The Combination of β-Glucan and Astragalus Polysaccharide Effectively Resists Nocardia seriolae Infection in Largemouth Bass (Micropterus salmoides)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Foundation and Preparation
2.2. Bacterial Culture and Challenge Test
2.3. Sampling and Testing
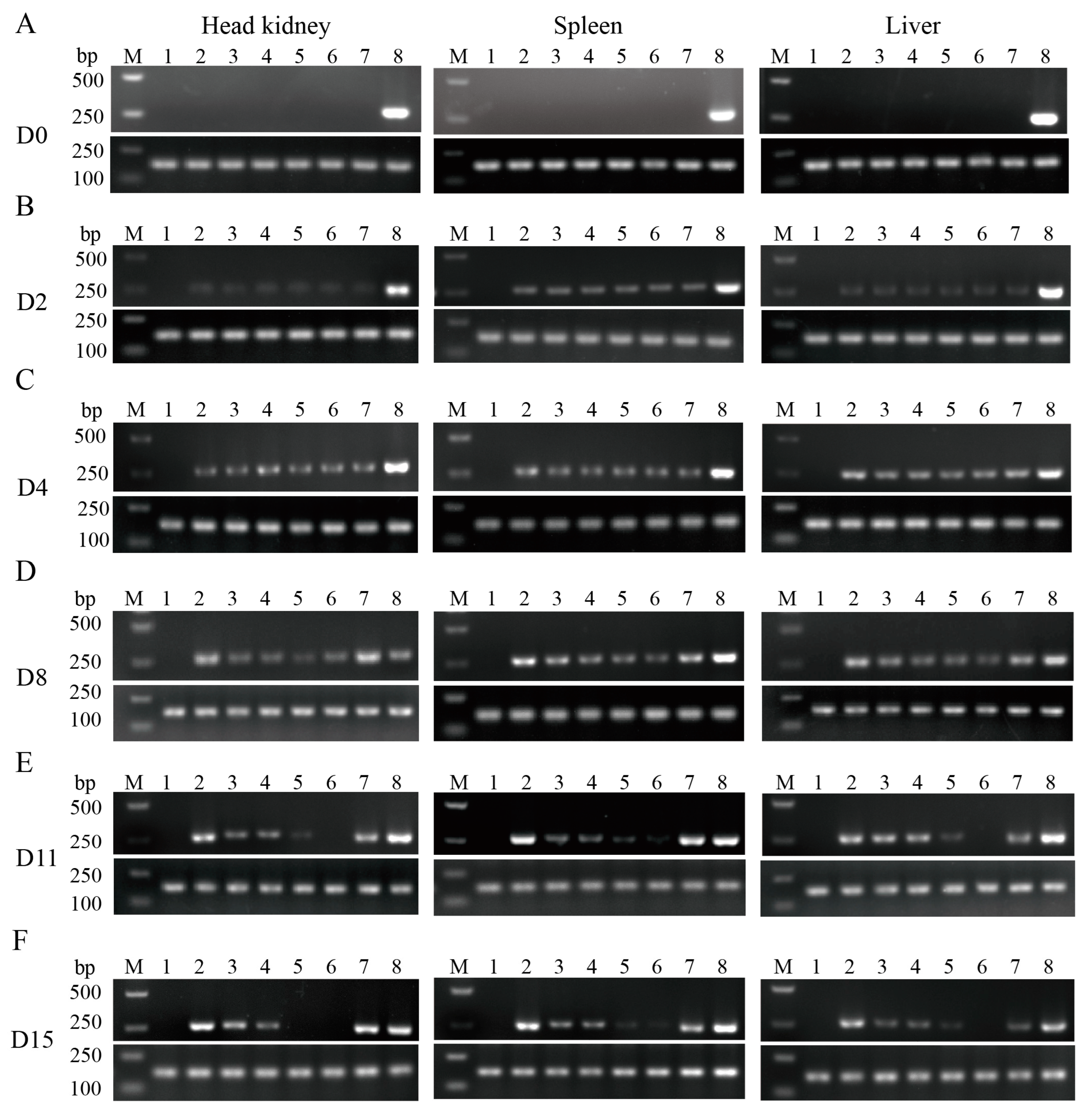
2.3.1. Semi-Quantitative PCR for the Detection of Pathogen
2.3.2. Determination of Serum Enzyme Activity
2.3.3. Quantitative Real-Time PCR for Detecting Tissue Pathogen Load and Immunity Genes
2.4. Histopathological Analysis
2.5. Spread Plate Method for Detecting Bacterial Drug Resistance
2.6. Statistical Analyses
3. Results
3.1. The Complex Immunostimulant of β-Glucan + APS Effectively Improves Protective Effect after N. seriolae Challenge
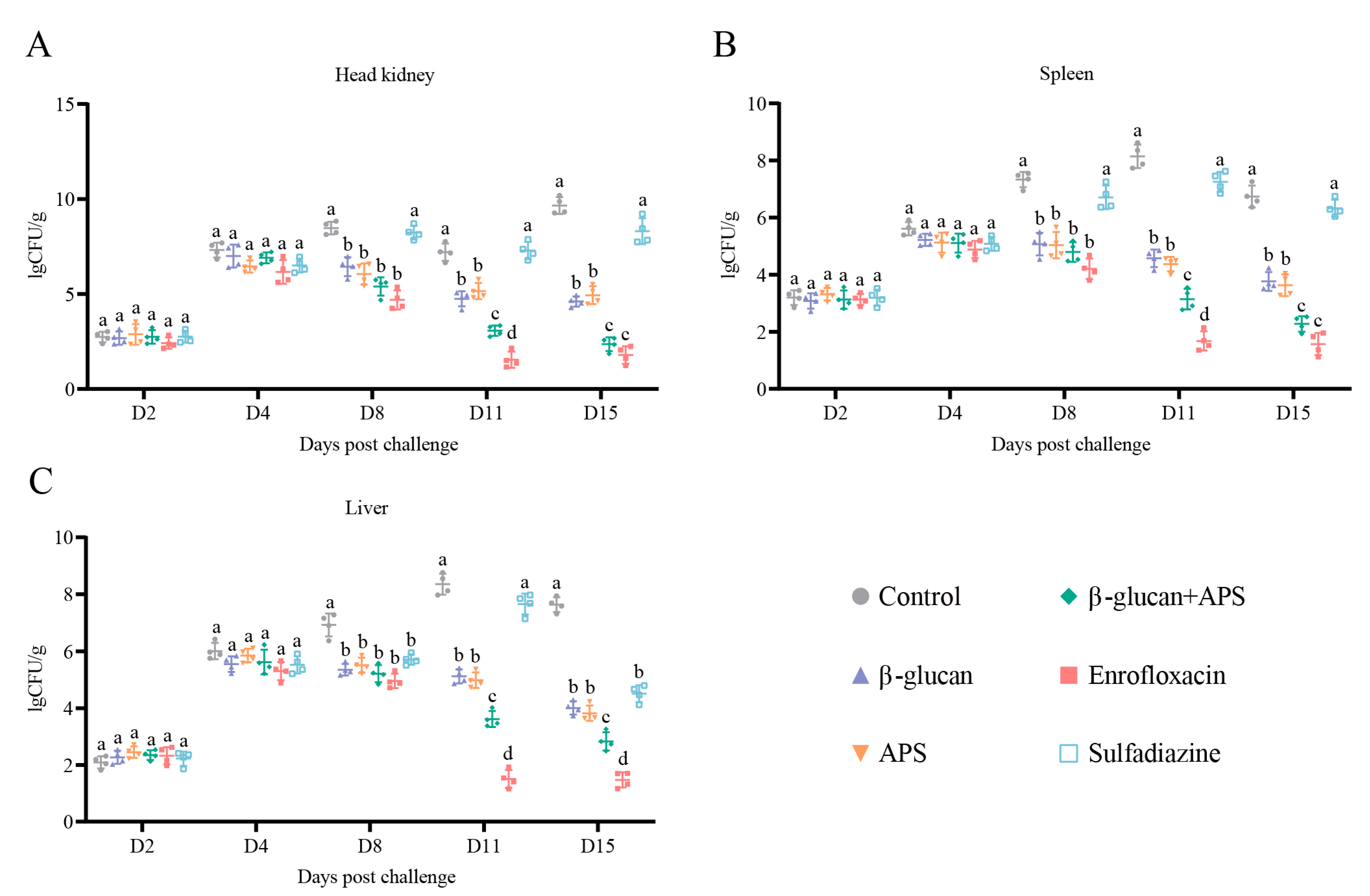
3.2. β-Glucan + APS Efficiently Inhibits N. seriolae Proliferation and Clears Bacterial Loading in Tissue
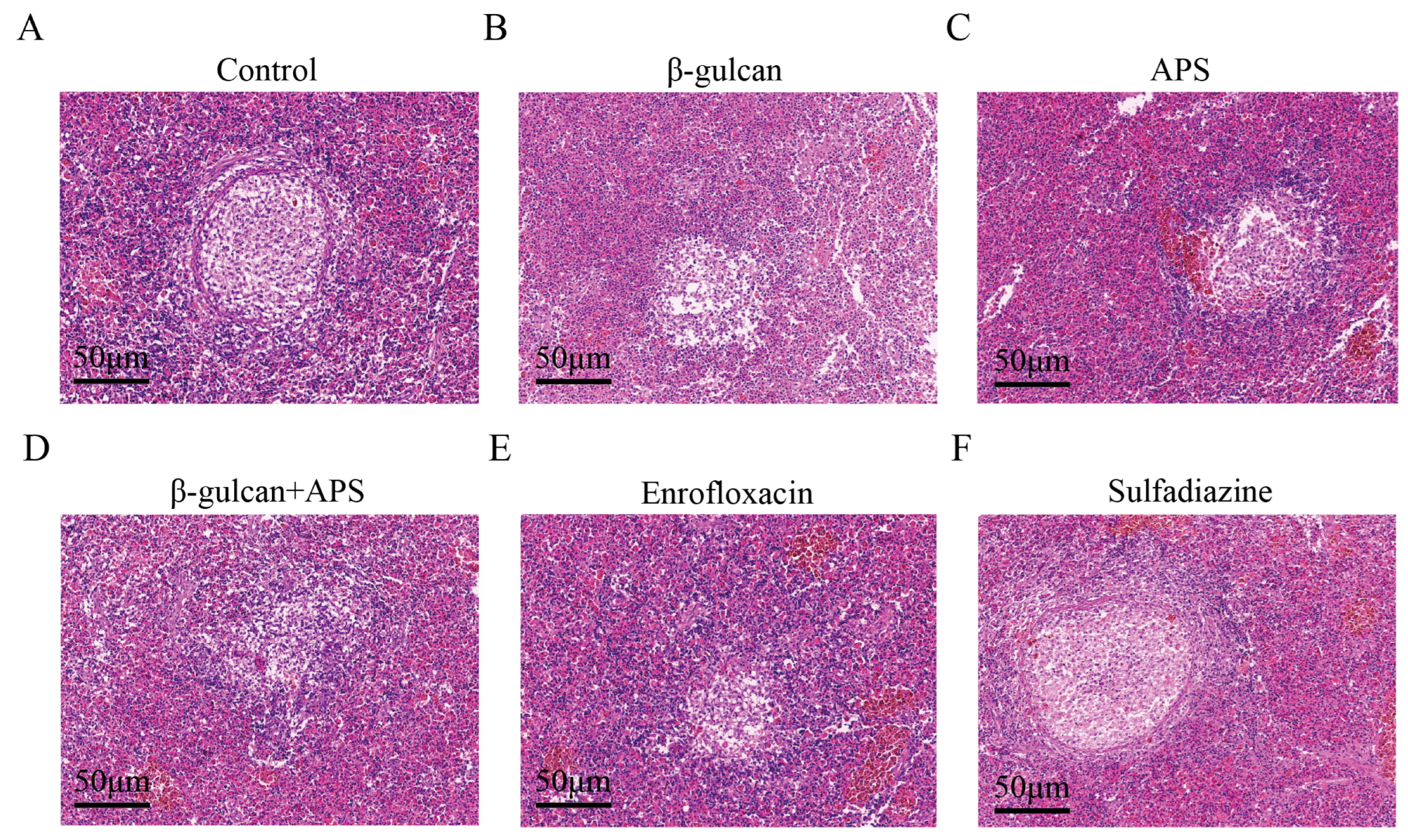
3.3. The Combination of β-Glucan and APS Effectively Suppresses Granuloma Formation and Alleviates Tissue Lesion during N. seriolae Infection
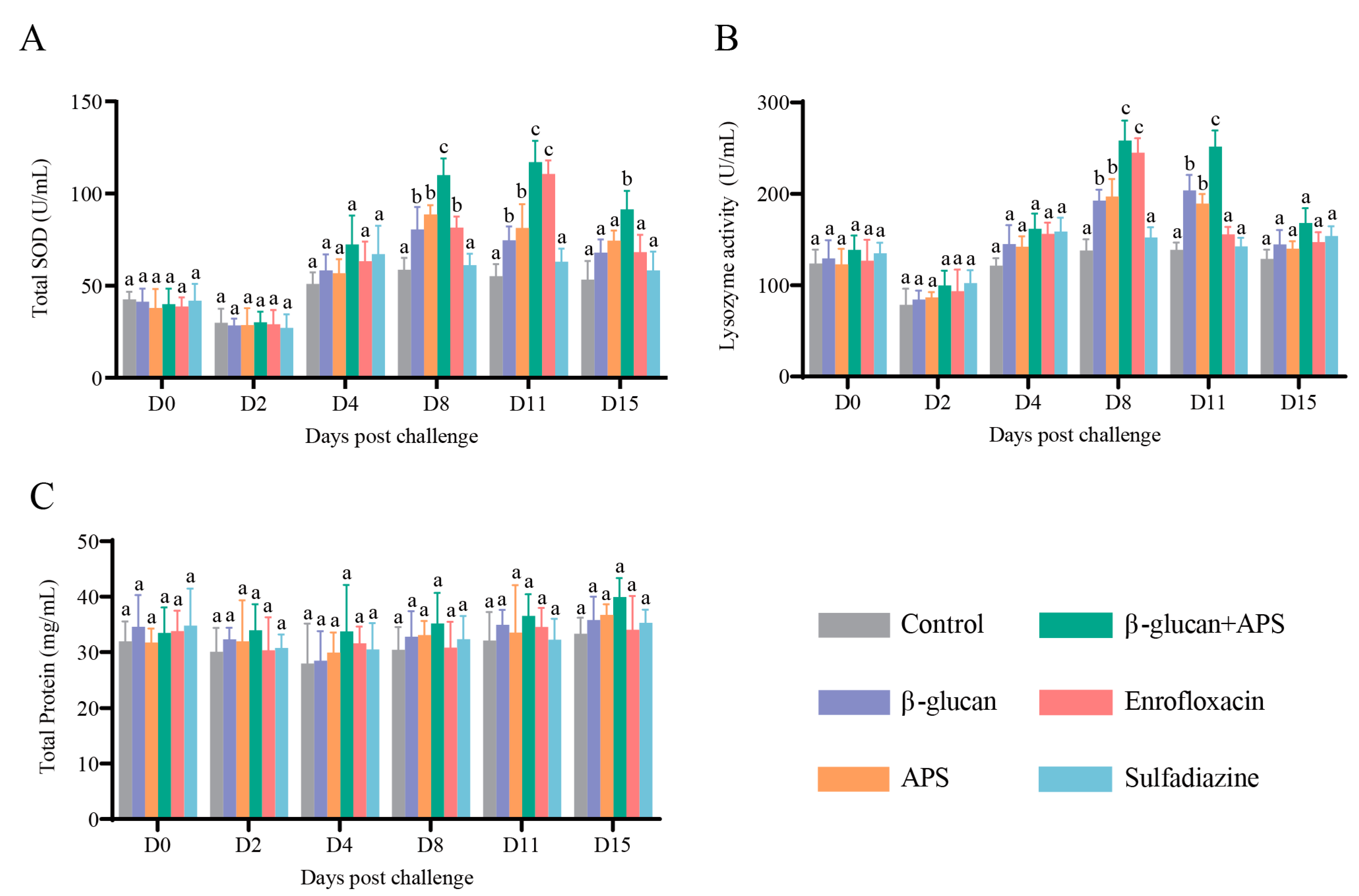
3.4. β-Glucan Complexed with APS Remarkably Enhances Serum Enzyme Activities
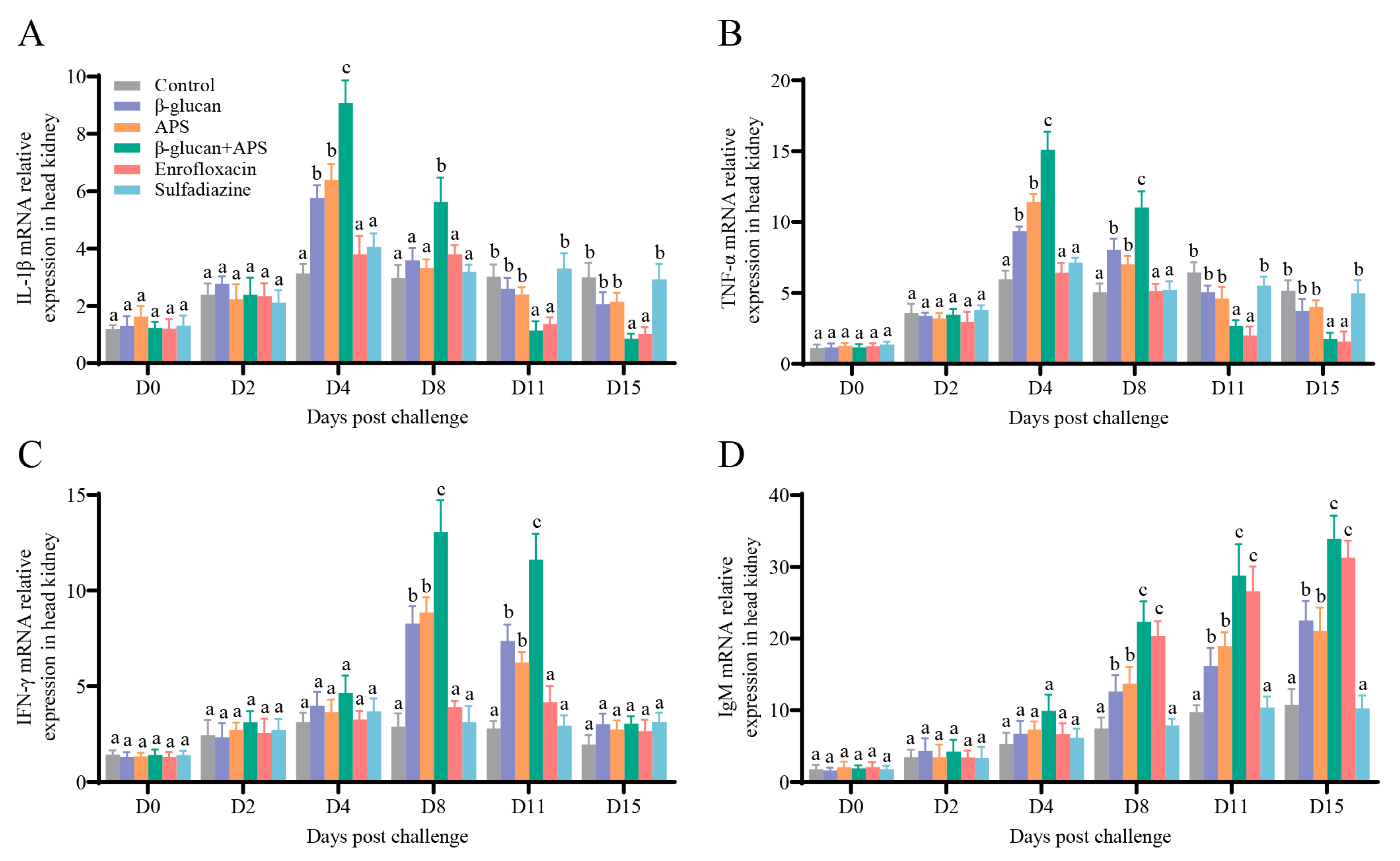
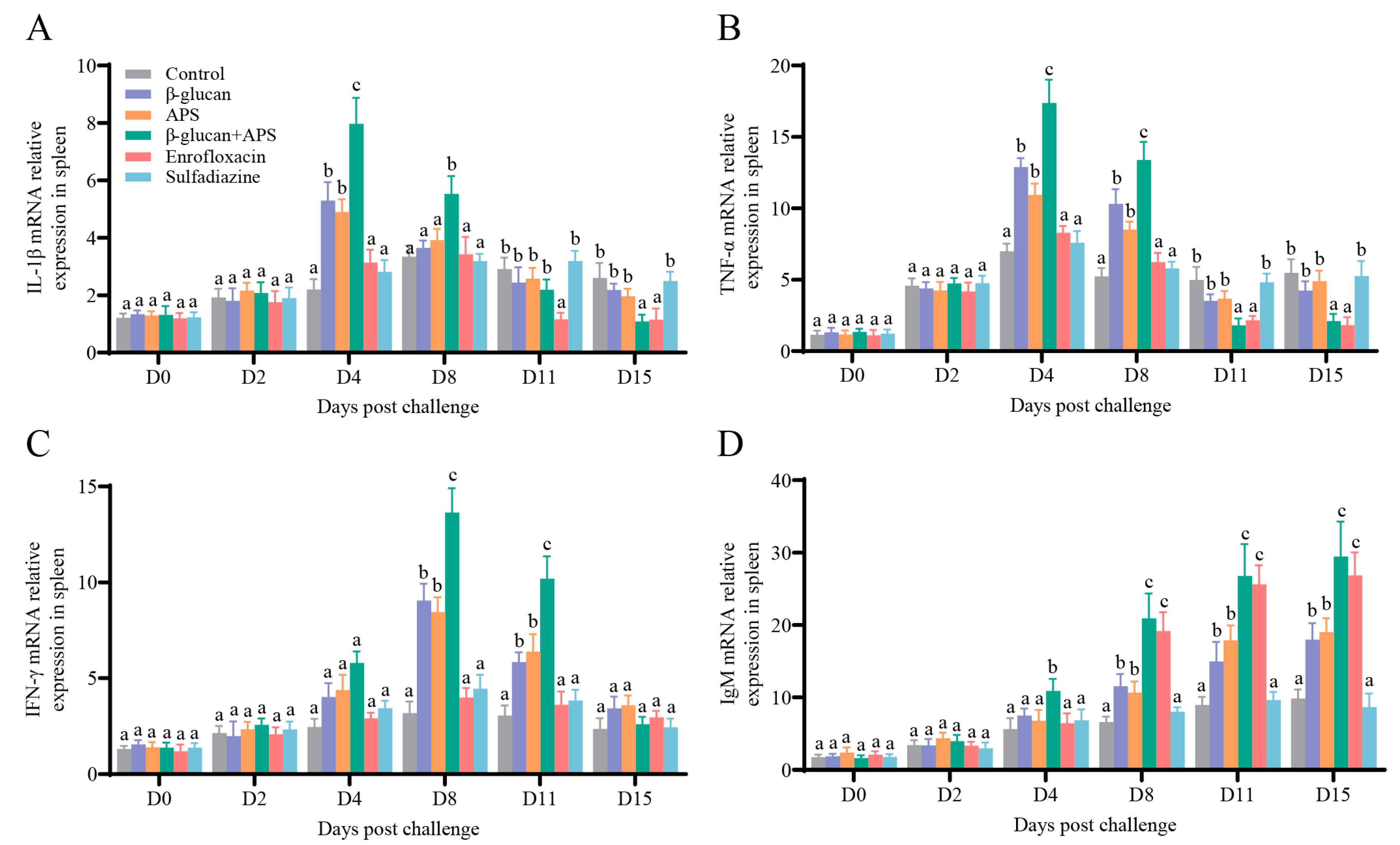
3.5. The Co-Application of β-Glucan and APS Activates the Expression of Immune-Related Genes
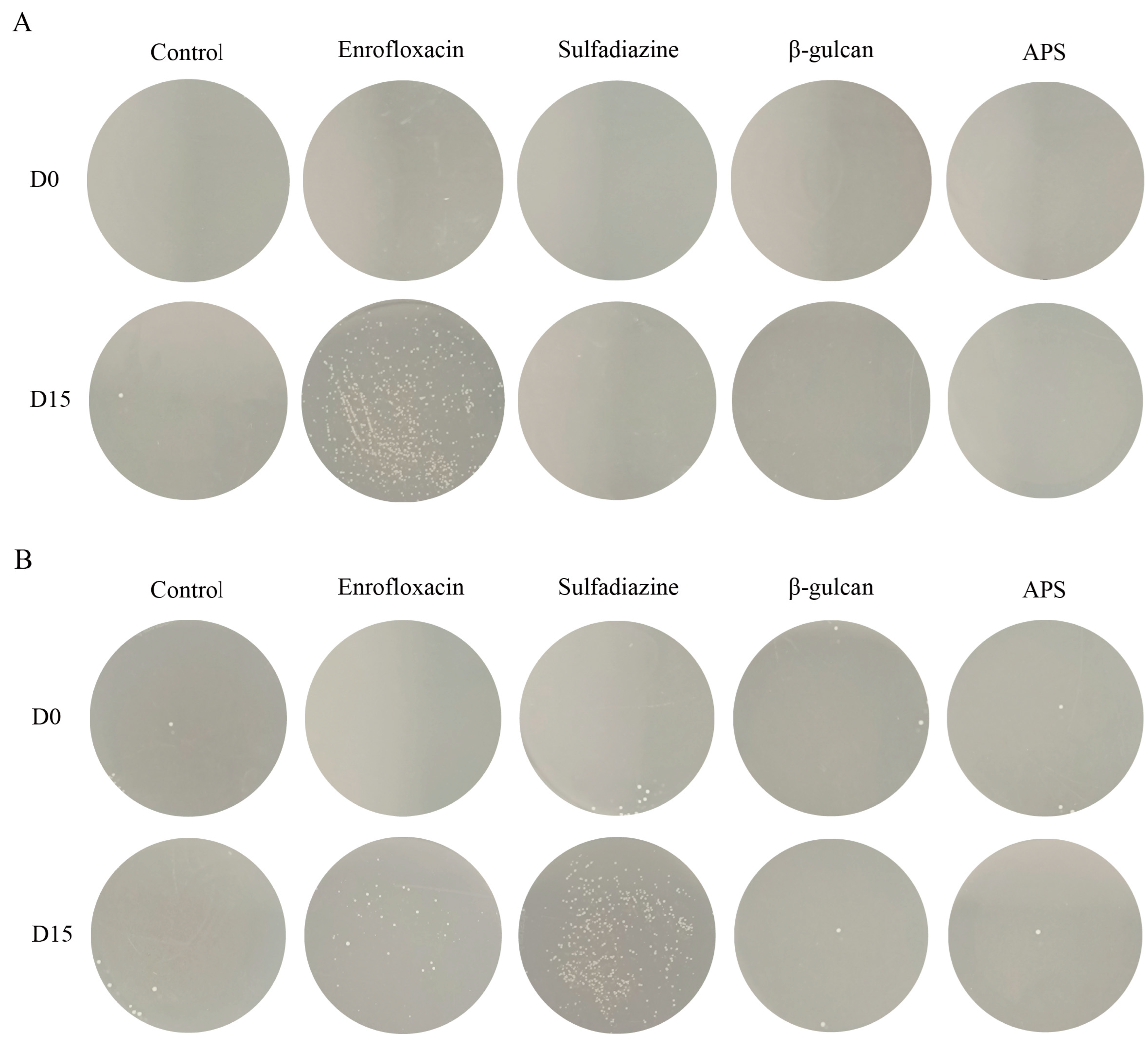
3.6. Antibiotic Treatment Leads to Bacterial Resistance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piazzon, M.C.; Calduch-Giner, J.A.; Fouz, B.; Estensoro, I.; Simo-Mirabet, P.; Puyalto, M.; Karalazos, V.; Palenzuela, O.; Sitja-Bobadilla, A.; Perez-Sanchez, J. Under control: How a dietary additive can restore the gut microbiome and proteomic profile, and improve disease resilience in a marine teleostean fish fed vegetable diets. Microbiome 2017, 5, 164. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zheng, S.; Li, Y.; Li, J.; Liu, H. Hemocyte-mediated phagocytosis in crustaceans. Front. Immunol. 2020, 11, 268. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhang, Z.; Wang, Y.; Li, S.; Ran, C.; Hu, J.; Xie, Y.; Li, W.; Zhou, Z. EPSP of L. casei BL23 protected against the infection caused by Aeromonas veronii via enhancement of immune response in zebrafish. Front. Microbiol. 2017, 8, 2406. [Google Scholar] [CrossRef]
- Liu, P.; Huang, D.; Hu, X.; Tang, Y.; Ma, X.; Yan, R.; Han, Q.; Guo, J.; Zhang, Y.; Sun, Q.; et al. Targeting inhibition of smpB by peptide aptamer attenuates the virulence to protect zebrafish against Aeromonas veronii infection. Front. Microbiol. 2017, 8, 1766. [Google Scholar] [CrossRef] [PubMed]
- Assefa, A.; Abunna, F. Maintenance of fish health in aquaculture: Review of epidemiological approaches for prevention and control of infectious disease of fish. Vet. Med. Int. 2018, 2018, 5432497. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Chen, X.; Wang, K.; Wang, J.; Chen, D.; Geng, Y.; Lai, W.; Wei, X. Plant polysaccharides used as immunostimulants enhance innate immune response and disease resistance against Aeromonas hydrophila infection in fish. Fish Shellfish Immunol. 2016, 59, 196–202. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, L.; Tiu, L.; Wang, H. Characterization of antibiotic resistance in commensal bacteria from an aquaculture ecosystem. Front. Microbiol. 2015, 6, 914. [Google Scholar] [CrossRef]
- Quesada, S.P.; Paschoal, J.A.; Reyes, F.G. Considerations on the aquaculture development and on the use of veterinary drugs: Special issue for fluoroquinolones—A review. J. Food Sci. 2013, 78, R1321–R1333. [Google Scholar] [CrossRef]
- Grabowski, L.; Gaffke, L.; Pierzynowska, K.; Cyske, Z.; Choszcz, M.; Wegrzyn, G.; Wegrzyn, A. Enrofloxacin-the ruthless killer of eukaryotic cells or the last hope in the fight against bacterial infections? Int. J. Mol. Sci. 2022, 23, 3648. [Google Scholar] [CrossRef]
- Stoffregen, D.A.; Backman, S.C.; Perham, R.E.; Bowser, P.R.; Babish, J.G. Initial disease report of Streptococcus iniae infection in hybrid striped (Sunshine) bass and successful therapeutic intervention with the fluoroquinolone antibacterial enrofloxacin. J. World Aquac. Soc. 1996, 27, 420–434. [Google Scholar] [CrossRef]
- Zhao, X.; Jin, Z.; Di, G.; Li, L.; Kong, X. Molecular characteristics, pathogenicity and medication regimen of Aeromonas hydrophila isolated from common carp (Cyprinus carpio L.). J. Vet. Med. Sci. 2019, 81, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Carraschi, S.P.; Barbuio, R.; Ikefuti, C.V.; Florêncio, T.; da Cruz, C.; Ranzani-Paiva, M.J.T. Effectiveness of therapeutic agents in disease treatment in Piaractus mesopotamicus. Aquaculture 2014, 431, 124–128. [Google Scholar] [CrossRef]
- Hsu, H.M.; Bowser, P.R.; Schachte, J.H.; Scarlett, J.M.; Babish, J.G. Winter field trials of enrofloxacin for the control of Aeromonas salmonicida infection in Salmonids. J. World Aquac. Soc. 1995, 26, 307–314. [Google Scholar] [CrossRef]
- Xu, N.; Li, M.; Lin, Z.; Ai, X. Comparative pharmacokinetics of sulfadiazine and its metabolite N4-acetyl sulfadiazine in grass carp (Ctenopharyngodon idella) at different temperatures after oral administration. Pharmaceutics 2022, 14, 712. [Google Scholar] [CrossRef] [PubMed]
- Plumb, J.A.; Maestrone, G.; Quinlan, E. Use of a potentiated sulfonamide to control Edwardsiella ictaluri infection in channel catfish (Ictalurus punctatus). Aquaculture 1987, 62, 187–194. [Google Scholar] [CrossRef]
- Miranda, C.D.; Godoy, F.A.; Lee, M.R. Current status of the use of antibiotics and the antimicrobial resistance in the chilean salmon farms. Front. Microbiol. 2018, 9, 1284. [Google Scholar] [CrossRef]
- Le, T.H.; Truong, T.; Tran, L.T.; Nguyen, D.; Pham, T.P.; Ng, C. Antibiotic resistance in the aquatic environments: The need for an interdisciplinary approach. Int. J. Environ. Sci. Technol. 2022, 20, 3395–3408. [Google Scholar] [CrossRef]
- Janecko, N.; Pokludova, L.; Blahova, J.; Svobodova, Z.; Literak, I. Implications of fluoroquinolone contamination for the aquatic environment-a review. Env. Toxicol. Chem. 2016, 35, 2647–2656. [Google Scholar] [CrossRef]
- Xie, T.; Xu, X.; Wu, Q.; Zhang, J.; Cheng, J. Prevalence, molecular characterization, and antibiotic susceptibility of Vibrio parahaemolyticus from ready-to-eat foods in China. Front. Microbiol. 2016, 7, 549. [Google Scholar] [CrossRef]
- Makrinos, D.L.; Bowden, T.J. Natural environmental impacts on teleost immune function. Fish Shellfish Immunol. 2016, 53, 50–57. [Google Scholar] [CrossRef]
- Wang, B.; Thompson, K.D.; Wangkahart, E.; Yamkasem, J.; Bondad-Reantaso, M.G.; Tattiyapong, P.; Jian, J.; Surachetpong, W. Strategies to enhance tilapia immunity to improve their health in aquaculture. Rev. Aquac. 2022, 15, 41–56. [Google Scholar] [CrossRef]
- Feng, J.; Cai, Z.; Zhang, X.; Chen, Y.; Chang, X.; Wang, X.; Qin, C.; Yan, X.; Ma, X.; Zhang, J.; et al. The effects of oral Rehmannia glutinosa polysaccharide administration on immune responses, antioxidant activity and resistance against Aeromonas hydrophila in the common carp, Cyprinus carpio L. Front. Immunol. 2020, 11, 904. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Pan, X.; Gong, Y.; Xia, A.; Wu, G.; Tang, J.; Han, X. Effects of Astragalus polysaccharides (APS) on the expression of immune response genes in head kidney, gill and spleen of the common carp, Cyprinus carpio L. Int. Immunopharmacol. 2008, 8, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ren, W.; Zhang, L.; Zhang, Y.; Liu, D.; Liu, Y. A Review of the pharmacological action of Astragalus polysaccharide. Front. Pharmacol. 2020, 11, 349. [Google Scholar] [CrossRef]
- Li, Y.; Ran, C.; Wei, K.; Xie, Y.; Xie, M.; Zhou, W.; Yang, Y.; Zhang, Z.; Lv, H.; Ma, X.; et al. The effect of Astragalus polysaccharide on growth, gut and liver health, and anti-viral immunity of zebrafish. Aquaculture 2021, 540, 736677. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Zhang, Y.; Li, J.; Lai, J. Astragalus polysaccharide: A review of its immunomodulatory effect. Arch. Pharm. Res. 2022, 45, 367–389. [Google Scholar] [CrossRef]
- Yu, W.; Yang, Y.; Zhou, Q.; Huang, X.; Huang, Z.; Li, T.; Wu, Q.; Zhou, C.; Ma, Z.; Lin, H. Effects of dietary Astragalus polysaccharides on growth, health and resistance to Vibrio harveyi of Lates calcarifer. Int. J. Biol. Macromol. 2022, 207, 850–858. [Google Scholar] [CrossRef]
- Lin, S.; Jiang, Y.; Chen, Y.; Luo, L.; Doolgindachbaporn, S.; Yuangsoi, B. Effects of Astragalus polysaccharides (APS) and chitooligosaccharides (COS) on growth, immune response and disease resistance of juvenile largemouth bass, Micropterus salmoides. Fish Shellfish Immunol. 2017, 70, 40–47. [Google Scholar] [CrossRef]
- Zekovic, D.B.; Kwiatkowski, S.; Vrvic, M.M.; Jakovljevic, D.; Moran, C.A. Natural and modified (1→3)-β-D-glucans in health promotion and disease alleviation. Crit. Rev. Biotechnol. 2005, 25, 205–230. [Google Scholar] [CrossRef]
- Bonaldo, A.; Thompson, K.; Manfrin, A.; Adams, A.; Murano, E.; Mordenti, A.L.; Gatta, P.P. The influence of dietary ß-glucans on the adaptive and innate immune responses of European sea bass(Dicentrarchus labrax)vaccinated against vibriosis. Ital. J. Anim. Sci. 2016, 6, 151–164. [Google Scholar] [CrossRef]
- Bagni, M.; Romano, N.; Finoia, M.G.; Abelli, L.; Scapigliati, G.; Tiscar, P.G.; Sarti, M.; Marino, G. Short- and long-term effects of a dietary yeast β-glucan (Macrogard) and alginic acid (Ergosan) preparation on immune response in sea bass (Dicentrarchus labrax). Fish Shellfish Immunol. 2005, 18, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Gopalakannan, A.; Arul, V. Enhancement of the innate immune system and disease-resistant activity in Cyprinus carpio by oral administration of β-glucan and whole cell yeast. Aquac. Res. 2009, 41, 884–892. [Google Scholar] [CrossRef]
- Chen, D.; Ainsworth, A.J. Glucan administration potentiates immune defence mechanisms of channel catfish, Ictalurus punctatus Rafinesque. J. Fish Dis. 1992, 15, 295–304. [Google Scholar] [CrossRef]
- Sherif, A.H.; Mahfouz, M.E. Immune status of Oreochromis niloticus experimentally infected with Aeromonas hydrophila following feeding with 1, 3 β-glucan and levamisole immunostimulants. Aquaculture 2019, 509, 40–46. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, M.; Li, N.; Dong, Z.; Cai, L.; Wu, B.; Xie, J.; Liu, L.; Ren, L.; Shi, B. New insights into β-glucan-enhanced immunity in largemouth bass Micropterus salmoides by transcriptome and intestinal microbial composition. Front. Immunol. 2022, 13, 1086103. [Google Scholar] [CrossRef]
- Wu, R.; Chi, Y.; Yu, J.; Ni, C.; Yao, J. Enhanced immersion vaccination through hyperosmotic treatment in the largemouth bass (Micropterus salmoides). Aquaculture 2021, 535, 736371. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, X.; Zhang, Z.; Jin, Z.; Wang, G.; Ling, F. Effects of dietary Cetobacterium somerae on the intestinal health, immune parameters and resistance against Nocardia seriolae of largemouth bass, Micropterus salmoides. Fish Shellfish Immunol. 2023, 135, 108693. [Google Scholar] [CrossRef]
- Nazareth, S.C.; Rao, S.; Cheng, L.; Wang, P.; Chen, S. Nocardia seriolae cell wall lipids: An effective protective mechanism in resistance and virulence. J. Fish. Dis. 2023, 46, 405–416. [Google Scholar] [CrossRef]
- Wang, F.; Wang, X.; Liu, C.; Chang, O.; Feng, Y.; Jiang, L.; Li, K. Nocardia seriolae infection in cultured jade perch, Scortum barcoo. Aquac. Int. 2017, 25, 2201–2212. [Google Scholar] [CrossRef]
- Liao, P.; Tsai, M.; See, M.; Wang, P.; Chen, S. Isolation and characterization of Nocardia seriolae, a causative agent of systematic granuloma in cultured East Asian four finger threadfin, Eleutheronema rhadinum, and red snapper, Lutjanus erythropterus. Aquac. Res. 2020, 52, 763–770. [Google Scholar] [CrossRef]
- Huo, X.; Zhang, Q.; Chang, J.; Yang, G.; He, S.; Yang, C.; Liang, X.; Zhang, Y.; Su, J. Nanopeptide C-I20 as a novel feed additive effectively alleviates detrimental impacts of soybean meal on mandarin fish by improving the intestinal mucosal barrier. Front. Immunol. 2023, 14, 1197767. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, W.; Zhang, Y.; Zhou, Y.; Xu, J.; Gao, X.; Zhang, S.; Zhang, X. Impact of Aeromonas hydrophila and infectious spleen and kidney necrosis virus infections on susceptibility and host immune response in Chinese perch (Siniperca chuatsi). Fish Shellfish Immunol. 2020, 105, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Gajic, I.; Kabic, J.; Kekic, D.; Jovicevic, M.; Milenkovic, M.; Mitic Culafic, D.; Trudic, A.; Ranin, L.; Opavski, N. Antimicrobial Susceptibility Testing: A Comprehensive Review of Currently Used Methods. Antibiotics 2022, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Zhu, X.; Ruan, H.; Yang, J.; Wei, W.; Wu, Y.; Zhou, L.; Jiang, H.; Ji, M.; Chen, J. Curcumin-stabilized silver nanoparticles encapsulated in biocompatible electrospun nanofibrous scaffold for sustained eradication of drug-resistant bacteria. J. Hazard. Mater. 2023, 452, 131290. [Google Scholar] [CrossRef]
- Santos, L.; Ramos, F. Antimicrobial resistance in aquaculture: Current knowledge and alternatives to tackle the problem. Int. J. Antimicrob. Agents 2018, 52, 135–143. [Google Scholar] [CrossRef]
- de Oliveira, T.F.; de Queiroz, G.A.; Pimenta Leibowitz, M.; Leal, C.A.G. Therapeutic efficacy of enrofloxacin in treatment of Francisella orientalis infections in juvenile Nile tilapia (Oreochromis niloticus L.). J. Vet. Pharmacol. Ther. 2023, 46, 344–352. [Google Scholar] [CrossRef]
- Rostang, A.; Peroz, C.; Fournel, C.; Thorin, C.; Calvez, S. Evaluation of the efficacy of enrofloxacin in rainbow trout (Oncorhynchus mykiss) following experimental challenge with Yersinia ruckeri. Vet. Rec. 2021, 188, e200. [Google Scholar] [CrossRef]
- Vijayaram, S.; Sun, Y.; Zuorro, A.; Ghafarifarsani, H.; Van Doan, H.; Hoseinifar, S.H. Bioactive immunostimulants as health-promoting feed additives in aquaculture: A review. Fish Shellfish Immunol. 2022, 130, 294–308. [Google Scholar] [CrossRef]
- Meena, D.K.; Das, P.; Kumar, S.; Mandal, S.C.; Prusty, A.K.; Singh, S.K.; Akhtar, M.S.; Behera, B.K.; Kumar, K.; Pal, A.K.; et al. Beta-glucan: An ideal immunostimulant in aquaculture (a review). Fish. Physiol. Biochem. 2013, 39, 431–457. [Google Scholar] [CrossRef]
- Li, Y.; Dong, X.; Zhang, Y.; Xiao, T.; Zhao, Y.; Wang, H. Astragalus polysaccharide improves the growth, meat quality, antioxidant capacity and bacterial resistance of Furong crucian carp (Furong carpfemale♀ × red crucian carpmale♂). Int. J. Biol. Macromol. 2023, 244, 124999. [Google Scholar] [CrossRef]
- Wilson, W.; Lowman, D.; Antony, S.P.; Puthumana, J.; Bright Singh, I.S.; Philip, R. Immune gene expression profile of Penaeus monodon in response to marine yeast glucan application and white spot syndrome virus challenge. Fish Shellfish Immunol. 2015, 43, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Bu, X.; Xiao, S.; Lin, Z.; Wang, X.; Jia, Y.; Wang, X.; Qin, J.; Chen, L. Effect of single and combined immunostimulants on growth, anti-oxidation activity, non-specific immunity and resistance to Aeromonas hydrophila in Chinese mitten crab (Eriocheir sinensis). Fish Shellfish Immunol. 2019, 93, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Pan, L.; Pan, S.; Song, M. Effects of dietary herbal formulae combined by Astragalus polysaccharides, chlorogenic acid and allicin in different combinations and proportions on growth performance, non-specific immunity, antioxidant status, vibriosis resistance and damage indexes of Litopenaeus vannamei. Aquac. Res. 2018, 49, 701–716. [Google Scholar] [CrossRef]
- Huo, X.; Wang, Z.; Xiao, X.; Yang, C.; Su, J. Nanopeptide CMCS-20H loaded by carboxymethyl chitosan remarkably enhances protective efficacy against bacterial infection in fish. Int. J. Biol. Macromol. 2022, 201, 226–241. [Google Scholar] [CrossRef]
- Yang, B.; Lei, Z.; Zhao, Y.; Ahmed, S.; Wang, C.; Zhang, S.; Fu, S.; Cao, J.; Qiu, Y. Combination susceptibility testing of common antimicrobials in vitro and the effects of sub-MIC of antimicrobials on Staphylococcus aureus biofilm formation. Front. Microbiol. 2017, 8, 2125. [Google Scholar] [CrossRef]
- Shan, Q.; Wang, J.; Wang, J.; Ma, L.; Yang, F.; Yin, Y.; Huang, R.; Liu, S.; Li, L.; Zheng, G. Pharmacokinetic/pharmacodynamic relationship of enrofloxacin against Aeromonas hydrophila in crucian carp (Carassius auratus gibelio). J. Vet. Pharmacol. Ther. 2018, 41, 887–893. [Google Scholar] [CrossRef]
- Chupani, L.; Erasmus, B.; Soldanová, M.; Zusková, E. Dietary immunostimulants reduced infectivity of Diplostomum spp. eye fluke in common carp, Cyprinus carpio L. Fish Shellfish Immunol. 2021, 119, 575–577. [Google Scholar] [CrossRef]
- Petit, J.; Wiegertjes, G.F. Long-lived effects of administering β-glucans: Indications for trained immunity in fish. Dev. Comp. Immunol. 2016, 64, 93–102. [Google Scholar] [CrossRef]
- Huang, X.; Liu, S.; Chen, X.; Zhang, H.; Yao, J.; Geng, Y.; Ou, Y.; Chen, D.; Yin, L.; Li, L.; et al. Comparative pathological description of nocardiosis in largemouth bass (Micropterus salmoides) and other Perciformes. Aquaculture 2021, 534, 736193. [Google Scholar] [CrossRef]
- Rajme-Manzur, D.; Gollas-Galvan, T.; Vargas-Albores, F.; Martinez-Porchas, M.; Hernandez-Onate, M.A.; Hernandez-Lopez, J. Granulomatous bacterial diseases in fish: An overview of the host’s immune response. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2021, 261, 111058. [Google Scholar] [CrossRef]
- Nawaz, M.; Gao, T.; Huang, K.; Gouife, M.; Chen, S.; Zhu, S.; Ma, R.; Jin, S.; Jiang, J.; Xie, J. Pathogenicity, diagnosis, prevention strategies and immune response of bacterium Nocardia seriolae: A critical review. Aquac. Res. 2022, 53, 4901–4918. [Google Scholar] [CrossRef]
- Ragland, S.A.; Criss, A.K. From bacterial killing to immune modulation: Recent insights into the functions of lysozyme. PLoS Pathog. 2017, 13, e1006512. [Google Scholar] [CrossRef]
- Hamed, M.; Soliman, H.A.M.; Osman, A.G.M.; Sayed, A.E.H. Antioxidants and molecular damage in Nile Tilapia (Oreochromis niloticus) after exposure to microplastics. Environ. Sci. Pollut. Res. Int. 2020, 27, 14581–14588. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Zhu, W.; Huo, X.; Qiao, M.; Yang, C.; Zhang, Y.; Su, J. Oral Lactobacillus casei expressing VP56310–500 and adjuvant flagellin C delivered by alginate-chitosan microcapsules remarkably enhances the immune protection against GCRV infection in grass carp. Aquaculture 2023, 567, 739301. [Google Scholar] [CrossRef]
- Tian, J.; Yang, Y.; Du, X.; Xu, W.; Zhu, B.; Huang, Y.; Ye, Y.; Zhao, Y.; Li, Y. Effects of dietary soluble β-1,3-glucan on the growth performance, antioxidant status, and immune response of the river prawn (Macrobrachium nipponense). Fish Shellfish Immunol. 2023, 138, 108848. [Google Scholar] [CrossRef]
- Bich Hang, B.T.; Phuong, N.T.; Kestemont, P. Can immunostimulants efficiently replace antibiotic in striped catfish (Pangasianodon hypophthalmus) against bacterial infection by Edwardsiella ictaluri? Fish Shellfish Immunol. 2014, 40, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Kong, L.; Zhao, F.; Tan, A.; Deng, Y.; Jiang, L. Two types of TNF-α and their receptors in snakehead (Channa argus): Functions in antibacterial innate immunity. Fish Shellfish Immunol. 2020, 104, 470–477. [Google Scholar] [CrossRef]
- Castro, R.; Jouneau, L.; Tacchi, L.; Macqueen, D.J.; Alzaid, A.; Secombes, C.J.; Martin, S.A.; Boudinot, P. Disparate developmental patterns of immune responses to bacterial and viral infections in fish. Sci. Rep. 2015, 5, 15458. [Google Scholar] [CrossRef]
- Su, J. The discovery of type IV interferon system revolutionizes interferon family and opens up a new frontier in jawed vertebrate immune defense. Sci. China Life Sci. 2022, 65, 2335–2337. [Google Scholar] [CrossRef]
- Ou, D.; Chen, B.; Bai, R.; Song, P.; Lin, H. Contamination of sulfonamide antibiotics and sulfamethazine-resistant bacteria in the downstream and estuarine areas of Jiulong River in Southeast China. Environ. Sci. Pollut. Res. Int. 2015, 22, 12104–12113. [Google Scholar] [CrossRef]

| Antibiotic | Disk Concentration | Zone of Inhibition (mm) | Result |
|---|---|---|---|
| Penicillin | 10 units | 0 | R |
| Erythromycin | 15 μg | 14 | I |
| Florfenicol | 30 μg | 19 | S |
| Enrofloxacin | 10 μg | 28 | S |
| Sulfadiazine | 300 μg | 0 | R |
| Gene Name | Primer Name | Primer Sequence (5′-3′) | Size (nt) | GenBank Number and Primer Application |
|---|---|---|---|---|
| ITS1 | N-1-a | TGAGTAGTGGCGAGCGAAAG | 260 | AB060282 |
| N-1-b | GTCCCGACAGATTCACAGCA | Semi-qPCR | ||
| 16S | N-2-a | TGCTACAATGGCCGGTACAGAG | 142 | AB060282 |
| N-2-b | TTCACGAGGTCGAGTTGCAGAC | qRT-PCR | ||
| IL-1β | IL-001 | CGTACATCCGTGCCAACAGT | 135 | XM038733429 |
| IL-002 | ATGCTCTTTAACTCCTCCT | qRT-PCR | ||
| TNF-α | TNF-009 | CTAGTGAAGAACCAGATTGT | 105 | XM038695351.1 |
| TNF-010 | AGGAGACTCTGAACGATG | qRT-PCR | ||
| IFN-γ | IFN-003 | TCCCTCTGAAGATGAACAAA | 146 | XM046040264.1 |
| IFN-004 | AACGCCACCCATAAACA | qRT-PCR | ||
| IgM | IgM-F | GTTACCTTCTCCTGCTTG | 137 | MN871984.1 |
| IgM-R | GTTCCGTTCTCATAGTTTC | qRT-PCR | ||
| β-actin | β-actin-a | AGCCGAGCCGCAGGAAA | 165 | XM038708956.1 |
| β-actin-b | TCGCACCACGCACCAAC | Semi-qPCR/qRT-PCR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, F.; Huo, X.; Wang, P.; Liu, Q.; Yang, C.; Su, J. The Combination of β-Glucan and Astragalus Polysaccharide Effectively Resists Nocardia seriolae Infection in Largemouth Bass (Micropterus salmoides). Microorganisms 2023, 11, 2529. https://doi.org/10.3390/microorganisms11102529
Zhao F, Huo X, Wang P, Liu Q, Yang C, Su J. The Combination of β-Glucan and Astragalus Polysaccharide Effectively Resists Nocardia seriolae Infection in Largemouth Bass (Micropterus salmoides). Microorganisms. 2023; 11(10):2529. https://doi.org/10.3390/microorganisms11102529
Chicago/Turabian StyleZhao, Fengxia, Xingchen Huo, Pengxu Wang, Qian Liu, Chunrong Yang, and Jianguo Su. 2023. "The Combination of β-Glucan and Astragalus Polysaccharide Effectively Resists Nocardia seriolae Infection in Largemouth Bass (Micropterus salmoides)" Microorganisms 11, no. 10: 2529. https://doi.org/10.3390/microorganisms11102529
APA StyleZhao, F., Huo, X., Wang, P., Liu, Q., Yang, C., & Su, J. (2023). The Combination of β-Glucan and Astragalus Polysaccharide Effectively Resists Nocardia seriolae Infection in Largemouth Bass (Micropterus salmoides). Microorganisms, 11(10), 2529. https://doi.org/10.3390/microorganisms11102529






