Effects of Varying Levels of Wheat Bran Dietary Fiber on Growth Performance, Fiber Digestibility and Gut Microbiota in Erhualian and Large White Pigs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Diets
2.2. Animals Slaughter and Samples Collecting
2.3. Experimental Chemical Analysis
2.4. 16S rRNA Gene Sequence and Bioinformatics Analysis
2.5. Calculations and Data Analysis
3. Results
3.1. Effect of Dietary Fiber Level in the Diet and Breed on the Growth Performance
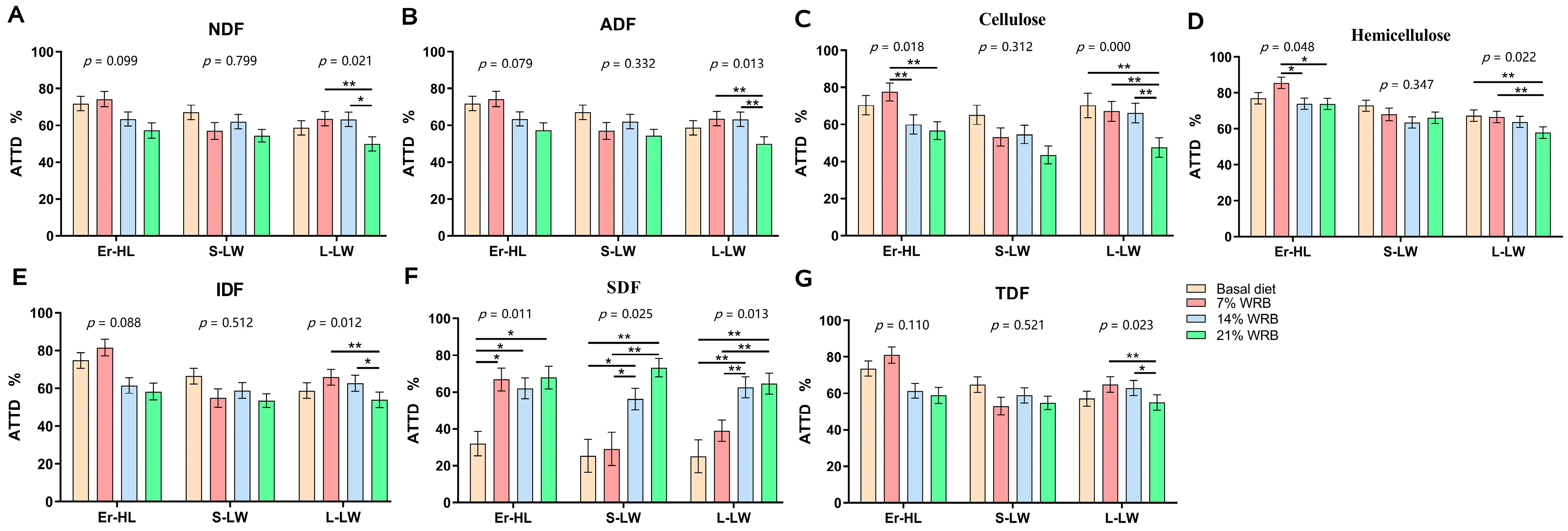
3.2. Effect of Dietary Fiber Level in the Diet and Breed on the Total Tract Apparent Digestibility
3.3. Effect of Dietary Fiber Level in the Diet and Breed on Gut Microbial Diversity and Community Structure
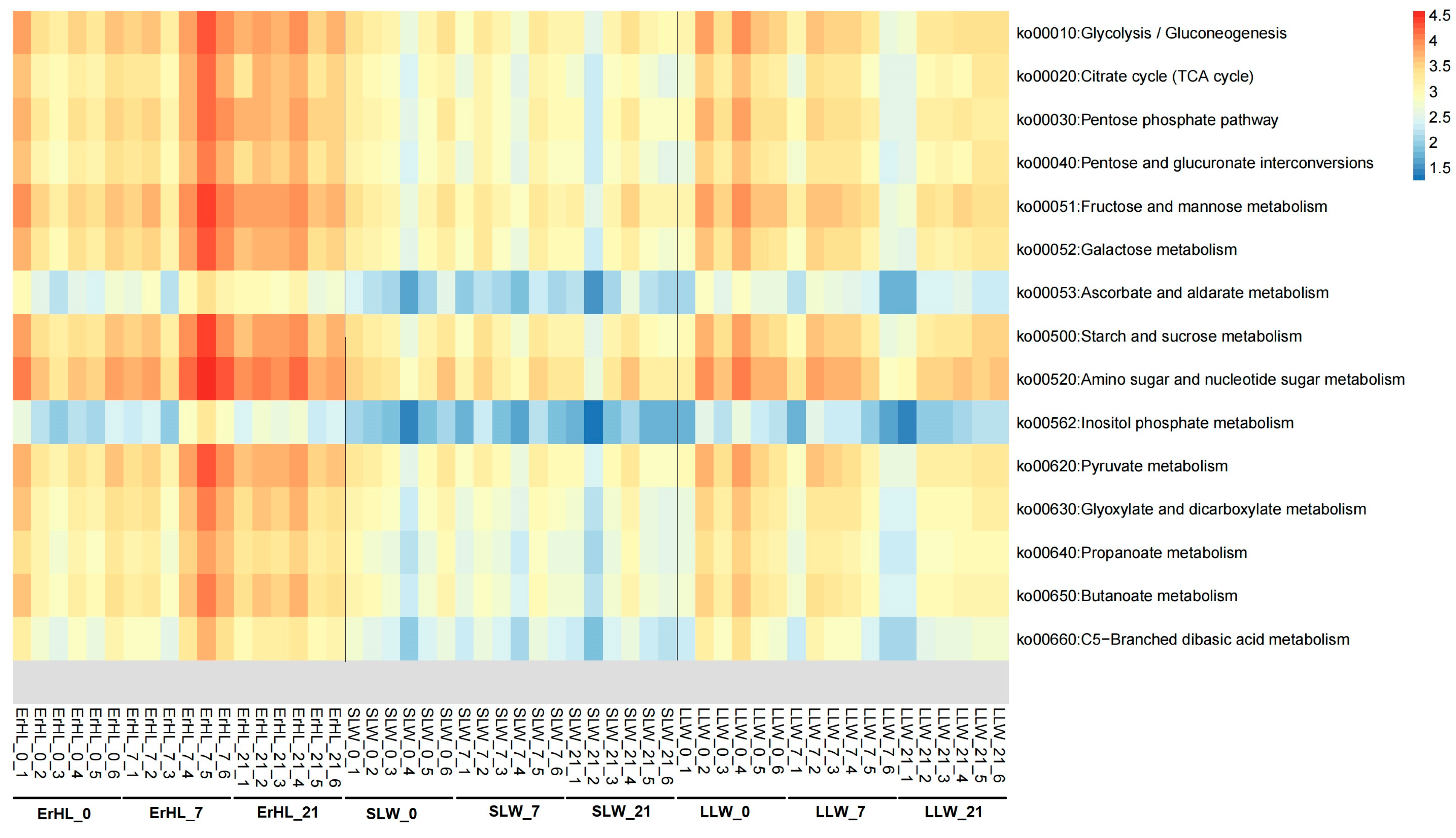
3.4. Effect of Dietary Fiber Level in the Diet and Breed on Phyla and Genera of Gut Microbiota
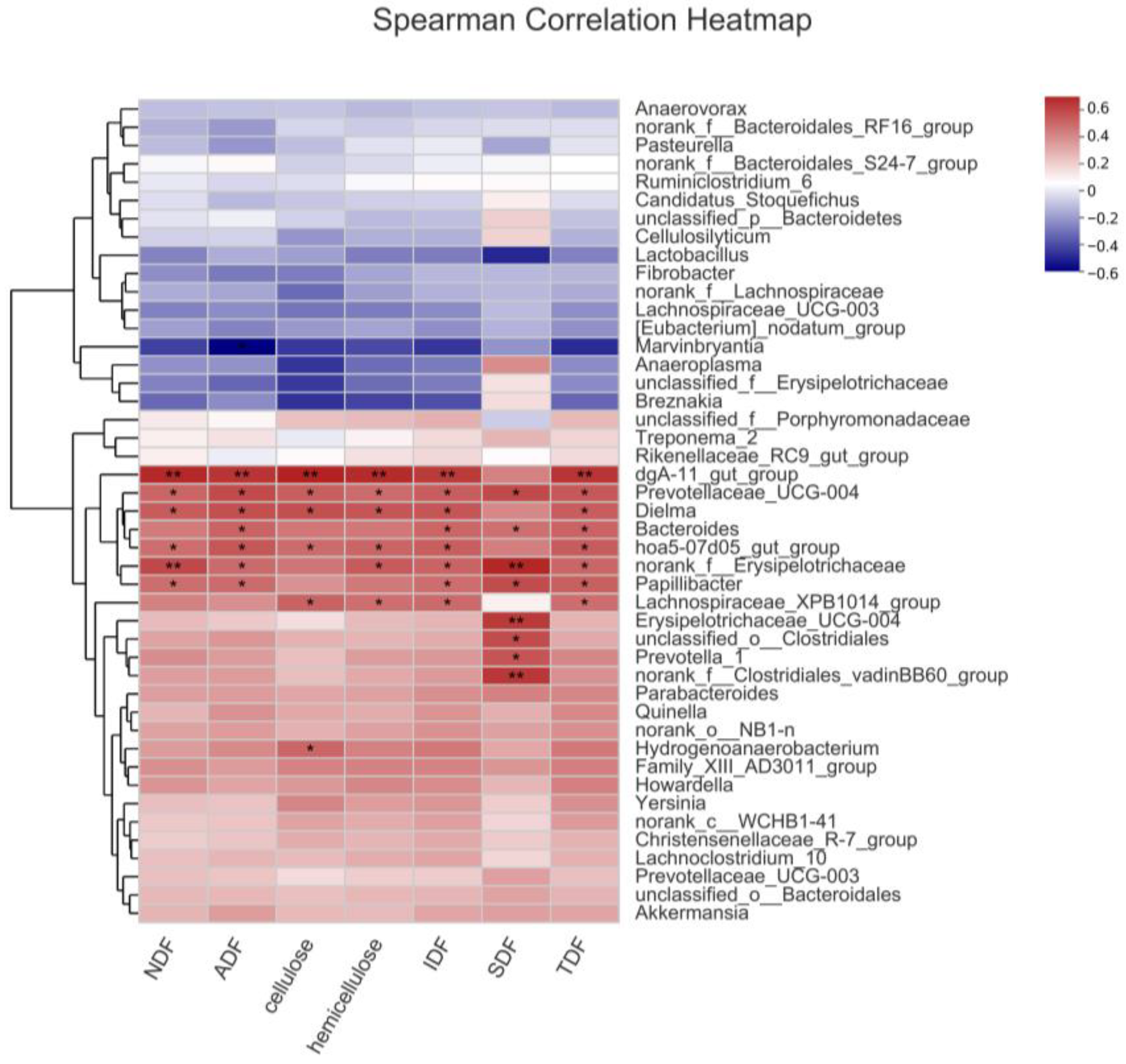
3.5. Selecting the Key Bacteria Related to the High Fiber Digestibility of Erhualian Pigs
3.6. Potential Core Members of Cecal Microbiome Related to Fiber Digestion and Metabolism in Pigs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, C.; Li, P.; Zhou, W.; Ma, X.; Wang, X.; Xu, Y.; Jiang, N.; Zhao, M.; Zhou, T.; Yin, Y. Genome data uncover conservation status, historical relatedness and candidate genes under selection in Chinese indigenous pigs in the Taihu Lake region. Front. Genet. 2020, 11, 591. [Google Scholar] [CrossRef] [PubMed]
- Hörtenhuber, S.J.; Lindenthal, T.; Zollitsch, W. Reduction of greenhouse gas emissions from feed supply chains by utilizing regionally produced protein sources: The case of Austrian dairy production. J. Sci. Food Agric. 2011, 91, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Meul, M.; Ginneberge, C.; Van Middelaar, C.E.; de Boer, I.J.; Fremaut, D.; Haesaert, G. Carbon footprint of five pig diets using three land use change accounting methods. Livest. Sci. 2012, 149, 215–223. [Google Scholar] [CrossRef]
- Lee, G.; Hedemann, M.; Jørgensen, H.; Knudsen, K.B. Influence of dietary fibre on nutrient digestibility and energy utilisation in growing pigs fed diets varying in soluble and insoluble fibres from co-products. Animal 2022, 16, 100511. [Google Scholar] [CrossRef]
- Urriola, P.E.; Stein, H.H. Comparative digestibility of energy and nutrients in fibrous feed ingredients fed to Meishan and Yorkshire pigs. J. Anim. Sci. 2012, 90, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Pu, G.; Hou, L.; Du, T.; Zhou, W.; Liu, C.; Niu, P.; Wu, C.; Bao, W.; Huang, R.; Li, P. Increased proportion of fiber-degrading microbes and enhanced cecum development jointly promote host to digest appropriate high-fiber diets. Msystems 2023, 8, e00937-22. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yin, J.; Tan, B.; Chen, J.; Zhang, H.; Li, Z.; Ma, X. Physiological function and application of dietary fiber in pig nutrition: A review. Anim. Nutr. 2021, 7, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Montagne, L.; Pluske, J.R.; Hampson, D.J. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim. Feed Sci. Technol. 2003, 108, 95–117. [Google Scholar] [CrossRef]
- Froidurot, A.; Julliand, V. Cellulolytic bacteria in the large intestine of mammals. Gut Microbes 2022, 14, 2031694. [Google Scholar] [CrossRef]
- Navarro, D.M.; Abelilla, J.J.; Stein, H.H. Structures and characteristics of carbohydrates in diets fed to pigs: A review. J. Anim. Sci. Biotechnol. 2019, 10, 39. [Google Scholar] [CrossRef]
- Lin, B.; Gong, J.; Wang, Q.; Cui, S.; Yu, H.; Huang, B. In-Vitro assessment of the effects of dietary fibers on microbial fermentation and communities from large intestinal digesta of pigs. Food Hydrocoll. 2011, 25, 180–188. [Google Scholar] [CrossRef]
- Tao, S.; Bai, Y.; Zhou, X.; Zhao, J.; Yang, H.; Zhang, S.; Wang, J. In Vitro fermentation characteristics for different ratios of soluble to insoluble dietary fiber by fresh fecal microbiota from growing pigs. ACS Omega 2019, 4, 15158–15167. [Google Scholar] [CrossRef] [PubMed]
- Dixit, Y.; Kanojiya, K.; Bhingardeve, N.; Ahire, J.J.; Saroj, D. In Vitro Human Gastrointestinal Tract Simulation Systems: A Panoramic Review. Probiotics Antimicrob. Proteins 2023, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, Y.; Liu, S.; Huang, J.; He, M. The Dynamic Distribution of Porcine Microbiota across Different Ages and Gastrointestinal Tract Segments. PLoS ONE 2015, 10, e0117441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shao, M.; Huang, H.; Wang, S.; Ma, L.; Wang, H.; Hu, L.; Wei, K.; Zhu, R. The dynamic distribution of small-tail han sheep microbiota across different intestinal segments. Front. Microbiol. 2018, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Pajarillo, E.A.B.; Chae, J.P.; Balolong, M.P.; Kim, H.B.; Seo, K.-S.; Kang, D.-K. Pyrosequencing-based analysis of fecal microbial communities in three purebred pig lines. J. Microbiol. 2014, 52, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, M.; Tiezzi, F.; Howard, J.; Huang, Y.J.; Gray, K.A.; Schillebeeckx, C.; McNulty, N.P.; Maltecca, C. Gut microbiome composition differences among breeds impact feed efficiency in swine. Microbiome 2020, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Li, P.; Hao, S.; Zhang, Y.; Kim, S.W.; Li, H.; Ma, X.; Gao, S.; He, L.; Wu, W.J. Dynamic Distribution of the Gut Microbiota and the Relationship with Apparent Crude Fiber Digestibility and Growth Stages in Pigs. Entific Rep. 2015, 5, 9938. [Google Scholar] [CrossRef]
- Barea, R.; Nieto, R.; Aguilera, J.F. Iberian vs. Landrace × large white pigs: Nutrient utilization, animal performance and relative organ weights at two stages of growth. Eaap Sci. 2010, 127, 391–392. [Google Scholar]
- Zhao, Y.; Liu, C.; Niu, J.; Cui, Z.; Zhao, X.; Li, W.; Zhang, Y.; Yang, Y.; Gao, P.; Guo, X. Impacts of dietary fiber level on growth performance, apparent digestibility, intestinal development, and colonic microbiota and metabolome of pigs. J. Anim. Sci. 2023, 101, skad174. [Google Scholar] [CrossRef]
- Villanueva, J.J. Comparative Digestibility of Nutrients, Energy and Dietary Fiber in Feed Ingredients Fed to Philippine Black Tiaong Native (Sus scrofa domesticus) and Hybrid Pigs. CLSU Int. J. Sci. Technol. 2022, 6, 1–15. [Google Scholar] [CrossRef]
- Yen, J.T.; Varel, V.; Nienaber, J. Metabolic and microbial responses in western crossbred and Meishan growing pigs fed a high-fiber diet. J. Anim. Sci. 2004, 82, 1740–1755. [Google Scholar] [CrossRef] [PubMed]
- Von Heimendahl, E.; Breves, G.; Abel, H. Fiber-related digestive processes in three different breeds of pigs. J. Anim. Sci. 2010, 88, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, P.H.; Zhu, M.X.; He, L.C.; Sui, S.P.; Gao, S.; Su, G.S.; Ding, N.S.; Huang, Y.; Lu, Z.Q. Genome-wide association analysis reveals genomic regions on Chromosome 13 affecting litter size and candidate genes for uterine horn length in Erhualian pigs. Anim. Int. J. Anim. Biosci. 2018, 12, 2453–2461. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, R.H.; Li, Q.X.; Gu, Y.Q. Research progress and development and utilization experience of germplasm resources of Erhualian pig. Chin. Pig Ind. 2013, S1. [Google Scholar]
- Han, P.; Li, P.; Zhou, W.; Fan, L.; Wang, B.; Liu, H.; Gao, C.; Du, T.; Pu, G.; Wu, C. Effects of various levels of dietary fiber on carcass traits, meat quality and myosin heavy chain I, IIa, IIx and IIb expression in muscles in Erhualian and Large White pigs. Meat Sci. 2020, 169, 108160. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, P.; Zhang, Q.; He, L.; Su, G.; Huang, Y.; Lu, Z.; Hu, W.; Ding, H.; Huang, R. Transcriptome analysis of the endometrium from Chinese Erhualian sows that differ in calcium ion concentration and litter size. Anim. Genet. 2019, 50, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Solanes, F.X.; Stern, S. Estimated mature weights and growth curves for large white sows. Acta Agric. Scand. Sect. A-Anim. Sci. 2001, 51, 142–147. [Google Scholar] [CrossRef]
- China NY/T 65–2004; Feeding Standard of Swine, China. China Agricultural Publisher: Beijing, China, 2004.
- Zhang, Y.; Yu, K.; Chen, H.; Su, Y.; Zhu, W. Caecal infusion of the short-chain fatty acid propionate affects the microbiota and expression of inflammatory cytokines in the colon in a fistula pig model. Microb. Biotechnol. 2018, 11, 859–868. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a Dual-Index Sequencing Strategy and Curation Pipeline for Analyzing Amplicon Sequence Data on the MiSeq Illumina Sequencing Platform. Appl. Environ. Microbiol 2013, 79, 5112–5120. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.R.; Wang, Q.; Cardenas, E.; Fish, J.; Chai, B.; Farris, R.J.; Kulam-Syed-Mohideen, A.S.; McGarrell, D.M.; Marsh, T.; Garrity, G.M.; et al. The Ribosomal Database Project: Improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2008, 37 (Suppl. 1), D141–D145. [Google Scholar] [CrossRef] [PubMed]
- Institute, S. SAS/ETS User’s Guide: Version 9; SAS Institute: Cary, NC, USA, 2004. [Google Scholar]
- Kirkpatrick, L.A.; Feeney, B.C. A Simple Guide to IBM SPSS: For Version 22.0; Wadsworth Publishing: Belmont, CA, USA, 2014. [Google Scholar]
- Wang, X.; Tsai, T.; Deng, F.; Wei, X.; Chai, J.; Knapp, J.; Apple, J.; Maxwell, C.V.; Lee, J.A.; Li, Y. Longitudinal investigation of the swine gut microbiome from birth to market reveals stage and growth performance associated bacteria. Microbiome 2019, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.; Hamady, M.; Knight, R. UniFrac—An online tool for comparing microbial community diversity in a phylogenetic context. BMC Bioinform. 2006, 7, 371. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Ndindana, W.; Dzama, K.; Ndiweni, P.N.B.; Maswaure, S.M.; Chimonyo, M. Digestibility of high fibre diets and performance of growing Zimbabwean indigenous Mukota pigs and exotic Large White pigs fed maize based diets with graded levels of maize cobs. Anim. Feed Sci. Technol. 2002, 97, 199–208. [Google Scholar] [CrossRef]
- Lei, L.; Wang, Z.; Li, J.; Yang, H.; Yin, Y.; Tan, B.; Chen, J. Comparative microbial profiles of colonic digesta between ningxiang pig and large white pig. Animals 2021, 11, 1862. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Luo, Y.; Kong, X.; Yu, B.; Zheng, P.; Huang, Z.; Mao, X.; Yu, J.; Luo, J.; Yan, H. Effects of dietary fiber on growth performance, nutrient digestibility and intestinal health in different pig breeds. Animals 2022, 12, 3298. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Cheng, Y.; Azad, M.A.K.; Dong, H.; He, J.; Huang, P.; Kong, X. Dietary fiber alters immunity and intestinal barrier function of different breeds of growing pigs. Front. Immunol. 2023, 14, 1104837. [Google Scholar] [CrossRef] [PubMed]
- White, B.; Lan, Y.; McKeith, F.; Novakofski, J.; Wheeler, M.; McLaren, D. Growth and body composition of Meishan and Yorkshire barrows and gilts. J. Anim. Sci. 1995, 73, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Fevrier, C.; Bourdon, D.; Aumaitre, A. Effects of level of dietary fibre from wheat bran on digestibility of nutrients, digestive enzymes and performance in the european Large White and Chinese Mei Shan pig. J. Anim. Physiol. Anim. Nutr. 1992, 68, 60–72. [Google Scholar] [CrossRef]
- Ngoc, T.T.B.; Len, N.T.; Lindberg, J.E. Impact of fibre intake and fibre source on digestibility, gut development, retention time and growth performance of indigenous and exotic pigs. Anim. Int. J. Anim. Biosci. 2013, 7, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Kemp, B.; Den Hartog, L.; Klok, J.; Zandstra, T. The digestibility of nutrients, energy and nitrogen in the Meishan and Dutch Landrace pig. J. Anim. Physiol. Anim. Nutr. 1991, 65, 263–266. [Google Scholar] [CrossRef]
- Chen, H.; Mao, X.B.; Che, L.Q.; Yu, B.; He, J.; Yu, J.; Han, G.Q.; Huang, Z.Q.; Zheng, P.; Chen, D.W. Impact of fiber types on gut microbiota, gut environment and gut function in fattening pigs. Anim. Feed Ence Technol. 2014, 195, 101–111. [Google Scholar] [CrossRef]
- Kim, H.B.; Borewicz, K.; White, B.A.; Singer, R.S.; Sreevatsan, S.; Tu, Z.J.; Isaacson, R.E. Longitudinal investigation of the age-related bacterial diversity in the feces of commercial pigs. Vet. Microbiol. 2011, 153, 124–133. [Google Scholar] [CrossRef]
- Lamendella, R.; Santo Domingo, J.W.; Ghosh, S.; Martinson, J.; Oerther, D.B. Comparative fecal metagenomics unveils unique functional capacity of the swine gut. BMC Microbiol. 2011, 11, 103. [Google Scholar] [CrossRef]
- Heinritz, S.N.; Weiss, E.; Eklund, M.; Aumiller, T.; Louis, S.; Rings, A.; Messner, S.; Camarinha-Silva, A.; Seifert, J.; Bischoff, S.C. Intestinal microbiota and microbial metabolites are changed in a pig model fed a high-fat/low-fiber or a low-fat/high-fiber diet. PLoS ONE 2016, 11, e0154329. [Google Scholar] [CrossRef]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the Genetic Basis of Fibrolytic Specialization by Lachnospiraceae and Ruminococcaceae in Diverse Gut Communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Reich, L.J.; Kung, L., Jr. Effects of combining Lactobacillus buchneri 40788 with various lactic acid bacteria on the fermentation and aerobic stability of corn silage. Anim. Feed Sci. Technol. 2010, 159, 105–109. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Shinkai, T.; Koike, S. Ecological and physiological characterization shows that Fibrobacter succinogenes is important in rumen fiber digestion. Folia Microbiol. 2008, 53, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Li, J.; Hu, F.Z.; Zhang, K.; Luo, Y.; Janto, B.; Boissy, R.; Ehrlich, G.; Dong, X. Cellulosilyticum ruminicola, a newly described rumen bacterium that possesses redundant fibrolytic-protein-encoding genes and degrades lignocellulose with multiple carbohydrate-borne fibrolytic enzymes. Appl. Environ. Microbiol. 2010, 76, 3818–3824. [Google Scholar] [CrossRef] [PubMed]
- Ravachol, J.; Borne, R.; Meynial-Salles, I.; Soucaille, P.; Tardif, C.; Fierobe, H.-P. Combining free and aggregated cellulolytic systems in the cellulosome-producing bacterium Ruminiclostridium cellulolyticum. Biotechnol. Biofuels 2015, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Liu, X.; Liu, S.; Li, Y.; Zhao, H.; Zhang, Y. Growth, rumen fermentation and plasma metabolites of Holstein male calves fed fermented corn gluten meal during the postweaning stage. Anim. Feed Sci. Technol. 2019, 249, 1–9. [Google Scholar] [CrossRef]
- Ungerfeld, E.M.; Leigh, M.B.; Forster, R.J.; Barboza, P.S. Influence of season and diet on fiber digestion and bacterial community structure in the rumen of muskoxen (Ovibos moschatus). Microorganisms 2018, 6, 89. [Google Scholar] [CrossRef]
- Pu, G.; Li, P.; Du, T.; Niu, Q.; Huang, R. Adding Appropriate Fiber in Diet Increases Diversity and Metabolic Capacity of Distal Gut Microbiota without Altering Fiber Digestibility and Growth Rate of Finishing Pig. Front. Microbiol. 2020, 11, 533. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lian, H.; Zheng, A.; Zhang, J.; Dai, P.; Niu, Y.; Gao, T.; Li, M.; Zhang, L.; Fu, T. Effects of Different Roughages on Growth Performance, Nutrient Digestibility, Ruminal Fermentation, and Microbial Community in Weaned Holstein Calves. Front. Vet. Sci. 2022, 9, 864320. [Google Scholar] [CrossRef]
- Teixeira, C.; Prykhodko, O.; Alminger, M.; Fåk Hållenius, F.; Nyman, M. Barley products of different fiber composition selectively change microbiota composition in rats. Mol. Nutr. Food Res. 2018, 62, 1701023. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Zhang, Z.; Zhao, J.; Ma, Q.; Liu, H.; Nie, C.; Ma, Z.; An, W.; Li, J. Dietary whole Goji berry (Lycium barbarum) intake improves colonic barrier function by altering gut microbiota composition in mice. Int. J. Food Sci. Technol. 2021, 56, 103–114. [Google Scholar] [CrossRef]
- Varel, V. Activity of fiber-degrading microorganisms in the pig large intestine. J. Anim. Sci. 1987, 65, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.; Yang, H.; Ren, M.; Wang, Y.; Xiao, M.; Tang, Q.; Zhu, M.; Xiao, Y. Identification of Enterotype and Its Effects on Intestinal Butyrate Production in Pigs. Animals 2021, 11, 730. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Peng, X.; Burrough, E.R.; Sahin, O.; Gould, S.A.; Gabler, N.K.; Loving, C.L.; Dorman, K.S.; Patience, J.F. Dietary soluble and insoluble fiber with or without enzymes altered the intestinal microbiota in weaned pigs challenged with enterotoxigenic E. coli F18. Front. Microbiol. 2020, 11, 1110. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Mikkelsen, D.; Yao, H.; Williams, B.A.; Flanagan, B.M.; Gidley, M.J. Wheat cell walls and constituent polysaccharides induce similar microbiota profiles upon in vitro fermentation despite different short chain fatty acid end-product levels. Food Funct. 2021, 12, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Nan, X.; Chu, K.; Tong, J.; Yang, L.; Zheng, S.; Zhao, G.; Jiang, L.; Xiong, B. Shifts of hydrogen metabolism from methanogenesis to propionate production in response to replacement of forage fiber with non-forage fiber sources in diets in vitro. Front. Microbiol. 2018, 9, 2764. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yan, Q.; Ren, A.; Zheng, M.; Zhang, P.; Tan, Z.; Zhou, C. Novel Linkages between Bacterial Composition of Hindgut and Host Metabolic Responses to SARA Induced by High-Paddy Diet in Young Goats. Front. Vet. Sci. 2021, 8, 791482. [Google Scholar] [CrossRef]
- Wu, X.; Elekwachi, C.O.; Bai, S.; Luo, Y.; Zhang, K.; Forster, R.J. Characterizing the Alteration in Rumen Microbiome and Carbohydrate-Active Enzymes Profile with Forage of Muskoxen Rumen through Comparative Metatranscriptomics. Microorganisms 2021, 10, 71. [Google Scholar] [CrossRef]
- Benítez-Páez, A.; Kjølbæk, L.; Gómez del Pulgar, E.M.; Brahe, L.K.; Astrup, A.; Matysik, S.; Schött, H.-F.; Krautbauer, S.; Liebisch, G.; Boberska, J. A multi-omics approach to unraveling the microbiome-mediated effects of arabinoxylan oligosaccharides in overweight humans. Msystems 2019, 4, e00209–e00219. [Google Scholar] [CrossRef]
- Jiang, G.; Liu, Y.; Oso, A.; Li, F.; Kong, X.; Geng, M.; Yang, H.; Yin, Y. The differences of bacteria and bacteria metabolites in the colon between fatty and lean pigs. J. Anim. Sci. 2016, 94, 349–353. [Google Scholar] [CrossRef]




| Item 1 | Breeds 2 | Diets 3 | RMSE | p-Values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Er-HL | L-LW | S-LW | Basal | 7% WRB | 14% WRB | 21% WRB | Breeds | Diets | Breeds × Diets | ||
| Initial body weight (kg) | 44.76 B | 68.29 A | 46.76 B | / | / | / | / | 1.38 | 0.000 | / | / |
| Final body weight (kg) | 57.57 C | 91.70 A | 65.80 B | 75.60 A | 71.73 AB | 67.37 B | 72.05 AB | 3.70 | 0.000 | 0.053 | 0.146 |
| ADFI (kg/d) | 1.67 | 1.92 | 1.93 | 2.07 a | 1.79 ab | 1.71 b | 1.78 ab | 0.16 | 0.056 | 0.015 | 0.012 |
| ADG (g/d) | 457.56 C | 835.81 A | 680.01 B | 776.52 a | 603.97 b | 615.37 b | 635.32 ab | 70.42 | 0.000 | 0.008 | 0.003 |
| F/G | 3.27 A | 2.66 B | 2.69 B | 2.81 | 3.01 | 2.68 | 2.99 | 0.22 | 0.001 | 0.236 | 0.195 |
| Item 1 | Breeds 2 | Diets 3 | RMSE | p-Values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Er-HL | L-LW | S-LW | Basal | 7% WRB | 14% WRB | 21% WRB | Breeds | Diets | Breeds × Diets | ||
| NDF | 66.08 A | 52.53 B | 55.77 B | 60.40 AB | 63.31 A | 57.85 AB | 50.95 B | 6.90 | 0.001 | 0.013 | 0.428 |
| ADF | 66.40 Aa | 56.78 Bb | 59.73 ABb | 63.78 ABa | 64.12 Aa | 62.08 ABab | 53.90 Bb | 5.74 | 0.004 | 0.003 | 0.320 |
| Cellulose | 66.20 A | 62.83 AB | 54.13 B | 68.63 Aa | 66.00 Aa | 60.28 ABa | 49.29 Bb | 6.73 | 0.000 | 0.000 | 0.212 |
| Hemicellulose | 77.60 A | 63.90 B | 67.64 B | 72.39 A | 73.38 A | 67.11 B | 65.98 B | 4.83 | 0.000 | 0.010 | 0.365 |
| IDF | 68.55 A | 57.08 B | 57.61 B | 63.42 ab | 65.82 a | 59.70 ab | 55.38 b | 6.01 | 0.000 | 0.015 | 0.104 |
| SDF | 58.05 | 44.48 | 47.23 | 27.55 C | 44.09 BC | 58.81 AB | 69.23 A | 8.47 | 0.033 | 0.000 | 0.085 |
| TDF | 71.61 A | 56.00 B | 56.24 B | 61.16 | 65.75 | 59.87 | 58.35 | 5.98 | 0.000 | 0.169 | 0.06 |
| EE | 84.51 | 80.75 | 81.05 | 84.41 a | 83.61 ab | 81.33 ab | 79.06 b | 3.16 | 0.043 | 0.018 | 0.074 |
| CP | 85.64 Aa | 84.64 ABa | 80.66 Bb | 87.94 Aa | 82.84 ABb | 82.67 Bab | 81.14 Bab | 2.78 | 0.001 | 0.001 | 0.036 |
| Item 1 | Breeds 2 | Diets 3 | RMSE | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Er-HL | L-LW | S-LW | Basal | 7% WRB | 21% WRB | Breeds | Diets | Breeds × Diets | ||
| Chao | 886.93 Aa | 847.24 ABb | 725.22 Cc | 889.03 a | 813.33 b | 757.02 c | 44.33 | 0.002 | 0.009 | 0.448 |
| Ace | 890.4 Aa | 829.79 ABb | 734.58 Cc | 882.49 a | 815.47 b | 756.81 c | 42.12 | 0.002 | 0.020 | 0.303 |
| Shannon | 4.24 a | 4.36 a | 3.88 b | 4.28 | 4.10 | 4.09 | 0.17 | 0.010 | 0.363 | 0.058 |
| Simpson | 21.85 | 11.13 | 11.13 | 13.98 | 12.42 | 10.6 | 1.10 | 0.057 | 0.896 | 0.001 |
| Characteristics | Df | SumsOfSqs | MeanSqs | F_Model | R2 | Pr (>F) |
|---|---|---|---|---|---|---|
| Breed | 2 | 1.3 | 0.65 | 3.92 | 0.13 | 0.0001 |
| Diet | 2 | 0.33 | 0.17 | 1 | 0.03 | 0.4254 |
| Body weight | 1 | 0.1 | 0.1 | 0.6 | 0.01 | 0.8485 |
| Average daily feed intake | 1 | 0.2 | 0.2 | 1.2 | 0.02 | 0.2648 |
| Residual | 46 | 7.65 | 0.17 | 0.74 | ||
| Total | 52 | 10.35 | 1 |
| Item 1 | Breeds 2 | Diets 3 | RMSE | p-Values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Er-HL | L-LW | S-LW | Basal | 7% WRB | 14% WRB | 21% WRB | Breeds | Diets | Breeds × Diets | ||
| Acetate | 241.67 | 300.60 | 185.08 | 235.17 | 215.45 | 278.37 | 240.81 | 33.84 | 0.08 | 0.54 | 0.16 |
| Propionate | 99.39 | 101.93 | 81.22 | 84.76 | 82.86 | 120.08 | 89.03 | 13.22 | 0.42 | 0.10 | 0.21 |
| Butyrate | 40.13 | 38.61 | 39.66 | 32.00 Bb | 34.40 Bb | 56.10 Aa | 35.36 ABb | 5.63 | 0.98 | 0.00 | 0.27 |
| Valerate | 7.65 | 7.80 | 6.51 | 5.33 Bb | 7.45 ABb | 10.25 Aa | 6.26 ABab | 1.21 | 0.67 | 0.01 | 0.17 |
| Total SCFAs | 397.80 | 457.49 | 317.14 | 365.61 | 346.08 | 473.31 | 378.24 | 50.38 | 0.16 | 0.21 | 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, T.; Li, P.; Niu, Q.; Pu, G.; Wang, B.; Liu, G.; Li, P.; Niu, P.; Zhang, Z.; Wu, C.; et al. Effects of Varying Levels of Wheat Bran Dietary Fiber on Growth Performance, Fiber Digestibility and Gut Microbiota in Erhualian and Large White Pigs. Microorganisms 2023, 11, 2474. https://doi.org/10.3390/microorganisms11102474
Du T, Li P, Niu Q, Pu G, Wang B, Liu G, Li P, Niu P, Zhang Z, Wu C, et al. Effects of Varying Levels of Wheat Bran Dietary Fiber on Growth Performance, Fiber Digestibility and Gut Microbiota in Erhualian and Large White Pigs. Microorganisms. 2023; 11(10):2474. https://doi.org/10.3390/microorganisms11102474
Chicago/Turabian StyleDu, Taoran, Pinghua Li, Qing Niu, Guang Pu, Binbin Wang, Gensheng Liu, Pinghui Li, Peipei Niu, Zongping Zhang, Chengwu Wu, and et al. 2023. "Effects of Varying Levels of Wheat Bran Dietary Fiber on Growth Performance, Fiber Digestibility and Gut Microbiota in Erhualian and Large White Pigs" Microorganisms 11, no. 10: 2474. https://doi.org/10.3390/microorganisms11102474
APA StyleDu, T., Li, P., Niu, Q., Pu, G., Wang, B., Liu, G., Li, P., Niu, P., Zhang, Z., Wu, C., Hou, L., Hedemann, M. S., Zhao, Q., & Huang, R. (2023). Effects of Varying Levels of Wheat Bran Dietary Fiber on Growth Performance, Fiber Digestibility and Gut Microbiota in Erhualian and Large White Pigs. Microorganisms, 11(10), 2474. https://doi.org/10.3390/microorganisms11102474








