Microbiota Transplant and Gynecological Disorders: The Bridge between Present and Future Treatments
Abstract
:1. Introduction
2. Methods
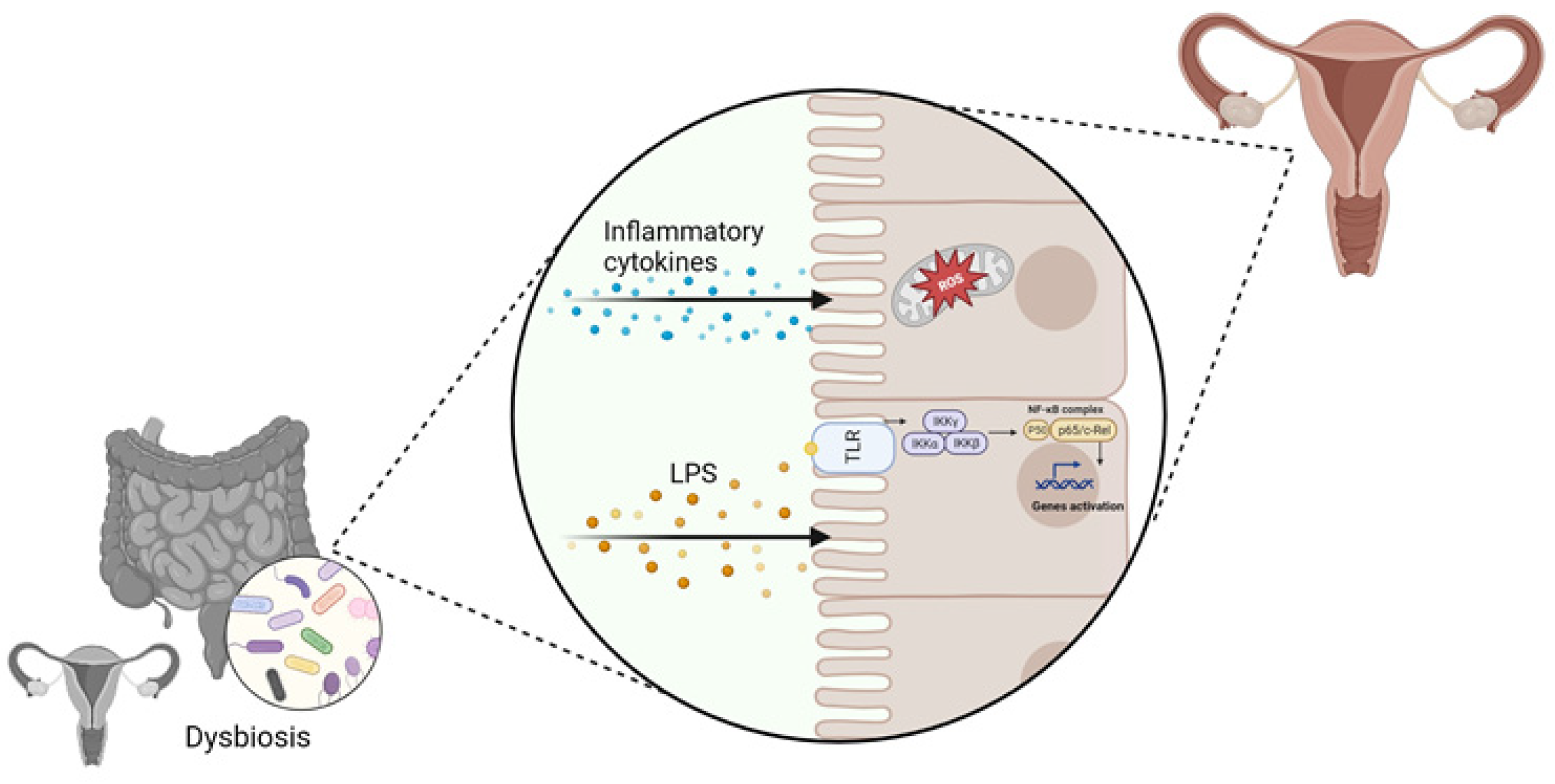
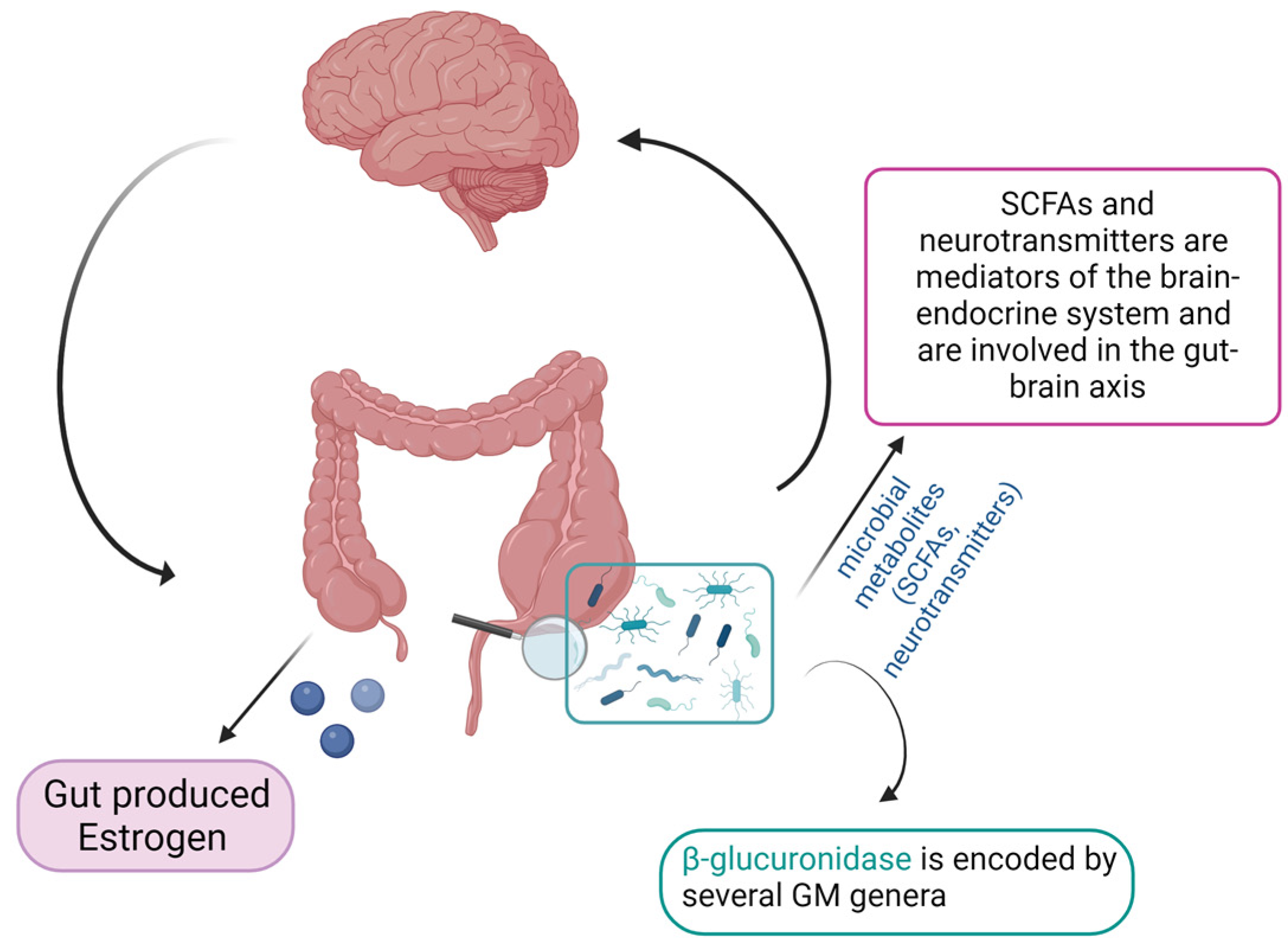
3. Role of the Gut Microbiome–Estrogen Axis in Gynecological Disorders
4. Fecal Microbiota Transplantation
5. FMT and Gynecological Disorders in Animal Models
6. The Human Vaginal Microbiota
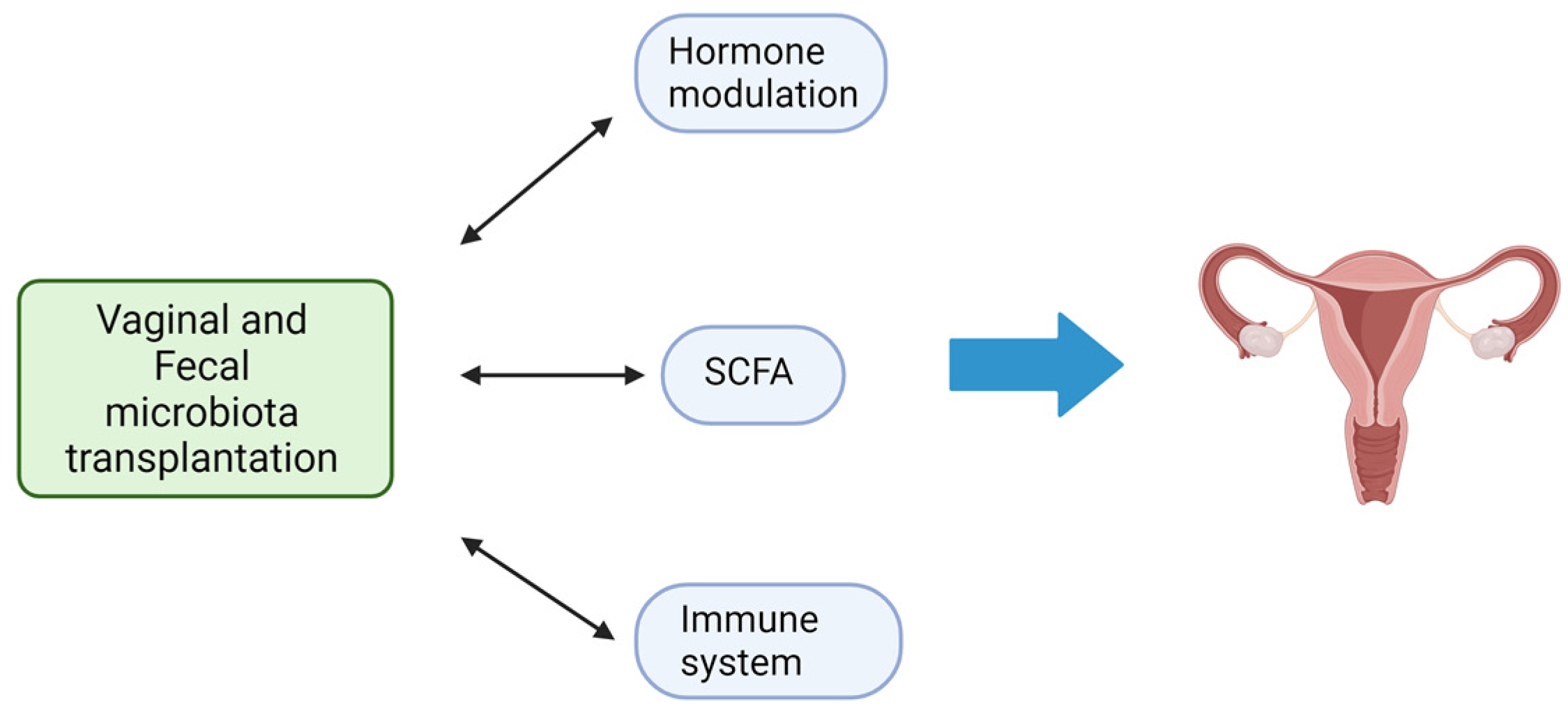
7. Vaginal Microbiota Transplantation and Gynecological Disorders
8. Other Microbiota-Changing Strategies and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hooper, L.V.; Gordon, J.I. Commensal host-bacterial relationships in the gut. Science 2001, 292, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Wilson, I.D. Gut microorganisms, mammalian metabolism and personalized health care. Nat. Rev. Microbiol. 2005, 3, 431–438. [Google Scholar] [CrossRef]
- Johnson, K.V. Gut microbiome composition and diversity are related to human personality traits. Hum. Microb. J. 2020, 15, 100069. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Engevik, M.A.; Spinler, J.K.; Versalovic, J. Healthy Human Gastrointestinal Microbiome: Composition and Function After a Decade of Exploration. Dig. Dis. Sci. 2020, 65, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Schloss, P.D. Dynamics and associations of microbial community types across the human body. Nature 2014, 509, 357–360. [Google Scholar] [CrossRef]
- Thomas, S.; Izard, J.; Walsh, E.; Batich, K.; Chongsathidkiet, P.; Clarke, G.; Sela, D.A.; Muller, A.J.; Mullin, J.M.; Albert, K.; et al. The Host Microbiome Regulates and Maintains Human Health: A Primer and Perspective for Non-Microbiologists. Cancer Res. 2017, 77, 1783–1812. [Google Scholar] [CrossRef]
- Li, P.; Shuai, P.; Shen, S.; Zheng, H.; Sun, P.; Zhang, R.; Lan, S.; Lan, Z.; Jayawardana, T.; Yang, Y.; et al. Perturbations in gut microbiota composition in patients with polycystic ovary syndrome: A systematic review and meta-analysis. BMC Med. 2023, 21, 302. [Google Scholar] [CrossRef]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef]
- Naghipour, A.; Amini-Salehi, E.; Orang Gorabzarmakhi, M.; Shahdkar, M.; Fouladi, B.; Alipourfard, I.; Sanat, Z.M. Effects of gut microbial therapy on lipid profile in individuals with non-alcoholic fatty liver disease: An umbrella meta-analysis study. Syst. Rev. 2023, 12, 144. [Google Scholar] [CrossRef]
- Moschen, A.R.; Kaser, S.; Tilg, H. Non-alcoholic steatohepatitis: A microbiota-driven disease. Trends Endocrinol. Metab. 2013, 24, 537–545. [Google Scholar] [CrossRef]
- Tripathi, A.; Debelius, J.; Brenner, D.A.; Karin, M.; Loomba, R.; Schnabl, B.; Knight, R. The gut-liver axis and the intersection with the microbiome. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Deng, Y.; Wang, J.; Zhao, H.; Zhang, J.; Xie, W. The association between NAFLD and risk of chronic kidney disease: A cross-sectional study. Ther. Adv. Chronic Dis. 2021, 12, 20406223211048649. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Yan, K.; Wang, Z.; Zhang, Q.; Gao, L.; Xu, T.; Sai, J.; Cheng, F.; Du, Y. The association between hypertension and nonalcoholic fatty liver disease (NAFLD): Literature evidence and systems biology analysis. Bioengineered 2021, 12, 2187–2202. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Corey, K.E.; Byrne, C.D.; Roden, M. The complex link between NAFLD and type 2 diabetes mellitus—Mechanisms and treatments. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 599–612. [Google Scholar] [CrossRef]
- Amabebe, E.; Anumba, D.O.C. Female Gut and Genital Tract Microbiota-Induced Crosstalk and Differential Effects of Short-Chain Fatty Acids on Immune Sequelae. Front. Immunol. 2020, 11, 2184. [Google Scholar] [CrossRef] [PubMed]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free Fatty Acid Receptors in Health and Disease. Physiol. Rev. 2020, 100, 171–210. [Google Scholar] [CrossRef]
- Baldi, S.; Menicatti, M.; Nannini, G.; Niccolai, E.; Russo, E.; Ricci, F.; Pallecchi, M.; Romano, F.; Pedone, M.; Poli, G.; et al. Free Fatty Acids Signature in Human Intestinal Disorders: Significant Association between Butyric Acid and Celiac Disease. Nutrients 2021, 13, 742. [Google Scholar] [CrossRef] [PubMed]
- Niccolai, E.; Baldi, S.; Ricci, F.; Russo, E.; Nannini, G.; Menicatti, M.; Poli, G.; Taddei, A.; Bartolucci, G.; Calabrò, A.S.; et al. Evaluation and comparison of short chain fatty acids composition in gut diseases. World J. Gastroenterol. 2019, 25, 5543–5558. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, F.; Munitic, I.; Amedei, A.; Berry, J.D.; Feldman, E.L.; Aronica, E.; Nardo, G.; Van Weehaeghe, D.; Niccolai, E.; Prtenjaca, N.; et al. Interplay between immunity and amyotrophic lateral sclerosis: Clinical impact. Neurosci. Biobehav. Rev. 2021, 127, 958–978. [Google Scholar] [CrossRef]
- Niccolai, E.; Di Pilato, V.; Nannini, G.; Baldi, S.; Russo, E.; Zucchi, E.; Martinelli, I.; Menicatti, M.; Bartolucci, G.; Mandrioli, J.; et al. The Gut Microbiota-Immunity Axis in ALS: A Role in Deciphering Disease Heterogeneity? Biomedicines 2021, 9, 753. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Barker, G.; Lappas, M. The TLR2 ligand FSL-1 and the TLR5 ligand Flagellin mediate pro-inflammatory and pro-labour response via MyD88/TRAF6/NF-κB-dependent signalling. Am. J. Reprod. Immunol. 2014, 71, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Lee, S.M.; Li, J.; Tran, G.; Jabri, B.; Chatila, T.A.; Mazmanian, S.K. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 2011, 332, 974–977. [Google Scholar] [CrossRef]
- Mirmonsef, P.; Zariffard, M.R.; Gilbert, D.; Makinde, H.; Landay, A.L.; Spear, G.T. Short-chain fatty acids induce pro-inflammatory cytokine production alone and in combination with toll-like receptor ligands. Am. J. Reprod. Immunol. 2012, 67, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.S.; Kao, C.Y. Current understanding of the gut microbiota shaping mechanisms. J. Biomed. Sci. 2019, 26, 59. [Google Scholar] [CrossRef]
- Flores, R.; Shi, J.; Fuhrman, B.; Xu, X.; Veenstra, T.D.; Gail, M.H.; Gajer, P.; Ravel, J.; Goedert, J.J. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: A cross-sectional study. J. Transl. Med. 2012, 10, 253. [Google Scholar] [CrossRef]
- Adlercreutz, H.; Pulkkinen, M.O.; Hämäläinen, E.K.; Korpela, J.T. Studies on the role of intestinal bacteria in metabolism of synthetic and natural steroid hormones. J. Steroid Biochem. 1984, 20, 217–229. [Google Scholar] [CrossRef]
- Candeliere, F.; Raimondi, S.; Ranieri, R.; Musmeci, E.; Zambon, A.; Amaretti, A.; Rossi, M. β-Glucuronidase Pattern Predicted from Gut Metagenomes Indicates Potentially Diversified Pharmacomicrobiomics. Front. Microbiol. 2022, 13, 826994. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Wu, J.; Chen, J. The Role of Gut Microbial β-Glucuronidase in Estrogen Reactivation and Breast Cancer. Front. Cell Dev. Biol. 2021, 9, 631552. [Google Scholar] [CrossRef] [PubMed]
- Chadchan, S.B.; Singh, V.; Kommagani, R. Female reproductive dysfunctions and the gut microbiota. J. Mol. Endocrinol. 2022, 69, R81–R94. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Makhlouf, Z.; Alharbi, A.M.; Alfahemi, H.; Khan, N.A. The Gut Microbiome and Female Health. Biology 2022, 11, 1683. [Google Scholar] [CrossRef]
- Ervin, S.M.; Li, H.; Lim, L.; Roberts, L.R.; Liang, X.; Mani, S.; Redinbo, M.R. Gut microbial β-glucuronidases reactivate estrogens as components of the estrobolome that reactivate estrogens. J. Biol. Chem. 2019, 294, 18586–18599. [Google Scholar] [CrossRef]
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Ata, B.; Yildiz, S.; Turkgeldi, E.; Brocal, V.P.; Dinleyici, E.C.; Moya, A.; Urman, B. The Endobiota Study: Comparison of Vaginal, Cervical and Gut Microbiota between Women with Stage 3/4 Endometriosis and Healthy Controls. Sci. Rep. 2019, 9, 2204. [Google Scholar] [CrossRef]
- Yuan, M.; Li, D.; Zhang, Z.; Sun, H.; An, M.; Wang, G. Endometriosis induces gut microbiota alterations in mice. Hum. Reprod. 2018, 33, 607–616. [Google Scholar] [CrossRef]
- Wei, Y.; Tan, H.; Yang, R.; Yang, F.; Liu, D.; Huang, B.; OuYang, L.; Lei, S.; Wang, Z.; Jiang, S.; et al. Gut dysbiosis-derived β-glucuronidase promotes the development of endometriosis. Fertil. Steril. 2023, 120, 682–694. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Li, X. Effects of intestinal flora on polycystic ovary syndrome. Front. Endocrinol. 2023, 14, 1151723. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2018, 14, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Lizneva, D.; Suturina, L.; Walker, W.; Brakta, S.; Gavrilova-Jordan, L.; Azziz, R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Tremellen, K.; Pearce, K. Dysbiosis of Gut Microbiota (DOGMA)--a novel theory for the development of Polycystic Ovarian Syndrome. Med. Hypotheses 2012, 79, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Sha, L.; Li, Y.; Zhu, L.; Wang, Z.; Li, K.; Lu, H.; Bao, T.; Guo, L.; Zhang, X.; et al. Dietary α-Linolenic Acid-Rich Flaxseed Oil Exerts Beneficial Effects on Polycystic Ovary Syndrome through Sex Steroid Hormones-Microbiota-Inflammation Axis in Rats. Front. Endocrinol. 2020, 11, 284. [Google Scholar] [CrossRef] [PubMed]
- Thackray, V.G. Sex, Microbes, and Polycystic Ovary Syndrome. Trends Endocrinol. Metab. 2019, 30, 54–65. [Google Scholar] [CrossRef]
- Zeng, X.; Xie, Y.J.; Liu, Y.T.; Long, S.L.; Mo, Z.C. Polycystic ovarian syndrome: Correlation between hyperandrogenism, insulin resistance and obesity. Clin. Chim. Acta 2020, 502, 214–221. [Google Scholar] [CrossRef]
- Zeng, B.; Lai, Z.; Sun, L.; Zhang, Z.; Yang, J.; Li, Z.; Lin, J. Structural and functional profiles of the gut microbial community in polycystic ovary syndrome with insulin resistance (IR-PCOS): A pilot study. Res. Microbiol. 2019, 170, 43–52. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, C.; Shi, Y.; Zhang, F.; Li, L.; Wang, X.; Ling, Y.; Fu, H.; Dong, W.; Shen, J.; et al. Dysbiosis of Gut Microbiota Associated with Clinical Parameters in Polycystic Ovary Syndrome. Front. Microbiol. 2017, 8, 324. [Google Scholar] [CrossRef]
- He, S.; Li, H.; Yu, Z.; Zhang, F.; Liang, S.; Liu, H.; Chen, H.; Lü, M. The Gut Microbiome and Sex Hormone-Related Diseases. Front. Microbiol. 2021, 12, 711137. [Google Scholar] [CrossRef]
- Semaan, J.; El-Hakim, S.; Ibrahim, J.N.; Safi, R.; Elnar, A.A.; El Boustany, C. Comparative effect of sodium butyrate and sodium propionate on proliferation, cell cycle and apoptosis in human breast cancer cells MCF-7. Breast Cancer 2020, 27, 696–705. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, B.; Zeng, P.; Zhu, H.; Sivaprakasam, S.; Li, S.; Xiao, H.; Dong, L.; Shiao, P.; Kolhe, R.; Patel, N.; et al. Gpr109a Limits Microbiota-Induced IL-23 Production To Constrain ILC3-Mediated Colonic Inflammation. J. Immunol. 2018, 200, 2905–2914. [Google Scholar] [CrossRef]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Licciardi, P.V.; Ververis, K.; Karagiannis, T.C. Histone deacetylase inhibition and dietary short-chain Fatty acids. ISRN Allergy 2011, 2011, 869647. [Google Scholar] [CrossRef]
- Liu, L.; Fu, Q.; Li, T.; Shao, K.; Zhu, X.; Cong, Y.; Zhao, X. Gut microbiota and butyrate contribute to nonalcoholic fatty liver disease in premenopause due to estrogen deficiency. PLoS ONE 2022, 17, e0262855. [Google Scholar] [CrossRef]
- Vallvé-Juanico, J.; Santamaria, X.; Vo, K.C.; Houshdaran, S.; Giudice, L.C. Macrophages display proinflammatory phenotypes in the eutopic endometrium of women with endometriosis with relevance to an infectious etiology of the disease. Fertil. Steril. 2019, 112, 1118–1128. [Google Scholar] [CrossRef]
- Chadchan, S.B.; Popli, P.; Ambati, C.R.; Tycksen, E.; Han, S.J.; Bulun, S.E.; Putluri, N.; Biest, S.W.; Kommagani, R. Gut microbiota-derived short-chain fatty acids protect against the progression of endometriosis. Life Sci. Alliance 2021, 4, e202101224. [Google Scholar] [CrossRef]
- Ratajczak, W.; Rył, A.; Mizerski, A.; Walczakiewicz, K.; Sipak, O.; Laszczyńska, M. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs). Acta Biochim. Pol. 2019, 66, 1–12. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Z.; Jiang, S.; Bai, X.; Ma, C.; Peng, Q.; Chen, K.; Chang, H.; Fang, T.; Zhang, H. Probiotic. mSystems 2019, 4, e00017-19. [Google Scholar] [CrossRef]
- Svensson, A.; Brunkwall, L.; Roth, B.; Orho-Melander, M.; Ohlsson, B. Associations between Endometriosis and Gut Microbiota. Reprod. Sci. 2021, 28, 2367–2377. [Google Scholar] [CrossRef] [PubMed]
- Lüll, K.; Arffman, R.K.; Sola-Leyva, A.; Molina, N.M.; Aasmets, O.; Herzig, K.H.; Plaza-Díaz, J.; Franks, S.; Morin-Papunen, L.; Tapanainen, J.S.; et al. The Gut Microbiome in Polycystic Ovary Syndrome and Its Association with Metabolic Traits. J. Clin. Endocrinol. Metab. 2021, 106, 858–871. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.S.; Nicholson, M.R.; Khoruts, A.; Kahn, S.A. Fecal Microbiota Transplantation Across the Lifespan: Balancing Efficacy, Safety, and Innovation. Am. J. Gastroenterol. 2023, 118, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Kuang, T.T.; Qiu, S.; Xu, T.; Gang Huan, C.L.; Fan, G.; Zhang, Y. Fecal medicines used in traditional medical system of China: A systematic review of their names, original species, traditional uses, and modern investigations. Chin. Med. 2019, 14, 31. [Google Scholar] [CrossRef] [PubMed]
- Paullini, C. Neu-Vermehrte Heylsame Dreck-Apothecke, wie Nemlich mit Koth und Urin Fast Alle, ja auch Die Schwerste, Gifftigste Kranckheiten, und Bezauberte Schäden vom Haupt Bis zun Füssen, Bnn-und Äusserlich, Glücklich Curiret Worden; Stuttgart, V.d.H.J.S., Ed.; Knoch: Frankfurt am Mayn, Germany, 1697; Volume VD17. [Google Scholar]
- Chandler, J.G.; Moore, E.E.; Paton, B.C.; Rainer, W.G.; Gallagher, J.Q.; Pomerantz, M.; Norton, L.W. Ben Eiseman, MD (1917–2012). J. Trauma Acute Care Surg. 2013, 75, 529–535. [Google Scholar] [CrossRef]
- EISEMAN, B.; SILEN, W.; BASCOM, G.S.; KAUVAR, A.J. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 1958, 44, 854–859. [Google Scholar]
- Borody, T.J.; Warren, E.F.; Leis, S.; Surace, R.; Ashman, O. Treatment of ulcerative colitis using fecal bacteriotherapy. J. Clin. Gastroenterol. 2003, 37, 42–47. [Google Scholar] [CrossRef]
- Rohlke, F.; Stollman, N. Fecal microbiota transplantation in relapsing Clostridium difficile infection. Therap Adv. Gastroenterol. 2012, 5, 403–420. [Google Scholar] [CrossRef]
- van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; de Vos, W.M.; Visser, C.E.; Kuijper, E.J.; Bartelsman, J.F.; Tijssen, J.G.; et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef]
- Food and Drug Administration. Guidance for Industry: Enforcement Policy Regarding Investigational New Drug Requirements for Use of Fecal Microbiota for Transplantation to Treat Clostridium difficile Infection Not Responsive to Standard Therapies; U.S. Food and Drug Administration: Washington, DC, USA, 2013; pp. 42965–42966.
- Mandrioli, J.; Amedei, A.; Cammarota, G.; Niccolai, E.; Zucchi, E.; D’Amico, R.; Ricci, F.; Quaranta, G.; Spanu, T.; Masucci, L. FETR-ALS Study Protocol: A Randomized Clinical Trial of Fecal Microbiota Transplantation in Amyotrophic Lateral Sclerosis. Front. Neurol. 2019, 10, 1021. [Google Scholar] [CrossRef]
- Quaranta, G.; Sanguinetti, M.; Masucci, L. Fecal Microbiota Transplantation: A Potential Tool for Treatment of Human Female Reproductive Tract Diseases. Front. Immunol. 2019, 10, 2653. [Google Scholar] [CrossRef]
- Guo, Y.; Qi, Y.; Yang, X.; Zhao, L.; Wen, S.; Liu, Y.; Tang, L. Association between Polycystic Ovary Syndrome and Gut Microbiota. PLoS ONE 2016, 11, e0153196. [Google Scholar] [CrossRef] [PubMed]
- Torres, P.J.; Ho, B.S.; Arroyo, P.; Sau, L.; Chen, A.; Kelley, S.T.; Thackray, V.G. Exposure to a Healthy Gut Microbiome Protects Against Reproductive and Metabolic Dysregulation in a PCOS Mouse Model. Endocrinology 2019, 160, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Chadchan, S.B.; Cheng, M.; Parnell, L.A.; Yin, Y.; Schriefer, A.; Mysorekar, I.U.; Kommagani, R. Antibiotic therapy with metronidazole reduces endometriosis disease progression in mice: A potential role for gut microbiota. Hum. Reprod. 2019, 34, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- DeLong, K.; Zulfiqar, F.; Hoffmann, D.E.; Tarzian, A.J.; Ensign, L.M. Vaginal Microbiota Transplantation: The Next Frontier. J. Law. Med. Ethics 2019, 47, 555–567. [Google Scholar] [CrossRef]
- Lev-Sagie, A.; Goldman-Wohl, D.; Cohen, Y.; Dori-Bachash, M.; Leshem, A.; Mor, U.; Strahilevitz, J.; Moses, A.E.; Shapiro, H.; Yagel, S.; et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat. Med. 2019, 25, 1500–1504. [Google Scholar] [CrossRef]
- Donders, G.G.; Bosmans, E.; Dekeersmaecker, A.; Vereecken, A.; Van Bulck, B.; Spitz, B. Pathogenesis of abnormal vaginal bacterial flora. Am. J. Obstet. Gynecol. 2000, 182, 872–878. [Google Scholar] [CrossRef]
- Gupta, K.; Stapleton, A.E.; Hooton, T.M.; Roberts, P.L.; Fennell, C.L.; Stamm, W.E. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli colonization in women with recurrent urinary tract infections. J. Infect. Dis. 1998, 178, 446–450. [Google Scholar] [CrossRef]
- Martin, H.L.; Richardson, B.A.; Nyange, P.M.; Lavreys, L.; Hillier, S.L.; Chohan, B.; Mandaliya, K.; Ndinya-Achola, J.O.; Bwayo, J.; Kreiss, J. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J. Infect. Dis. 1999, 180, 1863–1868. [Google Scholar] [CrossRef]
- Hillier, S.L.; Lau, R.J. Vaginal microflora in postmenopausal women who have not received estrogen replacement therapy. Clin. Infect. Dis. 1997, 25 (Suppl. S2), S123–S126. [Google Scholar] [CrossRef]
- Kaur, H.; Merchant, M.; Haque, M.M.; Mande, S.S. Crosstalk between Female Gonadal Hormones and Vaginal Microbiota Across Various Phases of Women’s Gynecological Lifecycle. Front. Microbiol. 2020, 11, 551. [Google Scholar] [CrossRef] [PubMed]
- Boskey, E.R.; Cone, R.A.; Whaley, K.J.; Moench, T.R. Origins of vaginal acidity: High D/L lactate ratio is consistent with bacteria being the primary source. Hum. Reprod. 2001, 16, 1809–1813. [Google Scholar] [CrossRef] [PubMed]
- Kaewsrichan, J.; Peeyananjarassri, K.; Kongprasertkit, J. Selection and identification of anaerobic lactobacilli producing inhibitory compounds against vaginal pathogens. FEMS Immunol. Med. Microbiol. 2006, 48, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, S.J.; Hillier, S.L.; Eschenbach, D.A.; Waltersdorph, A.M. Control of the microbial flora of the vagina by H2O2-generating lactobacilli. J. Infect. Dis. 1991, 164, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4680–4687. [Google Scholar] [CrossRef]
- Gajer, P.; Brotman, R.M.; Bai, G.; Sakamoto, J.; Schütte, U.M.; Zhong, X.; Koenig, S.S.; Fu, L.; Ma, Z.S.; Zhou, X.; et al. Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 2012, 4, 132ra52. [Google Scholar] [CrossRef]
- Schwebke, J.R. New concepts in the etiology of bacterial vaginosis. Curr. Infect. Dis. Rep. 2009, 11, 143–147. [Google Scholar] [CrossRef]
- Chen, T.; Xia, C.; Hu, H.; Wang, H.; Tan, B.; Tian, P.; Zhao, X.; Wang, L.; Han, Y.; Deng, K.Y.; et al. Dysbiosis of the rat vagina is efficiently rescued by vaginal microbiota transplantation or probiotic combination. Int. J. Antimicrob. Agents 2021, 57, 106277. [Google Scholar] [CrossRef]
- Lu, F.; Wei, J.; Zhong, Y.; Feng, Y.; Ma, B.; Xiong, Y.; Wei, K.; Tan, B.; Chen, T. Antibiotic Therapy and Vaginal Microbiota Transplantation Reduce Endometriosis Disease Progression in Female Mice. Front. Med. 2022, 9, 831115. [Google Scholar] [CrossRef]
- Reid, G. How Do Lactobacilli Search and Find the Vagina? Microorganisms 2023, 11, 148. [Google Scholar] [CrossRef]
- Pino, A.; Bartolo, E.; Caggia, C.; Cianci, A.; Randazzo, C.L. Detection of vaginal lactobacilli as probiotic candidates. Sci. Rep. 2019, 9, 3355. [Google Scholar] [CrossRef] [PubMed]
- Nasioudis, D.; Linhares, I.M.; Ledger, W.J.; Witkin, S.S. Bacterial vaginosis: A critical analysis of current knowledge. BJOG 2017, 124, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Mohankumar, B.; Shandil, R.K.; Narayanan, S.; Krishnan, U.M. Vaginosis: Advances in new therapeutic development and microbiome restoration. Microb. Pathog. 2022, 168, 105606. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.; Moreno, I.; Simón, C. Bacterial vaginosis and its association with infertility, endometritis, and pelvic inflammatory disease. Am. J. Obstet. Gynecol. 2021, 224, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Cerca, N. Addressing the challenges with bacterial vaginosis pharmacotherapy. Expert. Opin. Pharmacother. 2023, 24, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Bohbot, J.M.; Daraï, E.; Bretelle, F.; Brami, G.; Daniel, C.; Cardot, J.M. Efficacy and safety of vaginally administered lyophilized Lactobacillus crispatus IP 174178 in the prevention of bacterial vaginosis recurrence. J. Gynecol. Obstet. Hum. Reprod. 2018, 47, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Vladareanu, R.; Mihu, D.; Mitran, M.; Mehedintu, C.; Boiangiu, A.; Manolache, M.; Vladareanu, S. New evidence on oral L. plantarum P17630 product in women with history of recurrent vulvovaginal candidiasis (RVVC): A randomized double-blind placebo-controlled study. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 262–267. [Google Scholar] [CrossRef]
- Li, T.; Liu, Z.; Zhang, X.; Chen, X.; Wang, S. Local Probiotic. Front. Microbiol. 2019, 10, 1033. [Google Scholar] [CrossRef]
- Falagas, M.E.; Betsi, G.I.; Athanasiou, S. Probiotics for prevention of recurrent vulvovaginal candidiasis: A review. J. Antimicrob. Chemother. 2006, 58, 266–272. [Google Scholar] [CrossRef]
- Vahedpoor, Z.; Abastabar, M.; Sehat, M.; Talebian, P.; Felfelian Fini, T.; Dastanpour, Z.; Haghani, I.; Chelongarian, R.; Nazeri, M. Vaginal and oral use of probiotics as adjunctive therapy to fluconazole in patients with vulvovaginal candidiasis: A clinical trial on Iranian women. Curr. Med. Mycol. 2021, 7, 36–43. [Google Scholar] [CrossRef]
- Pericolini, E.; Gabrielli, E.; Ballet, N.; Sabbatini, S.; Roselletti, E.; Cayzeele Decherf, A.; Pélerin, F.; Luciano, E.; Perito, S.; Jüsten, P.; et al. Therapeutic activity of a Saccharomyces cerevisiae-based probiotic and inactivated whole yeast on vaginal candidiasis. Virulence 2017, 8, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Gaziano, R.; Sabbatini, S.; Roselletti, E.; Perito, S.; Monari, C. Saccharomyces cerevisiae-Based Probiotics as Novel Antimicrobial Agents to Prevent and Treat Vaginal Infections. Front. Microbiol. 2020, 11, 718. [Google Scholar] [CrossRef] [PubMed]
- Decherf, A.; Dehay, E.; Boyer, M.; Clément-Ziza, M.; Rodriguez, B.; Legrain-Raspaud, S. Recovery of Saccharomyces cerevisiae CNCM I-3856 in Vaginal Samples of Healthy Women after Oral Administration. Nutrients 2020, 12, 2211. [Google Scholar] [CrossRef] [PubMed]
- Yockey, L.J.; Hussain, F.A.; Bergerat, A.; Reissis, A.; Worrall, D.; Xu, J.; Gomez, I.; Bloom, S.M.; Mafunda, N.A.; Kelly, J.; et al. Screening and characterization of vaginal fluid donations for vaginal microbiota transplantation. Sci. Rep. 2022, 12, 17948. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Singh, V.P.; Sharma, J.; Babu, S.; Rizwanulla; Singla, A. Role of probiotics in health and disease: A review. J. Pak. Med. Assoc. 2013, 63, 253–257. [Google Scholar]
- González-Herrera, S.M.; Bermúdez-Quiñones, G.; Ochoa-Martínez, L.A.; Rutiaga-Quiñones, O.M.; Gallegos-Infante, J.A. Synbiotics: A technological approach in food applications. J. Food Sci. Technol. 2021, 58, 811–824. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Zheng, D.; Elinav, E. Diet-microbiota interactions and personalized nutrition. Nat. Rev. Microbiol. 2019, 17, 742–753. [Google Scholar] [CrossRef]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics-A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef]
- Klement, R.J.; Pazienza, V. Impact of Different Types of Diet on Gut Microbiota Profiles and Cancer Prevention and Treatment. Medicina 2019, 55, 84. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Kong, Q.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. A High-Fat Diet Increases Gut Microbiota Biodiversity and Energy Expenditure Due to Nutrient Difference. Nutrients 2020, 12, 3197. [Google Scholar] [CrossRef]
- Bryant, R.V.; Day, A.S.; McGrath, K.C.; Telfer, K.; Yao, C.K.; Costello, S.P. Fecal microbiota transplantation augmented by a sulfide-reducing diet for refractory ulcerative colitis: A case report with functional metagenomic analysis. JGH Open 2021, 5, 1099–1102. [Google Scholar] [CrossRef]
- Quaranta, G.; Guarnaccia, A.; Fancello, G.; Agrillo, C.; Iannarelli, F.; Sanguinetti, M.; Masucci, L. Fecal Microbiota Transplantation and Other Gut Microbiota Manipulation Strategies. Microorganisms 2022, 10, 2424. [Google Scholar] [CrossRef] [PubMed]
- Tanna, T.; Ramachanderan, R.; Platt, R.J. Engineered bacteria to report gut function: Technologies and implementation. Curr. Opin. Microbiol. 2021, 59, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Charbonneau, M.R.; Isabella, V.M.; Li, N.; Kurtz, C.B. Developing a new class of engineered live bacterial therapeutics to treat human diseases. Nat. Commun. 2020, 11, 1738. [Google Scholar] [CrossRef]
- Puurunen, M.K.; Vockley, J.; Searle, S.L.; Sacharow, S.J.; Phillips, J.A.; Denney, W.S.; Goodlett, B.D.; Wagner, D.A.; Blankstein, L.; Castillo, M.J.; et al. Publisher Correction: Safety and pharmacodynamics of an engineered E. coli Nissle for the treatment of phenylketonuria: A first-in-human phase 1/2a study. Nat. Metab. 2022, 4, 1214. [Google Scholar] [CrossRef]
- Tang, H.; Zhou, T.; Jin, W.; Zong, S.; Mamtimin, T.; Salama, E.S.; Jeon, B.H.; Liu, P.; Han, H.; Li, X. Tumor-targeting engineered probiotic Escherichia coli Nissle 1917 inhibits colorectal tumorigenesis and modulates gut microbiota homeostasis in mice. Life Sci. 2023, 324, 121709. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017, 66, 569–580. [Google Scholar] [CrossRef]
- Quaranta, G.; Fancello, G.; Ianiro, G.; Graffeo, R.; Gasbarrini, A.; Cammarota, G.; Sanguinetti, M.; Masucci, L. Laboratory handling practice for faecal microbiota transplantation. J. Appl. Microbiol. 2020, 128, 893–898. [Google Scholar] [CrossRef]
- Quaranta, G.; Ianiro, G.; De Maio, F.; Guarnaccia, A.; Fancello, G.; Agrillo, C.; Iannarelli, F.; Bibbo, S.; Amedei, A.; Sanguinetti, M.; et al. “Bacterial Consortium”: A Potential Evolution of Fecal Microbiota Transplantation for the Treatment of. Biomed. Res. Int. 2022, 2022, 5787373. [Google Scholar] [CrossRef] [PubMed]
- Louie, T.; Golan, Y.; Khanna, S.; Bobilev, D.; Erpelding, N.; Fratazzi, C.; Carini, M.; Menon, R.; Ruisi, M.; Norman, J.M.; et al. VE303, a Defined Bacterial Consortium, for Prevention of Recurrent Clostridioides difficile Infection: A Randomized Clinical Trial. JAMA 2023, 329, 1356–1366. [Google Scholar] [CrossRef] [PubMed]
| Study Title | NCT Number | Status | Conditions | Study Type |
|---|---|---|---|---|
| Vaginal Microbiota Transplant | NCT04046900 | Recruiting | Recurrent Bacterial Vaginosis | Interventional |
| Safety and Efficacy of Vaginal Microbiota Transplant (VMT) in Women With Bacterial Vaginosis (BV) | NCT03769688 | Withdraw | Bacterial Vaginosis | Interventional |
| Vaginal Microbiome Transplantation for Recurrent Bacterial Vaginosis | NCT04517487 | Recruiting | Bacterial Vaginosis | Interventional |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinelli, S.; Nannini, G.; Cianchi, F.; Staderini, F.; Coratti, F.; Amedei, A. Microbiota Transplant and Gynecological Disorders: The Bridge between Present and Future Treatments. Microorganisms 2023, 11, 2407. https://doi.org/10.3390/microorganisms11102407
Martinelli S, Nannini G, Cianchi F, Staderini F, Coratti F, Amedei A. Microbiota Transplant and Gynecological Disorders: The Bridge between Present and Future Treatments. Microorganisms. 2023; 11(10):2407. https://doi.org/10.3390/microorganisms11102407
Chicago/Turabian StyleMartinelli, Serena, Giulia Nannini, Fabio Cianchi, Fabio Staderini, Francesco Coratti, and Amedeo Amedei. 2023. "Microbiota Transplant and Gynecological Disorders: The Bridge between Present and Future Treatments" Microorganisms 11, no. 10: 2407. https://doi.org/10.3390/microorganisms11102407
APA StyleMartinelli, S., Nannini, G., Cianchi, F., Staderini, F., Coratti, F., & Amedei, A. (2023). Microbiota Transplant and Gynecological Disorders: The Bridge between Present and Future Treatments. Microorganisms, 11(10), 2407. https://doi.org/10.3390/microorganisms11102407









