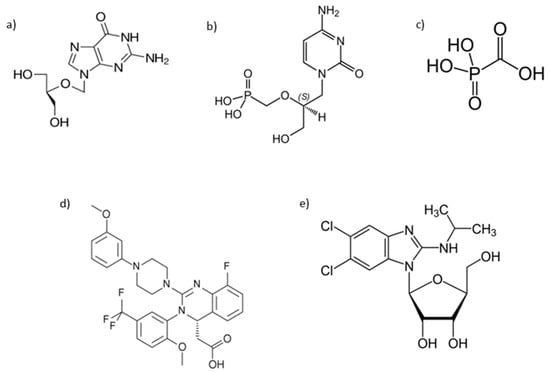
Abstract
Human cytomegalovirus (HCMV) is a herpesvirus capable of establishing a lifelong persistence in the host through a chronic state of infection and remains an essential global concern due to its distinct life cycle, mutations, and latency. It represents a life-threatening pathogen for immunocompromised patients, such as solid organ transplanted patients, HIV-positive individuals, and hematopoietic stem cell recipients. Multiple antiviral approaches are currently available and administered in order to prevent or manage viral infections in the early stages. However, limitations due to side effects and the onset of antidrug resistance are a hurdle to their efficacy, especially for long-term therapies. Novel antiviral molecules, together with innovative approaches (e.g., genetic editing and RNA interference) are currently in study, with promising results performed in vitro and in vivo. Since HCMV is a virus able to establish latent infection, with a consequential risk of reactivation, infection management could benefit from preventive treatment for critical patients, such as immunocompromised individuals and seronegative pregnant women. This review will provide an overview of conventional antiviral clinical approaches and their mechanisms of action. Additionally, an overview of proposed and developing new molecules is provided, including nucleic-acid-based therapies and immune-mediated approaches.
1. Introduction
Biological features. Human cytomegalovirus (HCMV), also known as human herpesvirus 5 (HHV-5), is the prototype member of the Betaherpesvirinae and the largest member of the virus family Herpesviridae [1]. It is a ubiquitous virus that infects almost all humans at some time in their lives. The virus was first isolated by three different groups of investigators: Rowe and colleagues, Weller and colleagues, and Smith simultaneously in 1956 [2]. The genome is a linear, double-stranded DNA molecule with a 236 ± 1.9 kbp size divided into a unique long (UL) and a unique short (US) region, both of which are flanked by terminal and internal repeats [3]. In detail, it contains more than 751 translated open reading frames (ORFs) of which 282 translationally active viral transcripts, 4 major long non-coding RNAs (lncRNAs) (RNA1.2, RNA2.7, RNA4.9, and RNA5.0) and at least 16 pre-miRNAs and 26 mature miRNAs [4]. Despite enclosing a much larger genome, the size of the HCMV capsid is similar to that of other herpesviruses (130 nm), structured as an icosahedral ordered nucleocapsid with triangulation number (T) = 16 and composed of 162 capsomers, divided into two distinct morphological units, 12 pentamers, and 150 hexamers [5,6,7]. Externally to the capsid, the tegument is located, which is generally thought to be unstructured and amorphous in nature. However, some structuring is observed with the binding of tegument proteins to the protein capsid. The tegument proteins are usually phosphorylated and comprise more than half of the total proteins found within infectious virions [8,9]. Finally, a lipidic bilayer envelope membrane, containing eighteen proteins including four viral G protein-coupled receptors (pUL33, pUL78, pUS27, and pUS28), covers the tegument and HCMV nucleocapsid. This envelope is similar in structure and composition to host cell membranes [10,11,12].
Life cycle and pathogenesis. Similar to other herpesviruses, HCMV establishes a persistent infection, remaining silent in the host and undergoing productive reactivation cycles that contribute to its efficient transmission. HCMV is able to replicate in a wide variety of cells (epithelial and mucosal cells, smooth muscle cells, fibroblasts, macrophages, dendritic cells, hepatocytes, and endothelial cells), thereby allowing for systemic spread in the human body and among host [13].
HCMV enters human cells either through direct fusion or an endocytic pathway. The virus attaches to the cell via interactions between viral anti-receptors (gH/gL/pUL128L pentamer complex, and gH/gL/gO trimer complex) and specific surface cell receptors (PDGFRα, Nrp2, and OR14I1), followed by gB activation to fuse the virus envelope with the cellular membrane [14]. Nucleocapsids are released into the cytoplasm and subsequently translocated to the nucleus, where they release viral DNA. HCMV genes are expressed in a sequential cascade, with temporal phases designated immediate-early (IE), early, and late. The major IE genes (MIE) UL123 and UL122 (IE1/IE2) are the first genes to be coded and, together with cellular host factors, coordinate the next level of gene expression (early (E) genes) involved in viral replication [15,16]. Typical early viral proteins include the DNA polymerase (pUL54), phosphotransferase (pUL97), and terminase components (pUL51, pUL52, pUL56, pUL77, pUL89, pUL93, and pUL104) [17]. Finally, HCMV encodes distinct categories of late genes, commonly referred to as leaky late (γ1) and true late (γ2): the former are expressed independently of viral DNA synthesis, while the latter are not expressed at all when viral DNA synthesis is blocked by specific inhibitors [18]. True late genes generally encode structural proteins required for the assembly of new virions, such as pUL77, pUL93 pUL115, pp28, and pp150 [19].
After DNA replication, the following steps are the encapsulation of the replicated viral DNA as capsids, which are then transported from the nucleus to the cytoplasm and coiling in the intermediate compartment of the endoplasmic reticulum (ER)-Golgi. This is then followed by a final complex two-stage envelopment and egress process that leads to virion release by exocytosis at the plasma membrane [20].
This lytic infection program leads to the release of infectious virions and can occur in an array of cells and tissues, while alternatively, in some cell types (CD14+ monocytes and their CD34+ progenitor), the virus can enter a latent life cycle that is associated with a much more limited viral transcription program and a lack of virion production [21].
Epidemiology and transmission routes. HCMV is a global herpesvirus highly prevalent worldwide with a prevalence of about 100% in both Africa and Asia and 45.6–95.7% in Europe and North America [22]. The heterogeneous HCMV seroprevalence appears to be related to race, ethnicity, socioeconomic status, and education level [23].
Cytomegalovirus infection can occur during pregnancy and alongside the entire lifetime along several transmission routes, as congenital HCMV infection (cHCMV) and maternal primary and non-primary infection (exogenous reinfection with a different strain or endogenous viral reactivation) of the virus during pregnancy can result in in utero transmission to the fetus (vertical road). Approximately 11% of live births born with cHCMV show abnormal clinical findings at birth: hematological disorders, cerebral malformation, chorioretinitis, and sensorineural hearing loss (SNHL), the most common sequela [24,25].
In the postnatal period, primary HCMV infections are acquired in several ways by infected fluids (e.g., saliva, breast milk, and blood products) as community exposure [26]. Breastfeeding is known to be the first close contact with a major impact, probably due to viral reactivation in the mammary glands and subsequent excretion of HCMV in milk without clinical or laboratory signs of systemic infection (negative serum IgM and negative viremia) [27]. Throughout childhood and early adulthood, HCMV is transmitted by exposure to saliva, stool, and urine [22]. Among adults, genital secretions are a common fluid for HCMV shedding, consistent with different studies that identified sexual risk factors for HCMV seropositivity or seroconversion [22,28,29]. Another important transmission route for primary infection consists of solid organ transplantation (SOT), especially in cases where there is a serological mismatch between the donor and the recipient (recipient HCMV seronegative/donor HCMV seropositive) [30]. Otherwise, infection can occur as reactivation in those patients with risk factors such as intense immunosuppression, use of lymphocyte-depleting antibodies or prednisolone, acute rejection, advanced age in the donor and/or recipient, concomitant viral infections, or genetic polymorphisms [31,32].
Clinical features. HCMV infection is generally asymptomatic in immunocompetent people, although clinical symptoms of primary infection may include a nonspecific glandular fever (mononucleosis) syndrome characterized by flu-like symptoms [33]. Instead, in immunocompromised or transplanted patients, HCMV primary infection or reactivation represents a fearsome complication resulting in a viral syndrome, characterized by fever and malaise as well as leukopenia, thrombocytopenia, and elevated liver enzymes. Rarely, pneumonia, hepatitis, meningoencephalitis, pancreatitis, or myocarditis could be present, requiring admission to an intensive care unit [30,34]. HCMV infection may also have indirect effects on graft dysfunction, acceleration of coronary atherosclerosis, renal artery stenosis, and the emergence of other opportunistic infections [35,36].
Laboratory diagnosis. Alongside serological tests and pp65 antigenemia, direct detection of HCMV DNA in clinical specimens is currently the standard method for the diagnosis of HCMV infection [2,37]. Particularly, quantitative polymerase chain reaction (qPCR) represents the gold standard, and several IVD kits based on HCMV conserved regions were developed in order to detect and quantify HCMV DNA [38,39,40,41,42]. Whole blood and plasma are the most common specimens for HCMV qPCR [43,44,45], although cerebrospinal fluid (CSF) and bronchoalveolar lavage fluid (BAL) are sometimes used [46]. Scheduled monitoring of HCMV viremia in immunocompromised individuals is pivotal to identifying patients at risk for HCMV disease, assessing preemptive therapy, and determining response to treatment [2]. Serological testing is often the reference diagnostic method for risk assessment in the prenatal/preconception field. The conventional approach of assessing the mother’s immune status using HCMV-specific antibodies entails the detection of anti-HCMV IgG and IgM antibodies as well as, in the case of IgG positivity, the measurement of IgG avidity [47]. Furthermore, the assessment of donor and recipient HCMV serologic status prior to transplantations is currently used as a marker for latent infection and the subsequent risk for donor-derived transmission and immune competence. The combination of donor and recipient serostatus allows the definition of several risk categories for HCMV disease from high-risk (serological mismatch (D+/R−)) to low-risk patients (seronegative donor and recipient (D−/R−)) [48]. In recent years, the measurement of HCMV-specific T cell activity via IFN-γ release assays (IGRAs) and enzyme-linked immunospot (ELISPOT) has gained interest for better risk stratification of immunocompromised patients or for guiding antiviral therapy. These tests can quantify HCMV cell-mediated immunity by measuring the IFN-γ that is released by CD4+ and CD8+ T lymphocytes in the presence of HCMV antigens, thus reflecting patients’ ability to control the virus and predicting the risk for post-transplant viral replication [49,50,51,52,53].
Prevention and treatment. The development of HCMV vaccines began in the 1970s, when the pathogenetic role of viruses on infants in utero and transplant recipients was settled, thus eliciting great interest in pharmacologic researchers. Both HCMV live vaccines (live-attenuated, chimeric, and viral-based) and non-living ones (subunit, RNA-based, virus-like particles, and plasmid-based DNA) were evaluated, but, to date, no effective candidate has been licensed. Several difficulties, such as virus immunological escape, undefined correlation with immune protection, the low number of available animal models, and insufficient general awareness, have been obstacles to the development of a satisfactory vaccine [54,55]. Moreover, epidemiological efforts should be performed in order to determine the best target populations for vaccine administration, even considering that a reduction in the birth prevalence of cHCMV and disease burden was estimated within 20 years of the start of intervention [56].
The antiviral approach for the treatment of HCMV infections relies on different drugs, such as inhibitors of viral DNA polymerase, nucleoside and nucleotide analogs, pyrophosphate analogs, and terminase inhibitors [57,58,59]. Currently, various strategies such as preemptive therapy, antiviral prophylaxis, hybrid approaches (continuous surveillance after prophylaxis for HCMV viremia with preemptive therapy), and HCMV-specific immunity-guided approaches could be used for the effective control of HCMV infection in transplanted patients [60]. However, antiviral prophylaxis and preemptive therapy are the most commonly used strategies worldwide. In antiviral prophylaxis, antiviral drugs are routinely administered to all transplant recipients at risk for HCMV disease, typically for 3 months or more immediately after transplantation, while in a preemptive therapy strategy, HCMV DNAemia is measured according to a predetermined time schedule, and antiviral drugs are administered to those transplant recipients in whom the HCMV DNA level reaches alert thresholds while the infection is still asymptomatic. Under such conditions, only a restricted cohort of patients is treated for a reduced period. [61,62]. The antiviral prophylaxis resulted in superior control of HCMV infection and prolonged time to HCMV disease in transplanted recipients without an increased risk of opportunistic infections, graft loss, drug-related adverse effects, the development of drug resistance, and mortality. For these reasons, it has become the recommended strategy by the American Society of Transplantation; however, post-prophylaxis HCMV disease (late onset of HCMV disease) remains a well-documented and widespread problem in patients receiving antiviral prophylaxis and is found to be independently associated with mortality. On the other hand, the preemptive strategy has been shown to reduce the incidence of late-onset HCMV disease and increase the HCMV-induced immune response, but it faces logistics challenges for medical centers and patient’s noncompliance with the monitoring of HCMV viremia [60,63].
This review resumes knowledge of currently available antiviral drugs and updated information on possible novel approaches to face HCMV infection.
3. Genome-Based Approach to HCMV Infection
RNAi-Based Therapeutics. RNA interference (RNAi) is an evolutionarily conserved mechanism of sequence-specific gene silencing that reduces the levels of protein products translated by a targeted mRNA [106]. The use of RNAi to reduce the levels of specific proteins not only helps to elucidate their function but also provides an opportunity to consider potential therapeutic targets that could be used to treat different diseases due to their antimicrobial activities and multiple roles in regulating gene expression. For this purpose, small interfering RNAs (siRNAs) and microRNAs (miRNAs) are the two main categories of RNAs widely investigated [107]. Since HCMV infection progresses through a well-characterized sequential process of immediate-early (IE), early (E), and late (L) viral gene expression, their product could be targeted by RNA interference approaches. To date, the available studies have investigated the role of siRNAs/miRNAs targeting the transcripts UL54 [108], UL123, and UL122 encoding the immediate-early proteins IE1 and IE2 [109,110,111], showing reduced levels of viral protein expression, DNA replication, and progeny virus production after siRNAs pretreatment.
Ribozyme-Based Therapeutics. Ribozymes are catalytically active RNA molecules (fewer than 100 nucleotides) or RNA–protein complexes in which solely the RNA provides catalytic activity. They are most often employed to knockdown gene expression and to inhibit infections [112]. The use of ribozymes appears to be a promising alternative to RNAi technology as no off-target hits have yet been observed [113]. They form base-pair-specific complexes and catalyze the hydrolysis of specific phosphodiester bonds, causing RNA strand cleavage. Differences exist between ribozymes in terms of size and structure, and although most naturally occurring ribozymes cleave intramolecularly at a cis linkage, the RNA component of RNase-P, which is involved in the processing of pre-tRNA molecules, acts in trans [114,115]. Several studies have been carried out to understand the catalytic mechanism and substrate binding of RNase P ribozymes and elucidated the structure of its active site, structured in a catalytic domain (C domain), with several conserved regions and a specificity domain (S domain) participating in the binding of tRNA substrate. Due to its properties, different engineered RNase-P-based ribozyme variants have been generated for the evaluation of in vitro activity towards HCMV. Kim et al. (2004) developed a functional ribozyme (M1GS RNA) that targets the overlapping mRNA region of two HCMV capsid proteins, capsid scaffolding protein (CSP) and Assemblin, which are essential for viral capsid formation. The ribozyme efficiently cleaved the target mRNA sequence in vitro, and a reduction in CSP/assembly expression levels by 85–90% was observed. Moreover, it inhibited viral growth by 4000-fold in cells that expressed the ribozyme, unlike virus-infected cells that either did not express the ribozyme or produced a ‘disabled’ ribozyme [116]. Other studies explored the efficiency of the RNase-P ribozyme variant (F-R228-IE and V718-A) towards HCMV targets such as capsid assembly protein (AP), protease, and immediate-early IE1/IE2 proteins. A 98–99% and 50,000-fold reduction was observed for protein expression and viral growth, respectively [117,118]. In all of these reports, the result suggests that the ribozyme does not interfere with host gene expression and does not exhibit cell cytotoxicity. Thus, improving the catalytic efficiency of RNase-P ribozyme could be a promising step toward developing a ribozyme-based technology for practical uses, but several limitations need to be resolved for successful delivery to targeted cells.
Aptamer-based approach. Aptamers are a class of nucleic acid (RNA/DNA) molecules that are beginning to be investigated for clinical use. These small molecules can form secondary and tertiary structures capable of binding cell targets [119]. Similarly to intracellular antibodies, aptamers can bind with high affinity and specificity to target proteins or mRNA under intracellular conditions and represent a powerful method to inactivate protein functions in vitro and in vivo [120]. Fomivirsen (C204H263N63O114P20S20) is a 21-nucleotide phosphorothioate oligonucleotide that inhibits HCMV replication through an antisense mechanism. Its oligonucleotide sequence (5′-GCGTTTGCTCTTCTTCTTGCG-3′) is complementary to a sequence in mRNA transcripts of the major immediate-early region 2 (IE2) of HCMV, which encodes for several proteins responsible for the viral gene expression that are essential for the production of infectious viral particles. The binding of Fomivirsen to target mRNA results in the inhibition of IE2 protein synthesis, with subsequent inhibition of viral replication [121]. It was the first in a class of antisense oligonucleotides approved by the FDA in August 1998 for the treatment of HCMV retinitis in AIDS patients who are intolerant of or have a contraindication to other HCMV regimens or who were insufficiently responsive to previous treatments for HCMV retinitis [122].
ZFNs and TALENs. In the 1990s, meganucleases and zinc finger nucleases (ZFNs) laid the groundwork for the concept of genome editing and initiated development in this field. These molecules comprise a chain of zinc finger proteins fused to a bacterial nuclease in order to obtain a system capable of making site-specific double-stranded DNA breaks, thus allowing gene editing. Zinc finger proteins provide site-specific targeting as they each recognize a 3–4 base pair DNA sequence [123,124]. Transcription activator-like effector nucleases (TALENs) are proteins secreted by plant pathogenic bacteria Xanthomonas with a core DNA binding domain of 12–28 repeats, a nuclear localization signal (NLS), an acidic domain for target gene transcription activation, and a Fok1 nuclease [125]. ZFNs and TALENs enable a broad range of genetic modifications by inducing DNA double-strand breaks that stimulate the joining of error-prone non-homologous ends or homology-directed repair at specific genomic locations [126]. One of the advantages of TALEN over ZFN is that its DNA-binding domain recognizes only one nucleotide in contrast to the three bps recognized by the first zinc finger domain of ZFN. Moreover, the TALEN system is more effective at creating double-stranded breaks and discriminating between nucleotides with different methylation states [124,127]. Targeted nucleases offer the potential to correct or disrupt gene products or sequences responsible for causing disorder, such as genetic diseases, but also to abolish the activity of viral genes [128]. This type of approach has been tested as an AIDS therapy in which ZFNs were targeted to disrupt the expression of the CCR5 gene product required by certain strains of HIV as a co-receptor to infect cells, thus resulting in HIV-1-resistant T-lymphocytes [129]. A similar approach could be applied to HCMV infection, as suggested by the discovery of the intracellular zinc finger antiviral protein (PARP13 and ZC3HAV1) and their cofactors (TRIM25) with antiviral activity. These molecules showed the ability to inhibit the replication of HCMV strain AD169 and TB40/E through the recognition of a high content of CpG dinucleotide in the viral mRNA encoding for HCMV proteins essential for virus replication. Furthermore, the production of HCMV strains from infected cells was reduced due to the destabilization of HCMV mRNA expression. However, the same results were not obtained on the HCMV Merlin strain, suggesting a specificity of the isolate for the ZFN action [130]. An interesting potential role of genetic editing could be to manage HCMV latent infection. Indeed, Chen et al. showed that three specific TALEN plasmids (MHCMV1–2, 3–4, and 5–6) were able to provide a negative regulation of latent murine HCMV infection (MHCMV) replication and gene expression through a decrease in IE1 gene expression [131].
CRISPR/Cas9. Clustered regularly interspaced palindromic repeats (CRISPR)/Cas9 is a gene-editing technology causing a major upheaval in biomedical research. It allows errors to be corrected in the genome and presents several promising laboratory applications such as the rapid generation of cellular and animal models, functional genomic screens, and gene therapy for the treatment of infectious diseases (e.g., HIV), malignancies, and other diseases (e.g., cystic fibrosis and Duchenne muscular dystrophy) [132]. The CRISPR/Cas-9 genome-editing mechanism contains three steps: recognition, cleavage, and repair. A designed guide RNA recognizes the target sequence in the gene of interest through a complementary base pair. While the Cas-9 nuclease makes double-stranded breaks in a site 3 base pair upstream to a protospacer adjacent motif, the double-stranded break is repaired by either non-homologous end-joining or homology-directed repair cellular mechanisms [133]. Focusing on HCMV, CRISPR/Cas-9 mechanisms also have the added advantages of inhibiting not only the reactivated virus but also the latent counterpart in cells, as well as stopping the dysregulation of innate immunity occurring during the early stages of HCMV infection. As for the RNAi and ribozyme approach abovementioned, the targeting of viral IE1 and IE2 genes led to a reduction in UL122, UL54, and UL83 transcripts with a consequential three-fold reduction in viral DNA load during both latency and reactivation phases [134]. As suggested by Natesan and Krishnan (2021), another promising target could be the major intermediate early gene promoter/enhancer MIEP/E, which is known to control all of the early genes involved in HCMV replication and reactivation from latency. Using a site-specific cleavage of the 930 bp segment, the MIEP/E gene was deleted in tested samples [135]. In another study, homologous recombination (HR) and non-homologous end-joining (NHEJ)-based methods were used to successfully mutate the HCMV genome, with optimal efficiencies of 42% and 81%, respectively, suggesting a framework for the use of CRISPR/Cas9 in a mutational analysis of the HCMV genome [136], thus also offering the high potential of genetic editing in clinical use. However, further studies are needed to fully understand the efficacy and safety of these gene-editing approaches (ZFNs, TALENs, and CRISPR/Cas-9), as well as their potential to eradicate latent infection, the major barrier for effective HCMV treatment and a long-term risk to the host.
In Figure 3 are resumed molecular mechanisms of genome-editing approaches for HCMV treatment.
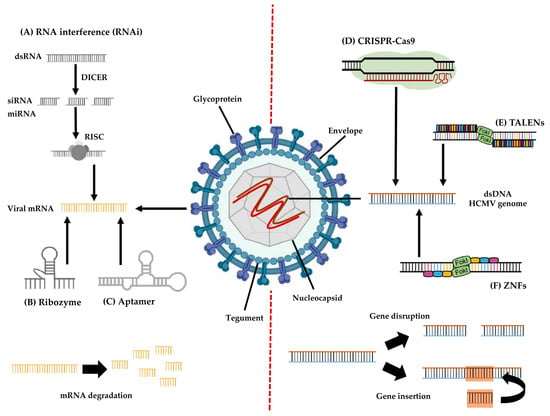
Figure 3.
Overview of genome-editing approaches to HCMV infection.
mRNA HCMV targeting: (A) RNA interference—double-stranded RNA (dsRNA) molecules are cleaved by the DICER enzyme into short double-stranded fragments of approximately 21 to 23 nucleotides (siRNAs or miRNA). Each siRNA/miRNA is subsequently unwound into two single-stranded RNAs (ssRNA) and combined with the molecules Argonaute2 (Ago2) and heat shock protein 70/90 (HSP79/90) to form the RNA-induced silencing complex (RISC). The RISC complex then binds and degrades the target viral mRNA. (B) Ribozyme—RNA-based structure that forms base-pair-specific complexes with viral mRNA and catalyzes its hydrolysis. (C) Aptamer—RNA or DNA molecules that bind with high affinity and specificity to viral target proteins or mRNA, inhibiting their functions.
dsDNA HCMV targeting: (D) CRISPR/Cas9—through a guide RNA able to recognize the target sequence in the viral gene of interest, Cas9 nuclease makes double-stranded breaks, disrupting the target gene and allowing the insertion of unrelated DNA sequences. (E) TALENs—two discrete TALENs recognizing single nucleotides bind to specific sites at opposite viral DNA strands; then, an assembled FokI nuclease dimer cleaves the target viral gene. (F) ZNF—two discrete ZFNs recognizing nucleotide triplets bind to specific sites at opposite viral DNA strands; then, the FokI nuclease dimer disrupts the viral genome.
4. Immune-Based Approach to HCMV Infection
Monoclonal antibodies (mAbs). Monoclonal antibodies (mABs) are safe and effective proteins produced in the laboratory that may be used to target a single epitope of a highly conserved protein of a virus or a bacterial pathogen. They are attractive as potential therapies and prophylaxis for viral infections due to characteristics such as their high specificity and their ability to enhance immune responses [137,138]. The COVID-19 pandemic has stimulated the development of neutralizing mAbs against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), with several mAbs authorized for emergency use. As a consequence, there has been a boost to harnessing mAbs in therapeutic and preventive settings for other infectious diseases [137]. However, only a handful of mAbs are currently approved for the management of infectious diseases (respiratory syncytial virus, Bacillus anthrasis, Clostridioides difficile, HIV-1, and Ebolavirus) [139].
MAbs anti-HCMV were developed in order to offer a safe and well-tolerated potential alternative to currently available therapies for the prevention and treatment of HCMV infection and to overcome several limitations of antiviral drugs (e.g., toxicity and development of antiviral resistance). The major antigen epitopes targeted by neutralizing mAbs are concentrated in glycoprotein B (gB) and glycoprotein H (gH). A phase 2 study evaluated the antiviral activity of CSJ148, consisting of two anti-HCMV mAbs (LJP538 and LJP539), both capable of inhibiting the function of essential viral glycoproteins. LJP538 binds to glycoprotein B (gB), while LJP539 binds to the pentameric complex (consisting of glycoproteins gH, gL, UL128, UL130, and UL131). CSJ148-treated patients showed trends toward decreased viral load, shorter median duration of preemptive therapy, and fewer courses of preemptive therapy. However, these mAbs did not prevent clinically significant HCMV reactivation in recipients of allogeneic hematopoietic cell transplants [140]. Another antibody (EV2038) was targeted to the most immunodominant epitope (AD-1) of HCMV gB. It was considered to play an essential role in gB oligomerization, which is required for gB folding and infectious virus assembly [141]. The results indicate that EV2038 is comparable or over five times more potent than other clinical candidate mAbs specific to gB and gH, also neutralizing the viral cell-to-cell spread [142]. Both studies imply that anti-HCMV mAbs can effectively limit viral dissemination, but the testing of mAbs cocktails and mAb/inhibitor combinations needs to be optimized to prevent virus dissemination and reactivation. Instead, Parsons et al. (2022) reported that a combination of anti-gH neutralizing mAbs with Ganciclovir significantly limited virus dissemination, thus supporting the use of mAbs in combination with small molecule inhibitors. Moreover, combined therapy may lower the required dose of antiviral drugs (Ganciclovir and Letermovir), reducing at the same time the associated toxicity and emergence of viral mutations [143].
Vaccines. The primary objectives of developing a vaccine against HCMV have been to eliminate congenital infection and to reduce morbidity and mortality in highly immunosuppressed individuals. Candidate vaccines in clinical evaluation include live attenuated, protein subunit, DNA, and viral-vectored approaches. Subunit approaches have focused on the HCMV proteins pp65 and IE-1 as important inducers of cytotoxic T cells and glycoprotein B (gB) as an important inducer of neutralizing antibodies [144,145]. DNA plasmids coding for pp65 and gB have shown preliminary evidence of efficacy in transplant recipients, with the ability to stimulate high levels of neutralizing antibodies [54]. Especially the gB surface protein combined with the MF59 oil-in-water adjuvant showed the most promising results. A regimen of three injections over a six-month period in young women naturally exposed to HCMV reduced infection acquisition, but antibodies and efficacy faded quickly. In addition, when the subunit gB protein was combined with the AS01 adjuvant that stimulates toll-like receptor 4, higher and more prolonged levels of anti-gB antibodies were elicited in humans [146]. More recently, a trial focused on the gB/MF59 vaccine observed an immune response towards the polypeptide AD-6 in more than 70% of vaccine recipients. These antibodies bind to gB and to infected cells but not the virion directly, suggesting a non-neutralizing mechanism of action and, instead, a mechanism preventing cell–cell spread of HCMV [147]. A number of HCMV vaccine candidates are currently in development, targeted to prevent congenital infection and post-transplant infections. There is evidence from Phase II trials that gB/MF59 vaccination can prevent the acquisition of HCMV in seronegative women exposed to the virus in nature, and there is solid evidence that HCMV disease in seronegative solid organ recipients and in hematogenous stem cell recipients can be prevented. However, even though no Phase III data are available yet, several candidate vaccines are moving forward. Moreover, the incorporation of epitopes derived from the pentameric complex may provide additional efficacy by inducing potent neutralizing/spread-inhibiting antibodies that target virus replication in a broad spectrum of cell types. The diversity of novel strategies being developed engenders optimism that a successful candidate will emerge [54,148].
5. Conclusions
HCMV infection is a significant global health problem, especially in immunocompromised individuals and congenitally infected infants. Moreover, depending on host physiology and the cell types infected, HCMV persistence comprises latent, chronic, and productive states that may occur concurrently, making its therapeutic management difficult [149]. The conventional antiviral approach relies on well-known molecules against pivotal HCMV targets. However, several side effects must be taken into consideration as well as the onset of antiviral resistance, especially in long-term therapies. For these reasons, novel antivirals against HCMV are strongly needed to treat HCMV disease. One such drug is Letermovir, an inhibitor of the viral DNA packaging, while other inhibitors (against both HCMV lytic replication and HCMV latent infection) have shown great promise [150]. Several reports suggest that RNAi, Ribozyme, CRISPR/Cas9, aptamers, and ZNFs are among the therapies that show good results, with lower toxicity, and may prove to be a promising milestone in developing therapeutic strategies for HCMV infection. However, the integration of these novel molecules in preemptive therapy, antiviral prophylaxis, or hybrid approaches must be defined in order to obtain the best clinical outcome for infected patients. Limitations to this approach include the mode of delivery, stability, and immunogenicity. Moreover, due to the ubiquity and lifelong nature of HCMV infection, efforts are addressed towards the development of any vaccine for the general population. Excellent results have been obtained, but it will likely take a very long time to eradicate the virus from the human population. The development of the vaccine could benefit from the widespread bioinformatic tools and artificial intelligence capable of predicting interaction between molecules and viral targets. Several direct anti-HCMV approaches are still available, while other drugs or molecules are currently under study with encouraging results. However, an integrated approach with immune modulation appears to be the future of controlling viral infection and managing HCMV latency/reactivation.
Author Contributions
P.B., L.P. and C.C. wrote the manuscript with input from co-authors A.C., A.B., F.S., E.Z., P.S. and R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by EU funding within the MUR PNRR Extended Partnership initiative on Emerging Infectious Diseases (Project no. PE00000007, INF-ACT).
Data Availability Statement
The data contained in this manuscript are available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Griffiths, P.; Reeves, M. Pathogenesis of human cytomegalovirus in the immunocompromised host. Nat. Rev. Microbiol. 2021, 19, 759–773. [Google Scholar] [CrossRef]
- Ross, S.A.; Novak, Z.; Pati, S.; Boppana, S.B. Diagnosis of Cytomegalovirus Infections. Infect. Disord. Drug Targets 2011, 11, 466. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, E.; Van Loock, M. Functional annotation of human cytomegalovirus gene products: An update. Front. Microbiol. 2014, 5, 218. [Google Scholar] [CrossRef]
- Ye, L.; Qian, Y.; Yu, W.; Guo, G.; Wang, H.; Xue, X. Functional Profile of Human Cytomegalovirus Genes and Their Associated Diseases: A Review. Front. Microbiol. 2020, 11, 2104. [Google Scholar] [CrossRef] [PubMed]
- Bhella, D.; Rixon, F.J.; Dargan, D.J. Cryomicroscopy of human cytomegalovirus virions reveals more densely packed genomic DNA than in herpes simplex virus type 1. J. Mol. Biol. 2000, 295, 155–161. [Google Scholar] [CrossRef]
- Yu, X.; Jih, J.; Jiang, J.; Hong Zhou, Z. Atomic structure of the human cytomegalovirus capsid with its securing tegument layer of pp150 HHS Public Access. Science 2017, 356, eaam6892. [Google Scholar] [CrossRef] [PubMed]
- Butcher, S.J.; Aitken, J.; Mitchell, J.; Gowen, B.; Dargan, D.J. Structure of the human cytomegalovirus B capsid by electron cryomicroscopy and image reconstruction. J. Struct. Biol. 1998, 124, 70–76. [Google Scholar] [CrossRef]
- Tomtishen, J. Human cytomegalovirus tegument proteins (pp65, pp71, pp150, pp28). Virol. J. 2012, 9, 22. [Google Scholar] [CrossRef]
- Kalejta, R.F. Tegument Proteins of Human Cytomegalovirus. Microbiol. Mol. Biol. Rev. 2008, 72, 249–265. [Google Scholar] [CrossRef]
- Garoff, H.; Hewson, R.; Opstelten, D.-J.E. Virus Maturation by Budding. Microbiol. Mol. Biol. Rev. 1998, 62, 1171. [Google Scholar] [CrossRef]
- Homman-Loudiyi, M.; Hultenby, K.; Britt, W.; Söderberg-Nauclér, C. Envelopment of Human Cytomegalovirus Occurs by Budding into Golgi-Derived Vacuole Compartments Positive for gB, Rab 3, Trans-Golgi Network 46, and Mannosidase II. J. Virol. 2003, 77, 3191–3203. [Google Scholar] [CrossRef]
- Foglierini, M.; Marcandalli, J.; Perez, L. HCMV envelope glycoprotein diversity demystified. Front. Microbiol. 2019, 10, 1005. [Google Scholar] [CrossRef]
- Jean Beltran, P.M.; Cristea, I.M. The life cycle and pathogenesis of human cytomegalovirus infection: Lessons from proteomics. Expert Rev. Proteom. 2014, 11, 697–711. [Google Scholar] [CrossRef]
- Gerna, G.; Kabanova, A.; Lilleri, D. Human cytomegalovirus cell tropism and host cell receptors. Vaccines 2019, 7, 70. [Google Scholar] [CrossRef]
- Isomura, H.; Stinski, M.F.; Murata, T.; Yamashita, Y.; Kanda, T.; Toyokuni, S.; Tsurumi, T. The Human Cytomegalovirus Gene Products Essential for Late Viral Gene Expression Assemble into Prereplication Complexes before Viral DNA Replication. J. Virol. 2011, 85, 6629–6644. [Google Scholar] [CrossRef] [PubMed]
- Garci´a, J.J.; Garci´a-Rami´rez, G.; Rami´rez, R.; Ruchti, F.; Huang, H.; Simmen, K.; Angulo, A.; Ghazal, P. Dominance of Virus over Host Factors in Cross-Species Activation of Human Cytomegalovirus Early Gene Expression. J. Virol. 2001, 75, 26–35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adamson, C.S.; Nevels, M.M. Bright and early: Inhibiting human cytomegalovirus by targeting major immediate-early gene expression or protein function. Viruses 2020, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Omoto, S.; Mocarski, E.S. Transcription of True Late (γ2) Cytomegalovirus Genes Requires UL92 Function That Is Conserved among Beta- and Gammaherpesviruses. J. Virol. 2014, 88, 120–130. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rozman, B.; Nachshon, A.; Levi Samia, R.; Lavi, M.; Schwartz, M.; Stern-Ginossar, N. Temporal dynamics of HCMV gene expression in lytic and latent infections. Cell Rep. 2022, 39, 110653. [Google Scholar] [CrossRef]
- Crough, T.; Khanna, R. Immunobiology of human cytomegalovirus: From bench to bedside. Clin. Microbiol. Rev. 2009, 22, 76–98. [Google Scholar] [CrossRef]
- Poole, E.; Wills, M.; Sinclair, J. Human Cytomegalovirus Latency: Targeting Differences in the Latently Infected Cell with a View to Clearing Latent Infection. New J. Sci. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Cannon, M.J.; Schmid, D.S.; Hyde, T.B. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev. Med. Virol. 2010, 20, 202–213. [Google Scholar] [CrossRef]
- Fowler, K.; Mucha, J.; Neumann, M.; Lewandowski, W.; Kaczanowska, M.; Grys, M.; Schmidt, E.; Natenshon, A.; Talarico, C.; Buck, P.O.; et al. A systematic literature review of the global seroprevalence of cytomegalovirus: Possible implications for treatment, screening, and vaccine development. BMC Public Health 2022, 22, 1659. [Google Scholar] [CrossRef] [PubMed]
- Lazzarotto, T.; Blázquez-Gamero, D.; Delforge, M.L.; Foulon, I.; Luck, S.; Modrow, S.; Leruez-Ville, M. Congenital Cytomegalovirus Infection: A Narrative Review of the Issues in Screening and Management From a Panel of European Experts. Front. Pediatr. 2020, 8, 13. [Google Scholar] [CrossRef]
- Kenneson, A.; Cannon, M.J. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 2007, 17, 253–276. [Google Scholar] [CrossRef]
- Gökçe, Ş. Human Cytomegalovirus Infection: Biological Features, Transmission, Symptoms, Diagnosis, and Treatment. In Human Herpesvirus Infection; Thomasini, R.L., Ed.; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Chiavarini, M.; Bragetti, P.; Sensini, A.; Cenci, E.; Castronari, R.; Rossi, M.J.; Fantauzzi, A.; Minelli, L. Breastfeeding and transmission of cytomegalovirus to preterm infants. Case report and kinetic of CMV-DNA in breast milk. Ital. J. Pediatr. 2011, 37, 6. [Google Scholar] [CrossRef]
- Hyde, T.B.; Schmid, D.S.; Cannon, M.J. Cytomegalovirus seroconversion rates and risk factors: Implications for congenital CMV. Rev. Med. Virol. 2010, 20, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Rubinacci, V.; Fumagalli, M.; Meraviglia, G.; Gianolio, L.; Sala, A.; Stracuzzi, M.; Dighera, A.; Zuccotti, G.V.; Giacomet, V. Congenital CMV, Lights and Shadows on Its Management: The Experience of a Reference Center in Northern Italy. Children 2022, 9, 655. [Google Scholar] [CrossRef]
- Azevedo, L.S.; Pierrotti, L.C.; Abdala, E.; Costa, S.F.; Strabelli, T.M.V.; Campos, S.V.; Ramos, J.F.; Latif, A.Z.A.; Litvinov, N.; Maluf, N.Z.; et al. Cytomegalovirus infection in transplant recipients. Clinics 2015, 70, 515. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Higashida-Konishi, M.; Izumi, K.; Hama, S.; Oshige, T.; Oshima, H.; Okano, Y. Risk factors associated with cytomegalovirus reactivation in patients receiving immunosuppressive therapy for rheumatic diseases: A retrospective study. Sci. Rep. 2022, 12, 20926. [Google Scholar] [CrossRef] [PubMed]
- Humar, A.; Snydman, D. Cytomegalovirus in solid organ transplant recipients. Am. J. Transplant 2009, 9 (Suppl. 4), S78–S86. [Google Scholar] [CrossRef] [PubMed]
- van Zuylen, W.J.; Hamilton, S.T.; Naing, Z.; Hall, B.; Shand, A.; Rawlinson, W.D. Congenital cytomegalovirus infection: Clinical presentation, epidemiology, diagnosis and prevention. Obstet. Med. 2014, 7, 140–146. [Google Scholar] [CrossRef]
- Requião-Moura, L.R.; de Matos, A.C.C.; Pacheco-Silva, A. Cytomegalovirus infection in renal transplantation: Clinical aspects, management and the perspectives. Einstein 2015, 13, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Paya, C.V. Indirect effects of CMV in the solid organ transplant patient. Transpl. Infect. Dis. Off. J. Transplant. Soc. 1999, 1 (Suppl. 1), 8–12. [Google Scholar]
- Diena, D.; Allesina, A.; Fop, F.; Mella, A.; Cavallo, R.; Costa, C.; Dolla, C.; Gallo, E.; De Rosa, F.G.; Lavacca, A.; et al. Relationship between Cytomegalovirus Viremia and Long-Term Outcomes in Kidney Transplant Recipients with Different Donor Ages. Microorganisms 2023, 11, 458. [Google Scholar] [CrossRef]
- Razonable, R.R.; Inoue, N.; Pinninti, S.G.; Boppana, S.B.; Lazzarotto, T.; Gabrielli, L.; Simonazzi, G.; Pellett, P.E.; Schmid, D.S. Clinical Diagnostic Testing for Human Cytomegalovirus Infections. J. Infect. Dis. 2020, 221, S74. [Google Scholar] [CrossRef]
- Gu, J.; Ji, H.; Liu, T.; Chen, C.; Zhao, S.; Cao, Y.; Wang, N.; Xiao, M.; Chen, L.; Cai, H. Detection of cytomegalovirus (CMV) by digital PCR in stool samples for the non-invasive diagnosis of CMV gastroenteritis. Virol. J. 2022, 19, 183. [Google Scholar] [CrossRef]
- Novak, Z.; Chowdhury, N.; Ross, S.A.; Pati, S.K.; Fowler, K.; Boppana, S.B. Diagnostic consequences of cytomegalovirus glycoprotein B polymorphisms. J. Clin. Microbiol. 2011, 49, 3033–3035. [Google Scholar] [CrossRef]
- Rasmussen, L.; Geissler, A.; Cowan, C.; Chase, A.; Winters, M. The genes encoding the gCIII complex of human cytomegalovirus exist in highly diverse combinations in clinical isolates. J. Virol. 2002, 76, 10841–10848. [Google Scholar] [CrossRef]
- Leruez-Ville, M.; Ducroux, A.; Rouzioux, C. Exon 4 of the human cytomegalovirus (CMV) major immediate-early gene as a target for CMV real-time PCR. J. Clin. Microbiol. 2008, 46, 1571–1572. [Google Scholar] [CrossRef]
- Chou, S.; Dennison, K.M. Analysis of interstrain variation in cytomegalovirus glycoprotein B sequences encoding neutralization-related epitopes. J. Infect. Dis. 1991, 163, 1229–1234. [Google Scholar] [CrossRef]
- Lazzarotto, T.; Chiereghin, A.; Piralla, A.; Piccirilli, G.; Girello, A.; Campanini, G.; Gabrielli, L.; Costa, C.; Prete, A.; Bonifazi, F.; et al. Cytomegalovirus and Epstein-Barr Virus DNA Kinetics in Whole Blood and Plasma of Allogeneic Hematopoietic Stem Cell Transplantation Recipients. Biol. Blood Marrow Transplant. 2018, 24, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Rzepka, M.; Depka, D.; Gospodarek-Komkowska, E.; Bogiel, T. Whole Blood versus Plasma Samples-How Does the Type of Specimen Collected for Testing Affect the Monitoring of Cytomegalovirus Viremia? Pathogens 2022, 11, 1384. [Google Scholar] [CrossRef] [PubMed]
- Rzepka, M.; Depka, D.; Gospodarek-Komkowska, E.; Bogiel, T. Diagnostic Value of Whole-Blood and Plasma Samples in Epstein-Barr Virus Infections. Diagnostics 2023, 13, 476. [Google Scholar] [CrossRef] [PubMed]
- Beam, E.; Germer, J.J.; Lahr, B.; Yao, J.D.C.; Limper, A.H.; Binnicker, M.J.; Razonable, R.R. Cytomegalovirus (CMV) DNA quantification in bronchoalveolar lavage fluid of immunocompromised patients with CMV pneumonia. Clin. Transplant. 2018, 32, e13149. [Google Scholar] [CrossRef]
- Müller, J.; Flindt, J.; Pollmann, M.; Saschenbrecker, S.; Borchardt-Lohölter, V.; Warnecke, J.M. Efficiency of CMV serodiagnosis during pregnancy in daily laboratory routine. J. Virol. Methods 2023, 314, 114685. [Google Scholar] [CrossRef]
- Navarro, D.; Fernández-Ruiz, M.; Aguado, J.M.; Sandonís, V.; Pérez-Romero, P. Going beyond serology for stratifying the risk of CMV infection in transplant recipients. Rev. Med. Virol. 2019, 29, e2017. [Google Scholar] [CrossRef]
- Clari, M.Á.; Munoz-Cobo, B.; Solano, C.; Benet, I.; Costa, E.; Remigia, M.J.; Bravo, D.; Amat, P.; Navarro, D. Performance of the QuantiFERON-cytomegalovirus (CMV) assay for detection and estimation of the magnitude and functionality of the CMV-specific gamma interferon-producing CD8 + T-cell response in allogeneic stem cell transplant recipients. Clin. Vaccine Immunol. 2012, 19, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, K.H.; Ryu, J.H.; Choi, A.R.; Yu, J.H.; Lim, J.; Han, K.; Kim, S., II; Yang, C.W.; Chung, B.H.; et al. Cytomegalovirus (CMV) immune monitoring with ELISPOTand QuantiFERON-CMV assay in seropositive kidney transplant recipients. PLoS ONE 2017, 12, e0189488. [Google Scholar] [CrossRef]
- Callens, R.; Colman, S.; Delie, A.; Schauwvlieghe, A.; Lodewyck, T.; Selleslag, D.; Reynders, M.; Kerre, T.; Padalko, E. Immunologic Monitoring after Allogeneic Stem Cell Transplantation: T-SPOT.CMV and QuantiFERON-CMV, Are They the Same? Transplant. Cell. Ther. 2023, 29, 392.e1–392.e7. [Google Scholar] [CrossRef]
- Costa, C.; Balloco, C.; Sidoti, F.; Mantovani, S.; Rittà, M.; Piceghello, A.; Fop, F.; Messina, M.; Cavallo, R. Evaluation of CMV-specific cellular immune response by EliSPOT assay in kidney transplant patien. J. Clin. Virol. 2014, 61, 523–528. [Google Scholar] [CrossRef]
- Costa, C.; Astegiano, S.; Terlizzi, M.E.; Sidoti, F.; Curtoni, A.; Solidoro, P.; Baldi, S.; Bergallo, M.; Cavallo, R. Evaluation and significance of cytomegalovirus-specific cellular immune response in lung transplant recipients. Transplant. Proc. 2011, 43, 1159–1161. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A.; Boppana, S.B. Vaccination against the human cytomegalovirus. Vaccine 2019, 37, 7437–7442. [Google Scholar] [CrossRef] [PubMed]
- Scarpini, S.; Morigi, F.; Betti, L.; Dondi, A.; Biagi, C.; Lanari, M. Development of a Vaccine against Human Cytomegalovirus: Advances, Barriers, and Implications for the Clinical Practice. Vaccines 2021, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Rozhnova, G.; Kretzschmar, M.E.; van der Klis, F.; van Baarle, D.; Korndewal, M.; Vossen, A.C.; van Boven, M. Short- and long-term impact of vaccination against cytomegalovirus: A modeling study. BMC Med. 2020, 18, 174. [Google Scholar] [CrossRef]
- Chen, S.J.; Wang, S.C.; Chen, Y.C. Antiviral agents as therapeutic strategies against cytomegalovirus infections. Viruses 2019, 12, 21. [Google Scholar] [CrossRef]
- Panda, K.; Parashar, D.; Viswanathan, R. An Update on Current Antiviral Strategies to Combat Human Cytomegalovirus Infection. Viruses 2023, 15, 1358. [Google Scholar] [CrossRef]
- Navarro, D.; San-Juan, R.; Manuel, O.; Giménez, E.; Fernández-Ruiz, M.; Hirsch, H.H.; Grossi, P.A.; Aguado, J.M. Cytomegalovirus infection management in solid organ transplant recipients across European centers in the time of molecular diagnostics: An ESGICH survey. Transpl. Infect. Dis. 2017, 19, e12773. [Google Scholar] [CrossRef]
- Yadav, D.K.; Adhikari, V.P.; Yadav, R.K.; Singh, A.; Huang, X.; Zhang, Q.; Pandit, P.; Ling, Q.; Liang, T. Antiviral prophylaxis or preemptive therapy for cytomegalovirus after liver transplantation?: A systematic review and meta-analysis. Front. Immunol. 2022, 13, 953210. [Google Scholar] [CrossRef]
- Razonable, R.R.; Humar, A. Cytomegalovirus in solid organ transplant recipients—Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13512. [Google Scholar] [CrossRef]
- Grossi, P.A.; Baldanti, F.; Andreoni, M.; Perno, C.F. CMV infection management in transplant patients in Italy. J. Clin. Virol. 2020, 123, 104211. [Google Scholar] [CrossRef]
- Singh, N.; Winston, D.J.; Razonable, R.R.; Lyon, G.M.; Silveira, F.P.; Wagener, M.M.; Stevens-Ayers, T.; Edmison, B.; Boeckh, M.; Limaye, A.P. Effect of Preemptive Therapy vs Antiviral Prophylaxis on Cytomegalovirus Disease in Seronegative Liver Transplant Recipients with Seropositive Donors: A Randomized Clinical Trial. JAMA 2020, 323, 1378. [Google Scholar] [CrossRef]
- Markham, A.; Faulds, D. Ganciclovir. An update of its therapeutic use in cytomegalovirus infection. Drugs 1994, 48, 455–484. [Google Scholar] [CrossRef]
- Crumpacker, C.S. Ganciclovir. N. Engl. J. Med. 1996, 335, 721–729. [Google Scholar] [CrossRef]
- Galar, A.; Valerio, M.; Catalán, P.; García-González, X.; Burillo, A.; Fernández-Cruz, A.; Zataráin, E.; Sousa-Casasnovas, I.; Anaya, F.; Rodríguez-Ferrero, M.L.; et al. Valganciclovir—Ganciclovir use and systematic therapeutic drug monitoring. An invitation to antiviral stewardship. Antibiotics 2021, 10, 77. [Google Scholar] [CrossRef]
- Kotton, C.N.; Kumar, D.; Caliendo, A.M.; Åsberg, A.; Chou, S.; Snydman, D.R.; Allen, U.; Humar, A.; Emery, V.; Lautenschlager, I.; et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation 2010, 89, 779–795. [Google Scholar] [CrossRef] [PubMed]
- Girmenia, C.; Lazzarotto, T.; Bonifazi, F.; Patriarca, F.; Irrera, G.; Ciceri, F.; Aversa, F.; Citterio, F.; Cillo, U.; Cozzi, E.; et al. Assessment and prevention of cytomegalovirus infection in allogeneic hematopoietic stem cell transplant and in solid organ transplant: A multidisciplinary consensus conference by the Italian GITMO, SITO, and AMCLI societies. Clin. Transplant. 2019, 33, e13666. [Google Scholar] [CrossRef] [PubMed]
- Samuel, E.; McNaught, K.A.; Mulbah, J.L.; HajiAlilou, H.; Mody, V.; Cates, D.W. Antiviral drugs. In Side Effects of Drugs Annual; Elsevier: Amsterdam, The Netherlands, 2022; Volume 44, pp. 291–301. ISBN 9780323989091. [Google Scholar]
- Boonsathorn, S.; Pasomsub, E.; Techasaensiri, C.; Apiwattanakul, N. Analysis of Ganciclovir-Resistant Cytomegalovirus Infection Caused by the UL97 Gene Mutation in Codons 460 and 520 in Pediatric Patients: A Case Series. Open Forum Infect. Dis. 2019, 6, ofz480. [Google Scholar] [CrossRef]
- Das, D.; Hong, J. Herpesvirus Polymerase Inhibitors. In Viral Polymerases; Academic Press: Cambridge, MA, USA, 2019; pp. 333–356. [Google Scholar] [CrossRef]
- Lea, A.P.; Bryson, H.M. Cidofovir. Drugs 1996, 52, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Paintsil, E.; Cheng, Y.C. Antiviral Agents. In Encyclopedia of Microbiology; Academic Press: Cambridge, MA, USA, 2009; pp. 223–257. [Google Scholar] [CrossRef]
- Meesing, A.; Razonable, R.R. New Developments in the Management of Cytomegalovirus Infection after Transplantation. Drugs 2018, 78, 1085–1103. [Google Scholar] [CrossRef]
- Chou, S.; Komazin-Meredith, G.; Williams, J.D.; Bowlin, T.L. Cytomegalovirus mutants resistant to ganciclovir and cidofovir differ in susceptibilities to synguanol and its 6-ether and 6-thioether derivatives. Antimicrob. Agents Chemother. 2014, 58, 1809–1812. [Google Scholar] [CrossRef]
- Bogner, E.; Egorova, A.; Makarov, V. Small Molecules—Prospective Novel HCMV Inhibitors. Viruses 2021, 13, 474. [Google Scholar] [CrossRef]
- Salvaggio, M.R.; Gnann, J.W. Drugs for Herpesvirus Infections. In Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1309–1317.e1. [Google Scholar] [CrossRef]
- Wagstaff, A.J.; Bryson, H.M. Foscarnet: A reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic use in immunocompromised patients with viral infections. Drugs 1994, 48, 199–226. [Google Scholar] [CrossRef] [PubMed]
- Avery, R.K.; Arav-Boger, R.; Marr, K.A.; Kraus, E.; Shoham, S.; Lees, L.; Trollinger, B.; Shah, P.; Ambinder, R.; Neofytos, D.; et al. Outcomes in Transplant Recipients Treated with Foscarnet for Ganciclovir-Resistant or Refractory Cytomegalovirus Infection. Transplantation 2016, 100, e74–e80. [Google Scholar] [CrossRef]
- Chrisp, P.; Clissold, S.P. Foscarnet: A review of its antiviral activity, pharmacokinetic properties and therapeutic use in immunocompromised patients with cytomegalovirus retinitis. Drugs 1991, 41, 104–129. [Google Scholar] [CrossRef]
- Chou, S. Foscarnet resistance mutations mapping to atypical domains of the cytomegalovirus DNA polymerase gene. Antivir. Res. 2017, 138, 57. [Google Scholar] [CrossRef] [PubMed]
- Kimberlin, D.W. Antiviral Agents. In Principles and Practice of Pediatric Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1583–1598.e6. [Google Scholar] [CrossRef]
- Lischka, P.; Hewlett, G.; Wunberg, T.; Baumeister, J.; Paulsen, D.; Goldner, T.; Ruebsamen-Schaeff, H.; Zimmermann, H. In vitro and in vivo activities of the novel anticytomegalovirus compound AIC246. Antimicrob. Agents Chemother. 2010, 54, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Melendez, D.P.; Razonable, R.R. Letermovir and inhibitors of the terminase complex: A promising new class of investigational antiviral drugs against human cytomegalovirus. Infect. Drug Resist. 2015, 8, 269. [Google Scholar] [CrossRef] [PubMed]
- Raglow, Z.; Kaul, D.R. A New Antiviral Option for Cytomegalovirus Prevention after Kidney Transplant. JAMA 2023, 330, 27–29. [Google Scholar] [CrossRef]
- Shigle, T.L.; Handy, V.W.; Chemaly, R.F. Letermovir and its role in the prevention of cytomegalovirus infection in seropositive patients receiving an allogeneic hematopoietic cell transplant. Ther. Adv. Hematol. 2020, 11, 204062072093715. [Google Scholar] [CrossRef]
- El Helou, G.; Razonable, R.R. Letermovir for the prevention of cytomegalovirus infection and disease in transplant recipients: An evidence-based review. Infect. Drug Resist. 2019, 12, 1481–1491. [Google Scholar] [CrossRef]
- Santos Bravo, M.; Tilloy, V.; Plault, N.; Palomino, S.S.; Mosquera, M.M.; Navarro Gabriel, M.; Fernández Avilés, F.; Suárez Lledó, M.; Rovira, M.; Moreno, A.; et al. Assessment of UL56 Mutations before Letermovir Therapy in Refractory Cytomegalovirus Transplant Recipients. Microbiol. Spectr. 2022, 10, e00191-22. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, E.; Sidler, D.; Dahdal, S.; Bittel, P.; Suter-Riniker, F.; Manuel, O.; Walti, L.N.; Hirzel, C. Emergence of letermovir resistance in solid organ transplant recipients with ganciclovir resistant cytomegalovirus infection: A case series and review of the literature. Transpl. Infect. Dis. 2021, 23, e13515. [Google Scholar] [CrossRef] [PubMed]
- Halpern-Cohen, V.; Blumberg, E.A. New Perspectives on Antimicrobial Agents: Maribavir. Antimicrob. Agents Chemother. 2022, 66, e02405-21. [Google Scholar] [CrossRef]
- Avery, R.K.; Alain, S.; Alexander, B.D.; Blumberg, E.A.; Chemaly, R.F.; Cordonnier, C.; Duarte, R.F.; Florescu, D.F.; Kamar, N.; Kumar, D.; et al. Maribavir for Refractory Cytomegalovirus Infections with or without Resistance Post-Transplant: Results From a Phase 3 Randomized Clinical Trial. Clin. Infect. Dis. 2022, 75, 690–701. [Google Scholar] [CrossRef]
- Kang, C. Maribavir: First Approval. Drugs 2022, 82, 335–340. [Google Scholar] [CrossRef]
- Maertens, J.; Cordonnier, C.; Jaksch, P.; Poiré, X.; Uknis, M.; Wu, J.; Wijatyk, A.; Saliba, F.; Witzke, O.; Villano, S. Maribavir for Preemptive Treatment of Cytomegalovirus Reactivation. N. Engl. J. Med. 2019, 381, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Peck, R.W.; Yin, Y.; Allanson, J.; Wiggs, R.; Wire, M.B. Phase I safety and pharmacokinetic trials of 1263W94, a novel oral anti-human cytomegalovirus agent, in healthy and human immunodeficiency virus-infected subjects. Antimicrob. Agents Chemother. 2003, 47, 1334–1342. [Google Scholar] [CrossRef]
- Chou, S.; Alain, S.; Cervera, C.; Chemaly, R.F.; Kotton, C.N.; Lundgren, J.; Papanicolaou, G.A.; Pereira, M.R.; Wu, J.J.; Murray, R.A.; et al. Drug Resistance Assessed in a Phase 3 Clinical Trial of Maribavir Therapy for Refractory or Resistant Cytomegalovirus Infection in Transplant Recipients. J. Infect. Dis. 2023, jiad293. [Google Scholar] [CrossRef]
- Chou, S.; Song, K.; Wu, J.; Bo, T.; Crumpacker, C. Drug Resistance Mutations and Associated Phenotypes Detected in Clinical Trials of Maribavir for Treatment of Cytomegalovirus Infection. J. Infect. Dis. 2022, 226, 576–584. [Google Scholar] [CrossRef]
- Komazin, G.; Townsend, L.B.; Drach, J.C. Role of a Mutation in Human Cytomegalovirus Gene UL104 in Resistance to Benzimidazole Ribonucleosides. J. Virol. 2004, 78, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Schleiss, M.R.; Bernstein, D.I.; Mcvoy, M.A.; Stroup, G.; Bravo, F.; Creasy, B.; Mcgregor, A.; Henninger, K.; Hallenberger, S. The non-nucleoside antiviral, BAY 38-4766, protects against cytomegalovirus (CMV) disease and mortality in immunocompromised guinea pigs. Antivir. Res. 2005, 65, 35–43. [Google Scholar] [CrossRef]
- Weber, O.; Bender, W.; Eckenberg, P.; Goldmann, S.; Haerter, M.; Hallenberger, S.; Henninger, K.; Reefschläger, J.; Trappe, J.; Witt-Laido, A.; et al. Inhibition of murine cytomegalovirus and human cytomegalovirus by a novel non-nucleosidic compound in vivo. Antivir. Res. 2001, 49, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Kosobucki, B.R.; Freeman, W.R. Retinal Disease in HIV-infected Patients. In Retina, 4th ed.; Mosby: Maryland Heights, MO, USA, 2006; Volume 2–3, pp. 1625–1672. [Google Scholar] [CrossRef]
- Townsend, L.B.; Devivar, R.V.; Turk, S.R.; Nassiri, M.R.; Drach, J.C. Design, synthesis, and antiviral activity of certain 2,5,6-trihalo-1-(beta-D-ribofuranosyl)benzimidazoles. J. Med. Chem. 1995, 38, 4098–4105. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.R.; Ferris, R.G.; Selleseth, D.W.; Davis, M.G.; Drach, J.C.; Townsend, L.B.; Biron, K.K.; Boyd, F.L. Mechanism of action of the ribopyranoside benzimidazole GW275175X against human cytomegalovirus. Antimicrob. Agents Chemother. 2004, 48, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- McVoy, M.A.; Nixon, D.E. Impact of 2-Bromo-5,6-Dichloro-1-β-d-Ribofuranosyl Benzimidazole Riboside and Inhibitors of DNA, RNA, and Protein Synthesis on Human Cytomegalovirus Genome Maturation. J. Virol. 2005, 79, 11115–11127. [Google Scholar] [CrossRef] [PubMed]
- Nixon, D.E.; McVoy, M.A. Dramatic effects of 2-bromo-5,6-dichloro-1-beta-D-ribofuranosyl benzimidazole riboside on the genome structure, packaging, and egress of guinea pig cytomegalovirus. J. Virol. 2004, 78, 1623–1635. [Google Scholar] [CrossRef]
- Gugliesi, F.; Coscia, A.; Griffante, G.; Galitska, G.; Pasquero, S.; Albano, C.; Biolatti, M. Where do we stand after decades of studying human cytomegalovirus? Microorganisms 2020, 8, 685. [Google Scholar] [CrossRef]
- Hannon, G.J. RNA interference. Nature 2002, 418, 244–251. [Google Scholar] [CrossRef]
- Carthew, R.W.; Sontheimer, E.J. Leading Edge Review Origins and Mechanisms of miRNAs and siRNAs. Cell 2009, 136, 642–655. [Google Scholar] [CrossRef]
- Wiebusch, L.; Truss, M.; Hagemeier, C. Inhibition of human cytomegalovirus replication by small interfering RNAs. J. Gen. Virol. 2004, 85, 179–184. [Google Scholar] [CrossRef]
- Xiaofei, E.; Stadler, B.M.; Debatis, M.; Wang, S.; Lu, S.; Kowalik, T.F. RNA Interference-Mediated Targeting of Human Cytomegalovirus Immediate-Early or Early Gene Products Inhibits Viral Replication with Differential Effects on Cellular Functions. J. Virol. 2012, 86, 5660–5673. [Google Scholar] [CrossRef]
- Balakrishnan, K.N.; Abdullah, A.A.; Bala, J.A.; Jesse, F.F.A.; Abdullah, C.A.C.; Noordin, M.M.; Mohd-Azmi, M.L. Multiple gene targeting siRNAs for down regulation of Immediate Early-2 (Ie2) and DNA polymerase genes mediated inhibition of novel rat Cytomegalovirus (strain All-03). Virol. J. 2020, 17, 164. [Google Scholar] [CrossRef]
- Møller, R.; Schwarz, T.M.; Noriega, V.M.; Panis, M.; Sachs, D.; Tortorella, D.; tenOever, B.R. miRNA-mediated targeting of human cytomegalovirus reveals biological host and viral targets of IE2. Proc. Natl. Acad. Sci. USA 2018, 115, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Skilandat, M.; Zelger-Paulus, S.; Sigel, R.K.O. Ribozymes☆. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-0-12-809633-8. [Google Scholar]
- Mulhbacher, J.; St-Pierre, P.; Lafontaine, D.A. Therapeutic applications of ribozymes and riboswitches. Curr. Opin. Pharmacol. 2010, 10, 551–556. [Google Scholar] [CrossRef] [PubMed]
- James, H.A.; Gibson, I. The Therapeutic Potential of Ribozymes. Blood 1998, 91, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Lilley, D.M.J. Classification of the nucleolytic ribozymes based upon catalytic mechanism [version 1; peer review: 3 approved]. F1000Research 2019, 8. [Google Scholar] [CrossRef]
- Kim, K.; Trang, P.; Umamoto, S.; Hai, R.; Liu, F. RNase P ribozyme inhibits cytomegalovirus replication by blocking the expression of viral capsid proteins. Nucleic Acids Res. 2004, 32, 3427–3434. [Google Scholar] [CrossRef]
- Sun, X.; Chen, W.; He, L.; Sheng, J.; Liu, Y.; Vu, G.P.; Yang, Z.; Li, W.; Trang, P.; Wang, Y.; et al. Inhibition of human cytomegalovirus immediate early gene expression and growth by a novel RNase P ribozyme variant. PLoS ONE 2017, 12, e0186791. [Google Scholar] [CrossRef]
- Yang, Z.; Reeves, M.; Ye, J.; Trang, P.; Zhu, L.; Sheng, J.; Wang, Y.; Zen, K.; Wu, J.; Liu, F. RNase P Ribozymes Inhibit the Replication of Human Cytomegalovirus by Targeting Essential Viral Capsid Proteins. Viruses 2015, 7, 3345–3360. [Google Scholar] [CrossRef]
- Ni, X.; Castanares, M.; Mukherjee, A.; Lupold, S.E. Nucleic Acid Aptamers: Clinical Applications and Promising New Horizons. Curr. Med. Chem. 2011, 18, 4206–4214. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, N.; Lischka, P.; Wagenknecht, N.; Stamminger, T. Inhibition of Human Cytomegalovirus Replication via Peptide Aptamers Directed against the Nonconventional Nuclear Localization Signal of the Essential Viral Replication Factor pUL84. J. Virol. 2009, 83, 11902–11913. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mondal, D. Fomivirsen. Ref. Modul. Biomed. Sci. 2016. [Google Scholar] [CrossRef]
- Kozak, I.; Allen McCutchan, J.; Freeman, W.R. HIV-Associated Infections. In Retina, 5th ed.; Saunders: Philadelphia, PA, USA, 2013; pp. 1441–1472. [Google Scholar] [CrossRef]
- Ede, D.R.; Farhang, N.; Stover, J.D.; Bowles, R.D. 4.32 Gene Editing Tools. Compr. Biomater. II 2017, 4, 589–599. [Google Scholar] [CrossRef]
- Becker, S.; Boch, J. TALE and TALEN genome editing technologies. Gene Genome Ed. 2021, 2, 100007. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Nain, V. TALENs-an indispensable tool in the era of CRISPR: A mini review. J. Genet. Eng. Biotechnol. 2021, 19, 125. [Google Scholar] [CrossRef] [PubMed]
- Gaj, T.; Gersbach, C.A.; Barbas Iii, C.F. ZFN, TALEN and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol 2013, 31, 397–405. [Google Scholar] [CrossRef]
- Khan, S.H. Genome-Editing Technologies: Concept, Pros, and Cons of Various Genome-Editing Techniques and Bioethical Concerns for Clinical Application. Mol. Ther. Nucleic Acids 2019, 16, 326–334. [Google Scholar] [CrossRef]
- Joung, J.K.; Sander, J.D. TALENs: A widely applicable technology for targeted genome editing. Nat. Rev. Mol. Cell Biol. 2013, 14, 49–55. [Google Scholar] [CrossRef]
- Perez, E.E.; Wang, J.; Miller, J.C.; Jouvenot, Y.; Kim, K.A.; Liu, O.; Wang, N.; Lee, G.; Bartsevich, V.V.; Lee, Y.L.; et al. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat. Biotechnol. 2008, 26, 808. [Google Scholar] [CrossRef]
- Lista, M.J.; Witney, A.A.; Nichols, J.; Davison, A.J.; Wilson, H.; Latham, K.A.; Ravenhill, B.J.; Nightingale, K.; Stanton, R.J.; Weekes, M.P.; et al. Strain-Dependent Restriction of Human Cytomegalovirus by Zinc Finger Antiviral Proteins. J. Virol. 2023, 97, e01846-22. [Google Scholar] [CrossRef]
- Chen, S.-J.; Chen, Y.-C. Potential Application of TALENs against Murine Cytomegalovirus Latent Infections. Viruses 2019, 11, 414. [Google Scholar] [CrossRef] [PubMed]
- Redman, M.; King, A.; Watson, C.; King, D. What is CRISPR/Cas9? Arch. Dis. Child.-Educ. Pract. 2016, 101, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Asmamaw, M.; Zawdie, B. Mechanism and Applications of CRISPR/Cas-9-Mediated Genome Editing. Biologics 2021, 15, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Deng, J.; Zhang, Q.; Ma, P.; Lv, L.; Zhang, Y.; Li, C.; Zhang, Y. Targeting human cytomegalovirus IE genes by CRISPR/Cas9 nuclease effectively inhibits viral replication and reactivation. Arch. Virol. 2020, 165, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Natesan, J.S. Suganthini Krishnan Using CRISPR technology to inhibit the replication of human cytomegalovirus by deletion of a gene promoter. J. Emerg. Investig. 2021, 4, 1–5. [Google Scholar]
- King, M.W.; Munger, J. Editing the human cytomegalovirus genome with the CRISPR/Cas9 system. Virology 2019, 529, 186–194. [Google Scholar] [CrossRef]
- Esposito, S.; Amirthalingam, G.; Bassetti, M.; Blasi, F.; De Rosa, F.G.; Halasa, N.B.; Hung, I.; Osterhaus, A.; Tan, T.; Torres, J.P.; et al. Monoclonal antibodies for prophylaxis and therapy of respiratory syncytial virus, SARS-CoV-2, human immunodeficiency virus, rabies and bacterial infections: An update from the World Association of Infectious Diseases and Immunological Disorders and the It. Front. Immunol. 2023, 14, 1162342. [Google Scholar] [CrossRef]
- Pantaleo, G.; Correia, B.; Fenwick, C.; Joo, V.S.; Perez, L. Antibodies to combat viral infections: Development strategies and progress. Nat. Rev. Drug Discov. 2022, 21, 676–696. [Google Scholar] [CrossRef]
- Crowe, J.E. Human Antibodies for Viral Infections. Annu. Rev. Immunol. 2022, 40, 349–386. [Google Scholar] [CrossRef]
- Maertens, J.; Logan, A.C.; Jang, J.; Long, G.; Tang, J.-L.; Hwang, W.Y.K.; Koh, L.P.; Chemaly, R.; Gerbitz, A.; Winkler, J.; et al. Phase 2 Study of Anti-Human Cytomegalovirus Monoclonal Antibodies for Prophylaxis in Hematopoietic Cell Transplantation. Antimicrob. Agents Chemother. 2020, 64, 10–128. [Google Scholar] [CrossRef]
- Britt, W.J.; Jarvis, M.A.; Drummond, D.D.; Mach, M. Antigenic domain 1 is required for oligomerization of human cytomegalovirus glycoprotein B. J. Virol. 2005, 79, 4066–4079. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Okamoto, M.; Kurino, R.; Miura, R.; Takada, K. A fully human neutralizing monoclonal antibody targeting a highly conserved epitope of the human cytomegalovirus glycoprotein B. PLoS ONE 2023, 18, e0285672. [Google Scholar] [CrossRef]
- Parsons, A.J.; Ophir, S.I.; Duty, J.A.; Kraus, T.A.; Stein, K.R.; Moran, T.M.; Tortorella, D. Development of broadly neutralizing antibodies targeting the cytomegalovirus subdominant antigen gH. Commun. Biol. 2022, 5, 387. [Google Scholar] [CrossRef] [PubMed]
- McVoy, M.A. Cytomegalovirus vaccines. Clin. Infect. Dis. 2013, 57 (Suppl. 4), S196–S199. [Google Scholar] [CrossRef] [PubMed]
- Moseley, P.; Klenerman, P.; Kadambari, S. Indirect effects of cytomegalovirus infection: Implications for vaccine development. Rev. Med. Virol. 2023, 33, e2405. [Google Scholar] [CrossRef]
- Griffiths, P.D.; Stanton, A.; McCarrell, E.; Smith, C.; Osman, M.; Harber, M.; Davenport, A.; Jones, G.; Wheeler, D.C.; O’Beirne, J.; et al. Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: A phase 2 randomised placebo-controlled trial. Lancet 2011, 377, 1256–1263. [Google Scholar] [CrossRef]
- Gomes, A.C.; Baraniak, I.A.; Lankina, A.; Moulder, Z.; Holenya, P.; Atkinson, C.; Tang, G.; Mahungu, T.; Kern, F.; Griffiths, P.D.; et al. The cytomegalovirus gB/MF59 vaccine candidate induces antibodies against an antigenic domain controlling cell-to-cell spread. Nat. Commun. 2023, 14, 1041. [Google Scholar] [CrossRef]
- Plotkin, S.A.; Wang, D.; Oualim, A.; Diamond, D.J.; Kotton, C.N.; Mossman, S.; Carfi, A.; Anderson, D.; Dormitzer, P.R. The Status of Vaccine Development Against the Human Cytomegalovirus. J. Infect. Dis. 2020, 221, S113–S122. [Google Scholar] [CrossRef]
- Goodrum, F. Human Cytomegalovirus Latency: Approaching the Gordian Knot. Annu. Rev. Virol. 2016, 3, 333–357. [Google Scholar] [CrossRef]
- Krishna, B.A.; Wills, M.R.; Sinclair, J.H. Advances in the treatment of cytomegalovirus. Br. Med. Bull. 2019, 131, 5–17. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).