agriculture and the food industry, given the long-time stability and non-specific toxicity of many active substances [82]. In this regard, several studies have demonstrated P. simplex’s ability to remove chemical pesticides from contaminated soils, as shown with the example of chlorsulfuron [83].
Finally, P. simplex isolates derived from bioaugmented oil contaminated soil have been classified as hydrocarbonoclastic bacteria, i.e., able to live on hydrocarbons as an energy source [84]. In addition to biodegradation, a P. simplex strain isolated from oil-contaminated sea sediment showed a high oil recovery efficiency through the production of a lipopeptide surfactant, including at a high salinity [85]. These features make P. simplex a particularly interesting candidate for the bioremediation of (crude) oil-contaminated sites via oil degradation and recovery.
6. EU Regulatory Aspects on the Use of Microorganisms in Sustainable Agriculture
Plant pathogens present a serious threat to agricultural productivity and can cause severe crop loss. For decades, chemical pesticides have been used to fight phytopathogens, including bacteria, fungi, or insects. However, with regulatory and food safety requirements becoming much stricter, a switch towards sustainable agriculture using biological alternatives to hazardous chemicals is gaining importance. In this regard, P. simplex and other members of the Bacillaceae family have shown promising traits that could be exploited in commercial agriculture, thus providing solutions to recent policy requests. Here, with the aim of contributing towards the objectives set under the Farm to Fork Strategy to reduce the overall use and risk of chemical pesticides by 50% and the use of more hazardous pesticides by 50% by 2030 [2], the European Union (EU) is facilitating the application of microorganisms in plant protection products. More specifically, it has developed four implementing regulations—applicable since 2022—regarding the approval of microorganisms as active substances in plant protection products (PPP). The first modification was Commission Regulation (EU) 2022/1438 amending Annex II of Regulation (EC) No 1107/2009 [86,87]. The latter provides rules for the authorization of PPPs and their placing on the market, while the amendment (amongst others) extends specific criteria related to microorganisms. Some of these main modifications and/or additions specifically refer to the requirement that the microorganism in question needs to be deposited at an internationally recognized culture collection and receive an accession number. It must be identified at minimum at the strain level and information must be provided about whether the biological materials are wild types, mutants, or genetically modified organisms. Regarding the safety aspects of the microorganisms, they must not be pathogenic to humans and must have no known functional and transferable gene coding for resistance to relevant antimicrobial agents. In this regard, the amendment further requires the microorganism to be susceptible to at least two classes of antimicrobial agents for it to be considered a low-risk active substance [87].
Other amendments related to the necessary information to be submitted for active substances and the specific data requirements for microorganisms were Commission Regulation (EU) 2022/1439 amending Regulation (EU) No 283/2013 [88,89]. We particularly highlight a modification referring to antimicrobial resistance (AMR), as well as the presence of antimicrobial resistance genes (ARG) [88]. Here, information is required on whether the bacterium shows any resistance to relevant antimicrobial agents or if ARG are acquired, transferable, and functional. These changes also relate to modifications in the data requirements for plant protection products containing microorganisms, as reflected in Commission Regulation (EU) 2022/1440 amending Part B of the Annex to Regulation (EU) No 284/2013 [90,91]. Thus, both amendments aim to update the data requirements for the latest scientific developments and adapt them to the specific biological properties of microorganisms.
Finally, given the abovementioned updated regulatory documents, Commission Regulation (EU) 2022/1441 amends Regulation (EU) No 546/2011 regarding the uniform principles for the evaluation and authorization of plant protection products containing microorganisms. Hence, data assessments are aligned across Member States, ensuring a high level of protection for human and animal health [92,93].
7. Conclusions
The advantages of Bacillus spp. in agriculture have long been recognized. That said, Peribacillus simplex has not received as much attention as other strains in this regard. However, recent efforts focusing on this spore former have shown its various beneficial effects for agricultural and environmental applications. These notably include plant-growth-promoting properties and excellent root colonization skills, as well as antimicrobial compound production and the induction of the plant systemic immune response. Regarding environmental functions, studies have begun to reveal highly promising properties of P. simplex as a bioremediation agent, for example, of heavy metals, pesticides, or oil removal and recovery. Future work will surely uncover further modes of action for this versatile bacterium.
A revision of the European regulatory landscape highlights changes in the legal frameworks to facilitate the use of microorganisms in sustainable plant protection products, while imposing strict safety rules to protect humans, animals, and the environment.
Author Contributions
Conceptualization, J.M., N.B. and H.A.; writing—original draft preparation, J.M., N.C.G., C.S.-R., N.B. and H.A.; writing—review and editing, J.M., N.C.G., C.S.-R., N.B. and H.A.; supervision, J.M., N.B. and H.A.; funding acquisition, J.M. and H.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union’s Horizon 2020 research and innovation program under Marie Skłodowska-Curie grant agreement No. 101029930.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge the research team at the University of Jaen (EI_BIO1_2023).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Velten, S.; Leventon, J.; Jager, N.; Newig, J. What Is Sustainable Agriculture? A Systematic Review. Sustainability 2015, 7, 7833–7865. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the European, the Council, the European Economic and Social and the Committee of the Regions—A Farm to Fork Strategy for a Fair, Healthy and Environmentally-Friendly Food System; European Commission: Brussels, Belgium, 2020.
- O’Callaghan, M.; Ballard, R.A.; Wright, D. Soil Microbial Inoculants for Sustainable Agriculture: Limitations and Opportunities. Soil Use Manag. 2022, 38, 1340–1369. [Google Scholar] [CrossRef]
- Mushtaq, Z.; Nazir, A.; Asghar, H.N.; Zahir, Z.A. Interactive Effect of Siderophore-Producing Bacteria and l-Tryptophan on Physiology, Tuber Characteristics, Yield, and Iron Concentration of Potato. Potato Res. 2022, 65, 1015–1027. [Google Scholar] [CrossRef]
- de Souza, R.; Ambrosini, A.; Passaglia, L.M.P. Plant Growth-Promoting Bacteria as Inoculants in Agricultural Soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.K.; Kumar, M.; Chakdar, H.; Anuroopa, N.; Bagyaraj, D.J. Bacillus Species in Soil as a Natural Resource for Plant Health and Nutrition. J. Appl. Microbiol. 2020, 128, 1583–1594. [Google Scholar] [CrossRef]
- Glick, B.R. The Enhancement of Plant Growth by Free-Living Bacteria. Can. J. Microbiol. 1995, 41, 109–117. [Google Scholar] [CrossRef]
- Manetsberger, J.; Caballero Gómez, N.; Benomar, N.; Christie, G.; Abriouel, H. Characterization of the Culturable Sporobiota of Spanish Olive Groves and Its Tolerance toward Environmental Challenges. Microbiol. Spectr. 2023, 11, e04013-22. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S.; Patel, S.; Saini, N.; Chen, S. Robust Demarcation of 17 Distinct Bacillus Species Clades, Proposed as Novel Bacillaceae Genera, by Phylogenomics and Comparative Genomic Analyses: Description of Robertmurraya kyonggiensis Sp. Nov. and Proposal for an Emended Genus Bacillus Limiting It Only to the Members of the Subtilis and Cereus Clades of Species. Int. J. Syst. Evol. Microbiol. 2020, 70, 5753–5798. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Hashem, A.; Abd Allah, E.F. Bacillus: A Biological Tool for Crop Improvement through Bio-Molecular Changes in Adverse Environments. Front. Physiol. 2017, 8, 667. [Google Scholar] [CrossRef] [PubMed]
- Setlow, P. Spores of Bacillus Subtilis: Their Resistance to and Killing by Radiation, Heat and Chemicals. J. Appl. Microbiol. 2006, 101, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, W.L. Roles of Bacillus Endospores in the Environment. Cell. Mol. Life Sci. 2002, 59, 410–416. [Google Scholar] [CrossRef]
- Driks, A. Bacillus Subtilis Spore Coat. Microbiol. Mol. Biol. Rev. 1999, 63, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Abriouel, H.; Franz, C.M.A.P.; Ben Omar, N.; Galvez, A. Diversity and Applications of Bacillus Bacteriocins. FEMS Microbiol. Rev. 2011, 35, 201–232. [Google Scholar] [CrossRef] [PubMed]
- Caulier, S.; Nannan, C.; Gillis, A.; Licciardi, F.; Bragard, C.; Mahillon, J. Overview of the Antimicrobial Compounds Produced by Members of the Bacillus Subtilis Group. Front. Microbiol. 2019, 10, 302. [Google Scholar] [CrossRef]
- Vlajkov, V.; Pajčin, I.; Loc, M.; Budakov, D.; Dodić, J.; Grahovac, M.; Grahovac, J. The Effect of Cultivation Conditions on Antifungal and Maize Seed Germination Activity of Bacillus-Based Biocontrol Agent. Bioengineering 2022, 9, 797. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Martínez-Hidalgo, P.; Ice, T.A.; Maymon, M.; Humm, E.A.; Nejat, N.; Sanders, E.R.; Kaplan, D.; Hirsch, A.M. Antifungal Activity of Bacillus Species against Fusarium and Analysis of the Potential Mechanisms Used in Biocontrol. Front. Microbiol. 2018, 9, 2363. [Google Scholar] [CrossRef] [PubMed]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The Significance of Bacillus Spp. in Disease Suppression and Growth Promotion of Field and Vegetable Crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef]
- Kumar, P.; Kamle, M.; Borah, R.; Mahato, D.K.; Sharma, B. Bacillus thuringiensis as Microbial Biopesticide: Uses and Application for Sustainable Agriculture. Egypt. J. Biol. Pest Control 2021, 31, 1–7. [Google Scholar] [CrossRef]
- Villarreal-Delgado, M.F.; Villa-Rodríguez, E.D.; Cira-Chávez, L.A.; Estrada-Alvarado, M.I.; Parra-Cota, F.I.; De los Santos-Villalobos, S. The Genus Bacillus as a Biological Control Agent and Its Implications in the Agricultural Biosecurity. Rev. Mex. De Fitopatol. Mex. J. Phytopathol. 2018, 36, 95–130. [Google Scholar] [CrossRef]
- Santoyo, G.; del Orozco-Mosqueda, M.C.; Govindappa, M. Mechanisms of Biocontrol and Plant Growth-Promoting Activity in Soil Bacterial Species of Bacillus and Pseudomonas: A Review. Biocontrol. Sci. Technol. 2012, 22, 855–872. [Google Scholar] [CrossRef]
- Akinrinlola, R.J.; Yuen, G.Y.; Drijber, R.A.; Adesemoye, A.O. Evaluation of Bacillus Strains for Plant Growth Promotion and Predictability of Efficacy by In Vitro Physiological Traits. Int. J. Microbiol. 2018, 2018, 5686874. [Google Scholar] [CrossRef] [PubMed]
- Miao, G.P.; Han, J.; Wang, C.R.; Zhang, K.G.; Wang, S. Chang Growth Inhibition and Induction of Systemic Resistance against Pythium aphanidermatum by Bacillus simplex Strain HS-2. Biocontrol. Sci. Technol. 2018, 28, 1114–1127. [Google Scholar] [CrossRef]
- Ongena, M.; Jourdan, E.; Adam, A.; Paquot, M.; Brans, A.; Joris, B.; Arpigny, J.L.; Thonart, P. Surfactin and Fengycin Lipopeptides of Bacillus Subtilis as Elicitors of Induced Systemic Resistance in Plants. Environ. Microbiol. 2007, 9, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Gupta, R.S. A Phylogenomic and Comparative Genomic Framework for Resolving the Polyphyly of the Genus Bacillus: Proposal for Six New Genera of Bacillus Species, Peribacillus Gen. Nov., Cytobacillus Gen. Nov., Mesobacillus Gen. Nov., Neobacillus Gen. Nov., Metabacillus Gen. Nov. and Alkalihalobacillus Gen. Nov. Int. J. Syst. Evol. Microbiol. 2020, 70, 406–438. [Google Scholar] [CrossRef] [PubMed]
- Parte, A.C.; Carbasse, J.S.; Meier-Kolthoff, J.P.; Reimer, L.C.; Göker, M. List of Prokaryotic Names with Standing in Nomenclature (LPSN) Moves to the DSMZ. Int. J. Syst. Evol. Microbiol. 2020, 70, 5607–5612. [Google Scholar] [CrossRef]
- Seuylemezian, A.; Ott, L.; Wolf, S.; Fragante, J.; Yip, O.; Pukall, R.; Schumann, P.; Vaishampayan, P. Bacillus glennii Sp. Nov. and Bacillus saganii Sp. Nov., Isolated from the Vehicle Assembly Building at Kennedy Space Center Where the Viking Spacecraft Were Assembled. Int. J. Syst. Evol. Microbiol. 2020, 70, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M.; Wickramasinghe, N.C.; Narlikar, J.V.; Rajaratnam, P. Microorganisms Cultured from Stratospheric Air Samples Obtained at 41 Km. FEMS Microbiol. Lett. 2003, 218, 161–165. [Google Scholar] [CrossRef]
- Ma, K.; Yin, Q.; Chen, L.; Lai, Q.; Xu, Y. Bacillus Acanthi Sp. Nov., Isolated from the Rhizosphere Soil of a Mangrove Plant Acanthus Ilicifolius. Int. J. Syst. Evol. Microbiol. 2018, 68, 3047–3051. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, M.; Zhang, X.H. Bacillus alkalitolerans Sp. Nov., Isolated from Marine Sediment near a Hydrothermal Vent. Int. J. Syst. Evol. Microbiol. 2018, 68, 1184–1189. [Google Scholar] [CrossRef]
- Yumoto, I.; Hirota, K.; Yamaga, S.; Nodasaka, Y.; Kawasaki, T.; Matsuyama, H.; Nakajima, K. Bacillus asahii Sp. Nov., a Novel Bacterium Isolated from Soil with the Ability to Deodorize the Bad Smell Generated from Short-Chain Fatty Acids. Int. J. Syst. Evol. Microbiol. 2004, 54, 1997–2001. [Google Scholar] [CrossRef]
- Kuisiene, N.; Raugalas, J.; Spröer, C.; Kroppenstedt, R.M.; Chitavichius, D. Bacillus butanolivorans Sp. Nov., a Species with Industrial Application for the Remediation of n-Butanol. Int. J. Syst. Evol. Microbiol. 2008, 58, 505–509. [Google Scholar] [CrossRef][Green Version]
- Rodríguez, M.; Reina, J.C.; Sampedro, I.; Llamas, I.; Martínez-Checa, F. Peribacillus castrilensis Sp. Nov.: A Plant-Growth-Promoting and Biocontrol Species Isolated From a River Otter in Castril, Granada, Southern Spain. Front. Plant Sci. 2022, 13, 896728. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Liu, D.; Sun, X.; Wang, G.; Li, M. Bacillus Cavernae Sp. Nov. Isolated from Cave Soil. Int. J. Syst. Evol. Microbiol. 2016, 66, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, G.L.; Wang, Y.; Dai, J.; Fang, C.X. Bacillus deserti Sp. Nov., a Novel Bacterium Isolated from the Desert of Xinjiang, China. Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2011, 99, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Chen, W.F.; Li, M.; Sui, X.H.; Liu, H.C.; Zhang, X.X.; Chen, W.X. Bacillus endoradicis Sp. Nov., an Endophytic Bacterium Isolated from Soybean Root. Int. J. Syst. Evol. Microbiol. 2012, 62, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Jung, W.Y.; Li, Z.; Lee, M.-K.; Kang, S.W.; Lee, J.-S.; Jung, H.; Hur, T.-Y.; Kim, H.B.; Kim, J.-K.; et al. Peribacillus faecalis Sp. Nov., a Moderately Halophilic Bacterium Isolated from the Faeces of a Cow. Int. J. Syst. Evol. Microbiol. 2019, 71, 004721. [Google Scholar] [CrossRef] [PubMed]
- Delaporte, B.; Sasson, A. Étude de Bactéries Des Sols Arides Du Maroc: Brevibacterium halotolerans n. Sp. et Brevibacterium Frigoritolerans n. Sp. Compte Rendu L’académie Sci. 1967, 264, 2257–2260. [Google Scholar]
- Montecillo, J.A.V.; Bae, H. Reclassification of Brevibacterium frigoritolerans as Peribacillus frigoritolerans Comb. Nov. Based on Phylogenomics and Multiple Molecular Synapomorphies. Int. J. Syst. Evol. Microbiol. 2022, 72, 005389. [Google Scholar] [CrossRef] [PubMed]
- Kämpfer, P.; Busse, H.J.; McInroy, J.A.; Glaeser, S.P. Bacillus gossypii Sp. Nov., Isolated from the Stem of Gossypium Hirsutum. Int. J. Syst. Evol. Microbiol. 2015, 65, 4163–4168. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, G.; Wu, M.; Zhao, Y.; Zhou, S. Bacillus huizhouensis Sp. Nov., Isolated from a Paddy Field Soil. Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2014, 106, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.M.; Jeon, C.O.; Lee, J.R.; Park, D.J.; Kim, C.J. Bacillus kribbensis Sp. Nov., Isolated from a Soil Sample in Jeju, Korea. Int. J. Syst. Evol. Microbiol. 2007, 57, 2912–2916. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, G.H.; Zhu, Y.J.; Wang, J.P.; Che, J.M.; Chen, Q.Q.; Chen, Z. Bacillus loiseleuriae Sp. Nov., Isolated from Rhizosphere Soil from a Loiseleuria Plant. Int. J. Syst. Evol. Microbiol. 2016, 66, 2678–2683. [Google Scholar] [CrossRef] [PubMed]
- Afouda, P.; Dubourg, G.; Cadoret, F.; Fournier, P.E.; Raoult, D. ‘Bacillus massiliglaciei’, a New Bacterial Species Isolated from Siberian Permafrost. New Microbes New Infect. 2017, 15, 92–93. [Google Scholar] [CrossRef] [PubMed]
- Heyrman, J.; Logan, N.A.; Rodríguez-Díaz, M.; Scheldeman, P.; Lebbe, L.; Swings, J.; Heyndrickx, M.; De Vos, P. Study of Mural Painting Isolates, Leading to the Transfer of “Bacillus maroccanus” and “Bacillus carotarum” to Bacillus Simplex, Emended Description of Bacillus Simplex, Re-Examination of the Strains Previously Attributed to “Bacillus macroides” and Description of Bacillus muralis Sp. Nov. Int. J. Syst. Evol. Microbiol. 2005, 55, 119–131. [Google Scholar] [CrossRef]
- Priest, F.G.; Goodfellow, M.; Todd, C. A Numerical Classification of the Genus Bacillus. J. Gen. Microbiol. 1988, 134, 1847–1882. [Google Scholar] [CrossRef] [PubMed]
- Narsing Rao, M.P.; Dhulappa, A.; Banerjee, A.; Thamchaipenet, A. Transfer of Bacillus tepidiphilus Narsing Rao et al. 2021 to the Genus Peribacillus as Peribacillus tepidiphilus Comb. Nov. Arch. Microbiol. 2022, 204, 545. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hidalgo, P.; Flores-Félix, J.D.; Sánchez-Juanes, F.; Rivas, R.; Mateos, P.F.; Regina, I.S.; Peix, Á.; Martínez-Molina, E.; Igual, J.M.; Velázquez, E. Identification of Canola Roots Endophytic Bacteria and Analysis of Their Potential as Biofertilizers for Canola Crops with Special Emphasis on Sporulating Bacteria. Agronomy 2021, 11, 1796. [Google Scholar] [CrossRef]
- Chandra, P.; Khobra, R.; Sundha, P.; Sharma, R.K.; Jasrotia, P.; Chandra, A.; Singh, D.P.; Singh, G.P. Plant Growth Promoting Bacillus-Based Bio Formulations Improve Wheat Rhizosphere Biological Activity, Nutrient Uptake and Growth of the Plant. Acta Physiol. Plant 2021, 43, 1–12. [Google Scholar] [CrossRef]
- Cochard, B.; Giroud, B.; Crovadore, J.; Chablais, R.; Arminjon, L.; Lefort, F. Endophytic PGPR from Tomato Roots: Isolation, In Vitro Characterization and In vivo Evaluation of Treated Tomatoes (Solanum lycopersicum L.). Microorganisms 2022, 10, 765. [Google Scholar] [CrossRef]
- Hassen, A.I.; Labuschagne, N. Root Colonization and Growth Enhancement in Wheat and Tomato by Rhizobacteria Isolated from the Rhizoplane of Grasses. World J. Microbiol. Biotechnol. 2010, 26, 1837–1846. [Google Scholar] [CrossRef]
- Gutiérrez-Luna, F.M.; López-Bucio, J.; Altamirano-Hernández, J.; Valencia-Cantero, E.; De La Cruz, H.R.; Macías-Rodríguez, L. Plant Growth-Promoting Rhizobacteria Modulate Root-System Architecture in Arabidopsis Thaliana through Volatile Organic Compound Emission. Symbiosis 2010, 51, 75–83. [Google Scholar] [CrossRef]
- Schwartz, A.R.; Ortiz, I.; Maymon, M.; Herbold, C.W.; Fujishige, N.A.; Vijanderan, J.A.; Villella, W.; Hanamoto, K.; Diener, A.; Sanders, E.R.; et al. Bacillus Simplex—A Little Known Pgpb with Anti-Fungal Activity—Alters Pea Legume Root Architecture and Nodule Morphology When Coinoculated with Rhizobium Leguminosarum Bv. Viciae. Agronomy 2013, 3, 595–620. [Google Scholar] [CrossRef]
- Al-Sman, K.M.; Abo-Elyousr, K.; Eraky, A.; El-Zawahry, A. Potential Activities of Bacillus simplex as a Biocontrol Agent against Root Rot of Nigella sativa Caused by Fusarium camptoceras. Egypt. J. Biol. Pest Control 2019, 29, 79. [Google Scholar] [CrossRef]
- Hansen, V.; Bonnichsen, L.; Nunes, I.; Sexlinger, K.; Lopez, S.R.; van der Bom, F.J.T.; Nybroe, O.; Nicolaisen, M.H.; Jensen, L.S. Seed Inoculation with Penicillium bilaiae and Bacillus simplex Affects the Nutrient Status of Winter Wheat. Biol. Fertil. Soils 2020, 56, 97–109. [Google Scholar] [CrossRef]
- Erturk, Y.; Ercisli, S.; Haznedar, A.; Cakmakci, R. Effects of Plant Growth Promoting Rhizobacteria (PGPR) on Rooting and Root Growth of Kiwifruit (Actinidia deliciosa) Stem Cuttings. Biol. Res. 2010, 43, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Senger, M.; Moresco, E.; Dalbosco, M.; Santin, R.; Inderbitzin, P.; Barrocas, E.N. Methods to Quantify Bacillus Simplex-Based Inoculant and Its Effect as a Seed Treatment on Field-Grown Corn and Soybean in Brazil. J. Seed Sci. 2022, 44, e202244040. [Google Scholar] [CrossRef]
- Rezakhani, L.; Motesharezadeh, B.; Tehrani, M.M.; Etesami, H.; Mirseyed Hosseini, H. Phosphate–Solubilizing Bacteria and Silicon Synergistically Augment Phosphorus (P) Uptake by Wheat (Triticum aestivum L.) Plant Fertilized with Soluble or Insoluble P Source. Ecotoxicol. Environ. Saf. 2019, 173, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Sözer Bahadir, P.; Liaqat, F.; Eltem, R. Plant Growth Promoting Properties of Phosphate Solubilizing Bacillus Species Isolated from the Aegean Region of Turkey. Turk. J. Botany 2018, 42, 183–196. [Google Scholar] [CrossRef]
- Yao, Z.; Chen, Y.; Luo, S.; Wang, J.; Zhang, J.; Zhang, J.; Tian, C.; Tian, L. Culturable Screening of Plant Growth-Promoting and Biocontrol Bacteria in the Rhizosphere and Phyllosphere of Wild Rice. Microorganisms 2022, 10, 1468. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Liza, S.E.; Sanchez, L.L.; Davila, D.E.Z. Agronomical Performance of Potato (Solanum tuberosum L.) Cv. “Unica” under Inoculation with Native Rhizobacteria and Application of Acetyl Salicylic Acid. Rev. Cienc. Agrovet. 2017, 16, 456–462. [Google Scholar] [CrossRef][Green Version]
- Mesanza, N.; Crawford, B.D.; Coulson, T.J.D.; Iturritxa, E.; Patten, C.L. Colonization of Pinus radiata D. Don Seedling Roots by Biocontrol Bacteria Erwinia billingiae and Bacillus simplex. Forests 2019, 10, 552. [Google Scholar] [CrossRef]
- Parikh, L.; Eskelson, M.J.; Adesemoye, A.O. Relationship of in Vitro and in Planta Screening: Improving the Selection Process for Biological Control Agents against Fusarium Root Rot in Row Crops. Arch. Phytopathol. Plant Prot. 2018, 51, 156–169. [Google Scholar] [CrossRef]
- Gu, Y.Q.; Mo, M.H.; Zhou, J.P.; Zou, C.S.; Zhang, K.Q. Evaluation and Identification of Potential Organic Nematicidal Volatiles from Soil Bacteria. Soil Biol. Biochem. 2007, 39, 2567–2575. [Google Scholar] [CrossRef]
- Allioui, N.; Driss, F.; Dhouib, H.; Jlail, L.; Tounsi, S.; Frikha-Gargouri, O. Two Novel Bacillus Strains (Subtilis and Simplex Species) with Promising Potential for the Biocontrol of Zymoseptoria Tritici, the Causal Agent of Septoria Tritici Blotch of Wheat. Biomed Res. Int. 2021, 2021, 6611657. [Google Scholar] [CrossRef] [PubMed]
- Adiyaman, T.; Schisler, D.A.; Slininger, P.J.; Sloan, J.M.; Jackson, M.A.; Rooney, A.P. Selection of Biocontrol Agents of Pink Rot Based on Efficacy and Growth Kinetics Index Rankings. Plant Dis. 2011, 95, 24–30. [Google Scholar] [CrossRef] [PubMed]
- des Essarts, Y.R.; Cigna, J.; Quêtu-Laurent, A.; Caron, A.; Munier, E.; Beury-Cirou, A.; Hélias, V.; Faure, D. Biocontrol of the Potato Blackleg and Soft Rot Diseases Caused by Dickeya Dianthicola. Appl. Environ. Microbiol. 2016, 82, 268–278. [Google Scholar] [CrossRef]
- Manetsberger, J.; Caballero Gómez, N.; Benomar, N.; Christie, G.; Abriouel, H. Antimicrobial profile of the culturable olive sporobiota and its potential as a source of biocontrol agents for major phytopathogens in olive agriculture. Pest Manag. Sci. 2023; preprint. [Google Scholar] [CrossRef]
- Mesanza, N.; Iturritxa, E.; Patten, C.L. Native Rhizobacteria as Biocontrol Agents of Heterobasidion Annosum s.s. and Armillaria Mellea Infection of Pinus Radiata. Biol. Control 2016, 101, 8–16. [Google Scholar] [CrossRef]
- Iturritxa, E.; Trask, T.; Mesanza, N.; Raposo, R.; Elvira-Recuenco, M.; Patten, C.L. Biocontrol of Fusarium Circinatum Infection of Young Pinus Radiata Trees. Forests 2017, 8, 32. [Google Scholar] [CrossRef]
- Xing, Z.; Wu, X.; Zhao, J.; Zhao, X.; Zhu, X.; Wang, Y.; Fan, H.; Chen, L.; Liu, X.; Duan, Y. Isolation and Identification of Induced Systemic Resistance Determinants from Bacillus Simplex Sneb545 against Heterodera glycines. Sci. Rep. 2020, 10, 11586. [Google Scholar] [CrossRef]
- Kang, W.; Zhu, X.; Wang, Y.; Chen, L.; Duan, Y. Transcriptomic and Metabolomic Analyses Reveal That Bacteria Promote Plant Defense during Infection of Soybean Cyst Nematode in Soybean. BMC Plant. Biol. 2018, 18, 86. [Google Scholar] [CrossRef]
- Kang, W.S.; Chen, L.J.L.J.; Wang, Y.Y.; Zhu, X.F.; Liu, X.Y.; Fan, H.; Duan, Y.X. Bacillus Simplex Treatment Promotes Soybean Defence against Soybean Cyst Nematodes: A Metabolomics Study Using GC-MS. PLoS ONE 2020, 15, e0237194. [Google Scholar] [CrossRef]
- Khayi, S.; des Essarts, Y.R.; Mondy, S.; Moumni, M.; Hélias, V.; Beury-Cirou, A.; Faure, D. Draft Genome Sequences of the Three Pectobacterium-Antagonistic Bacteria Pseudomonas Brassicacearum PP1-210F and PA1G7 and Bacillus Simplex BA2H3. Genome. Announc. 2015, 3, e01497-14. [Google Scholar] [CrossRef]
- Valentine, N.; Bolton Jr, H.; Kingsley, M.; Drake, G.; BalkwilF, D.; Plymale, A. Biosorption of Cadmium, Cobalt, Nickel, and Strontium by a Bacillus simplex Strain Isolated from the Vadose Zone. J. Ind. Microbiol. Biotechnol. 1996, 16, 189–196. [Google Scholar] [CrossRef]
- Chamekh, A.; Kharbech, O.; Driss-Limam, R.; Fersi, C.; Khouatmeya, M.; Chouari, R. Evidences for Antioxidant Response and Biosorption Potential of Bacillus simplex Strain 115 against Lead. World J. Microbiol. Biotechnol. 2021, 37, 44. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Shao, W.; Zhang, K.; Huo, Y.; Li, M. Characterization of Phosphate Solubilizing Bacteria Isolated from Heavy Metal Contaminated Soils and Their Potential for Lead Immobilization. J. Environ. Manag. 2019, 231, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Arce-Inga, M.; González-Pérez, A.R.; Hernandez-Diaz, E.; Chuquibala-Checan, B.; Chavez-Jalk, A.; Llanos-Gomez, K.J.; Leiva-Espinoza, S.T.; Oliva-Cruz, S.M.; Cumpa-Velasquez, L.M. Bioremediation Potential of Native Bacillus Sp. Strains as a Sustainable Strategy for Cadmium Accumulation of Theobroma Cacao in Amazonas Region. Microorganisms 2022, 10, 2108. [Google Scholar] [CrossRef]
- Seo, J.S.; Keum, Y.S.; Li, Q.X. Bacterial Degradation of Aromatic Compounds. Int. J. Environ. Res. Public Health 2009, 6, 278–309. [Google Scholar] [CrossRef] [PubMed]
- Mandree, P.; Masika, W.; Naicker, J.; Moonsamy, G.; Ramchuran, S.; Lalloo, R. Bioremediation of Polycyclic Aromatic Hydrocarbons from Industry Contaminated Soil Using Indigenous bacillus spp. Processes 2021, 9, 1606. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, T.; Shi, Y.; Xin, Y.; Zhang, L.; Gu, Z.; Li, Y.; Ding, Z.; Shi, G. The Nitrogen Removal Characterization of a Cold-Adapted Bacterium: Bacillus simplex H-b. Bioresour. Technol. 2021, 323, 124554. [Google Scholar] [CrossRef]
- Silva, V.; Mol, H.G.J.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide Residues in European Agricultural Soils—A Hidden Reality Unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef]
- Erguven, G.O.; Yildirim, N. Efficiency of Some Soil Bacteria for Chemical Oxygen Demand Reduction of Synthetic Chlorsulfuron Solutions under Agiated Culture Conditions. Cell Mol. Biol. 2016, 62, 92–96. [Google Scholar] [CrossRef]
- Kansour, M.K.; Al-Mailem, D.M. Bioremediation of Two Oil-Contaminated Kuwaiti Hyper-Saline Soils by Cross Bioaugmentation and the Role of Indigenous Halophilic/Halotolerant Hydrocarbonoclastic Bacteria. Environ. Technol. Innov. 2023, 32, 103259. [Google Scholar] [CrossRef]
- Mani, P.; Sivakumar, P.; Balan, S.S. Economic Production and Oil Recovery Efficiency of a Lipopeptide Biosurfactant from a Novel Marine Bacterium Bacillus simplex. Achiev. Life Sci. 2016, 10, 102–110. [Google Scholar] [CrossRef][Green Version]
- European Parliament and the Council of the European Union Regulation. (EC) No 1107/2009 of the European Parliament and the Council of 21 October 2009 Concerning the Placing of Plant Protection Products on the Market and Repealing Council Directives/117/EEC and 91/414/EEC. Off. J. Eur. Union 2009, 309, 1–50. [Google Scholar]
- European Commission. Commission Regulation (EU) 2022/1438 of 31 August 2022 Annex II to Regulation (EC) No 1107/2009 of the European Parliament and of the Council as Regards Specific Criteria for the Approval of Active Substances That Are Micro-Organisms. Off. J. Eur. Union 2022, 227, 2–7. [Google Scholar]
- European Commission. Commission Regulation (EU) 2022/1439 of 31 August 2022 Amending Regulation (EU) No 283/2013 as Regards the Information to Be Submitted for Active and the Specific Data Requirements for Micro-Organisms. Off. J. Eur. Union 2022, 227, 8–37. [Google Scholar]
- European Commission. Commission Regulation (EU) No 283/2013 of 1 March 2013 Setting out the Data Requirements for Active Substances, in Accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council Concerning the Placing of Plant Protection Products on the Market. Off. J. Eur. Union 2013, 93, 1–84. [Google Scholar]
- European Commission. Commission Regulation (EU) 2022/1440 of 31 August 2022 Regulation (EU) No 284/2013 as Regards the Information to Be Submitted for Plant Protection Products and the Specific Data Requirements for Plant Protection Products Containing Micro-Organisms. Off. J. Eur. Union 2022, 227, 38–69. [Google Scholar]
- European Commission. Commission Regulation (EU) No 284/2013 of 1 March 2013 Setting out the Data Requirements for Plant Protection Products, in Accordance with Regulation (EC) of the European Parliament and of the Council Concerning the Placing of Plant Protection Products on the Market. Off. J. Eur. Union 2013, 93, 85–152. [Google Scholar]
- European Commission. Commission Regulation (EU) No 546/2011 of 10 June 2011 Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards Uniform Principles for Evaluation and Authorisation of Plant Protection Products. Off. J. Eur. Union 2011, 155, 127–175. [Google Scholar]
- European Commission. Commission Regulation (EU) 2022/1441 of 31 August 2022 Regulation (EU) No 546/2011 as Regards Specific Uniform Principles for Evaluation and Authorisation of Plant Protection Products Containing Micro-Organisms. Off. J. Eur. Union 2022, 227, 70–116. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
