Signaling and Detoxification Strategies in Plant-Microbes Symbiosis under Heavy Metal Stress: A Mechanistic Understanding
Abstract
1. Introduction
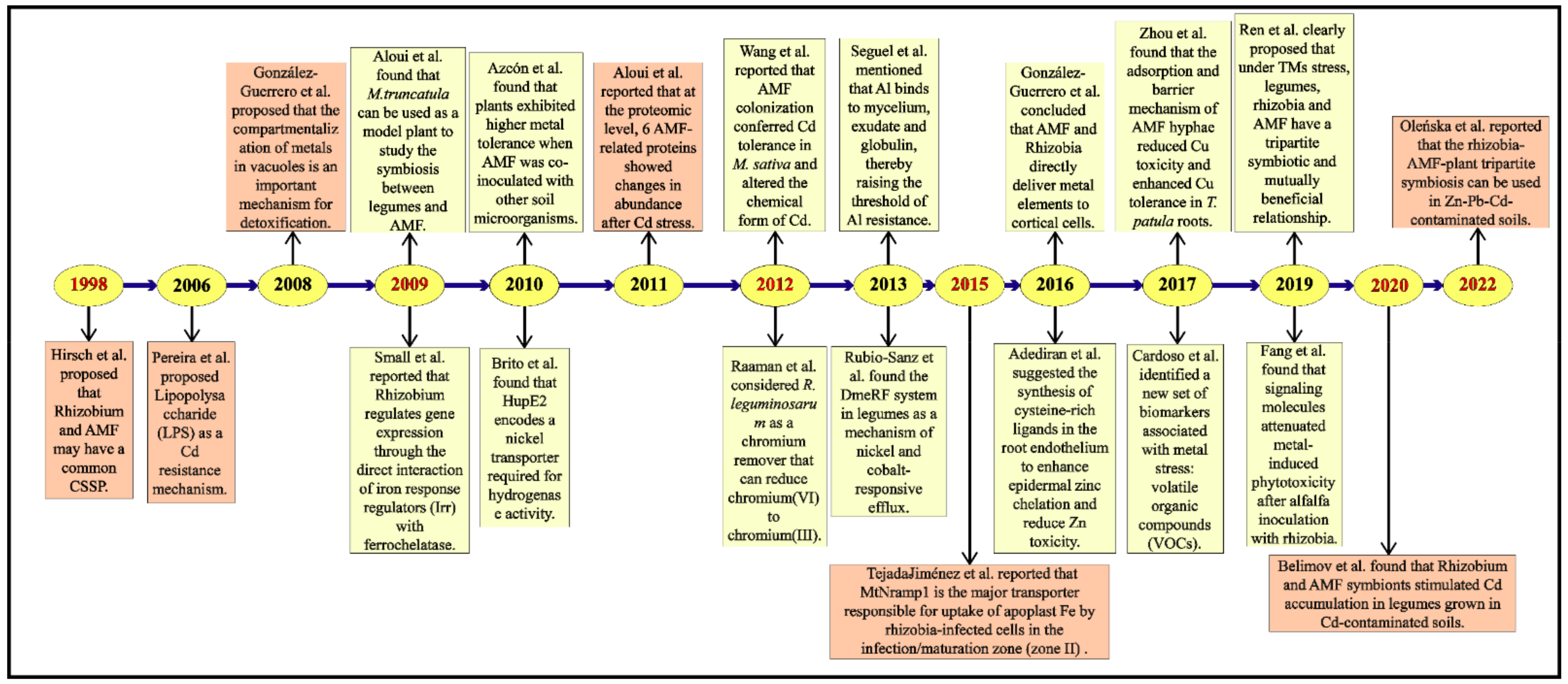
2. AMF and Rhizobium Co-Mediate Signal Transduction Pathway in Plant Cells
2.1. The Entry Point of the Symbiotic Pathway: SYMRK
2.2. Extranuclear Cation Channels: CASTOR and POLLUX
2.3. Co-Utilization of Nucleoporin Channels
2.4. Intranuclear Signaling: CCaMK and the Coiled-Coil Protein CYCLOPS
3. Detoxification Regulation Strategies of Rhizobium Symbiosis and AMF Symbiosis under Toxic Metal Stress
3.1. Cell Wall Adsorption and Siderophore Chelation
3.2. Gating Guards: The Extracellular Polymer EPS
3.3. Binding of TMs to Metallothioneins
3.4. Metal Detoxification by Redox
4. Plant-AMF-Rhizobium Work Together to Regulate Metal Homeostasis
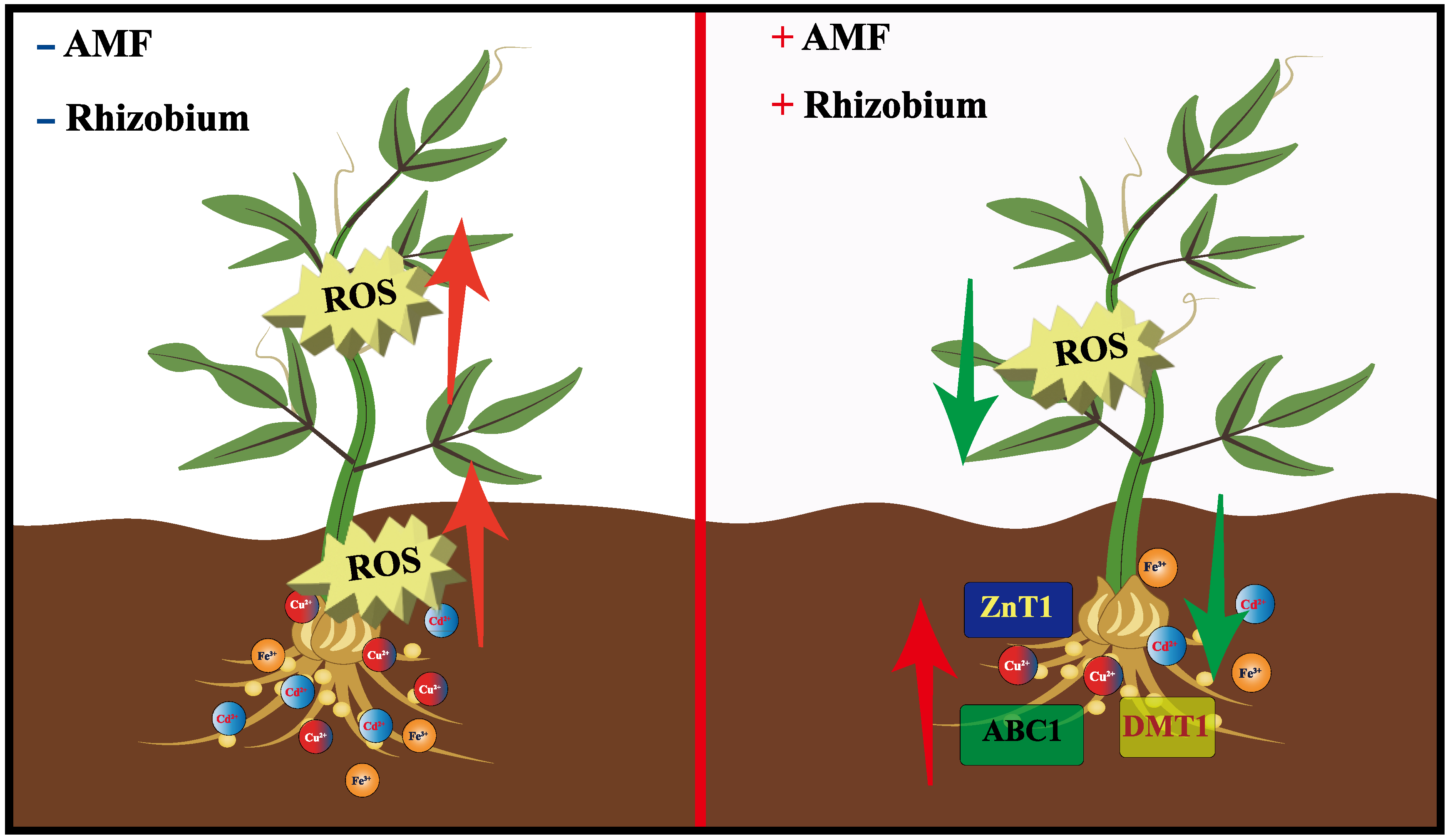
4.1. Effects of the Synergistic Antagonistic Relationship between Rhizobium and AMF on Plants
4.2. AMF-Rhizobium Activates Plant Transporter Proteins to Enhance Metal Tolerance
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, G.; Niu, Z.; Yu, J.; Li, Z.; Ma, J.; Xiang, P. Soil heavy metal pollution and food safety in China: Effects, sources and removing technology. Chemosphere 2021, 267, 129205. [Google Scholar] [CrossRef]
- Li, K.; Wang, J.; Zhang, Y. Heavy metal pollution risk of cultivated land from industrial production in China: Spatial pattern and its enlightenment. Sci. Total Environ. 2022, 828, 154382. [Google Scholar] [CrossRef] [PubMed]
- Tufail, M.A.; Iltaf, J.; Zaheer, T.; Tariq, L.; Amir, M.B.; Fatima, R.; Asbat, A.; Kabeer, T.; Fahad, M.; Naeem, H.; et al. Recent advances in bioremediation of heavy metals and persistent organic pollutants: A review. Sci. Total Environ. 2022, 850, 157961. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, J.; Yang, Y.; Li, S.; Wang, T.; Oleksak, P.; Chrienova, Z.; Wu, Q.; Nepovimova, E.; Zhang, X.; et al. Phytoremediation of heavy metal pollution: Hotspots and future prospects. Ecotoxicol. Environ. Saf. 2022, 234, 113403. [Google Scholar] [CrossRef]
- Zhu, Y.; Dong, Y.; Zhu, N.; Jin, H. Foliar application of biosynthetic nano-selenium alleviates the toxicity of Cd, Pb, and Hg in Brassica chinensis by inhibiting heavy metal adsorption and improving antioxidant system in plant. Ecotoxicol. Environ. Saf. 2022, 240, 113681. [Google Scholar] [CrossRef]
- Ojuederie, O.B.; Babalola, O.O. Microbial and Plant-Assisted Bioremediation of Heavy Metal Polluted Environments: A Review. Int. J. Environ. Res. Public Health 2017, 14, 1504. [Google Scholar] [CrossRef]
- Zakaria, Z.; Zulkafflee, N.S.; Mohd Redzuan, N.A.; Selamat, J.; Ismail, M.R.; Praveena, S.M.; Tóth, G.; Abdull Razis, A.F. Understanding Potential Heavy Metal Contamination, Absorption, Translocation and Accumulation in Rice and Human Health Risks. Plants 2021, 10, 1070. [Google Scholar] [CrossRef]
- Asgari, K.; Cornelis, W.M. Heavy metal accumulation in soils and grains, and health risks associated with use of treated municipal wastewater in subsurface drip irrigation. Environ. Monit. Assess. 2015, 187, 410. [Google Scholar] [CrossRef]
- Mao, Y.; Tan, H.; Wang, M.; Jiang, T.; Wei, H.; Xu, W.; Jiang, Q.; Bao, H.; Ding, Y.; Wang, F.; et al. Research Progress of Soil Microorganisms in Response to Heavy Metals in Rice. J. Agric. Food Chem. 2022, 70, 8513–8522. [Google Scholar] [CrossRef]
- Abhilash, P.C.; Powell, J.R.; Singh, H.B.; Singh, B.K. Plant-microbe interactions: Novel applications for exploitation in multipurpose remediation technologies. Trends Biotechnol. 2012, 30, 416–420. [Google Scholar] [CrossRef]
- Shetty, K.G.; Hetrick, B.A.; Figge, D.A.; Schwab, A.P. Effects of mycorrhizae and other soil microbes on revegetation of heavy metal contaminated mine spoil. Environmental pollution (Barking, Essex: 1987) 1994, 86, 181–188. [Google Scholar] [CrossRef]
- Bonfante, P.; Venice, F.; Lanfranco, L. The mycobiota: Fungi take their place between plants and bacteria. Curr. Opin. Microbiol. 2019, 49, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.J. Signaling in the arbuscular mycorrhizal symbiosis. Annu. Rev. Microbiol. 2005, 59, 19–42. [Google Scholar] [CrossRef] [PubMed]
- Brundrett, M.C. Coevolution of roots and mycorrhizas of land plants. New Phytol. 2002, 154, 275–304. [Google Scholar] [CrossRef]
- Kistner, C.; Parniske, M. Evolution of signal transduction in intracellular symbiosis. Trends Plant Sci. 2002, 7, 511–518. [Google Scholar] [CrossRef]
- Op den Camp, R.; Streng, A.; De Mita, S.; Cao, Q.; Polone, E.; Liu, W.; Ammiraju, J.S.; Kudrna, D.; Wing, R.; Untergasser, A.; et al. LysM-type mycorrhizal receptor recruited for rhizobium symbiosis in nonlegume Parasponia. Science 2011, 331, 909–912. [Google Scholar] [CrossRef]
- Aloui, A.; Recorbet, G.; Gollotte, A.; Robert, F.; Valot, B.; Gianinazzi-Pearson, V.; Aschi-Smiti, S.; Dumas-Gaudot, E. On the mechanisms of cadmium stress alleviation in Medicago truncatula by arbuscular mycorrhizal symbiosis: A root proteomic study. Proteomics 2009, 9, 420–433. [Google Scholar] [CrossRef]
- Ferrol, N.; Tamayo, E.; Vargas, P. The heavy metal paradox in arbuscular mycorrhizas: From mechanisms to biotechnological applications. J. Exp. Bot. 2016, 67, 6253–6265. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.Q.; Yan, X.W.; Wei, G.H.; Zhang, J.H.; Fang, L.C. Rhizobium inoculation enhances copper tolerance by affecting copper uptake and regulating the ascorbate-glutathione cycle and phytochelatin biosynthesis-related gene expression in Medicago sativa seedlings. Ecotoxicol. Environ. Saf. 2018, 162, 312–323. [Google Scholar] [CrossRef]
- Ju, W.; Liu, L.; Fang, L.; Cui, Y.; Duan, C.; Wu, H. Impact of co-inoculation with plant-growth-promoting rhizobacteria and rhizobium on the biochemical responses of alfalfa-soil system in copper contaminated soil. Ecotoxicol. Environ. Saf. 2019, 167, 218–226. [Google Scholar] [CrossRef]
- Lanfranco, L.; Bonfante, P.; Genre, A. The Mutualistic Interaction between Plants and Arbuscular Mycorrhizal Fungi. Microbiol. Spectr. 2016, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Cardini, A.; Pellegrino, E.; Declerck, S.; Calonne-Salmon, M.; Mazzolai, B.; Ercoli, L. Direct transfer of zinc between plants is channelled by common mycorrhizal network of arbuscular mycorrhizal fungi and evidenced by changes in expression of zinc transporter genes in fungus and plant. Environ. Microbiol. 2021, 23, 5883–5900. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Gao, H.; Wang, H.; Guo, Y.; He, M.; Peng, Y.; Wang, X. GSK3-mediated stress signaling inhibits legume-rhizobium symbiosis by phosphorylating GmNSP1 in soybean. Mol. Plant 2021, 14, 488–502. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lan, L.; Jin, Y.; Yu, N.; Wang, D.; Wang, E. Mechanisms underlying legume-rhizobium symbioses. J. Integr. Plant Biol. 2022, 64, 244–267. [Google Scholar] [CrossRef] [PubMed]
- Colombo, R.P.; Benavidez, M.E.; Fernandez Bidondo, L.; Silvani, V.A.; Bompadre, M.J.; Statello, M.; Scorza, M.V.; Scotti, A.; Godeas, A.M. Arbuscular mycorrhizal fungi in heavy metal highly polluted soil in the Riachuelo river basin. Rev. Argent. De Microbiol. 2020, 52, 145–149. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, Z.; Hao, B.; Diao, F.; Zhang, J.; Bao, Z.; Guo, W. Arbuscular mycorrhizal fungi alter microbiome structure of rhizosphere soil to enhance maize tolerance to La. Ecotoxicol. Environ. Saf. 2021, 212, 111996. [Google Scholar] [CrossRef]
- Hao, X.; Taghavi, S.; Xie, P.; Orbach, M.J.; Alwathnani, H.A.; Rensing, C.; Wei, G. Phytoremediation of heavy and transition metals aided by legume-rhizobia symbiosis. Int. J. Phytoremediation 2014, 16, 179–202. [Google Scholar] [CrossRef]
- Fan, M.; Xiao, X.; Guo, Y.; Zhang, J.; Wang, E.; Chen, W.; Lin, Y.; Wei, G. Enhanced phytoremdiation of Robinia pseudoacacia in heavy metal-contaminated soils with rhizobia and the associated bacterial community structure and function. Chemosphere 2018, 197, 729–740. [Google Scholar] [CrossRef]
- Gomes, M.P.; Marques, R.Z.; Nascentes, C.C.; Scotti, M.R. Synergistic effects between arbuscular mycorrhizal fungi and rhizobium isolated from As-contaminated soils on the As-phytoremediation capacity of the tropical woody legume Anadenanthera peregrina. Int. J. Phytoremediation 2020, 22, 1362–1371. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, A.; Wang, F.; Han, X.; Wang, D.; Li, S. Arbuscular mycorrhizal fungi and rhizobium facilitate nitrogen uptake and transfer in soybean/maize intercropping system. Front. Plant Sci. 2015, 6, 339. [Google Scholar] [CrossRef]
- Ortíz, J.; Sanhueza, C.; Romero-Munar, A.; Hidalgo-Castellanos, J.; Castro, C.; Bascuñán-Godoy, L.; Coba de la Peña, T.; López-Gómez, M.; Florez-Sarasa, I.; Del-Saz, N.F. In Vivo Metabolic Regulation of Alternative Oxidase under Nutrient Deficiency-Interaction with Arbuscular Mycorrhizal Fungi and Rhizobium Bacteria. Int. J. Mol. Sci. 2020, 21, 4201. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, M.M.; Azcón, R.; Barea, J.M. Compatibility of a wild type and its genetically modified Sinorhizobium strain with two mycorrhizal fungi on Medicago species as affected by drought stress. Plant Sci. Int. J. Exp. Plant Biol. 2001, 161, 347–358. [Google Scholar] [CrossRef]
- Hirsch, A.M.; Kapulnik, Y. Signal Transduction Pathways in Mycorrhizal Associations: Comparisons with the Rhizobium-Legume Symbiosis. Fungal Genet. Biol. FG B 1998, 23, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.I.; Lima, A.I.; Figueira, E.M. Screening possible mechanisms mediating cadmium resistance in Rhizobium leguminosarum bv. viciae isolated from contaminated Portuguese soils. Microb. Ecol. 2006, 52, 176–186. [Google Scholar] [CrossRef] [PubMed]
- González-Guerrero, M.; Melville, L.H.; Ferrol, N.; Lott, J.N.; Azcón-Aguilar, C.; Peterson, R.L. Ultrastructural localization of heavy metals in the extraradical mycelium and spores of the arbuscular mycorrhizal fungus Glomus intraradices. Can. J. Microbiol. 2008, 54, 103–110. [Google Scholar] [CrossRef]
- Small, S.K.; Puri, S.; O’Brian, M.R. Heme-dependent metalloregulation by the iron response regulator (Irr) protein in Rhizobium and other Alpha-proteobacteria. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2009, 22, 89–97. [Google Scholar] [CrossRef]
- Azcón, R.; Perálvarez Mdel, C.; Roldán, A.; Barea, J.M. Arbuscular mycorrhizal fungi, Bacillus cereus, and Candida parapsilosis from a multicontaminated soil alleviate metal toxicity in plants. Microb. Ecol. 2010, 59, 668–677. [Google Scholar] [CrossRef]
- Brito, B.; Prieto, R.I.; Cabrera, E.; Mandrand-Berthelot, M.A.; Imperial, J.; Ruiz-Argüeso, T.; Palacios, J.M. Rhizobium leguminosarum hupE encodes a nickel transporter required for hydrogenase activity. J. Bacteriol. 2010, 192, 925–935. [Google Scholar] [CrossRef]
- Aloui, A.; Recorbet, G.; Robert, F.; Schoefs, B.; Bertrand, M.; Henry, C.; Gianinazzi-Pearson, V.; Dumas-Gaudot, E.; Aschi-Smiti, S. Arbuscular mycorrhizal symbiosis elicits shoot proteome changes that are modified during cadmium stress alleviation in Medicago truncatula. BMC Plant Biol. 2011, 11, 75. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J.; Gao, Y. Arbuscular mycorrhizal colonization alters subcellular distribution and chemical forms of cadmium in Medicago sativa L. and resists cadmium toxicity. PLoS ONE 2012, 7, e48669. [Google Scholar] [CrossRef]
- Raaman, N.; Mahendran, B.; Jaganathan, C.; Sukumar, S.; Chandrasekaran, V. Removal of chromium using Rhizobium leguminosarum. World J. Microbiol. Biotechnol. 2012, 28, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Seguel, A.; Cumming, J.R.; Klugh-Stewart, K.; Cornejo, P.; Borie, F. The role of arbuscular mycorrhizas in decreasing aluminium phytotoxicity in acidic soils: A review. Mycorrhiza 2013, 23, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Sanz, L.; Prieto, R.I.; Imperial, J.; Palacios, J.M.; Brito, B. Functional and expression analysis of the metal-inducible dmeRF system from Rhizobium leguminosarum bv. viciae. Appl. Environ. Microbiol. 2013, 79, 6414–6422. [Google Scholar] [CrossRef] [PubMed]
- Tejada-Jiménez, M.; Castro-Rodríguez, R.; Kryvoruchko, I.; Lucas, M.M.; Udvardi, M.; Imperial, J.; González-Guerrero, M. Medicago truncatula natural resistance-associated macrophage Protein1 is required for iron uptake by rhizobia-infected nodule cells. Plant. Physiol. 2015, 168, 258–272. [Google Scholar] [CrossRef] [PubMed]
- González-Guerrero, M.; Escudero, V.; Saéz, Á.; Tejada-Jiménez, M. Transition Metal Transport in Plants and Associated Endosymbionts: Arbuscular Mycorrhizal Fungi and Rhizobia. Front. Plant Sci. 2016, 7, 1088. [Google Scholar] [CrossRef]
- Adediran, G.A.; Ngwenya, B.T.; Mosselmans, J.F.; Heal, K.V. Bacteria-zinc co-localization implicates enhanced synthesis of cysteine-rich peptides in zinc detoxification when Brassica juncea is inoculated with Rhizobium leguminosarum. New Phytol. 2016, 209, 280–293. [Google Scholar] [CrossRef]
- Zhou, X.; Fu, L.; Xia, Y.; Zheng, L.; Chen, C.; Shen, Z.; Chen, Y. Arbuscular mycorrhizal fungi enhance the copper tolerance of Tagetes patula through the sorption and barrier mechanisms of intraradical hyphae. Met. Integr. Biometal Sci. 2017, 9, 936–948. [Google Scholar] [CrossRef]
- Cardoso, P.; Santos, M.; Freitas, R.; Rocha, S.M.; Figueira, E. Response of Rhizobium to Cd exposure: A volatile perspective. Environ. Pollut. 2017, 231, 802–811. [Google Scholar] [CrossRef]
- Ren, C.G.; Kong, C.C.; Wang, S.X.; Xie, Z.H. Enhanced phytoremediation of uranium-contaminated soils by arbuscular mycorrhiza and rhizobium. Chemosphere 2019, 217, 773–779. [Google Scholar] [CrossRef]
- Fang, L.; Ju, W.; Yang, C.; Duan, C.; Cui, Y.; Han, F.; Shen, G.; Zhang, C. Application of signaling molecules in reducing metal accumulation in alfalfa and alleviating metal-induced phytotoxicity in Pb/Cd-contaminated soil. Ecotoxicol. Environ. Saf. 2019, 182, 109459. [Google Scholar] [CrossRef]
- Belimov, A.A.; Shaposhnikov, A.I.; Azarova, T.S.; Makarova, N.M.; Safronova, V.I.; Litvinskiy, V.A.; Nosikov, V.V.; Zavalin, A.A.; Tikhonovich, I.A. Microbial Consortium of PGPR, Rhizobia and Arbuscular Mycorrhizal Fungus Makes Pea Mutant SGECd(t) Comparable with Indian Mustard in Cadmium Tolerance and Accumulation. Plants 2020, 9, 975. [Google Scholar] [CrossRef] [PubMed]
- Oleńska, E.; Małek, W.; Sujkowska-Rybkowska, M.; Szopa, S.; Włostowski, T.; Aleksandrowicz, O.; Swiecicka, I.; Wójcik, M.; Thijs, S.; Vangronsveld, J. An Alliance of Trifolium repens-Rhizobium leguminosarum bv. trifolii-Mycorrhizal Fungi From an Old Zn-Pb-Cd Rich Waste Heap as a Promising Tripartite System for Phytostabilization of Metal Polluted Soils. Front. Microbiol. 2022, 13, 853407. [Google Scholar] [CrossRef]
- Suzaki, T.; Takeda, N.; Nishida, H.; Hoshino, M.; Ito, M.; Misawa, F.; Handa, Y.; Miura, K.; Kawaguchi, M. LACK OF SYMBIONT ACCOMMODATION controls intracellular symbiont accommodation in root nodule and arbuscular mycorrhizal symbiosis in Lotus japonicus. PLoS Genet. 2019, 15, e1007865. [Google Scholar] [CrossRef]
- Gherbi, H.; Markmann, K.; Svistoonoff, S.; Estevan, J.; Autran, D.; Giczey, G.; Auguy, F.; Péret, B.; Laplaze, L.; Franche, C.; et al. SymRK defines a common genetic basis for plant root endosymbioses with arbuscular mycorrhiza fungi, rhizobia, and Frankiabacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 4928–4932. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Yoshikawa, M.; Yano, K.; Miwa, H.; Uchida, H.; Asamizu, E.; Sato, S.; Tabata, S.; Imaizumi-Anraku, H.; Umehara, Y.; et al. NUCLEOPORIN85 is required for calcium spiking, fungal and bacterial symbioses, and seed production in Lotus japonicus. Plant Cell 2007, 19, 610–624. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, N.; Madsen, L.H.; Radutoiu, S.; Frantescu, M.; Quistgaard, E.M.; Miwa, H.; Downie, J.A.; James, E.K.; Felle, H.H.; Haaning, L.L.; et al. A nucleoporin is required for induction of Ca2+ spiking in legume nodule development and essential for rhizobial and fungal symbiosis. Proc. Natl. Acad. Sci. USA 2006, 103, 359–364. [Google Scholar] [CrossRef]
- Groth, M.; Takeda, N.; Perry, J.; Uchida, H.; Dräxl, S.; Brachmann, A.; Sato, S.; Tabata, S.; Kawaguchi, M.; Wang, T.L.; et al. NENA, a Lotus japonicus homolog of Sec13, is required for rhizodermal infection by arbuscular mycorrhiza fungi and rhizobia but dispensable for cortical endosymbiotic development. Plant Cell 2010, 22, 2509–2526. [Google Scholar] [CrossRef]
- Kim, S.; Zeng, W.; Bernard, S.; Liao, J.; Venkateshwaran, M.; Ane, J.M.; Jiang, Y. Ca(2+)-regulated Ca(2+) channels with an RCK gating ring control plant symbiotic associations. Nat. Commun. 2019, 10, 3703. [Google Scholar] [CrossRef]
- Oldroyd, G.E. Speak, friend, and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 2013, 11, 252–263. [Google Scholar] [CrossRef]
- Kosuta, S.; Held, M.; Hossain, M.S.; Morieri, G.; Macgillivary, A.; Johansen, C.; Antolín-Llovera, M.; Parniske, M.; Oldroyd, G.E.; Downie, A.J.; et al. Lotus japonicus symRK-14 uncouples the cortical and epidermal symbiotic program. Plant J. Cell Mol. Biol. 2011, 67, 929–940. [Google Scholar] [CrossRef]
- Antolín-Llovera, M.; Petutsching, E.K.; Ried, M.K.; Lipka, V.; Nürnberger, T.; Robatzek, S.; Parniske, M. Knowing your friends and foes--plant receptor-like kinases as initiators of symbiosis or defence. New Phytol. 2014, 204, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.T.; Zhang, L.; He, S.Y. Plant-Microbe Interactions Facing Environmental Challenge. Cell Host Microbe 2019, 26, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Montiel, J.; Reid, D.; Grønbæk, T.H.; Benfeldt, C.M.; James, E.K.; Ott, T.; Ditengou, F.A.; Nadzieja, M.; Kelly, S.; Stougaard, J. Distinct signaling routes mediate intercellular and intracellular rhizobial infection in Lotus japonicus. Plant Physiol. 2021, 185, 1131–1147. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wu, P.; Liu, C.; Peng, L.; Wang, T.; Wang, C.; Tan, Q.; Li, B.; Ou, Y.; Zhu, H.; et al. Suppression of LjBAK1-mediated immunity by SymRK promotes rhizobial infection in Lotus japonicus. Mol. Plant 2021, 14, 1935–1950. [Google Scholar] [CrossRef]
- Holsters, M. SYMRK, an enigmatic receptor guarding and guiding microbial endosymbioses with plant roots. Proc. Natl. Acad. Sci. USA 2008, 105, 4537–4538. [Google Scholar] [CrossRef]
- Vasan, S.; Srivastava, D.; Cahill, D.; Singh, P.P.; Adholeya, A. Important innate differences in determining symbiotic responsiveness in host and non-hosts of arbuscular mycorrhiza. Sci. Rep. 2021, 11, 14444. [Google Scholar] [CrossRef]
- Sánchez-López, R.; Jáuregui, D.; Quinto, C. SymRK and the nodule vascular system: An underground connection. Plant Signal. Behav. 2012, 7, 691–693. [Google Scholar] [CrossRef][Green Version]
- Endre, G.; Kereszt, A.; Kevei, Z.; Mihacea, S.; Kaló, P.; Kiss, G.B. A receptor kinase gene regulating symbiotic nodule development. Nature 2002, 417, 962–966. [Google Scholar] [CrossRef]
- Stracke, S.; Kistner, C.; Yoshida, S.; Mulder, L.; Sato, S.; Kaneko, T.; Tabata, S.; Sandal, N.; Stougaard, J.; Szczyglowski, K.; et al. A plant receptor-like kinase required for both bacterial and fungal symbiosis. Nature 2002, 417, 959–962. [Google Scholar] [CrossRef]
- Liang, P.; Stratil, T.F.; Popp, C.; Marín, M.; Folgmann, J.; Mysore, K.S.; Wen, J.; Ott, T. Symbiotic root infections in Medicago truncatula require remorin-mediated receptor stabilization in membrane nanodomains. Proc. Natl. Acad. Sci. USA 2018, 115, 5289–5294. [Google Scholar] [CrossRef]
- Indrasumunar, A.; Wilde, J.; Hayashi, S.; Li, D.; Gresshoff, P.M. Functional analysis of duplicated Symbiosis Receptor Kinase (SymRK) genes during nodulation and mycorrhizal infection in soybean (Glycine max). J. Plant Physiol. 2015, 176, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Wang, C.; Gao, Q.; Li, L.; Luan, S. Calcium spikes, waves and oscillations in plant development and biotic interactions. Nat. Plants 2020, 6, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, M.; Sun, J.; Vaz Martins, T.; Radhakrishnan, G.V.; Findlay, K.; Soumpourou, E.; Thouin, J.; Véry, A.A.; Sanders, D.; Morris, R.J.; et al. Nuclear-localized cyclic nucleotide-gated channels mediate symbiotic calcium oscillations. Science 2016, 352, 1102–1105. [Google Scholar] [CrossRef] [PubMed]
- Capoen, W.; Sun, J.; Wysham, D.; Otegui, M.S.; Venkateshwaran, M.; Hirsch, S.; Miwa, H.; Downie, J.A.; Morris, R.J.; Ané, J.M.; et al. Nuclear membranes control symbiotic calcium signaling of legumes. Proc. Natl. Acad. Sci. USA 2011, 108, 14348–14353. [Google Scholar] [CrossRef] [PubMed]
- Radutoiu, S.; Madsen, L.H.; Madsen, E.B.; Felle, H.H.; Umehara, Y.; Grønlund, M.; Sato, S.; Nakamura, Y.; Tabata, S.; Sandal, N.; et al. Plant recognition of symbiotic bacteria requires two LysM receptor-like kinases. Nature 2003, 425, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.H.; Hoelz, A. The Structure of the Nuclear Pore Complex (An Update). Annu. Rev. Biochem. 2019, 88, 725–783. [Google Scholar] [CrossRef]
- Shimoda, Y.; Imaizumi-Anraku, H.; Hayashi, M. Kinase activity-dependent stability of calcium/calmodulin-dependent protein kinase of Lotus japonicus. Planta 2019, 250, 1773–1779. [Google Scholar] [CrossRef]
- Bapaume, L.; Reinhardt, D. How membranes shape plant symbioses: Signaling and transport in nodulation and arbuscular mycorrhiza. Front. Plant Sci. 2012, 3, 223. [Google Scholar] [CrossRef]
- Wang, T.; Guo, J.; Peng, Y.; Lyu, X.; Liu, B.; Sun, S.; Wang, X. Light-induced mobile factors from shoots regulate rhizobium-triggered soybean root nodulation. Science 2021, 374, 65–71. [Google Scholar] [CrossRef]
- Yano, K.; Yoshida, S.; Müller, J.; Singh, S.; Banba, M.; Vickers, K.; Markmann, K.; White, C.; Schuller, B.; Sato, S.; et al. CYCLOPS, a mediator of symbiotic intracellular accommodation. Proc. Natl. Acad. Sci. USA 2008, 105, 20540–20545. [Google Scholar] [CrossRef] [PubMed]
- Capoen, W.; Oldroyd, G. How CYCLOPS keeps an eye on plant symbiosis. Proc. Natl. Acad. Sci. USA 2008, 105, 20053–20054. [Google Scholar] [CrossRef] [PubMed]
- Kudla, J.; Becker, D.; Grill, E.; Hedrich, R.; Hippler, M.; Kummer, U.; Parniske, M.; Romeis, T.; Schumacher, K. Advances and current challenges in calcium signaling. New Phytol. 2018, 218, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Manoj, S.R.; Karthik, C.; Kadirvelu, K.; Arulselvi, P.I.; Shanmugasundaram, T.; Bruno, B.; Rajkumar, M. Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: A review. J. Environ. Manag. 2020, 254, 109779. [Google Scholar] [CrossRef] [PubMed]
- Göhre, V.; Paszkowski, U. Contribution of the arbuscular mycorrhizal symbiosis to heavy metal phytoremediation. Planta 2006, 223, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 2015, 756120. [Google Scholar] [CrossRef]
- Huang, D.; Wang, Q.; Zou, Y.; Ma, M.; Jing, G.; Ma, F.; Li, C. Silencing MdGH3-2/12 in apple reduces cadmium resistance via the regulation of AM colonization. Chemosphere 2021, 269, 129407. [Google Scholar] [CrossRef]
- Chang, Q.; Diao, F.W.; Wang, Q.F.; Pan, L.; Dang, Z.H.; Guo, W. Effects of arbuscular mycorrhizal symbiosis on growth, nutrient and metal uptake by maize seedlings (Zea mays L.) grown in soils spiked with Lanthanum and Cadmium. Environ. Pollut. 2018, 241, 607–615. [Google Scholar] [CrossRef]
- Kullu, B.; Patra, D.K.; Acharya, S.; Pradhan, C.; Patra, H.K. AM fungi mediated bioaccumulation of hexavalent chromium in Brachiaria mutica-a mycorrhizal phytoremediation approach. Chemosphere 2020, 258, 127337. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, Y.; Hu, N.; Shi, Y.; Li, T.; Zhao, Z. Differential responses of 23 maize cultivar seedlings to an arbuscular mycorrhizal fungus when grown in a metal-polluted soil. Sci. Total Environ. 2021, 789, 148015. [Google Scholar] [CrossRef]
- Lu, R.R.; Hu, Z.H.; Zhang, Q.L.; Li, Y.Q.; Lin, M.; Wang, X.L.; Wu, X.N.; Yang, J.T.; Zhang, L.Q.; Jing, Y.X.; et al. The effect of Funneliformis mosseae on the plant growth, Cd translocation and accumulation in the new Cd-hyperaccumulator Sphagneticola calendulacea. Ecotoxicol. Environ. Saf. 2020, 203, 110988. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, R.; Favas, P.J.C.; Pratas, J.; Varun, M.; Paul, M.S. Harnessing Pisum sativum-Glomus mosseae symbiosis for phytoremediation of soil contaminated with lead, cadmium, and arsenic. Int. J. Phytoremediation 2021, 23, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ren, W.; Zheng, Y.; Li, Y.; Zhu, M.; Tang, M. Arbuscular Mycorrhizal Fungi Increase Pb Uptake of Colonized and Non-Colonized Medicago truncatula Root and Deliver Extra Pb to Colonized Root Segment. Microorganisms 2021, 9, 1203. [Google Scholar] [CrossRef]
- Chaturvedi, R.; Favas, P.J.C.; Pratas, J.; Varun, M.; Paul, M.S. Effect of Glomus mossae on accumulation efficiency, hazard index and antioxidant defense mechanisms in tomato under metal(loid) Stress. Int. J. Phytoremediation 2018, 20, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, H.; Lou, X.; Tang, M. Mycorrhizal and non-mycorrhizal Medicago truncatula roots exhibit differentially regulated NADPH oxidase and antioxidant response under Pb stress. Environ. Exp. Bot. 2019, 164, 10–19. [Google Scholar] [CrossRef]
- Singh, G.; Pankaj, U.; Chand, S.; Verma, R.K. Arbuscular Mycorrhizal Fungi-Assisted Phytoextraction of Toxic Metals by Zea mays L. From Tannery Sludge. Soil Sediment Contam. Int. J. 2019, 28, 729–746. [Google Scholar] [CrossRef]
- Zhan, F.; Li, B.; Jiang, M.; Li, T.; He, Y.; Li, Y.; Wang, Y. Effects of arbuscular mycorrhizal fungi on the growth and heavy metal accumulation of bermudagrass [Cynodon dactylon (L.) Pers.] grown in a lead–zinc mine wasteland. Int. J. Phytoremediation 2019, 21, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Ai, S.; Chen, K.; Wang, X. Arbuscular mycorrhiza augments cadmium tolerance in soybean by altering accumulation and partitioning of nutrient elements, and related gene expression. Ecotoxicol. Environ. Saf. 2019, 171, 231–239. [Google Scholar] [CrossRef]
- Zhang, J.; Su, L.; Yan, K.; Li, M.; He, Y.; Zu, Y.; Zhan, F.; Li, T. An arbuscular mycorrhizal fungus increased the macroaggregate proportion and reduced cadmium leaching from polluted soil. Int. J. Phytoremediation 2021, 23, 684–692. [Google Scholar] [CrossRef]
- Abdelhameed, R.E.; Metwally, R.A. Alleviation of cadmium stress by arbuscular mycorrhizal symbiosis. Int. J. Phytoremediation 2019, 21, 663–671. [Google Scholar] [CrossRef]
- Baghaie, A.H.; Aghili, F.; Jafarinia, R. Soil-indigenous arbuscular mycorrhizal fungi and zeolite addition to soil synergistically increase grain yield and reduce cadmium uptake of bread wheat (through improved nitrogen and phosphorus nutrition and immobilization of Cd in roots). Environ. Sci. Pollut. Res. Int. 2019, 26, 30794–30807. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.T.; Wang, L.; Zhao, L.; Huang, X.C.; Ma, F. Arbuscular mycorrhizal fungi effect growth and photosynthesis of Phragmites australis (Cav.) Trin ex. Steudel under copper stress. Plant Biol. 2020, 22, 62–69. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Wang, L.; Ju, C.; Wang, G.; Ma, F.; Wang, Y.; Yang, D. Effects of arbuscular mycorrhizal fungi on the growth and toxic element uptake of Phragmites australis (Cav.) Trin. ex Steud under zinc/cadmium stress. Ecotoxicol. Environ. Saf. 2021, 213, 112023. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Han, X.; Liang, Y.; Ghosh, A.; Chen, J.; Tang, M. The Combined Effects of Arbuscular Mycorrhizal Fungi (AMF) and Lead (Pb) Stress on Pb Accumulation, Plant Growth Parameters, Photosynthesis, and Antioxidant Enzymes in Robinia pseudoacacia L. PLoS ONE 2015, 10, e0145726. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Thokchom, S.D.; Kapoor, R. Arbuscular Mycorrhiza Improves Photosynthesis and Restores Alteration in Sugar Metabolism in Triticum aestivum L. Grown in Arsenic Contaminated Soil. Front. Plant Sci. 2021, 12, 640379. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Mohamad, O.A.; Deng, Z.; Liu, X.; Glick, B.R.; Wei, G. Rhizobial symbiosis effect on the growth, metal uptake, and antioxidant responses of Medicago lupulina under copper stress. Environ. Sci. Pollut. Res. Int. 2015, 22, 12479–12489. [Google Scholar] [CrossRef] [PubMed]
- Dhali, S.; Pradhan, M.; Sahoo, R.K.; Mohanty, S.; Pradhan, C. Alleviating Cr(VI) stress in horse gram (Macrotyloma uniflorum Var. Madhu) by native Cr-tolerant nodule endophytes isolated from contaminated site of Sukinda. Environ. Sci. Pollut. Res. Int. 2021, 28, 31717–31730. [Google Scholar] [CrossRef]
- Fan, M.; Liu, Z.; Nan, L.; Wang, E.; Chen, W.; Lin, Y.; Wei, G. Isolation, characterization, and selection of heavy metal-resistant and plant growth-promoting endophytic bacteria from root nodules of Robinia pseudoacacia in a Pb/Zn mining area. Microbiol. Res. 2018, 217, 51–59. [Google Scholar] [CrossRef]
- Abdollahi, S.; Golchin, A.; Shahryari, F. Lead and cadmium-resistant bacterial species isolated from heavy metal-contaminated soils show plant growth-promoting traits. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2020, 23, 625–640. [Google Scholar] [CrossRef]
- Jebara, S.H.; Abdelkerim, S.; Fatnassi, I.C.; Chiboub, M.; Saadani, O.; Jebara, M. Identification of effective Pb resistant bacteria isolated from Lens culinaris growing in lead contaminated soils. J. Basic Microbiol. 2015, 55, 346–353. [Google Scholar] [CrossRef]
- Chiboub, M.; Jebara, S.H.; Saadani, O.; Fatnassi, I.C.; Abdelkerim, S.; Jebara, M. Physiological responses and antioxidant enzyme changes in Sulla coronaria inoculated by cadmium resistant bacteria. J. Plant Res. 2018, 131, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Fatnassi, I.C.; Chiboub, M.; Saadani, O.; Jebara, M.; Jebara, S.H. Impact of dual inoculation with Rhizobium and PGPR on growth and antioxidant status of Vicia faba L. under copper stress. Comptes Rendus Biol. 2015, 338, 241–254. [Google Scholar] [CrossRef]
- Guefrachi, I.; Rejili, M.; Mahdhi, M.; Mars, M. Assessing genotypic diversity and symbiotic efficiency of five rhizobial legume interactions under cadmium stress for soil phytoremediation. Int. J. Phytoremediation 2013, 15, 938–951. [Google Scholar] [CrossRef] [PubMed]
- Oleńska, E.; Małek, W. Genomic polymorphism of Trifolium repens root nodule symbionts from heavy metal-abundant 100-year-old waste heap in southern Poland. Arch. Microbiol. 2019, 201, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Sujkowska-Rybkowska, M.; Ważny, R. Metal resistant rhizobia and ultrastructure of Anthyllis vulneraria nodules from zinc and lead contaminated tailing in Poland. Int. J. Phytoremediation 2018, 20, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Ajeesh Krishna, T.P.; Maharajan, T.; Victor Roch, G.; Ignacimuthu, S.; Antony Ceasar, S. Structure, Function, Regulation and Phylogenetic Relationship of ZIP Family Transporters of Plants. Front. Plant Sci. 2020, 11, 662. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.D.; Huang, S.; Yamaji, N.; Zhang, W.; Ma, J.F.; Zhao, F.J. OsNRAMP1 transporter contributes to cadmium and manganese uptake in rice. Plant Cell Environ. 2020, 43, 2476–2491. [Google Scholar] [CrossRef]
- Kowalkowski, T.; Krakowska, A.; Złoch, M.; Hrynkiewicz, K.; Buszewski, B. Cadmium-affected synthesis of exopolysaccharides by rhizosphere bacteria. J. Appl. Microbiol. 2019, 127, 713–723. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, W.; Xie, X.; Wu, Y.; Liang, F.; Tang, M. Arbuscular mycorrhizal fungi promote lead immobilization by increasing the polysaccharide content within pectin and inducing cell wall peroxidase activity. Chemosphere 2021, 267, 128924. [Google Scholar] [CrossRef]
- Erbs, G.; Silipo, A.; Aslam, S.; De Castro, C.; Liparoti, V.; Flagiello, A.; Pucci, P.; Lanzetta, R.; Parrilli, M.; Molinaro, A.; et al. Peptidoglycan and muropeptides from pathogens Agrobacterium and Xanthomonas elicit plant innate immunity: Structure and activity. Chem. Biol. 2008, 15, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.D.; He, Z.L.; Yang, X.E. Role of soil rhizobacteria in phytoremediation of heavy metal contaminated soils. J. Zhejiang Univ. Sci. B 2007, 8, 192–207. [Google Scholar] [CrossRef]
- Hufton, J.; Harding, J.; Smith, T.; Romero-González, M.E. The importance of the bacterial cell wall in uranium(VI) biosorption. Phys. Chem. Chem. Phys. PCCP 2021, 23, 1566–1576. [Google Scholar] [CrossRef]
- Park, D.; Park, J.M.; Yun, Y.S. Mechanisms of the removal of hexavalent chromium by biomaterials or biomaterial-based activated carbons. J. Hazard. Mater. 2006, 137, 1254–1257. [Google Scholar] [CrossRef] [PubMed]
- Arévalo-Rangel, D.L.; Cárdenas-González, J.F.; Martínez-Juárez, V.M.; Acosta-Rodríguez, I. Hexavalent Chromate Reductase Activity in Cell Free Extracts of Penicillium sp. Bioinorg. Chem. Appl. 2013, 2013, 909412. [Google Scholar] [CrossRef] [PubMed]
- Neilands, J.B. Siderophores. Arch. Biochem. Biophys. 1993, 302, 1–3. [Google Scholar] [CrossRef]
- Miethke, M.; Marahiel, M.A. Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. MMBR 2007, 71, 413–451. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.C.; Garcia-Herrero, A.; Johanson, T.H.; Krewulak, K.D.; Lau, C.K.; Peacock, R.S.; Slavinskaya, Z.; Vogel, H.J. Siderophore uptake in bacteria and the battle for iron with the host; a bird’s eye view. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2010, 23, 601–611. [Google Scholar] [CrossRef]
- Illmer, P.; Buttinger, R. Interactions between iron availability, aluminium toxicity and fungal siderophores. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2006, 19, 367–377. [Google Scholar] [CrossRef]
- Khan, A.; Gupta, A.; Singh, P.; Mishra, A.K.; Ranjan, R.K.; Srivastava, A. Siderophore-assisted cadmium hyperaccumulation in Bacillus subtilis. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2020, 23, 277–286. [Google Scholar] [CrossRef]
- Koh, E.I.; Henderson, J.P. Microbial Copper-binding Siderophores at the Host-Pathogen Interface. J. Biol. Chem. 2015, 290, 18967–18974. [Google Scholar] [CrossRef]
- Braud, A.; Hoegy, F.; Jezequel, K.; Lebeau, T.; Schalk, I.J. New insights into the metal specificity of the Pseudomonas aeruginosa pyoverdine-iron uptake pathway. Environ. Microbiol. 2009, 11, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.; Hernlem, L.M.; Vane, G.D.; Sayles. Stability constants for complexes of the siderophore desferrioxamine B with selected heavy metal cations. Inorg. Chim. Acta 1996, 24, 179–184. [Google Scholar] [CrossRef]
- Wilson, B.R.; Bogdan, A.R.; Miyazawa, M.; Hashimoto, K.; Tsuji, Y. Siderophores in Iron Metabolism: From Mechanism to Therapy Potential. Trends Mol. Med. 2016, 22, 1077–1090. [Google Scholar] [CrossRef] [PubMed]
- Jaskula, J.C.; Letain, T.E.; Roof, S.K.; Skare, J.T.; Postle, K. Role of the TonB amino terminus in energy transduction between membranes. J. Bacteriol. 1994, 176, 2326–2338. [Google Scholar] [CrossRef] [PubMed]
- Postle, K. TonB protein and energy transduction between membranes. J. Bioenerg. Biomembr. 1993, 25, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Braun, V. Energy-coupled transport and signal transduction through the gram-negative outer membrane via TonB-ExbB-ExbD-dependent receptor proteins. FEMS Microbiol. Rev. 1995, 16, 295–307. [Google Scholar] [CrossRef]
- Ren, X.M.; Guo, S.J.; Tian, W.; Chen, Y.; Han, H.; Chen, E.; Li, B.L.; Li, Y.Y.; Chen, Z.J. Effects of Plant Growth-Promoting Bacteria (PGPB) Inoculation on the Growth, Antioxidant Activity, Cu Uptake, and Bacterial Community Structure of Rape (Brassica napus L.) Grown in Cu-Contaminated Agricultural Soil. Front. Microbiol. 2019, 10, 1455. [Google Scholar] [CrossRef]
- Sepehri, M.; Khatabi, B. Combination of Siderophore-Producing Bacteria and Piriformospora indica Provides an Efficient Approach to Improve Cadmium Tolerance in Alfalfa. Microb. Ecol. 2021, 81, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Winkelmann, G. A search for glomuferrin: A potential siderophore of arbuscular mycorrhizal fungi of the genus Glomus. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2017, 30, 559–564. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. Relevance of microbial extracellular polymeric substances (EPSs)--Part I: Structural and ecological aspects. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2001, 43, 1–8. [Google Scholar] [CrossRef]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.S.; Silva, I.N.; Oliveira, V.H.; Cunha, R.; Moreira, L.M. Insights into the role of extracellular polysaccharides in Burkholderia adaptation to different environments. Front. Cell. Infect. Microbiol. 2011, 1, 16. [Google Scholar] [CrossRef] [PubMed]
- Lian, Z.; Yang, Z.; Song, W.; Sun, M.; Gan, Y.; Bai, X. Effects of different exogenous cadmium compounds on the chemical composition and adsorption properties of two gram-negative bacterial EPS. Sci. Total Environ. 2022, 806, 150511. [Google Scholar] [CrossRef] [PubMed]
- Kopycińska, M.; Lipa, P.; Cieśla, J.; Kozieł, M.; Janczarek, M. Extracellular polysaccharide protects Rhizobium leguminosarum cells against zinc stress in vitro and during symbiosis with clover. Environ. Microbiol. Rep. 2018, 10, 355–368. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, X.; Sun, Y.; Wu, Z.; Li, T.; Hu, Y.; Lv, J.; Li, G.; Zhang, Z.; Zhang, J.; et al. Chromium immobilization by extra- and intraradical fungal structures of arbuscular mycorrhizal symbioses. J. Hazard. Mater. 2016, 316, 34–42. [Google Scholar] [CrossRef]
- Pandit, A.; Adholeya, A.; Cahill, D.; Brau, L.; Kochar, M. Microbial biofilms in nature: Unlocking their potential for agricultural applications. J. Appl. Microbiol. 2020, 129, 199–211. [Google Scholar] [CrossRef]
- Frey-Klett, P.; Burlinson, P.; Deveau, A.; Barret, M.; Tarkka, M.; Sarniguet, A. Bacterial-fungal interactions: Hyphens between agricultural, clinical, environmental, and food microbiologists. Microbiol. Mol. Biol. Rev. MMBR 2011, 75, 583–609. [Google Scholar] [CrossRef] [PubMed]
- Osińska-Jaroszuk, M.; Jaszek, M.; Starosielec, M.; Sulej, J.; Matuszewska, A.; Janczarek, M.; Bancerz, R.; Wydrych, J.; Wiater, A.; Jarosz-Wilkołazka, A. Bacterial exopolysaccharides as a modern biotechnological tool for modification of fungal laccase properties and metal ion binding. Bioprocess Biosyst. Eng. 2018, 41, 973–989. [Google Scholar] [CrossRef]
- Armendariz, A.L.; Talano, M.A.; Wevar Oller, A.L.; Medina, M.I.; Agostini, E. Effect of arsenic on tolerance mechanisms of two plant growth-promoting bacteria used as biological inoculants. J. Environ. Sci. 2015, 33, 203–210. [Google Scholar] [CrossRef]
- Nocelli, N.; Bogino, P.C.; Banchio, E.; Giordano, W. Roles of Extracellular Polysaccharides and Biofilm Formation in Heavy Metal Resistance of Rhizobia. Materials 2016, 9, 418. [Google Scholar] [CrossRef]
- More, T.T.; Yadav, J.S.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Extracellular polymeric substances of bacteria and their potential environmental applications. J. Environ. Manag. 2014, 144, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Sen, I.K.; Kati, A.; Some, S.; Mandal, A.K.; Islam, S.S.; Bhattacharyya, R.; Mukhopadhyay, A. Flocculating, emulsification and metal sorption properties of a partial characterized novel exopolysaccharide produced by Rhizobium tropici SRA1 isolated from Psophocarpus tetragonolobus (L) D.C. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2019, 22, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Wang, Q.; Li, A.; Yang, J.; Ma, F.; Pi, S.; Wu, D. Biosorption of Pb (II) from aqueous solution by extracellular polymeric substances extracted from Klebsiella sp. J1: Adsorption behavior and mechanism assessment. Sci. Rep. 2016, 6, 31575. [Google Scholar] [CrossRef]
- Avelar Ferreira, P.A.; Bomfeti, C.A.; Lima Soares, B.; de Souza Moreira, F.M. Efficient nitrogen-fixing Rhizobium strains isolated from amazonian soils are highly tolerant to acidity and aluminium. World J. Microbiol. Biotechnol. 2012, 28, 1947–1959. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Nayuki, K.; Kuga, Y.; Zhang, X.; Wu, S.; Ohtomo, R. Uptake and Intraradical Immobilization of Cadmium by Arbuscular Mycorrhizal Fungi as Revealed by a Stable Isotope Tracer and Synchrotron Radiation μX-Ray Fluorescence Analysis. Microbes Environ. 2018, 33, 257–263. [Google Scholar] [CrossRef]
- Acosta-Jurado, S.; Fuentes-Romero, F.; Ruiz-Sainz, J.E.; Janczarek, M.; Vinardell, J.M. Rhizobial Exopolysaccharides: Genetic Regulation of Their Synthesis and Relevance in Symbiosis with Legumes. Int. J. Mol. Sci. 2021, 22, 6233. [Google Scholar] [CrossRef]
- Barnett, M.J.; Long, S.R. The Sinorhizobium meliloti SyrM regulon: Effects on global gene expression are mediated by syrA and nodD3. J. Bacteriol. 2015, 197, 1792–1806. [Google Scholar] [CrossRef]
- Janczarek, M.; Skorupska, A. Rhizobium leguminosarum bv. trifolii rosR gene expression is regulated by catabolic repression. FEMS Microbiol. Lett. 2009, 291, 112–119. [Google Scholar] [CrossRef]
- Glazebrook, J.; Reed, J.W.; Reuber, T.L.; Walker, G.C. Genetic analyses of Rhizobium meliloti exopolysaccharides. Int. J. Biol. Macromol. 1990, 12, 67–70. [Google Scholar] [CrossRef]
- Reuber, T.L.; Reed, J.; Glazebrook, J.; Glucksmann, M.A.; Ahmann, D.; Marra, A.; Walker, G.C. Rhizobium meliloti exopolysaccharides: Genetic analyses and symbiotic importance. Biochem. Soc. Trans. 1991, 19, 636–641. [Google Scholar] [CrossRef][Green Version]
- Li, C.; Yu, Y.; Fang, A.; Feng, D.; Du, M.; Tang, A.; Chen, S.; Li, A. Insight into biosorption of heavy metals by extracellular polymer substances and the improvement of the efficacy: A review. Lett. Appl. Microbiol. 2021, 75, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Liu, Z.; Zhao, Z.; Wang, Z.; Hu, X.; Jiang, Y.; Yan, J.; Li, Z.; Zheng, Z.; Zhan, X. Exopolysaccharide synthesis repressor genes (exoR and exoX) related to curdlan biosynthesis by Agrobacterium sp. Int. J. Biol. Macromol. 2022, 205, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Janczarek, M.; Skorupska, A. The Rhizobium leguminosarum bv. trifolii RosR: Transcriptional regulator involved in exopolysaccharide production. Mol. Plant-Microbe Interact. MPMI 2007, 20, 867–881. [Google Scholar] [CrossRef] [PubMed]
- Ziller, A.; Fraissinet-Tachet, L. Metallothionein diversity and distribution in the tree of life: A multifunctional protein. Met. Integr. Biometal Sci. 2018, 10, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.L.; Singh, S.; Chen, W. Arsenic metabolism by microbes in nature and the impact on arsenic remediation. Curr. Opin. Biotechnol. 2009, 20, 659–667. [Google Scholar] [CrossRef]
- Tsyganov, V.E.; Tsyganova, A.V.; Gorshkov, A.P.; Seliverstova, E.V.; Kim, V.E.; Chizhevskaya, E.P.; Belimov, A.A.; Serova, T.A.; Ivanova, K.A.; Kulaeva, O.A.; et al. Efficacy of a Plant-Microbe System: Pisum sativum (L.) Cadmium-Tolerant Mutant and Rhizobium leguminosarum Strains, Expressing Pea Metallothionein Genes PsMT1 and PsMT2, for Cadmium Phytoremediation. Front. Microbiol. 2020, 11, 15. [Google Scholar] [CrossRef]
- Pérez-Palacios, P.; Romero-Aguilar, A.; Delgadillo, J.; Doukkali, B.; Caviedes, M.A.; Rodríguez-Llorente, I.D.; Pajuelo, E. Double genetically modified symbiotic system for improved Cu phytostabilization in legume roots. Environ. Sci. Pollut. Res. Int. 2017, 24, 14910–14923. [Google Scholar] [CrossRef]
- Lanfranco, L.; Bolchi, A.; Ros, E.C.; Ottonello, S.; Bonfante, P. Differential expression of a metallothionein gene during the presymbiotic versus the symbiotic phase of an arbuscular mycorrhizal fungus. Plant Physiol. 2002, 130, 58–67. [Google Scholar] [CrossRef]
- Chen, B.; Xiao, X.; Zhu, Y.G.; Smith, F.A.; Xie, Z.M.; Smith, S.E. The arbuscular mycorrhizal fungus Glomus mosseae gives contradictory effects on phosphorus and arsenic acquisition by Medicago sativa Linn. Sci. Total Environ. 2007, 379, 226–234. [Google Scholar] [CrossRef]
- Li, J.; Sun, Y.; Jiang, X.; Chen, B.; Zhang, X. Arbuscular mycorrhizal fungi alleviate arsenic toxicity to Medicago sativa by influencing arsenic speciation and partitioning. Ecotoxicol. Environ. Saf. 2018, 157, 235–243. [Google Scholar] [CrossRef]
- Hall, J.L. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef]
- Bruins, M.R.; Kapil, S.; Oehme, F.W. Microbial resistance to metals in the environment. Ecotoxicol. Environ. Saf. 2000, 45, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Corticeiro, S.; Freitas, R.; Figueira, E. The role of GSTs in the tolerance of Rhizobium leguminosarum to cadmium. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2013, 26, 879–886. [Google Scholar] [CrossRef]
- Adamis, P.D.; Gomes, D.S.; Pinto, M.L.; Panek, A.D.; Eleutherio, E.C. The role of glutathione transferases in cadmium stress. Toxicol. Lett. 2004, 154, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Dixon, D.P.; Davis, B.G.; Edwards, R. Functional divergence in the glutathione transferase superfamily in plants. Identification of two classes with putative functions in redox homeostasis in Arabidopsis thaliana. J. Biol. Chem. 2002, 277, 30859–30869. [Google Scholar] [CrossRef]
- Bianucci, E.; Furlan, A.; Castro, S. Importance of Glutathione in the Legume-Rhizobia Symbiosis. In Glutathione in Plant Growth, Development, and Stress Tolerance; Springer: Cham, Switzerland, 2017; pp. 373–396. [Google Scholar] [CrossRef]
- Pal, R.; Rai, J.P. Phytochelatins: Peptides involved in heavy metal detoxification. Appl. Biochem. Biotechnol. 2010, 160, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Hu, Z.H.; Yan, T.X.; Lu, R.R.; Peng, C.L.; Li, S.S.; Jing, Y.X. Arbuscular mycorrhizal fungi alleviate Cd phytotoxicity by altering Cd subcellular distribution and chemical forms in Zea mays. Ecotoxicol. Environ. Saf. 2019, 171, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Most, P.; Papenbrock, J. Possible roles of plant sulfurtransferases in detoxification of cyanide, reactive oxygen species, selected heavy metals and arsenate. Molecules 2015, 20, 1410–1423. [Google Scholar] [CrossRef]
- Sharma, M.P.; Grover, M.; Chourasiya, D.; Bharti, A.; Agnihotri, R.; Maheshwari, H.S.; Pareek, A.; Buyer, J.S.; Sharma, S.K.; Schütz, L.; et al. Deciphering the Role of Trehalose in Tripartite Symbiosis Among Rhizobia, Arbuscular Mycorrhizal Fungi, and Legumes for Enhancing Abiotic Stress Tolerance in Crop Plants. Front. Microbiol. 2020, 11, 509919. [Google Scholar] [CrossRef]
- Liu, A.; Ku, Y.S.; Contador, C.A.; Lam, H.M. The Impacts of Domestication and Agricultural Practices on Legume Nutrient Acquisition Through Symbiosis With Rhizobia and Arbuscular Mycorrhizal Fungi. Front. Genet. 2020, 11, 583954. [Google Scholar] [CrossRef]
- Larimer, A.L.; Clay, K.; Bever, J.D. Synergism and context dependency of interactions between arbuscular mycorrhizal fungi and rhizobia with a prairie legume. Ecology 2014, 95, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.A.L.; Abreu, C.A.; de Abreu, M.F.; Silveira, A.P.D. Influence of lead additions on arbuscular mycorrhiza and Rhizobium symbioses under soybean plants. Appl. Soil Ecol. 2004, 26, 123–131. [Google Scholar] [CrossRef]
- Gupta Sood, S. Chemotactic response of plant-growth-promoting bacteria towards roots of vesicular-arbuscular mycorrhizal tomato plants. FEMS Microbiol. Ecol. 2003, 45, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Denison, R.F.; Kiers, E.T. Life histories of symbiotic rhizobia and mycorrhizal fungi. Curr. Biol. CB 2011, 21, R775–R785. [Google Scholar] [CrossRef]
- Wang, X.; Fang, L.; Beiyuan, J.; Cui, Y.; Peng, Q.; Zhu, S.; Wang, M.; Zhang, X. Improvement of alfalfa resistance against Cd stress through rhizobia and arbuscular mycorrhiza fungi co-inoculation in Cd-contaminated soil. Environ. Pollut. 2021, 277, 116758. [Google Scholar] [CrossRef]
- Moreira, H.; Pereira, S.I.; Marques, A.P.; Rangel, A.O.; Castro, P.M. Mine land valorization through energy maize production enhanced by the application of plant growth-promoting rhizobacteria and arbuscular mycorrhizal fungi. Environ. Sci. Pollut. Res. Int. 2016, 23, 6940–6950. [Google Scholar] [CrossRef]
- Ren, C.G.; Kong, C.C.; Bian, B.; Liu, W.; Li, Y.; Luo, Y.M.; Xie, Z.H. Enhanced phytoremediation of soils contaminated with PAHs by arbuscular mycorrhiza and rhizobium. Int. J. Phytoremediation 2017, 19, 789–797. [Google Scholar] [CrossRef]
- Jian, L.; Bai, X.; Zhang, H.; Song, X.; Li, Z. Promotion of growth and metal accumulation of alfalfa by coinoculation with Sinorhizobium and Agrobacterium under copper and zinc stress. PeerJ 2019, 7, e6875. [Google Scholar] [CrossRef]
- Mashiguchi, K.; Seto, Y.; Yamaguchi, S. Strigolactone biosynthesis, transport and perception. Plant J. Cell Mol. Biol. 2021, 105, 335–350. [Google Scholar] [CrossRef]
- Hellsberg, E.; Montanari, F.; Ecker, G.F. The ABC of Phytohormone Translocation. Planta Med. 2015, 81, 474–487. [Google Scholar] [CrossRef]
- Borghi, L.; Liu, G.W.; Emonet, A.; Kretzschmar, T.; Martinoia, E. The importance of strigolactone transport regulation for symbiotic signaling and shoot branching. Planta 2016, 243, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Broughton, W.J.; Perret, X. Genealogy of legume-Rhizobium symbioses. Curr. Opin. Plant Biol. 1999, 2, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Murakami, E.; Cheng, J.; Gysel, K.; Bozsoki, Z.; Kawaharada, Y.; Hjuler, C.T.; Sørensen, K.K.; Tao, K.; Kelly, S.; Venice, F.; et al. Epidermal LysM receptor ensures robust symbiotic signalling in Lotus japonicus. eLife 2018, 7, e33506. [Google Scholar] [CrossRef] [PubMed]
- Catford, J.G.; Staehelin, C.; Lerat, S.; Piché, Y.; Vierheilig, H. Suppression of arbuscular mycorrhizal colonization and nodulation in split-root systems of alfalfa after pre-inoculation and treatment with Nod factors. J. Exp. Bot. 2003, 54, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Rival, P.; Bono, J.J.; Gough, C.; Bensmihen, S.; Rosenberg, C. Cell autonomous and non-cell autonomous control of rhizobial and mycorrhizal infection in Medicago truncatula. Plant Signal. Behav. 2013, 8, e22999. [Google Scholar] [CrossRef]
- Liang, Y.; Tóth, K.; Cao, Y.; Tanaka, K.; Espinoza, C.; Stacey, G. Lipochitooligosaccharide recognition: An ancient story. New Phytol. 2014, 204, 289–296. [Google Scholar] [CrossRef]
- Richard, W.; Zobel, S.F.; Wright. Communication in the Tripartite Symbiosis Formed by Arbuscular Mycorrhizal Fungi, Rhizobia and Legume Plants: A Review. Roots Soil Manag. 2005, 48, 199–222. [Google Scholar] [CrossRef]
- Hogekamp, C.; Arndt, D.; Pereira, P.A.; Becker, J.D.; Hohnjec, N.; Küster, H. Laser microdissection unravels cell-type-specific transcription in arbuscular mycorrhizal roots, including CAAT-box transcription factor gene expression correlating with fungal contact and spread. Plant Physiol. 2011, 157, 2023–2043. [Google Scholar] [CrossRef]
- Banasiak, J.; Jamruszka, T.; Murray, J.D.; Jasiński, M. A roadmap of plant membrane transporters in arbuscular mycorrhizal and legume-rhizobium symbioses. Plant Physiol. 2021, 187, 2071–2091. [Google Scholar] [CrossRef]
- Gavrin, A.; Loughlin, P.C.; Brear, E.; Griffith, O.W.; Bedon, F.; Suter Grotemeyer, M.; Escudero, V.; Reguera, M.; Qu, Y.; Mohd-Noor, S.N.; et al. Soybean Yellow Stripe-like 7 is a symbiosome membrane peptide transporter important for nitrogen fixation. Plant Physiol. 2021, 186, 581–598. [Google Scholar] [CrossRef]
- Krusell, L.; Krause, K.; Ott, T.; Desbrosses, G.; Krämer, U.; Sato, S.; Nakamura, Y.; Tabata, S.; James, E.K.; Sandal, N.; et al. The sulfate transporter SST1 is crucial for symbiotic nitrogen fixation in Lotus japonicus root nodules. Plant Cell 2005, 17, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Senovilla, M.; Castro-Rodríguez, R.; Abreu, I.; Escudero, V.; Kryvoruchko, I.; Udvardi, M.K.; Imperial, J.; González-Guerrero, M. Medicago truncatula copper transporter 1 (MtCOPT1) delivers copper for symbiotic nitrogen fixation. New Phytol. 2018, 218, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Brear, E.M.; Day, D.A.; Smith, P.M. Iron: An essential micronutrient for the legume-rhizobium symbiosis. Front. Plant Sci. 2013, 4, 359. [Google Scholar] [CrossRef] [PubMed]
- Römheld, V. Different strategies for iron acquisition in higher plants. Physiol. Plant. 1987, 70, 231–234. [Google Scholar] [CrossRef]
- Kaiser, B.N.; Moreau, S.; Castelli, J.; Thomson, R.; Lambert, A.; Bogliolo, S.; Puppo, A.; Day, D.A. The soybean NRAMP homologue, GmDMT1, is a symbiotic divalent metal transporter capable of ferrous iron transport. Plant J. Cell Mol. Biol. 2003, 35, 295–304. [Google Scholar] [CrossRef]
- Kryvoruchko, I.S.; Routray, P.; Sinharoy, S.; Torres-Jerez, I.; Tejada-Jiménez, M.; Finney, L.A.; Nakashima, J.; Pislariu, C.I.; Benedito, V.A.; González-Guerrero, M.; et al. An Iron-Activated Citrate Transporter, MtMATE67, Is Required for Symbiotic Nitrogen Fixation. Plant Physiol. 2018, 176, 2315–2329. [Google Scholar] [CrossRef]
- González-Guerrero, M.; Azcón-Aguilar, C.; Mooney, M.; Valderas, A.; MacDiarmid, C.W.; Eide, D.J.; Ferrol, N. Characterization of a Glomus intraradices gene encoding a putative Zn transporter of the cation diffusion facilitator family. Fungal Genet. Biol. 2005, 42, 130–140. [Google Scholar] [CrossRef]
- González-Guerrero, M.; Benabdellah, K.; Valderas, A.; Azcón-Aguilar, C.; Ferrol, N. GintABC1 encodes a putative ABC transporter of the MRP subfamily induced by Cu, Cd, and oxidative stress in Glomus intraradices. Mycorrhiza 2010, 20, 137–146. [Google Scholar] [CrossRef]






| Types | Host Latin Name | Repaired Heavy Metals | The Mechanism of Repairing HMs | Citation |
|---|---|---|---|---|
| Types of Mycorrhizal | ||||
| Rhizophagus irregularis | Malus pumila | Cd | Changes in AMF colonization rate led to up-regulation of MdGH3-2 and MdGH3-12 genes. | [87] |
| Claroideoglomus etunicatum | Zea mays and Trifolium repens | La, Cd | AMF mycelium binds to HMs, chelates substances to fix HMs, mediates antioxidant activity, and regulates the distribution of HMs in host plants. | [88] |
| Claroideoglomus etunicatum | Zea mays L. | La | AMF changes the structure of the rhizosphere microbiome through root signals to enhance the tolerance of corn to La. | [26] |
| Rhizophagus irregularis | Brachiaria eruciformis (J. E. Smith) Griseb. | Cr | AMF protects plants from oxidative stress induced by hexavalent chromium by changing the metabolic processes of plants. | [89] |
| Funneliformis mosseae | Zea mays | Pb, Zn, Cd | Phyto-stabilization | [90] |
| Funneliformis mosseae | Sphagneticola calendulacea | Cd | AMF symbiosis enhances the transport of Cd to the cell wall and the conversion of Cd to a less toxic chemical form, reducing the phytotoxicity of Cd. | [91] |
| Glomus mosseae | Pisum sativum | Pb, Cd | AMF increases plant’s enzymatic and non-enzymatic defense strategies. | [92] |
| Rhizophagus irregularis | Medicago sativa | Pb | Ri transfer Pb to the planted root segment and be isolated and isolated in the root. | [93] |
| Glomus mosseae | Lycopersicon esculentum | Cd, Pb | AMF symbiosis for better growth, chlorophyll synthesis, and stronger osmotic regulation and antioxidant defense mechanisms. | [94] |
| Rhizophagus irregularis | Medicago sativa | Pb | AMF inoculation reduces the content of water-soluble Pb complexes. | [95] |
| Rhizophagus fasciculatus;Glomus agregatum | Zea mays | Cd, Cr, Ni, Pb | Plant extracts. | [96] |
| Diversispora spurcum | Cynodon dactylon | Pb, Zn, Cd | AMF increases nutrient absorption to reduce the translocation of HMs to buds to improve the growth of bermudagrass. | [97] |
| Rhizophagus irregularis | Glycine max | Cd | AMF symbiosis reduces the toxicity of Cd in soybeans by enhancing P nutrition and up-regulating the expression of AMF-inducible GmPTs and GmHMA19. | [98] |
| Funneliformis mosseae | Zea mays | Cd | AMF increases the proportion of large aggregates and reduces the leaching of Cd in the soil. | [99] |
| Glomus clarum;Glomus monosporum; Gigaspora nigra | Trigonella foenum-graecum | Cd | Phyto-stabilization. | [100] |
| Rhizophagus irregularis; Claroideoglomus claroideum | Medicago sativa | Cd | Reduce the absorption of cadmium by Medicago sativa growth in cadmium contaminated soil. | [101] |
| Rhizophagus irregularis | Phragmites australis | Cu | AMF can promote the growth and photosynthesis of P. australis under copper stress. | [102] |
| Rhizophagus irregularis | Phragmites australis | Zn, Cd | AMF uses the activity of antioxidant enzymes to enhance the tolerance of P. australis and the ability to enrich Zn and Cd | [103] |
| Rhizophagus intraradices; Funneliformis mosseae | Robinia pseudoacacia | Pb | AMF reduces the lead concentration in host leaves, accumulates biomass, and increases photosynthetic pigment content. | [104] |
| Rhizophagus intraradices | Triticum aestivum | As | AMF symbiosis increased the concentration of photosynthetic pigments, enhances Hill reaction activity, regulates the activity of various enzymes in leaves, and restores As-mediated changes in sugar metabolism. | [105] |
| Types of Rhizobium | ||||
| Medicago lupulina | Sinorhizobium meliloti | Cu | The total amount of Cu uptake by shoots and roots of inoculated plants was significantly increased, and the increase in roots was much higher than that in shoots, which reduced the translocation factor and contributed to the stability of Cu plants. | [106] |
| Rhizobium pusense | Macrotyloma uniflorum | Cr | Plants inoculated with R. pusense showed tolerance to Cr (VI), a large accumulation of Cr (VI) in roots, and a decreasing trend of reactive oxygen species and antioxidant enzymes. | [107] |
| Sinorhizobium meliloti | Medicago sativa | Cu | Rhizobium inoculation enhanced copper tolerance by affecting copper uptake, modulating antioxidant enzyme activity and ascorbic acid-glutathione cycle, and affecting the expression of PC biosynthesis-related genes in rice. | [19] |
| Mesorhizobium loti | Robinia pseudoacacia | Pb/Zn/Cd/Cu | Inoculation with M. loti significantly increased shoot biomass, and TMs were mainly accumulated in roots through plant extraction and plant stabilization. | [108] |
| Rhizobium pusense | Brassica oleracea | Pb/Cd | The concentrations of Pb and Cd in B. oleracea decreased significantly after inoculation, and the biomass of the plants also increased significantly. | [109] |
| Rhizobium sp | Lens culinaris | Pb | Inoculated plant roots accumulated more Pb than shoots and decreased Pb uptake by plants, suggesting that this symbiotic relationship should be investigated for plant stabilization of lead-contaminated soils. | [110] |
| Rhizobium sullae | Sulla coronaria | Cd | Symbiosis of host plants with Rhizobium increases plant biomass and increases Cd uptake, especially in roots. | [111] |
| Rhizobium leguminosarum | Vicia faba | Cu | V. faba inoculation with Rhizobium can help relieve copper stress and plant stability under hydroponic conditions. | [112] |
| Sinorhizobium | Lens culinaris | Cd | L. culinaris plant-Rhizobium interactions were the most tolerant to Cd. | [113] |
| Rhizobium leguminosarum | Trifolium repens | Zn/Pb/Cd | TMs have specific selective properties for genotypes of Rhizobium, conferring tolerance to TMs in plants. | [114] |
| Rhizobium metallidurans | Anthyllis vulneraria | Zn/Pb | The thickening of the cell wall and the synthesis of phenolic substances in the vacuole resist the oppression of the plant by the metal. | [115] |
| EPS Producer | Adsorbed Metal | Remark | References |
|---|---|---|---|
| Rhizobium and AMF | |||
| Bradyrhizobium japonicum USDA110 | Mg (II), Fe (III) | Mg (II) and Fe (III) bind to positively charged EPS or react with hydroxyl groups. | [148] |
| Bradyrhizobium japonicum E109 | As (III) | Under As (III) treatment, EPS content was significantly increased, which induced biofilm formation. | [149] |
| Sinorhizobium meliloti | Hg (II) and As (III) | EPS type II exoY strains pump As (III) from plant cells to the extracellular, thereby increasing As (III) metal resistance. | [150] |
| Rhizobium leguminosarum bv. trifolii | Zn (II) | Zn (II) stimulates the production of EPS and biofilms, and EPS promotes the sequestration of extracellular metals, limiting the influx of metals. | [144] |
| Pseudomonas aeruginosa | Pb (II) | EPS utilizes an ion exchange mechanism to displace intracellular Pb (II) to extracellular. | [151] |
| Rhizobium metallidurans | Cd (II) | Cd causes a conformational transition of EPS produced by R. metallidurans from larger spherical particles to smaller planar particles, thereby reducing damage to plant cells. | [118] |
| Rhizobium tropici SRA1 | Zn/Hg/Mn/Mg/Co | FT-IR analysis of EPS indicated that the carboxyl group of uronic acid may be the binding site for divalent cations, contributing to the bridging between EPS and cations. | [152] |
| Klebsiella sp. J1 | Pb (II) | Carboxylic acids, uronic acids, and esters in EPS combine with Pb (II) through ion exchange and complexation. | [153] |
| Rhizobium tropici CIAT899T | Al (III) | The higher the EPS yield, the higher the tolerance to Al (III). | [154] |
| Rhizophagus irregularis DAOM 197198 | Cr (VI) | Under Cr (VI) stress, a large of EPS was generated on the surface of AMF to adsorb Cr (VI), Cr (VI) was reduced to Cr (III) in the cell wall, and Cr (III)-phosphate analogs were formed on the surface of AMF. | [145] |
| Rhizophagus irregularis | Cd (II) | After extra-root hyphae uptake and Cd transfer into root hyphae, Cd was mainly retained in the AMF structure and was not transmitted to plant cells. | [155] |
| Host Species | Type of Vaccination | Pollutants | Main Findings | Citations |
|---|---|---|---|---|
| Medicago sativa | Sinorhizobium meliloti, Glomus mosseae | Cd | Enhanced resistance of M. sativa to Cd stress after co-inoculation by increasing antioxidant enzyme activity and reducing plant photosynthetically-derived C partitioning into soil. | [186] |
| Sesbania rostrata | Glomus etunicatum, Azorhizobium caulinodans | Ur | Phytochelatase synthase (PCS) gene expression and organic acid content increased after co-inoculation of AMF with Rhizobium, which improved the phytoremediation efficiency of Ur. | [49] |
| Anadenanthera peregrina | Acaulospora scrobiculata, Rhizobium BH-ICB-A8 | As | Co-inoculation of Rhizobium and AMF increased the growth and As-phytoremediation capacity of A. peregrina. | [29] |
| Zea mays | Pseudomonas reactans EDP28, Rhizophagusregulations | Cd/Zn | When co-inoculation increased root and stem biomass and stem elongation, Cd and Zn accumulation in maize tissues decreased. | [187] |
| Glycine max | Glomus macrocarpum, Bradyrhizobium | Pb | AMF enhances the uptake of Pb, which interferes with the establishment of a dual symbiotic relationship between AMF and Rhizobium and interferes with the survival of fungi in the soil. | [183] |
| Sesbania cannabina | Glomus mosseae, Ensifer mexicanus | PAHs | The triple symbiosis stimulates microbial development and soil enzyme activity to promote PAHs degradation. | [188] |
| Medicago lupulina | Sinorhizobium meliloti, Agrobacterium tumefaciens | Cu/Zn | Co-inoculation increased the growth and antioxidant activity of plants under Cu/Zn stress and thus enhanced the extraction capacity of metal plants. | [189] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; He, G.; He, T.; Saleem, M. Signaling and Detoxification Strategies in Plant-Microbes Symbiosis under Heavy Metal Stress: A Mechanistic Understanding. Microorganisms 2023, 11, 69. https://doi.org/10.3390/microorganisms11010069
Liu Y, He G, He T, Saleem M. Signaling and Detoxification Strategies in Plant-Microbes Symbiosis under Heavy Metal Stress: A Mechanistic Understanding. Microorganisms. 2023; 11(1):69. https://doi.org/10.3390/microorganisms11010069
Chicago/Turabian StyleLiu, Yao, Guandi He, Tengbing He, and Muhammad Saleem. 2023. "Signaling and Detoxification Strategies in Plant-Microbes Symbiosis under Heavy Metal Stress: A Mechanistic Understanding" Microorganisms 11, no. 1: 69. https://doi.org/10.3390/microorganisms11010069
APA StyleLiu, Y., He, G., He, T., & Saleem, M. (2023). Signaling and Detoxification Strategies in Plant-Microbes Symbiosis under Heavy Metal Stress: A Mechanistic Understanding. Microorganisms, 11(1), 69. https://doi.org/10.3390/microorganisms11010069






