Phenotypic and Genotypic Characterization of Recently Isolated Multidrug-Resistant Acinetobacter baumannii Clinical and Aquatic Strains and Demonstration of Silver Nanoparticle Potency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Antibiotic Susceptibility Profiles
2.3. Biofilm Formation Assay
2.4. Characterization of Resistance and Virulence Profiles of AB Strains
2.5. Synthesis of Ag NPs
2.6. Ag NP Characterization Techniques
2.7. Qualitative Screening of the Antibacterial Activity of the Ag NPs
2.8. Quantitative Evaluation of the Antibacterial Activity of the Ag NPsol
2.9. Effect of Ag NPsol on the Adherence Capacity and Soluble Virulence Factor Production
- C1—colony diameter of strain control;
- D1—clear/brown/white/yellow precipitate/ring surrounding the culture spot/yellow zone diameter of strain control;
- C2—colony diameter of sample;
- D2—clear/brown/precipitation/yellow zone diameter of sample.
- As = the absorbance of the microbial adherence of AB strains treated with Ag NPsol;
- Ac = the absorbance of the microbial adherence of untreated AB strains.
2.10. Extracellular Nitric Oxide Quantification
2.11. Determination of the Interaction between the Ag NPs and Conventional Antibiotics
2.12. Statistical Analysis
3. Results
3.1. Characterization of Ag NPs
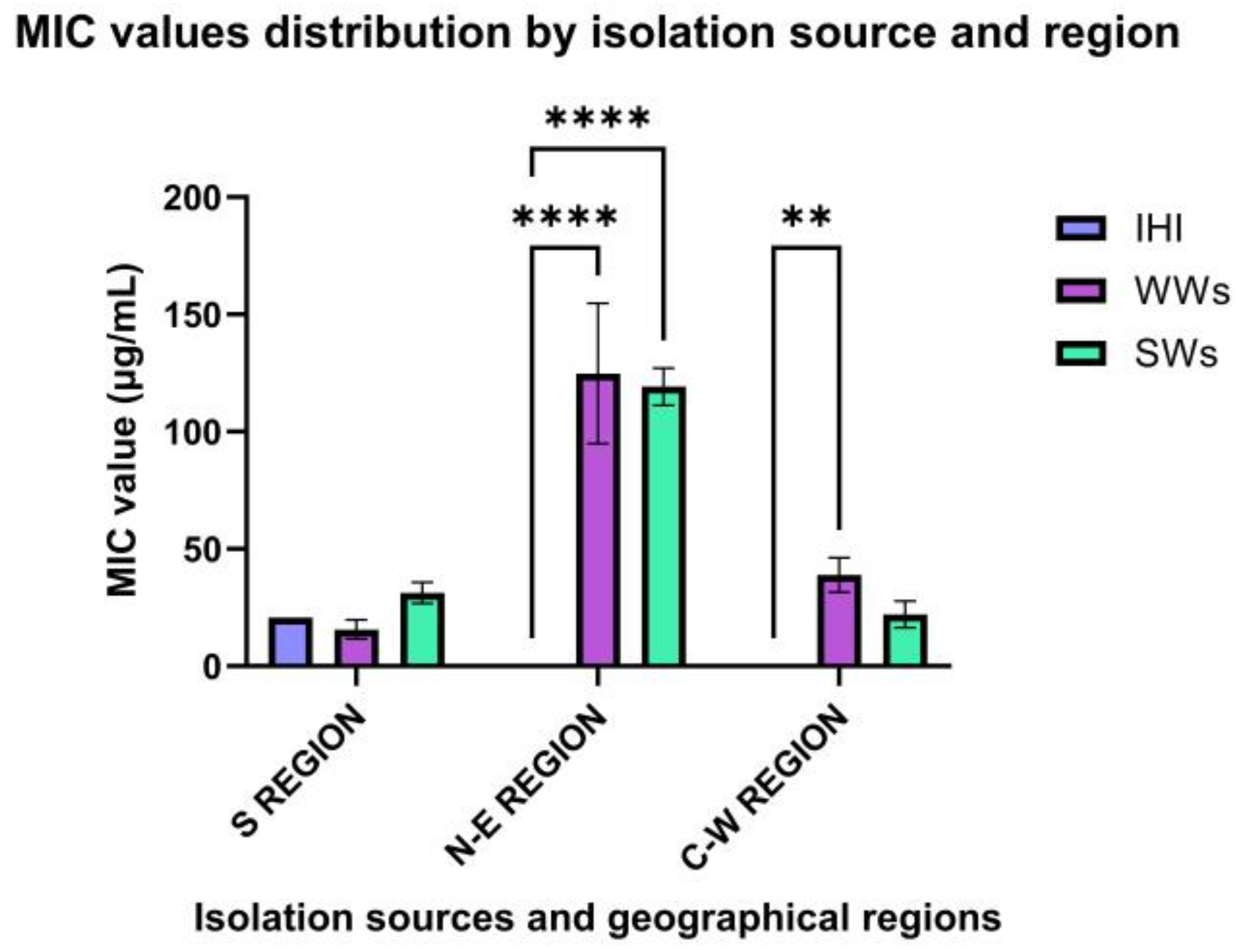
3.2. Phenotypic Antimicrobial Resistance (AR) Profiles
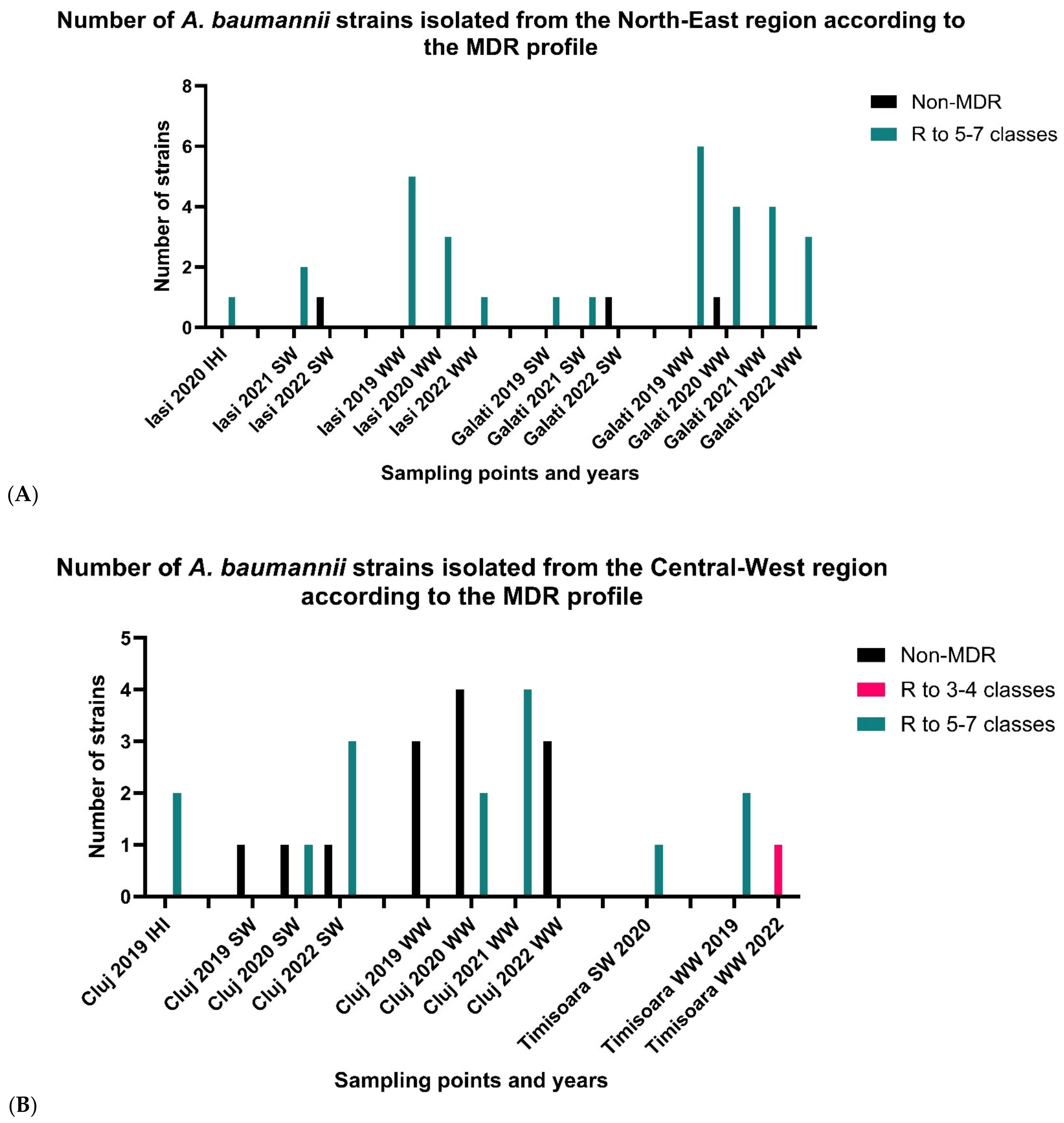
3.2.1. North-East Region
3.2.2. Central-Western Regions
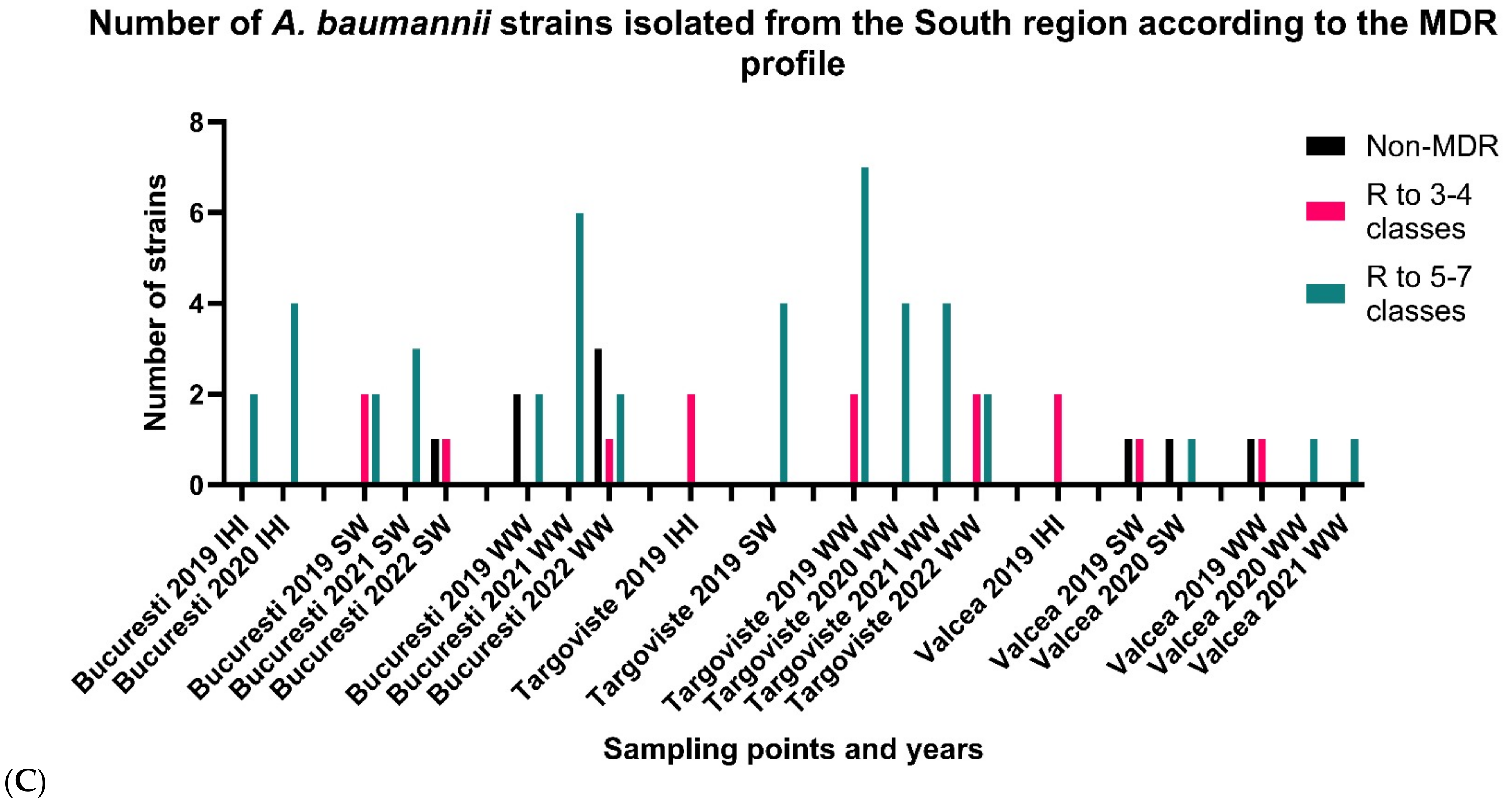
3.2.3. South Romania
3.3. Genotypic AR Profiles
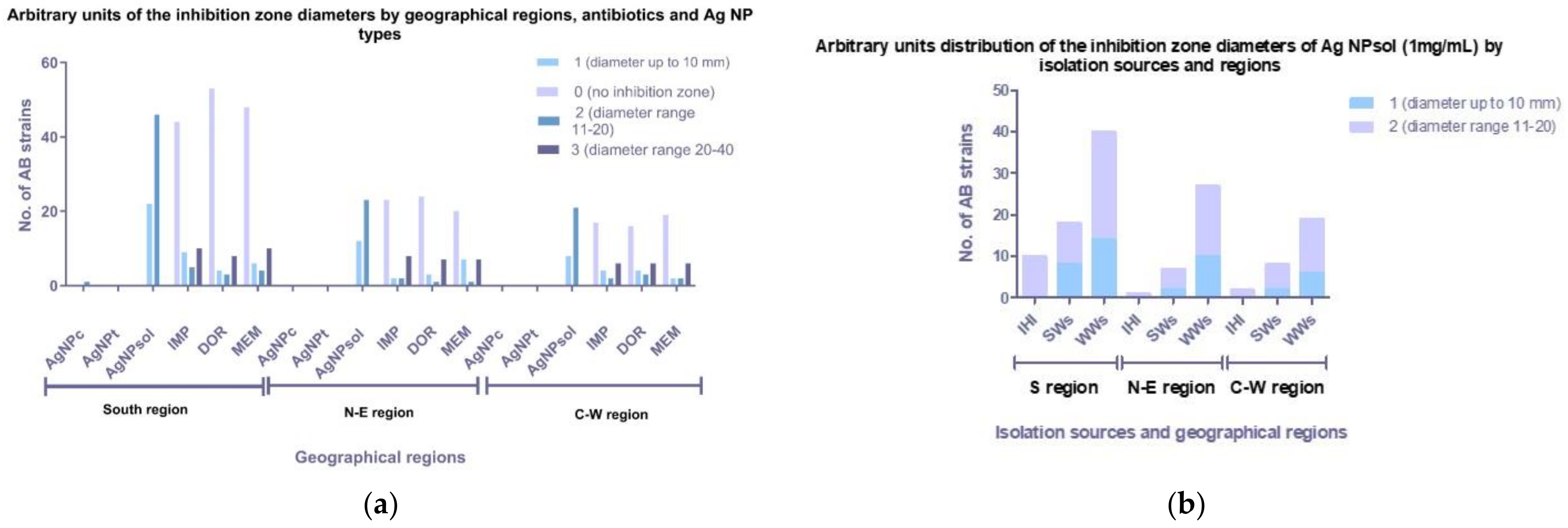
3.4. Antimicrobial Activity of Ag NPs
3.5. Ag NPsol Effect on Adherence Capacity
3.6. The Modulation of Virulence Factor Capacity by Ag NPsol
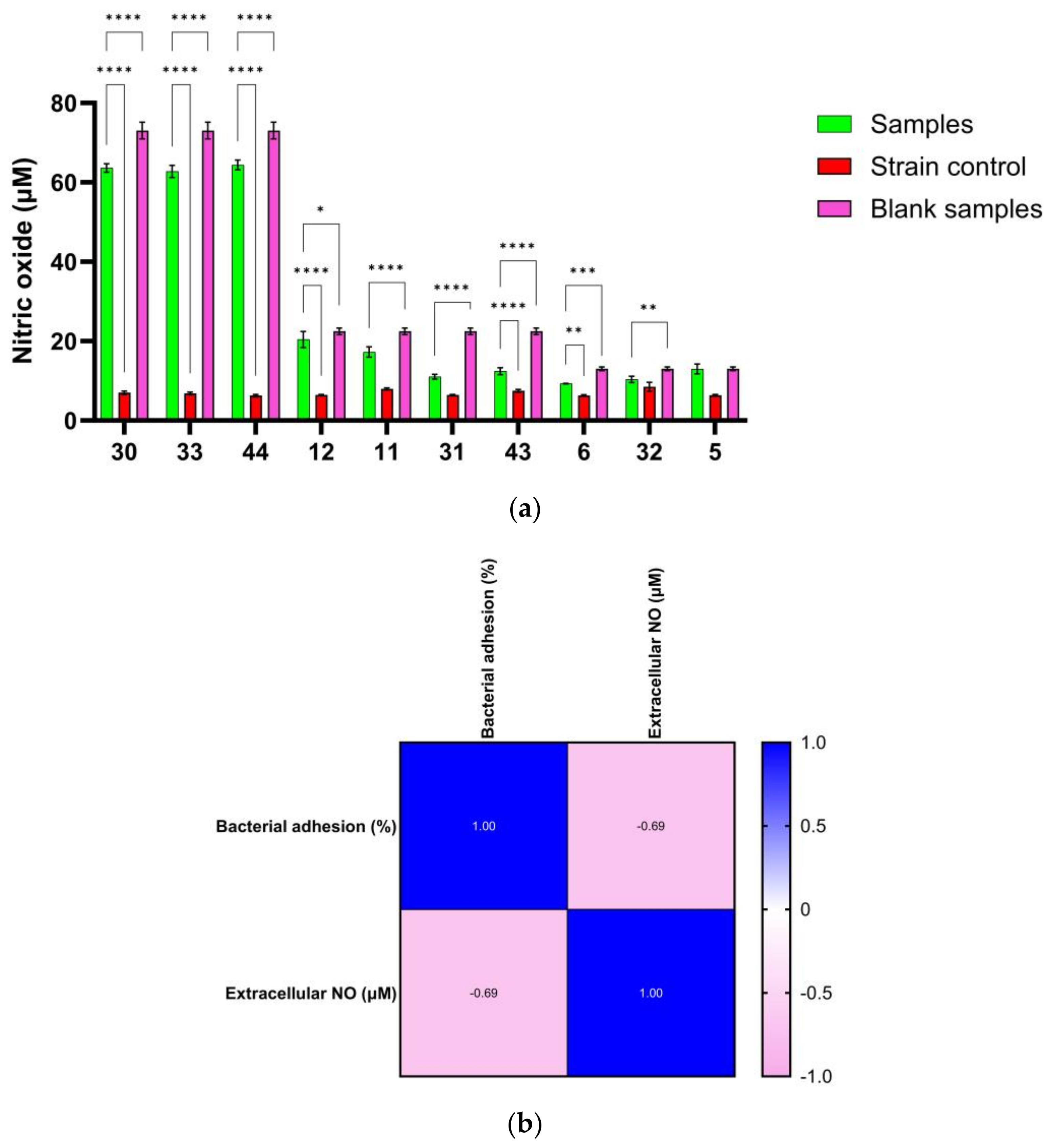
3.7. Correlation between NO Release and Antibiofilm Effect of Ag NPs
3.8. The Interaction between the Ag NPsol and Conventional Antibiotics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, C.R.; Lee, J.H.; Park, M.; Park, K.S.; Bae, I.K.; Kim, Y.B.; Cha, C.J.; Jeong, B.C.; Lee, S.H. Biology of Acinetobacter baumannii: Pathogenesis, Antibiotic Resistance Mechanisms, and Prospective Treatment Options. Front. Cell Infect. Microbiol. 2017, 7, 55. [Google Scholar] [CrossRef]
- Khazaal, S.S.; Al-Kadmy, I.M.; Aziz, S.N. Mechanism of Pathogenesis in Multidrug Resistant Acinetobacter baumannii Isolated from Intensive Care Unit. Gene Rep. 2020, 18, 100557. [Google Scholar] [CrossRef]
- Lima, W.G.; Brito, J.C.M.; Cruz Nizer, W.S. Ventilator-Associated Pneumonia (VAP) Caused by Carbapenem-Resistant Acinetobacter baumannii in Patients with COVID-19: Two Problems, One Solution? Med. Hypotheses 2020, 144, 110139. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Available online: https://atlas.ecdc.europa.eu/public/index.aspx (accessed on 12 June 2023).
- Kumar, S.; Anwer, R.; Azzi, A. Virulence Potential and Treatment Options of Multidrug-Resistant (MDR) Acinetobacter baumannii. Microorganisms 2021, 9, 2104. [Google Scholar] [CrossRef]
- Chakravarty, B. Genetic mechanisms of antibiotic resistance and virulence in Acinetobacter baumannii: Background, challenges and future prospects. Mol. Biol. Rep. 2020, 47, 4037–4046. [Google Scholar] [CrossRef]
- Vázquez-López, R.; Solano-Gálvez, S.G.; Juárez Vignon-Whaley, J.J.; Abello Vaamonde, J.A.; Padró Alonzo, L.A.; Rivera Reséndiz, A.; Muleiro Álvarez, M.; Vega López, E.N.; Franyuti-Kelly, G.; Álvarez-Hernández, D.A.; et al. Acinetobacter baumannii Resistance: A Real Challenge for Clinicians. Antibiotics 2020, 9, 205. [Google Scholar] [CrossRef]
- Zeighami, H.; Valadkhani, F.; Shapouri, R. Virulence Characteristics of Multidrug Resistant Biofilm Forming Acinetobacter baumannii Isolated from Intensive Care Unit Patients. BMC Infect. Dis. 2019, 19, 629. [Google Scholar] [CrossRef]
- Eid, A.M.; Fouda, A.; Niedbała, G.; Hassan, S.E.-D.; Salem, S.S.; Abdo, A.M.; Hetta, H.F.; Shaheen, T.I. Endophytic Streptomyces laurentii Mediated Green Synthesis of Ag-NPs with Antibacterial and Anticancer Properties for Developing Functional Textile Fabric Properties. Antibiotics 2020, 9, 641. [Google Scholar] [CrossRef]
- Hetta, H.F.; Al-Kadmy, I.M.S.; Khazaal, S.S.; Abbas, S.; Suhail, A.; El-Mokhtar, M.A.; Ellah, N.H.A.; Ahmed, E.A.; Abd-Ellatief, R.B.; El-Masry, E.A.; et al. Antibiofilm and antivirulence potential of silver nanoparticles against multidrug-resistant Acinetobacter baumannii. Sci. Rep. 2021, 11, 10751. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Vora, J.; Nadhe, S.B.; Wadhwani, S.A.; Shedbalkar, U.U.; Chopade, B.A. Antibacterial Activities of Bacteriagenic Silver Nanoparticles against Nosocomial Acinetobacter baumannii. J. Nanosci. Nanotechnol. 2018, 18, 3806–3815. [Google Scholar] [CrossRef] [PubMed]
- Blejan, I.E.; Arsene, A.L. Efectele Antibacteriene Ale Nanoparticulelor de Argint. Mecanisme de Acţiune. Farmacist.ro 2018, 183, 39–43. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef]
- Rinna, A.; Magdolenova, Z.; Hudecova, A.; Kruszewski, M.; Refsnes, M.; Dusinska, M. Effect of Silver Nanoparticles on Mitogen-Activated Protein Kinases Activation: Role of Reactive Oxygen Species and Implication in DNA Damage. Mutagenesis 2015, 30, 59–66. [Google Scholar] [CrossRef]
- Yassin, M.T.; Mostafa, A.A.-F.; Al-Askar, A.A.; Al-Otibi, F.O. Facile Green Synthesis of Silver Nanoparticles Using Aqueous Leaf Extract of Origanum majorana with Potential Bioactivity against Multidrug Resistant Bacterial Strains. Crystals 2022, 12, 603. [Google Scholar] [CrossRef]
- Yassin, M.T.; Mostafa, A.A.-F.; Al-Askar, A.A.; Al-Otibi, F.O. Synergistic Antibacterial Activity of Green Synthesized Silver Nanomaterials with Colistin Antibiotic against Multidrug-Resistant Bacterial Pathogens. Crystals 2022, 12, 1057. [Google Scholar] [CrossRef]
- Allend, S.O.; Garcia, M.O.; Cunha, K.F.; Albernaz, D.T.F.; Silva, M.E.; Ishikame, R.Y.; Panagio, L.A.; Nakazaro, G.; Reis, G.F.; Pereira, D.B.; et al. Biogenic Silver Nanoparticle (Bio-AgNP) Has an Antibacterial Effect against Carbapenem-Resistant Acinetobacter baumannii with Synergism and Additivity When Combined with Polymyxin B. J. Appl. Microbiol. 2022, 132, 1036–1047. [Google Scholar] [CrossRef] [PubMed]
- Camargo, L.O.; Fontoura, I.; Veriato, T.S.; Raniero, L.; Castilho, M.L. Antibacterial Activity of Silver Nanoparticles Functionalized with Amikacin Applied against Multidrug-Resistant Acinetobacter baumannii. Am. J. Infect. Control 2023, 51, 871–878. [Google Scholar] [CrossRef]
- Dove, A.S.; Dzurny, D.I.; Dees, W.R.; Qin, N.; Nunez Rodriguez, C.C.; Alt, L.A.; Ellward, G.L.; Best, J.A.; Rudawski, N.G.; Fujii, K.; et al. Silver nanoparticles enhance the efficacy of aminoglycosides against antibiotic-resistant bacteria. Front. Microbiol. 2023, 13, 1064095. [Google Scholar] [CrossRef]
- Al-Otibi, F.O.; Yassin, M.T.; Al-Askar, A.A.; Maniah, K. Green Biofabrication of Silver Nanoparticles of Potential Synergistic Activity with Antibacterial and Antifungal Agents against Some Nosocomial Pathogens. Microorganisms 2023, 11, 945. [Google Scholar] [CrossRef]
- Gautam, D.; Dolma, K.G.; Khandelwal, B.; Gupta, M.; Singh, M.; Mahboob, T.; Teotia, A.; Thota, P.; Bhattacharya, J.; Goyal, R.; et al. Green Synthesis of Silver Nanoparticles Using Ocimum sanctum Linn. and Its Antibacterial Activity against Multidrug Resistant Acinetobacter baumannii. PeerJ 2023, 11, e15590. [Google Scholar] [CrossRef]
- Khan, S.; Fiaz, M.; Yasmin, H.; Ahmad, J.; Ullah, A.; Niaz, Z.; Hayat, S.; Ahmad, A.; Kaushik, P.; Farid, A. Molecular Profiling, Characterization and Antimicrobial Efficacy of Silver Nanoparticles Synthesized from Calvatia gigantea and Mycena leaiana against Multidrug-Resistant Pathogens. Molecules 2023, 28, 6291. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Guo, Y.; Han, W.; Zhou, Y.; Netala, V.R.; Li, H.; Li, H.; Zhang, Z. Exploring the Biomedical Applications of Biosynthesized Silver Nanoparticles Using Perilla frutescens Flavonoid Extract: Antibacterial, Antioxidant, and Cell Toxicity Properties against Colon Cancer Cells. Molecules 2023, 28, 6431. [Google Scholar] [CrossRef]
- Gheorghe-Barbu, I.; Barbu, I.C.; Popa, L.I. Temporo-spatial variations in resistance determinants and clonality of Acinetobacter baumannii and Pseudomonas aeruginosa strains from Romanian hospitals and wastewaters. Antimicrob. Resist. Infect. Control 2022, 11, 115. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. In CLSI Supplement M100 ([Electronic]); Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022; ISBN 978-1-68440-170-3. [Google Scholar]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Stepanović, S.; Vuković, D.; Hola, V.; Bonaventura, G.D.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of Biofilm in Microtiter Plates: Overview of Testing Conditions and Practical Recommendations for Assessment of Biofilm Production by Staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Gheorghe, I.; Cristea, V.C.; Marutescu, L.; Popa, M.; Murariu, C.; Trusca, B.S.; Borcan, E.; Ghiță, M.C.; Lazăr, V.; Chifiriuc, M.C. Resistance and virulence features in carbapenem-resistant Acinetobacter baumannii community acquired and nosocomial isolates in Romania. Rev. Chim. 2019, 10, 3502–3507. [Google Scholar] [CrossRef]
- Corbu, V.M.; Dumbravă, A.S.; Marinescu, L.; Chircov, C.; Surdu, A.S.; Gheorghe-Barbu, I.; Pecete, I.; Popa, M.; Marinas, I.C.; Ianovici, N.; et al. Alternative mitigating solutions based on inorganic nanoparticles for the preservation of cultural heritage objects. Front. Mater. 2023, 10, 1272869. [Google Scholar] [CrossRef]
- Corbu, V.M.; Gheorghe, I.; Marinaș, I.C.; Geană, E.I.; Moza, M.I.; Csutak, O.; Chifiriuc, M.C. Demonstration of Allium sativum Extract Inhibitory Effect on Biodeteriogenic Microbial Strain Growth, Biofilm Development, and Enzymatic and Organic Acid Production. Molecules 2021, 26, 7195. [Google Scholar] [CrossRef]
- Georgescu, M.; Gheorghe, I.; Curutiu, C.; Lazar, V.; Bleotu, C.; Chifiriuc, M.C. Virulence and Resistance Features of Pseudomonas aeruginosa Strains Isolated from Chronic Leg Ulcers. BMC Infect. Dis. 2016, 16, 92. [Google Scholar] [CrossRef]
- Truşcă, B.S.; Gheorghe-Barbu, I.; Manea, M.; Ianculescu, E.; Barbu, I.C.; Măruțescu, L.G.; Dițu, L.-M.; Chifiriuc, M.-C.; Lazăr, V. Snapshot of Phenotypic and Molecular Virulence and Resistance Profiles in Multidrug-Resistant Strains Isolated in a Tertiary Hospital in Romania. Pathogens 2023, 12, 609. [Google Scholar] [CrossRef]
- Quinteros, M.A.; Cano Aristizábal, V.; Dalmasso, P.R.; Paraje, M.G.; Páez, P.L. Oxidative Stress Generation of Silver Nanoparticles in Three Bacterial Genera and Its Relationship with the Antimicrobial Activity. Toxicol. In Vitro 2016, 36, 216–223. [Google Scholar] [CrossRef]
- Nweze, E.I.; Eze, E.E. Justification for the Use of Ocimum gratissimum L. in Herbal Medicine and Its Interaction with Disc Antibiotics. BMC Complement. Altern. Med. 2009, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Khanra, K.; Panja, S.; Choudhuri, I.; Chakraborty, A.; Bhattacharyya, N. Evaluation of Antibacterial Activity and Cytotoxicity of Green Synthesized Silver Nanoparticles Using Scoparia dulcis. Nano Biomed. Eng. 2015, 7, 128–133. [Google Scholar] [CrossRef]
- Devadiga, A.; Shetty, K.V.; Saidutta, M.B. Timber Industry Waste-Teak (Tectona grandis Linn.) Leaf Extract Mediated Synthesis of Antibacterial Silver Nanoparticles. Int. Nano Lett. 2015, 5, 205–214. [Google Scholar] [CrossRef]
- Hochgräfe, F.; Wolf, C.; Fuchs, S.; Liebeke, M.; Lalk, M.; Engelmann, S.; Hecker, M. Nitric Oxide Stress Induces Different Responses but Mediates Comparable Protein Thiol Protection in Bacillus subtilis and Staphylococcus aureus. J. Bacteriol. 2008, 190, 4997–5008. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A Common Mechanism of Cellular Death Induced by Bactericidal Antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef]
- Hu, Z.; Wessels, H.J.C.T.; van Alen, T.; Jetten, M.S.M.; Kartal, B. Nitric Oxide-Dependent Anaerobic Ammonium Oxidation. Nat. Commun. 2019, 10, 1244. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Feura, E.S.; Ahonen, M.J.; Schoenfisch, M.H. Nitric Oxide–Releasing Macromolecular Scaffolds for Antibacterial Applications. Adv. Healthc. Mater. 2018, 7, 1800155. [Google Scholar] [CrossRef]
- Thummeepak, R.; Kongthai, P.; Leungtongkam, U.; Sitthisak, S. Distribution of Virulence Genes Involved in Biofilm Formation in Multi-Drug Resistant Acinetobacter baumannii Clinical Isolates. Int. Microbiol. 2016, 19, 121–129. [Google Scholar] [PubMed]
- Ghasemi, E.; Ghalavand, Z.; Goudarzi, H.; Yeganeh, F.; Hashemi, A.; Dabiri, H.; Mirsamadi, E.S.; Foroumand, M. Phenotypic and Genotypic Investigation of Biofilm Formation in Clinical and Environmental Isolates of Acinetobacter baumannii. Arch. Clin. Infect. Dis. 2018, 13, e12914. [Google Scholar] [CrossRef]
- Sung, J.Y. Molecular Characterization and Antimicrobial Susceptibility of Biofilm-Forming Acinetobacter baumannii Clinical Isolates from Daejeon, Korea. Korean J. Clin. Lab. Sci. 2018, 50, 100–109. [Google Scholar] [CrossRef]
- Rezania, N.; Rahmati, P.; Noorbakhsh, F.; Farhadyar, N.; Lotfali, E. Investigation the Effects of Silver Nanoparticles and Gold Nanoparticles on Expression of Bap and Csu Genes in Biofilm Formation of Acinetobacter baumannii. Iran. J. Microbiol. 2022, 14, 510–517. [Google Scholar] [CrossRef]
- Khaled, J.M.; Alharbi, N.S.; Siddiqi, M.Z.; Alobaidi, A.S.; Nauman, K.; Alahmedi, S.; Almazyed, A.O.; Almosallam, M.A.; Al Jurayyan, A.N. A Synergic Action of Colistin, Imipenem, and Silver Nanoparticles against Pandrug-Resistant Acinetobacter baumannii Isolated from Patients. J. Infect. Public Health 2021, 14, 1679–1685. [Google Scholar] [CrossRef] [PubMed]
- Zendegani, E.; Dolatabadi, S. The Efficacy of Imipenem Conjugated with Synthesized Silver Nanoparticles Against Acinetobacter baumannii Clinical Isolates, Iran. Biol. Trace Elem. Res. 2020, 197, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Haji, S.H.; Ali, F.A.; Aka, S.T.H. Synergistic antibacterial activity of silver nanoparticles biosynthesized by carbapenem-resistant Gram-negative bacilli. Sci. Rep. 2022, 12, 15254. [Google Scholar] [CrossRef]
- Barabadi, H.; Mohammadzadeh, A.; Vahidi, H. Penicillium Chrysogenum-Derived Silver Nanoparticles: Exploration of Their Antibacterial and Biofilm Inhibitory Activity against the Standard and Pathogenic Acinetobacter baumannii Compared to Tetracycline. J. Clust. Sci. 2022, 33, 1929–1942. [Google Scholar] [CrossRef]
- Shah, A.A.; Ahmad, I.; Shafique, M.; Siddique, A.B.; Aslam, B.; Qamar, M.U. Antibacterial Activity of Silver Nanoparticles against Carbapenem-Resistant Acinetobacter baumannii Clinical Isolates. Pak. J. Pharm. Sci. 2022, 35 (Suppl. 1), 203–208. [Google Scholar]

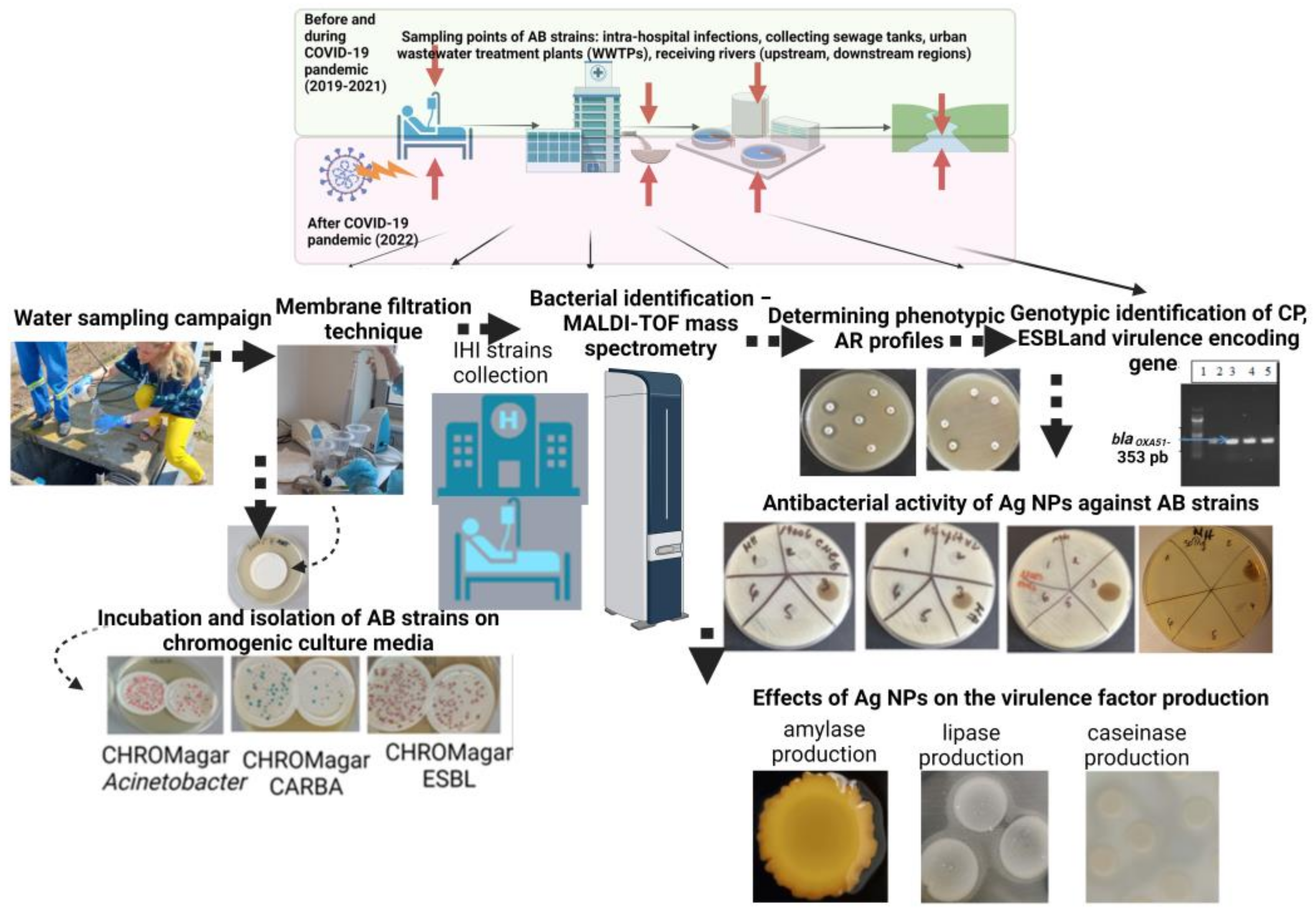






| Target Gene | Primer Sequences | Amplicon Size (bp) | Second Amplification Cycle |
|---|---|---|---|
| Primers used for CP (OXA-23; OXA-24; OXA51; OXA-58; VIM; IMP; NDM; KPC) and ESBL detection (TEM; SHV; CTX-M; VEB; GES; PER) | |||
| blaOXA-51 | OXA-51F: TAATGCTTTGATCGG CCT TG OXA-51R: TGGATTGCACTTCATCTTGG | 353 | 30× (95°—30 s, 52°—40 s, 72°—50 s) |
| blaOXA-23 | OXA-23F: GAT CGG ATT GGA GAA CCA GA OXA-23R: ATT TCT GAC CGC ATT TCC AT | 501 | |
| blaOXA-24 | OXA-24F: GGT TAG TTG GCC CCC TTA AA OXA-24R: AGT TGA GCG AAA AGG GGA TT | 242 | |
| blaOXA58 | OXA-58F:AAGTATTGGGGCTTGTGCTG OXA-58R:CCCCTCTGCGCTCTACATAC | 599 | |
| blaOXA-235 | OXA-235F:TTGTTGCCTTTACTTAGTTGC OXA-235R:CAAAATTTTAAGACGGATCG | 700 | |
| blaTEM | TEM-F: ATA AAA TTC TTG AAG AC TEM-R: TTA CCA ATG CTT AAT CA | 1080 | 30× (95°—30 s, 52°—40 s, 72°—70 s) |
| blaSHV | SHV-F: TGG TTA TGC GTT ATA TTC GCC SHV-R: GGT TAG CGT TGC CAG TGC T | 868 | 30× (95°—30 s, 56°—40 s, 72°—60 s) |
| blaCTX-M | CTX-M-F: TCG TCT CTT CCA GAA TAA GG CTX-M-R: AAG GAG AAC CAG GAA CCA CG | 754 | 30× (95°—30 s, 56°—40 s, 72°—60 s) |
| blaVIM | VIM-F: GAT GGT GTT TGG TCG CAT A VIM-R: CGA ATG CGC AGC ACC AG | 389 | 30× (95°—30 s, 55°—40 s, 72°—50 s) |
| blaIMP | IMP-F-GGAATAGAGTGGCTTAAYTCTC IMP-R-GGTTTAAYAAAACAACCACC | 232 | |
| blaNDM | NDM-F: GCA GCT TGT CGG CCA TGC GG GC NDM-R: GGT CGC GAA GCT GAG CAC CGC AT | 621 | 30× (95°—30 s, 55°—40 s, 72°—50 s) |
| blaKPC | KPC-F: ATGTCACTGTATCGCCGTCT KPC-R: TTTTCAGAGCCTTACTGCCC | 883 | 30× (95°—30 s, 55°—40 s, 72°—50 s) |
| blaVEB | VEB-F:CGA CTT CCA TTT CCC GAT GC VEB-R: GGA CTC TGC AAC AAA TAC GC | 643 | 30× (95°—30 s, 58.5°—40 s, 72°—50 s) |
| blaGES | GES-F: GTT TTG CAA TGT GCT CAA CG GES-R: TCG CAT AGC AAT AGG CGT AG | 371 | 30× (95°—30 s, 56°—40 s, 72°—50 s) |
| blaPER | PER-F: AAT TTG GGC TTA GGG CAG AA PER-R: ATGAAT GTC ATT ATA AAA GC | 925 | 30× (95°—30 s, 50°—40 s, 72°—50 s) |
| Primers used for VM detection: ompA; epsA; csuE; bap; bfmS | |||
| ompA | ompA-F: CGCTTCTGCTGGTGCTGAAT ompA-R: CGTGCAGTAGCGTTAGGGTA | 531 | 30× (95°—30 s, 55°—40 s, 72°—50 s) |
| epsA | epsA-F: AGCAAGTGGTTATCCAATCG epsA-R: ACCAGACTCACCCATTACAT | 451 | |
| bap | bap-F: TACTTCCAATCCAATGCTAGGGAGGGTACCAATGCAG bap-R: TTATCCACTTCCAATGATCAGCAACCAAACCGCTAC | 1225 | 35× (96°—60 s, 56.5°—60 s, 72°—60 s) |
| csuE | csuE-F: ATGCATGTTCTCTGGACTGATGTTGAC csuE-R: CGACTTGTACCGTGACCGTATCTTGATAAG | 976 | 35× (96°—60 s, 57°—60 s, 72°—60 s) |
| bfmS | bfmS-F: TTGCTCGAACTTCCAATTTATTATAC bfmS-R: TTATGCAGGTGCTTTTTTATTGGTC | 1368 | 35× (94°—60 s, 55°—60 s, 72°—45s) |
| Geographical Region | Isolation Sources (n) | Phenotype | β-Lactamases | VMs | Virulence Factors | Biofilm Production Capacity |
|---|---|---|---|---|---|---|
| S Romania (22) | IHI (3) | MDR (2) | OXA-23, OXA-24, OXA-51 | ompA, csuE, bfmS, bap | lecithinase, amylase, caseinase | Moderate |
| lecithinase, amylase, caseinase, lipase | Moderate | |||||
| Non-MDR (1) | OXA-24, OXA-51 | ompA, bfmS | lecithinase, amylase, caseinase | Moderate | ||
| SW (7) | MDR (3) | OXA-23, OXA-51 | ompA, csuE, bfmS, bap | lecithinase, amylase, caseinase | Weak | |
| lecithinase, caseinase | Moderate | |||||
| lecithinase, amylase, caseinase, lipase | Weak | |||||
| MDR (2) | OXA-24, OXA-51, TEM | ompA, csuE, bfmS, bap | lecithinase, amylase, caseinase | Weak | ||
| Moderate | ||||||
| Non-MDR (2) | OXA-51 | ompA, bfmS | lecithinase, caseinase | Strong | ||
| lecithinase, amylase, caseinase, lipase | Moderate | |||||
| WW (15) | MDR (7) | OXA-23, OXA-51 | ompA, csuE, bfmS, bap | lecithinase, amylase, caseinase | Weak | |
| Weak | ||||||
| Weak | ||||||
| Moderate | ||||||
| Moderate | ||||||
| Moderate | ||||||
| Weak | ||||||
| MDR (3) | OXA-24, OXA-51 | ompA, csuE, bfmS | lecithinase, amylase, caseinase | Non-biofilm producer | ||
| lecithinase, amylase, caseinase | Weak | |||||
| lecithinase, caseinase | Moderate | |||||
| MDR (3) | OXA-23, OXA-24, OXA-51 | ompA, csuE | lecithinase, amylase, caseinase | Non-biofilm producer | ||
| lecithinase, amylase, caseinase | Weak | |||||
| lecithinase, amylase | Weak | |||||
| MDR (2) | OXA-51 | lecithinase, amylase, caseinase | Strong | |||
| lecithinase, caseinase | Strong | |||||
| N-E region (13) | SW (5) | MDR (3) | OXA-24, OXA-51, PER | ompA, csuE, bfmS, bap | lecithinase, amylase, caseinase | Weak |
| lecithinase, amylase, caseinase | Weak | |||||
| lecithinase, amylase, caseinase, lipase | Moderate | |||||
| MDR (1) | OXA-24, OXA-51, TEM | lecithinase, amylase, caseinase, lipase | Weak | |||
| Non-MDR (1) | OXA-51, TEM | csuE, bfmS | lecithinase, caseinase, | Moderate | ||
| WW (8) | MDR (5) | OXA-23, OXA-51 | ompA, csuE, bfmS | lecithinase, amylase, caseinase | Weak | |
| lecithinase, amylase, caseinase, lipase | Moderate | |||||
| lecithinase, amylase, caseinase, lipase | Moderate | |||||
| lecithinase, amylase, caseinase | Moderate | |||||
| lecithinase, amylase, caseinase | Moderate | |||||
| MDR (1) | OXA-24, OXA-51 | lecithinase, caseinase, lipase | Moderate | |||
| MDR (1) | OXA-24, OXA-51, TEM | lecithinase, amylase, caseinase | Moderate | |||
| Non-MDR (1) | OXA-51 | ompA, bfmS | lecithinase, amylase, caseinase, lipase | Strong | ||
| C-W Romania (9) | SW (4) | MDR (2) | OXA-23, OXA-51 | ompA, csuE, bfmS, bap | lecithinase, amylase, caseinase, lipase | Weak |
| lecithinase, amylase, caseinase | Non-biofilm producer | |||||
| Non-MDR (2) | OXA-51 | ompA, csuE, bfmS | amylase, caseinase, | Non-biofilm producer | ||
| lecithinase, amylase, caseinase | Strong | |||||
| WW (5) | MDR (1) | OXA-24, OXA-51 | ompA, csuE, bfmS | lecithinase, caseinase, lipase | Non-biofilm producer | |
| MDR (2) | OXA-23, OXA-51 | ompA, csuE, bfmS | lecithinase, caseinase, lipase | Non-biofilm producer | ||
| lecithinase, amylase, lipase | Moderate | |||||
| Non-MDR (2) | OXA-51 | csuE, bfmS | lecithinase, amylase, caseinase, lipase | Moderate | ||
| lecithinase, caseinase, lipase | Weak |
| Caseinase Activity (%) | Lipase Activity (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Region | Isolation Source | Strain | Control | MIC/2 | p-Value | Control | MIC/2 | p-Value |
| N-E region | WW | 4 | 100 ± 16.6 | 127.77 ± 9.6 | 0.5785 | - | - | - |
| 18 | 100 ± 14.2 | 109.52 ± 29.7 | >0.9999 | - | - | - | ||
| 19 | 100 ± 8.6 | 95 ± 31.2 | >0.9999 | 100 ± 21.65 | 100 ± 21.65 | >0.9999 | ||
| 29 | 100 ± 16.3 | 82.14 ± 16.3 | 0.9975 | 100 ± 34.64 | 80 ± 34.64 | >0.9999 | ||
| 30 | 100 ± 11.1 | 40.74 ± 6.4 | <0.0001 | 100 ± 43.3 | 0 ± 0 | 0.0069 | ||
| 31 | 100 ± 18.3 | 100 ± 6.9 | >0.9999 | 100 ± 24.74 | 57.14 ± 24.74 | 0.9254 | ||
| 33 | 100 ± 6.92 | 40 ± 6.9 | <0.0001 | - | - | - | ||
| SW | 32 | 100 ± 11.1 | 100 ± 11.1 | >0.9999 | - | - | - | |
| 1 | 100 ± 10.18 | 100 ± 20.37 | >0.9999 | 100 ± 21.65 | 75 ± 37.5 | >0.9999 | ||
| 2 | 100 ± 16.6 | 111.11 ± 9.62 | >0.9999 | - | - | - | ||
| 3 | 100 ± 14.2 | 104.76 ± 8.24 | >0.9999 | - | - | - | ||
| 35 | 100 ± 7.35 | 104.34 ± 0 | >0.9999 | - | - | - | ||
| 44 | 100 ± 20 | 0 ± 0 | <0.0001 | 100 ± 0 | 0 ± 0 | 0.0069 | ||
| S region | SW | 5 | 100 ± 10.1 | 105.88 ± 17.6 | >0.9999 | - | - | - |
| 7 | 100 ± 27.1 | 73.91 ± 7.53 | 0.7113 | 100 ± 0 | 88,88 ± 38.4 | >0.9999 | ||
| 12 | 100 ± 12.5 | 95.83 ± 7.53 | >0.9999 | - | - | - | ||
| 13 | 100 ± 24.7 | 104.76 ± 8.24 | >0.9999 | - | - | - | ||
| 20 | 100 ± 10.8 | 112.5 ± 18.75 | >0.9999 | 100 ± 13.8 | 96 ± 12 | >0.9999 | ||
| 21 | 100 ± 6.6 | 96.15 ± 6.66 | >0.9999 | - | - | - | ||
| WW | 22 | 100 ± 7.5 | 91.30 ± 13.3 | >0.9999 | - | - | - | |
| 34 | 100 ± 5.2 | 140 ± 20 | 0.039 | - | - | - | ||
| 36 | 100 ± 14.2 | 90.47 ± 16.49 | >0.9999 | - | - | - | ||
| 37 | 100 + 8.6 | 100 ± 8.66 | >0.9999 | - | - | - | ||
| 14 | 100 ± 7.5 | 95.65 ± 7.53 | >0.9999 | - | - | - | ||
| 39 | 100 ± 0 | 116.66 ± 16.6 | 0.9993 | - | - | - | ||
| 40 | 100 ± 7.8 | 95.45 ± 7.53 | >0.9999 | - | - | - | ||
| 41 | 100 ± 28.6 | 118.75 ± 10.8 | 0.994 | 100 ± 24.74 | 85.71 ± 0 | >0.9999 | ||
| 42 | 100 ± 10.8 | 118.75 ± 10.8 | 0.994 | - | - | - | ||
| 43 | 100 ± 6.6 | 92.30 ± 0 | >0.9999 | - | - | - | ||
| 6 | 100 ± 12.5 | 91.66 ± 26.0 | >0.9999 | - | - | - | ||
| 8 | 100 ± 0 | 91.66 ± 7.21 | >0.9999 | - | - | - | ||
| IHI | 9 | 100 ± 19.92 | 95.65 ± 7.53 | >0.9999 | 100 ± 34.6 | 180 ± 0 | 0.0739 | |
| 11 | 100 ± 7.53 | 95.65 ± 7.53 | >0.9999 | - | - | - | ||
| 38 | 100 ± 12.5 | 91.66 ± 7.21 | >0.9999 | - | - | - | ||
| C-W region | WW | 16 | 100 ± 7.87 | 109.09 ± 1.75 | >0.9999 | 100 ± 34.6 | 60 ± 0 | 0.9608 |
| 17 | 100 ± 7.53 | 86.95 ± 7.53 | >0.9999 | 100 ± 34.6 | 60 ± 0 | 0.9608 | ||
| 28 | 100 ± 15.06 | 100 ± 7.53 | >0.9999 | 100 ± 15.74 | 109.09 ± 1.74 | >0.9999 | ||
| 25 | - | - | 100 ± 0 | 66.66 ± 14.43 | 0.995 | |||
| SW | 23 | 100 ± 12.5 | 87.5 ± 12.5 | >0.9999 | - | - | - | |
| 24 | 100 ± 7.53 | 82.60 ± 7.53 | 0.9985 | - | - | - | ||
| 26 | 100 ± 18.23 | 131.57 ± 9.11 | 0.3033 | 100 ± 17.32 | 60 ± 30 | 0.9608 | ||
| 15 | 100 ± 6.66 | 84.61 ± 17.62 | 0.9999 | - | - | - | ||
| Sample | The Percentage of NO Consumed (%) |
|---|---|
| 30 | 12.93 ± 1.46 |
| 12 | 9.23 ± 3.92 |
| 11 | 23.20 ± 5.67 |
| 31 | 50.99 ± 2.68 |
| 6 | 28.58 ± 0.44 |
| 43 | 44.75 ± 3.87 |
| 32 | 20.48 ± 6.22 |
| 5 | 0.44 ± 9.51 |
| 33 | 14.08 ± 2.08 |
| 44 | 11.85 ± 1.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheorghe-Barbu, I.; Corbu, V.M.; Vrancianu, C.O.; Marinas, I.C.; Popa, M.; Dumbravă, A.Ș.; Niță-Lazăr, M.; Pecete, I.; Muntean, A.A.; Popa, M.I.; et al. Phenotypic and Genotypic Characterization of Recently Isolated Multidrug-Resistant Acinetobacter baumannii Clinical and Aquatic Strains and Demonstration of Silver Nanoparticle Potency. Microorganisms 2023, 11, 2439. https://doi.org/10.3390/microorganisms11102439
Gheorghe-Barbu I, Corbu VM, Vrancianu CO, Marinas IC, Popa M, Dumbravă AȘ, Niță-Lazăr M, Pecete I, Muntean AA, Popa MI, et al. Phenotypic and Genotypic Characterization of Recently Isolated Multidrug-Resistant Acinetobacter baumannii Clinical and Aquatic Strains and Demonstration of Silver Nanoparticle Potency. Microorganisms. 2023; 11(10):2439. https://doi.org/10.3390/microorganisms11102439
Chicago/Turabian StyleGheorghe-Barbu, Irina, Viorica Maria Corbu, Corneliu Ovidiu Vrancianu, Ioana Cristina Marinas, Marcela Popa, Andreea Ștefania Dumbravă, Mihai Niță-Lazăr, Ionut Pecete, Andrei Alexandru Muntean, Mircea Ioan Popa, and et al. 2023. "Phenotypic and Genotypic Characterization of Recently Isolated Multidrug-Resistant Acinetobacter baumannii Clinical and Aquatic Strains and Demonstration of Silver Nanoparticle Potency" Microorganisms 11, no. 10: 2439. https://doi.org/10.3390/microorganisms11102439
APA StyleGheorghe-Barbu, I., Corbu, V. M., Vrancianu, C. O., Marinas, I. C., Popa, M., Dumbravă, A. Ș., Niță-Lazăr, M., Pecete, I., Muntean, A. A., Popa, M. I., Marinescu, L., Ficai, D., Ficai, A., & Czobor Barbu, I. (2023). Phenotypic and Genotypic Characterization of Recently Isolated Multidrug-Resistant Acinetobacter baumannii Clinical and Aquatic Strains and Demonstration of Silver Nanoparticle Potency. Microorganisms, 11(10), 2439. https://doi.org/10.3390/microorganisms11102439











